Abstract
Vascularized fibular grafting has been used for treatment of osteonecrosis of the femoral head and although some reports demonstrate successful short- to mid-term outcomes, long-term results are still unknown. We retrospectively reviewed 135 patients (151 hips) who underwent vascularized fibular grafting for osteonecrosis of the femoral head. One-hundred and ten patients (124 hips) were followed for a minimum 10 years (mean, 13.9 years; range, 10–23.7 years). The mean Harris hip score improved from 72 to 88. At the latest followup, we found improved or unchanged radiographs in 37 of 59 hips initially Stage II hips and 39 of 65 Stage III hips. Thirteen hips (13 patients) (10.5%) failed treatment and underwent total hip arthroplasty. The location and size of the necrotic lesion and the patient’s age influenced long-term survival of the graft. Postoperative complications included clawing of the big toe in 17 patients, partial peroneal nerve palsy in two, and superficial infection in two. Subtrochanteric fracture occurred in two hips. The data suggest free vascularized fibular grafting was successful in maintaining joint function and delaying the need for joint replacement procedure. Graft survival was associated with the patient’s age and size and location of the lesion but not etiology and stages of the disease.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Osteonecrosis of the femoral head (ONFH) typically affects younger patients [23, 43]. Treatment options include joint preserving procedures such as electrical stimulation, drilling, core decompression, vascularized or non-vascularized fibular grafting, and osteotomy; and joint replacement procedures such as resurfacing and hemi- or total hip arthroplasty (THA) [1, 2, 3, 11, 18, 28, 32, 34–36, 38, 41–44]. Early diagnosis and appropriate surgery may reduce the risk of progression and improve the outcome [6, 12, 18, 19, 21, 24, 29]. Among the joint-preserving surgical procedures, free vascularized fibular grafting (VFG) reportedly has a survival of 61%–96% at mid-term (4–7 years) followup [4, 16, 17, 20, 28, 31, 43, 44]. Although VFG appears successful in the short- to mid-term [4, 16, 17, 19, 20, 28, 31, 43–45], its long-term benefits are not known. While most authors report their results in relation to preoperative etiology or collapse stage, the influence of other variables (e.g., the radiographic extent or location of the necrotic lesion) on long-term graft survival are also unknown.
We addressed the following questions: (1) Does this procedure provide a long-term (10 years or more) improvement in function (as measured by Harris hip score)?; (2) Does the procedure avert the need for THA and, if so, for how long?; (3) Do factors such as age, size and location of the lesion, or etiology of the disease influence long-term survivorship?; and (4) Does radiographic appearance change over time?
Materials and Methods
We retrospectively reviewed 135 patients (151 hips) who underwent free VFG in for Ficat and Arlet stage II and III ONFH between August 1979 and December 1995 in a single institution. Important clinical and imaging data including patient age (two groups: younger than and older than 35 years at the time of operation), etiology, Harris hip score, and various radiographic parameters (collapse stage, extent of involvement, and location of the necrotic lesion) were collected for all the patients. The outcome and survivorship of VFG was evaluated using conversion to THA as the endpoint. Seventeen patients (19 hips) died and eight patients (eight hips) were lost to followup. This left 110 patients (81%) with 124 hips for review. The minimum followup was 10 years (mean, 13.9 years; range, 10–23.7 years). There were 94 men and 16 women with a mean age at surgery of 35.5 years (range, 13–63 years). The diagnosis of osteonecrosis was confirmed in all cases by a histologic examination of the subchondral bone that was obtained from a core biopsy of the femoral head during the operation. We divided hips into four groups based on etiology of the disease: idiopathic (n = 59), alcoholic (n = 31), posttraumatic (n = 21), and steroid-induced (n = 13) (Table 1).
Table 1.
Demographic data
| Variables | n |
|---|---|
| Average patient age (years) | 35.5 (range, 13–63) |
| Number of hips ≤ 35 years old | 68 |
| Number of hips > 36 years old | 56 |
| Male:female | 94:16 |
| Mean patient weight (kg) | 63.5 (range, 45–97) |
| Etiology (number of hips) | |
| Idiopathic | 59 |
| Alcoholic | 31 |
| Posttraumatic | 21 |
| Steroid-induced | 13 |
We (KKI, PSW) examined the preoperative radiographs of the patients to determine the location and size of the necrosis as well as the presence or absence of collapse. For the presence of radiographic collapse, we used the Ficat and Arlet system [9] and for quantifying the lesion, the Steinberg classification [37] was used. Size A lesions involved less than 15% of the femoral head, Size B involved 15% to 30%, and Size C involved more than 30% of the femoral head. Also, for evaluating the location of the lesion, the method by Sugano et al. [40] was used, which was adopted as a classification by Ohzono et al. [27]. In Type A, the lesion involved the medial third of the weight-bearing dome (sourcil) of the acetabulum. Type B lesions involved the middle third (medial two-thirds or less) of the weight-bearing dome, and Type C lesions involved the lateral third or more of the dome. Preoperatively, 59 hips were classified as Ficat Stage II and 65 hips as Stage III. Based on the Steinberg classification, 27 hips were Size A, 38 hips were Size B, and 59 hips were Size C. Based on location of the lesion, nine hips were classified as Type A, 35 hips were Type B, and 80 hips were Type C (Table 2).
Table 2.
Classifications of osteonecrosis of the femoral head
The operative technique has been previously described [44] and was originally designed by the senior author (YMC) [44]. One surgical team by the senior author operates on the femoral side. Briefly, this involves exposure of the femur using a Watson-Jones approach. Following release of the gluteus maximus insertion and release of the vastus lateralis from linea aspera the first or the second perforating branch of the profunda femoris artery was dissected carefully. In general, the second perforating branch was preferable for the recipient vessels owing to enough length and diameter of the vessels. After complete dissection of the recipient vessels, a large hole 2 cm in diameter in the lateral cortex just beneath the flare of the trochanter was made. At this point we performed a biopsy of the subchondral bone using an 8-mm trephine directed towards the necrotic lesion. A tunnel was then created in the femoral neck to admit the fibula and its vessels without compressing them. The tunnel was directed towards the lesion and as much of the subchondral bone as possible was removed. At this point we brought the fibular graft harvested by another team (HCS) to the field and performed the vascular anastomosis. The fibula was harvested using a curvilinear incision over the fibula of the contralateral leg. The dissection then proceeded between the peroneus longus and the soleus muscles. With careful dissection of the flexor hallucis longus muscle, we exposed the entire course of the peroneal artery. Then the anterolateral musculature attached to the fibula was released and fibular freed from the interosseous membrane on the medial side. We used the middle third of the fibula as the graft. The peroneal vessels supplying this part of the fibula are usually cut in a sufficient length after checking the vascularity of the fibula with deflation of the tourniquet. The average length of the harvested fibula usually ranged from 8 to 10 cm. Our technique differs from others in some respects. First, we have used the first or the second perforating branch of the profunda femoris artery rather than a branch of lateral femoral circumflex artery. We think it is easier and has less morbidity to the hip joint. We do not routinely perform angiography to assess patency of the anastomosed vessels. Instead we raise a small area of the skin overlying fibula during harvest that allows monitoring the vascular patency of the grafted fibula. We also perform autografting of subchondral region using cancellous bone chips obtained from the greater trochanter region.
We gave all patients antibiotic prophylaxis. Postoperative thromboembolic prophylaxis included intravenous infusion of dextran for 3 days after surgery and application of compression stockings. Passive range-of-motion exercises were encouraged after removal of suction drains on Day 3. Postoperative rehabilitation included complete nonweightbearing (wheelchair-bound) for 1 week, minimal weightbearing and ambulation with crutches for 10 weeks, followed by partial weightbearing for a total of 6 months. Patients were encouraged to bear full weight after this period. Because of the potential injury to the flexor hallucis longus and the risk of clawing of the big toe from the fibular harvest side, patients used a short leg splint including the big toe for 3 weeks and we encouraged the patient to do physiotherapy (active and passive dorsiflexion and plantarflexion of the toe) for 6 months to prevent toe clawing.
Clinical evaluation was performed by the senior surgeon (YMC) whenever the patient visited his outpatient clinic. Clinical results were recorded preoperatively and postoperatively using the Harris hip score [13] (HHS). Radiographic evaluation was performed by two (KKI, PSW) individuals who were blinded to the functional results. We categorized final radiographs (AP, lateral, and frog leg view) in one of three classes: (1) Improved—Those cases in which the osteonecrosis had healed or was being replaced with new bone formation. For the Stage II lesion, the crescent had disappeared or the density of cystic lesion had increased with trabecular formation of the tip of the vascularized fibula. For the Stage III lesion, the collapsed lesion healed or became more rounded with trabecular formation of the tip of the vascularized fibula; (2) No change—compared with the preoperative status; and (3) Progressed—Those cases with progression observed based on stage or those with more than 3-mm of collapse. We confirmed any definite change on any of three radiographs. For the exact measurement of a collapsed lesion, we used Mose’s template of concentric circles. To evaluate interobserver validity we compared the radiographic results that were made by the two different observers. Of 124 total cases, there was agreement between observers in five, 61, and 48 cases with improved, unchanged and progressed respectively. The level of agreement was tested by Kappa statistics (k = 0.85, p < 0.0001) which we considered highly in agreement.
We recorded the number of cases converted to THA during followup resulting from progression of osteonecrosis or degenerative change. We then calculated survival with THA as an endpoint for each radiographic group, etiology, and patient’s age using the Kaplan-Meier method. The changes in HHS were evaluated with the Wilcoxon signed rank test. We used Cox proportional hazard model to assess the independent effects of location and size of lesion on survivorship; for the independent variables with a categorical characteristics, we created ‘dummy variables’ if the variables had more than 2 groups. We performed all analyses using SPSS (version 12.0; SPSS Inc, Chicago, IL).
Results
Preoperative HHS improved from 72 (range, 52–81) to 88 (range, 62–100) at the latest followup (p < 0.001). There were 85 hips with HHS of more than 90 points, 13 with HHS between 80 and 90, 13 with HHS between 70 and 79, and 13 with HHS of less than 69. HHS over 80 was observed in 48 of 59 Stage II hips (81%) and in 50 of 65 Stage III hips (77%).
Thirteen patients with 13 hips (10%) had undergone THA resulting in a survivorship of 93% at ten years and 83% at 20 years. Moreover, the rate of graft survival at ten years of the patients without preoperative femoral collapse was 93% and 92% in patients with collapse. The time interval between fibular grafting and THA averaged 8.4 years (range, 1.3–18.8 years). Conversion to THA was in 7 hips with Ficat Stage II and 6 hips with stage III at the time of fibular grafting. The conversion rate to THA was not statistically different between Stage II or Stage III hips at 12% and 9% respectively.
We observed a higher (p = 0.019) survival rate in patients younger than 35 years of age compared with those older than 35 years. The location of lesion (p = 0.032), as well as the extent of involvement (p = 0.015) independently influence survivorship. Survivorship was not influenced by Ficat stage of the hip (p = 0.574) or the etiology (p = 0.204).
Radiographically seven hips (6%) improved, 69 hips (56%) were unchanged, and 48 hips (39%) progressed (Fig. 1A–D). We observed improved or unchanged radiographs in 37 of 59 (63%) hips at Ficat-Arlet Stage II and 39 of 65 (60%) hips at Stage III (Table 3). According to the location of femoral head necrosis, improved or unchanged results were seen in seven of nine hips with osteonecrosis in the medial region, 22 of 35 (63%) hips in the central region, and 47 of 80 (59%) hips in the lateral region. Improved or unchanged results were found in 22 of 27 (82%) hips with osteonecrosis less than 15%, 22 of 38 (58%) hips with osteonecrosis less than 30%, and 32 of 59 (54%) hips with osteonecrosis more than 30%. According to etiology, improved or unchanged results were observed in 31 of 59 (53%) hips (53 patients) with idiopathic necrosis, 18 of 31 (58%) hips (26 patients) with alcoholic necrosis, 17 of 21 hips (21 patients) with traumatic necrosis, and 10 of 13 hips (10 patients) with steroid-induced osteonecrosis.
Fig. 1A–D.
(A) Preoperative radiograph shows a 19-year-old man with collapse of the femoral head secondary to osteonecrosis. Vascularized fibular grafting was performed resulting in an excellent outcome. Anteroposterior radiograph of the same hip is shown at (B) 3 months, (C) 7 years, and (D) 15 years.
Table 3.
Summary of the outcomes
| Variables | “Improved” or “Unchanged,” number of hips (%) | HHS over 80, number of hips (%) | Conversion to THA, number of hips (%) |
|---|---|---|---|
| Ficat stage [9] | |||
| II | 37 (62.7) | 48 (81.3) | 7 (11.8) |
| III | 39 (60.0) | 50 (76.9) | 6 (9.2) |
| p-value* | NS | NS | NS |
| Ohzono classification [27] | |||
| A | 7 (77.8) | 9 (100) | 0 (0) |
| B | 22 (62.8) | 31 (88.6) | 1 (2.8) |
| C | 47 (58.7) | 58 (72.5) | 12 (15.0) |
| p-value* | NS | 0.011 | 0.039 |
| Steinberg classification [37] | |||
| A | 22 (81.5) | 27 (100) | 0 (0) |
| B | 22 (57.9) | 30 (78.9) | 3 (78.9) |
| C | 32 (54.2) | 41 (69.5) | 10 (16.9) |
| p-value* | 0.026 | 0.004 | 0.012 |
| Etiology | |||
| Idiopathic | 31 (52.5) | 47 (79.6) | 8 (13.5) |
| Alcoholic | 18 (58.0) | 22 (70.9) | 4 (12.9) |
| Posttraumatic | 17 (80.9) | 18 (85.7) | 0 (0) |
| Steroid-induced | 10 (76.9) | 11 (84.6) | 1 (7.7) |
| p-value* | 0.039 | NS | NS |
| Age | |||
| ≤ 35 years | 50 (73.5) | 57 (83.8) | 3 (4.4) |
| > 36 years | 26 (46.4) | 41 (73.2) | 10 (17.8) |
| p-value* | 0.009 | NS | 0.014 |
* The chi square test was used to test significance among the groups.
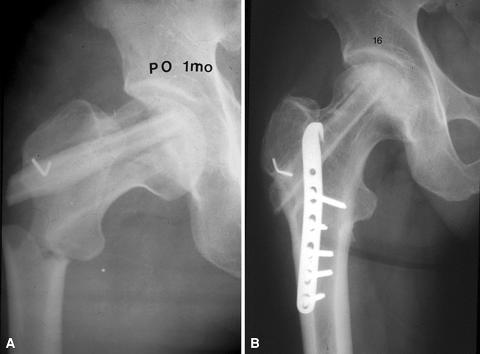
Clawing of the big toe developed in 17 cases and most of the patients were treated nonoperatively including physiotherapy except three cases having surgical release of the flexor hallucis longus tendon. This complication was caused by the extensive dissection and injury of the flexor hallucis longus muscle. Partial peroneal nerve palsy developed in two patients; their symptoms resolved within 1.5 years. Superficial infections developed in two patients and were successfully resolved with adequate antibiotics therapy. Subtrochanteric fractures occurred in two patients; these were successfully treated with open reduction and internal fixation (Fig. 2A–B).
Fig. 2A–B.
(A) Preoperative radiograph of the right hip of a 46-year-old man who slipped and fell 1 month after VFG shows subtrochanteric fracture. (B) 16-year followup radiograph shows excellent bony union and well-maintained fibular graft without substantial collapse of the femoral head.
Discussion
Although free VFG in the treatment of ONFH is reported to have encouraging short to mid-term results, long-term outcome of this procedure is largely unknown [4, 6, 7, 10, 12, 14, 15, 17–21, 31, 43, 44]. Furthermore, some studies suggest the success rate after VFG decreases with time [3, 20], various results have been reported both clinically and radiographically [5, 7, 19, 31, 43, 44] (Table 4). Most previous studies report the outcome based on etiology and radiographic stage of the disease [4, 7, 14, 16, 20, 31, 43, 44]. However, size and location of necrotic involvement also influence outcomes [25–27, 39, 40]. Most of these reports reflect short- or mid-term followup and rather than the long-term followup. We therefore addressed the following questions: (1) Does this procedure provide a long-term (10 years or more) improvement in function (as measured by Harris hip score)?; (2) Does the procedure avert the need for THA and, if so, for how long?; (3) Do factors such as age, size and location of the lesion, or etiology of the disease influence long-term survivorship?; and (4) Does radiographic appearance change over time?
Table 4.
Comparison of our results with those in the literature
| Study | Number of hips | Followup (years) | HHS > 80 (%) | Radiographic progression (%) | Survival (%) |
|---|---|---|---|---|---|
| Malizos et al. [19] | 40 | 2.7 | 87.5 | 12.5 | 92.5 |
| Louie et al. [17] | 59 | 4.2 | NA | 48 | 73 |
| Soucacos et al. [31] | 184 | 4.7 | NA | 37.5 | 92.4 |
| Plakseychuk et al. [28] | 50 | 5 | 70 | 24 | 86 |
| Yoo et al. [44] | 81 | 5 | 91 | 11 | 96 |
| Marciniak et al. [20] | 101 | 5 | NA | 57.4 | 61 |
| Berend et al. [4] | 121 | 5.7 | 63 | NA | 64.5 |
| Urbaniak et al. [43] | 103 | 7 | 81 | NA | 70 |
| Brunelli and Brunelli [5] | 18 | 7.7 | 78 | 46 | NA |
| Judet and Gilbert [14] | 68 | 18 | 52* | NA | 73.5 |
| Current study | 124 | 13.9 | 79 | 38.7 | 89.6 |
* Merle d’Aubigne score > 15.
We are aware of some limitations of our study. First, we had no control group treated with alternative joint-preserving procedures. Second, because the criteria for determining both clinical and radiographic assessment is different in each article, direct comparison of our findings to those reported by others is difficult. We also recognize that even for appropriately experienced surgeons VFG is a complex operation often with long operation time, the necessity of two surgical teams to reduce the time, donor site morbidity, and substantial postoperative complications.
Marcus et al. [21] proposed the most desirable time for joint-sparing surgery for ONFH is before collapse of the femoral head. Springfield and Enneking [32], on the other hand, suggested the possibility for regeneration of the femoral head still exists even in some cases with collapse and subchondral fracture. Others have also reiterated that collapse does not necessarily imply a poor prognosis and cessation of collapse can be expected in a certain percentage of hips [25]. Marciniak et al. [20] found no correlation between the initial radiographic stage and clinical outcomes or the overall rate of graft survival. Their 5-year results were even more encouraging for the Marcus-Enneking Stage 3 and 4 hips than for the hips with Stage 2. Moreover, in the large series of mid-term followup, Scully et al. [30] also concluded VFG could delay or prevent collapse in hips that have Ficat Stage II or III. Our long-term data also suggests similar survival in Ficat Stage II and III, findings similar to those of Judet and Gilbert [14]. We obtained a rate of 92.1% of graft survival at 10 years in 65 patients with femoral head collapse. These findings confirm VFG can be used successfully even in the period of early collapse such as Ficat Stage III.
Previous reports have suggested a direct correlation between the size of necrotic lesion and the outcome of VFG [33]. While others have refuted the latter being unable to identify an association between lesion size and survivorship of VFG at a mean followup of 4.3 years [4]. The classification system they used was different from the widely used Steinberg classification because they classified femoral involvement as less than 25%, 25% to 50%, or more than 50%. A direct comparison between the two results is thus impossible. Our data showed a relationship between the size of the lesion and survivorship. Our data support the quantitative analysis of lesion morphology by Nishii et al. [26], demonstrated lesion volume correlated with progression.
Currently, the importance of lesion location and size is well accepted [22, 26]. A laterally located lesion on the weight-bearing surface of the acetabulum predicts poor outcomes [25–27, 39, 40]. In such types, regardless of size, head collapse is likely to occur soon after the onset of the disease [27]. Thus, joint-preserving surgery may have a limited role for treatment of lateral lesions, particularly when they are large. Our data showed a relationship between the size of the lesion and outcome. Twelve of 13 failures in our series occurred in patients with lateral lesion (Type C). On the contrary, we had a 100% rate of graft survival with nine cases of Type A lesions, but such lesions are believed less predictive than Type B or C [25]. Thus, we no longer perform this procedure in patients with Type A lesions unless a simpler procedure of core decompression fails to alleviate consistent pain.
A few studies considered patient age at the time of operation as a possible factor predicting survival [8, 14, 20, 43]. Several authors [7, 20, 43] suggest age does not affect the results in mid-term followup, whereas Judet and Gilbert [14] reported, in 68 cases of VFG with an average followup of 18 years, that better results were obtained in patients younger than 40 years of age. Another report [8] suggests a trend toward a lower rate of failure in younger patients. Our long-term results suggest a better outcome in patients younger than 35 years of age. Thus, the results of Judet and Gilbert [14] and our long-term results support the view that the patient’s age can be one of the key factors in long-term survival. Because this procedure involves a microvascular repair, it is generally agreed younger patients undergoing microvascular surgery have a higher success rate than older patients.
Berend et al. [4] and others [43, 44] reported etiology was not a factor in the success of VFG in their group. Urbaniak et al. [43] also found no difference in survival rate according to etiology. Our long-term study confirms those findings.
We conclude this method as a joint-preserving treatment for osteonecrosis is a reasonable option. Preoperative evaluation should include not only the stage, but also the extent and location of the necrosis to predict long-term graft survival. This modality appears especially effective in young patients.
Acknowledgment
We thank Dr. Sung-Woo Park for the radiographic assessment.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution either has waived or does not require approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aaron RK. Treatment of osteonecrosis of the femoral head with electrical stimulation. Instr Course Lect. 1994;43:495–498. [PubMed]
- 2.Arai K, Toh S, Tsubo K, Nishikawa S, Narita S, Miura H. Complications of vascularized fibula graft for reconstruction of long bones. Plast Reconstr Surg. 2002;1097:2301–2306. [DOI] [PubMed]
- 3.Berend KR, Gunneson E, Urbaniak JR, Vail TP. Hip arthroplasty after failed free vascularized fibular grafting for osteonecrosis in young patients. J Arthroplasty. 2003;184:411–419. [DOI] [PubMed]
- 4.Berend KR, Gunneson EE, Urbaniak JR. Free vascularized fibular grafting for the treatment of postcollapse osteonecrosis of the femoral head. J Bone Joint Surg Am. 2003;85:987–993. [DOI] [PubMed]
- 5.Brunelli G, Brunelli G. Free microvascular fibular transfer for idiopathic femoral head necrosis: long-term follow-up. J Reconstr Microsurg. 1991;7:285–295. [DOI] [PubMed]
- 6.Chillag KJ. Survival analysis of hips treated with core decompression or vascularized fibular grafting because of avascular necrosis. J Bone Joint Surg Am. 2000;82:289–290. [PubMed]
- 7.Cho BC, Kim SY, Lee JH, Ramasastry SS, Weinzweig N, Baik BS. Treatment of osteonecrosis of the femoral head with free vascularized fibular transfer. Ann Plast Surg. 1998;40:586–593. [DOI] [PubMed]
- 8.Dean GS, Kime RC, Fitch RD, Gunneson E, Urbaniak JR. Treatment of osteonecrosis in the hip of pediatric patients by free vascularized fibular graft. Clin Orthop Relat Res. 2001;386:106–113. [DOI] [PubMed]
- 9.Ficat P, Arlet J, Vidal R, Ricci A, Fournial JC. Therapeutic results of drill biopsy in primary osteonecrosis of the femoral head (100 cases) [in French]. Rev Rhum Mal Osteoartic. 1971;38:269–276. [PubMed]
- 10.Garberina MJ, Berend KR, Gunneson EE, Urbaniak JR. Results of free vascularized fibular grafting for femoral head osteonecrosis in patients with systemic lupus erythematosus. Orthop Clin North Am. 2004;353:353–357. [DOI] [PubMed]
- 11.Gonzalez Della Valle A, Bates J, Di Carlo E, Salvati EA. Failure of free vascularized fibular graft for osteonecrosis of the femoral head: a histopathologic study of 6 cases. J Arthroplasty. 2005;203:331–336. [DOI] [PubMed]
- 12.Goodman SB. Survival analysis of hips treated with core decompression or vascularized fibular grafting because of avascular necrosis. J Bone Joint Surg Am. 2000;82:289. [PubMed]
- 13.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed]
- 14.Judet H, Gilbert A. Long-term results of free vascularized fibular grafting for femoral head necrosis. Clin Orthop Relat Res. 2001;386:114–119. [DOI] [PubMed]
- 15.Kane SM, Ward WA, Jordan LC, Guilford WB, Hanley EN Jr. Vascularized fibular grafting compared with core decompression in the treatment of femoral head osteonecrosis. Orthopedics. 1996;19:869–872. [DOI] [PubMed]
- 16.Kim SY, Kim YG, Kim PT, Ihn JC, Cho BC, Koo KH. Vascularized compared with nonvascularized fibular grafts for large osteonecrotic lesions of the femoral head. J Bone Joint Surg Am. 2005;879:2012–2018. [DOI] [PubMed]
- 17.Louie BE, McKee MD, Richards RR, Mahoney JL, Waddell JP, Beaton DE, Schemitsch EH, Yoo DJ. Treatment of osteonecrosis of the femoral head by free vascularized fibular grafting: an analysis of surgical outcome and patient health status. Can J Surg. 1999;42:274–283. [PMC free article] [PubMed]
- 18.Malizos KN, Quarles LD, Dailiana ZH, Rizk WS, Seaber AV, Urbaniak JR. Analysis of failures after vascularized fibular grafting in femoral head necrosis. Orthop Clin North Am. 2004;353:305–314. [DOI] [PubMed]
- 19.Malizos KN, Soucacos PN, Beris AE. Osteonecrosis of the femoral head. Hip salvaging with implantation of a vascularized fibular graft. Clin Orthop Relat Res. 1995;314:67–75. [PubMed]
- 20.Marciniak D, Furey C, Shaffer JW. Osteonecrosis of the femoral head. A study of 101 hips treated with vascularized fibular grafting. J Bone Joint Surg Am. 2005;874:742–747. [DOI] [PubMed]
- 21.Marcus ND, Enneking WF, Massam RA. The silent hip in idiopathic aseptic necrosis. Treatment by bone-grafting. J Bone Joint Surg Am. 1973;55:1351–1366. [PubMed]
- 22.Mont MA, Jones LC, Einhorn TA, Hungerford DS, Reddi AH. Osteonecrosis of the femoral head. Potential treatment with growth and differentiation factors. Clin Orthop Relat Res. 1998;355(Suppl):S314–S335. [DOI] [PubMed]
- 23.Mont MA, Jones LC, Hungerford DS. Survival analysis of hips treated with core decompression or vascularized fibular grafting because of avascular necrosis. J Bone Joint Surg Am. 2000;82:290–291. [DOI] [PubMed]
- 24.Mulliken BD. Osteonecrosis of the femoral head: current concepts and controversies. Iowa Orthop J. 1993;13:160–166. [PMC free article] [PubMed]
- 25.Nishii T, Sugano N, Ohzono K, Sakai T, Haraguchi K, Yoshikawa H. Progression and cessation of collapse in osteonecrosis of the femoral head. Clin Orthop Relat Res. 2002;400:149–157. [DOI] [PubMed]
- 26.Nishii T, Sugano N, Ohzono K, Sakai T, Sato Y, Yoshikawa H. Significance of lesion size and location in the prediction of collapse of osteonecrosis of the femoral head: a new three-dimensional quantification using magnetic resonance imaging. J Orthop Res. 2002;201:130–136. [DOI] [PubMed]
- 27.Ohzono K, Saito M, Takaoka K, Ono K, Saito S, Nishina T, Kadowaki T. Natural history of nontraumatic avascular necrosis of the femoral head. J Bone Joint Surg Br. 1991;73:68–72. [DOI] [PubMed]
- 28.Plakseychuk AY, Kim SY, Park BC, Varitimidis SE, Rubash HE, Sotereanos DG. Vascularized compared with nonvascularized fibular grafting for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2003;85:589–596. [DOI] [PubMed]
- 29.Plancher KD, Razi A. Management of osteonecrosis of the femoral head. Orthop Clin North Am. 1997;283:461–477. [DOI] [PubMed]
- 30.Scully SP, Aaron RK, Urbaniak JR. Survival analysis of hips treated with core decompression or vascularized fibular grafting because of avascular necrosis. J Bone Joint Surg Am. 1998;80:1270–1275. [DOI] [PubMed]
- 31.Soucacos PN, Beris AE, Malizos K, Koropilias A, Zalavras H, Dailiana Z. Treatment of avascular necrosis of the femoral head with vascularized fibular transplant. Clin Orthop Relat Res. 2001;386:120–130. [DOI] [PubMed]
- 32.Springfield DS, Enneking WJ. Surgery for aseptic necrosis of the femoral head. Clin Orthop Relat Res. 1978;130:175–185. [PubMed]
- 33.Steinberg ME, Bands RE, Parry S, Hoffman E, Chan T, Hartman KM. Does lesion size affect the outcome in avascular necrosis? Clin Orthop Relat Res. 1999;367:262–271. [DOI] [PubMed]
- 34.Steinberg ME, Brighton CT, Corces A, Hayken GD, Steinberg DR, Strafford B, Tooze SE, Fallon M. Osteonecrosis of the femoral head. Results of core decompression and grafting with and without electrical stimulation. Clin Orthop Relat Res. 1989;249:199–208. [PubMed]
- 35.Steinberg ME, Brighton CT, Hayken GD, Tooze SE, Steinberg DR. Electrical stimulation in the treatment of osteonecrosis of the femoral head—a 1-year follow-up. Orthop Clin North Am. 1985;16:747–756. [PubMed]
- 36.Steinberg ME, Brighton CT, Steinberg DR, Tooze SE, Hayken GD. Treatment of avascular necrosis of the femoral head by a combination of bone grafting, decompression, and electrical stimulation. Clin Orthop Relat Res. 1984;186:137–153. [PubMed]
- 37.Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed]
- 38.Steinberg ME, Larcom PG, Strafford B, Hosick WB, Corces A, Bands RE, Hartman KE. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001;386:71–78. [DOI] [PubMed]
- 39.Sugano N, Atsumi T, Ohzono K, Kubo T, Hotokebuchi T, Takaoka K. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci. 2002;75:601–605. [DOI] [PubMed]
- 40.Sugano N, Takaoka K, Ohzono K, Matsui M, Masuhara K, Ono K. Prognostication of nontraumatic avascular necrosis of the femoral head. Significance of location and size of the necrotic lesion. Clin Orthop Relat Res. 1994;303:155–164. [PubMed]
- 41.Sugioka Y, Hotokebuchi T, Tsutsui H. Transtrochanteric anterior rotational osteotomy for idiopathic and steroid-induced necrosis of the femoral head. Indications and long-term results. Clin Orthop Relat Res. 1992;277:111–120. [PubMed]
- 42.Trancik T, Lunceford E, Strum D. The effect of electrical stimulation on osteonecrosis of the femoral head. Clin Orthop Relat Res. 1990;256:120–124. [PubMed]
- 43.Urbaniak JR, Coogan PG, Gunneson EB, Nunley JA. Treatment of osteonecrosis of the femoral head with free vascularized fibular grafting. A long-term follow-up study of one hundred and three hips. J Bone Joint Surg Am. 1995;77:681–694. [DOI] [PubMed]
- 44.Yoo MC, Chung DW, Hahn CS. Free vascularized fibula grafting for the treatment of osteonecrosis of the femoral head. Clin Orthop Relat Res. 1992;277:128–138. [PubMed]
- 45.Zhang C, Zeng B, Xu Z, Sui S, Song W, Jin D, Shi H, Wang K. Treatment of osteonecrosis of femoral head with free vascularized fibula grafting [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2004;18:367–369. [PubMed]