Abstract
A variety of nonvascularized bone grafting techniques have been proposed with varying degrees of success as treatment alternatives for osteonecrosis of the femoral head. The success of these procedures may be enhanced using ancillary growth and differentiation factors. We retrospectively reviewed 33 patients (39 hips) with osteonecrosis of the hip who had nonvascularized bone grafting procedures with supplemental OP-1. We compared the outcomes in this cohort to similar patients treated nonoperatively or with other nonvascularized bone grafting procedures. We used a trapdoor to make a window at the head-neck junction to remove necrotic bone and packed the excavated area with autogenous cancellous bone graft, marrow, and OP-1. The minimum followup was 24 months (mean, 36 months; range, 24–50 months). We performed no further surgery in 25 of 30 small- and medium-sized lesions (80%) but did in two of nine large lesions. Hips with Ficat Stage II disease were not reoperated in 18 of 22 cases during the followup periods. Our short-term results compare similarly to nonoperative treatment and other reports of nonvascularized bone grafting. With the addition of ancillary growth factors, these procedures effectively reduce donor site morbidity and may defer joint arthroplasty in selected patients.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-008-0211-x) contains supplementary material, which is available to authorized users.
Introduction
Osteonecrosis of the femoral head is a devastating disease that often leads to destruction of the hip and the need for total hip arthroplasty [32, 35]. Annual reports from various joint registries such as the Canadian Joint Replacement Registry, the Australian National Joint Replacement Registry, and the Swedish Hip Arthroplasty Register have demonstrated that the diagnosis of osteonecrosis accounts for between 2.8% to 6% of all primary total hip arthroplasties performed [1, 7, 60]. In early stages of the disease, head-preserving treatment modalities such as core decompression, osteotomy, and vascularized or nonvascularized bone grafting are often utilized to defer head-replacing options such as total hip arthroplasty [32, 35].
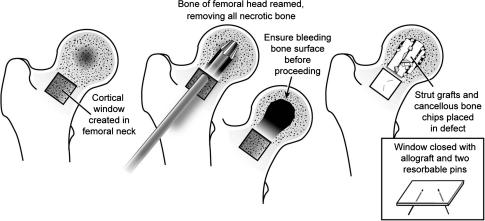
The rationale for the use of nonvascularized bone grafting is to remove necrotic bone and replace it with cancellous and cortical autografts that support the subchondral bone and articular cartilage of the femoral head and may stimulate bone formation [11, 43]. Three different surgical techniques have been popularized for nonvascularized bone grafting: (1) grafting through a core decompression tract (Phemister technique) (Appendix 1 -Supplemental Website Materials; supplemental materials are available with the online version of CORR ) [2–4, 24, 43, 55]; (2) grafting through a window or trapdoor in the articular cartilage (Appendix 2 - Supplemental Website Materials; supplemental materials are available with the online version of CORR) [21, 28–30]; and (3) grafting through a window made in the femoral neck or femoral head-neck junction (Fig. 1) [31, 48]. Each of these techniques has its advantages and its limitations. While earlier studies of nonvascularized bone grafting through a core tract or cartilage window reported promising clinical results [2, 6, 24, 29, 48], later studies using this technique reported less favorable outcomes [9, 39].
Fig. 1.
The five key steps for vascularized bone grafting through a window made in the femoral neck or femoral head-neck junction are illustrated.
The use of OP-1 (BMP 7) in combination with allograft has been applied in various bone healing applications (nonunions, trauma, spine fusion) [36]. For osteonecrosis, it was used in a canine model in which defects treated with OP-1 and bone grafting healed faster radiographically than defects simply treated with bone grafting [34]. This study provided a rationale for the possible use of OP-1 in combination with allograft to heal human osteonecrotic defects.
We describe the principles, indications, and surgical techniques for nonvascularized bone grafting through a window made at the femoral head-neck junction. We asked whether this technique effectively and similarly deferred further surgical treatment options when compared to those reported in studies using nonoperative treatment. In addition, we questioned how these outcomes compared to other studies of nonvascularized bone grafting.
Materials and Methods
We retrospectively reviewed 33 patients (39 hips) with osteonecrosis of the femoral head who had nonvascularized bone grafting procedures with supplemental OP-1 performed consecutively for the appropriate indications between December 1, 2002, and January 1, 2004. Indications for the procedure were Ficat and Arlet Stage II or III lesions that met various intraoperative criteria (described later). There were 15 women (16 hips) and 18 men (23 hips) who had a mean age of 35 years (range, 18–52 years). The mean body mass index was 27.2 kg/m2 (range, 19.4–36.0 kg/m2). No patients were lost to followup. Minimum followup was 24 months (mean, 36 months; range, 24–50 months). After obtaining institutional review board approval, a prospective database was used to collect relevant surgical, clinical, and radiographic data.
We identified the following risk factors and associated conditions with osteonecrosis of the femoral head: corticosteroid usage (defined as a dose greater than 2 g prednisone or its equivalent per month for 3 months minimum [42]) in 9 patients (12 hips), alcohol abuse (defined as alcohol consumption of more than 400 mL per week [25]) in 8 patients (eight hips), systemic lupus erythematosus in 6 patients (seven hips), tobacco abuse (defined as 20 cigarettes or more per day [25]) in 3 patients (four hips), hepatitis C in 2 patients (three hips), and HIV infection in 2 patients (two hips). Of the remaining 4 patients (four hips), one each had an underlying diagnosis of ulcerative colitis, sickle cell disease, high levels of plasminogen activator inhibitor with hypofibrinolysis, and chronic obstructive pulmonary disease. Three patients (four hips) had no apparent associated risk factors and were deemed idiopathic osteonecrosis. Some patients had more than one associated risk factor.
We (TMS, SDU) assessed patients using the Harris hip rating system [14]. We defined failure as patients who underwent total hip arthroplasty surgery.
Anteroposterior and lateral radiographs were made preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. We determined Ficat and Arlet stage [10], combined Kerboul angle [12], presence or absence of new bone formation, location of the lesion, and disease progression. Preoperative radiographs were evaluated by two of us (TMS, SDU) to determine the staging according to the system by Ficat and Arlet. The size of the lesions was measured using the combined necrotic angle technique described by Kerboul et al. [19]. The combined angle is derived from evaluating anteroposterior and lateral radiographs by adding the sums of the angle of the lesions delineated on each view. In vague cases in which the lesion was not clearly demarcated on plain radiographs, MRI and computed tomography evaluations were used to assist in the evaluation of lesion size. Using this method, the extent of the necrosis was stratified into three categories: (1) large lesions, when the combined necrotic angle was 200° or greater; (b) medium lesions, when the angle was between 150° and 200°; and (c) small lesions, when the angle was 150° or less. The location of the lesion was defined using a system initially described by Ohzono et al. [41]. Lesions were classified as type A, B, C1, or C2. A Type A lesion was one that occupied the medial third or less of the weight-bearing portion. A Type B lesion occupied the medial two-thirds or less of the weight-bearing portion. A Type C lesion occupied more than the medial two-thirds of the weight-bearing portion. The subtypes C1 and C2 were used to further stratify the C type lesions with a Type C2 lesion extending laterally to the acetabular edge, whereas a Type C1 lesion did not. Because of the possible introduction of error assessing radiographic measurements, an evaluation of interobserver and intraobserver error in radiographic assessment was performed by two of us (TMS, SDU) before reviewing study-related radiographs. The intraobserver agreement was 100% in the 10 pilot cases and the interobserver agreement was an exact match in 90% of the pilot cases. To guarantee objectivity and avoid the problem of intraobserver and interobserver variability in assessing the various radiographic parameters, two of the authors (TMS, SDU) independently evaluated the radiographs 2 weeks apart. If there was a disagreement, the senior author (MAM) interpreted the films until a unanimous decision could be made regarding the best estimate at staging, size, or extent of collapse of lesion evaluation. The various radiographic variables (Ficat and Arlet stage and Kerboul angle) were assessed to see whether they had any prognostic value. Of the 39 hips, 22 hips were classified as Ficat and Arlet Stage II and 17 hips were classified as Ficat and Arlet Stage III preoperatively. The assessment of lesions size using the Kerboul technique revealed seven small lesions, 23 medium lesions, and nine large lesions.
All procedures were performed by the senior author (MAM) using a trapdoor made at the femoral head-neck junction (Fig. 1). Large lesions were not considered a contraindication for the present patient cohort. The technique [48, 49] was performed using the anterolateral approach (Watson-Jones [62]) with the patient lying in the lateral decubitus position. The skin incision was started just distal to the anterosuperior iliac spine and carried out to a point just posterior to the greater trochanter. The incision was then angled at about 110° anteriorly and extended distally to parallel the femoral shaft for 8 to 10 cm. In the next step, the interval between the tensor fascia latae muscle and the gluteus medius muscle was identified by dividing the gluteus maximus fascia and the fascia latae. The dissection was carefully extended proximally to expose but protect the superior gluteal nerve. The fascia latae was then split in the direction of the skin incision and the anterior 40% of the gluteus medius was detached and retracted posteriorly. The gluteus minimus muscle was detached fully revealing the capsule with the head of the rectus femoris muscle attached to the upper part of acetabular rim. The capsule was then excised with the labrum left intact and the capsule peeled anteriorly to preserve the medial circumflex artery and its branches posteriorly. This approach allowed for preservation of the blood supply to the femoral head. We inspected the femoral head cartilage in situ by rotating and distracting the femur without dislocating the femoral head. The femoral head cartilage was then inspected to ascertain whether there were any full-thickness defects or areas of delaminated cartilage. We considered a defect of 1 cm or greater, cartilage delamination, or erosive areas as contraindications for performing this bone grafting procedure. This occurred in five cases during the time period of the study and these patients received a total hip arthroplasty. An approximate 2-cm × 2-cm window was then made at the femur head-neck junction (trapdoor) using a microoscillating saw and osteotomes. The window segment was preserved and stored in normal saline-wrapped gauze for replacement at the end of the procedure. A 6-mm mushroom-tipped burr was used to débride necrotic bone in the femoral head using the trapdoor as an entrance point. If 70% or more of the femoral head was involved with the disease (necrotic bone), the procedure was abandoned and a hip arthroplasty was performed. Accidental head penetration with the burr was avoided. The cavity was filled with cancellous bone chips and bone marrow. In addition, recombinant human bone morphogenetic protein 7 was added to promote new bone formation. Each sterile unit of implant contained 3.5 mg OP-1 (purchased from Stryker Biotech, Hopkinton, MA) mixed with 1 g Type I bovine bone-derived collagen. The material was tightly packed into the cavity with a layered approach and the saved bony window segment was put back and fixed with three, 2-mm poly-p-dioxanone resorbable pins (Orthosorb®, Johnson and Johnson, New Brunswick, NJ). Finally, the hip was relocated, and the gluteus minimus muscle was reattached to bone and the gluteus medius muscle and fascia latae were repaired with interrupted sutures. The procedure had a mean operative time of 62 minutes (range, 37–102 minutes).
All patients were maintained at toe-touch weightbearing with two crutches or a walker for 5 to 6 weeks. For the next 5 to 6 weeks, patients were advanced to approximately 50% weightbearing using a cane or crutch in the opposite hand. Patients were then advised to start full weightbearing as tolerated at 10 weeks postoperatively. Participation in sports and higher impact loading activities such as running were not recommended for the first 10 months postoperatively.
Survival was defined by whether the patient had subsequent surgery on the hip.
To assess how the results of the procedures for our cohort compared other nonvascularized bone grafting procedures in similarly aged patients at a similar length of followup, the authors carried out an extensive literature review of the databases of the National Library of Medicine, the National Institutes of Health, and EMBASE. We identified all articles concerning nonvascularized bone grafting for osteonecrosis of the femoral head. The key words used in the search were “hip,” “femoral head,” “osteonecrosis,” “avascular necrosis,” and “necrosis.” The initial search was refined with the addition of the keywords “core decompression,” “bone grafting,” “nonvascularized,” “trapdoor,” and “lightbulb” [49]. All articles identified in this manner were then subject to a review by two of us (TMS, DRM, MAM, or TF). The search revealed 26 published studies. A similar review was conducted to identify reports of nonoperative treatment for osteonecrosis of the head. This search revealed 11 reports. For both the nonoperative and nonvascularized bone grafting literature reviews we collected the following data: failure rates (in terms of later receiving a total hip arthroplasty), surgical technique, bone grafting procedure, and demographic variables.
Results
Overall, 26 of the hips survived out of the 39 hips treated (67%). At most recent followup, 24 of the 30 hips (80%) with small- or medium-sized lesions had avoided further surgery. Patients with large lesions fared poorly with only two of nine hips avoiding further surgery. When stratified by Ficat and Arlet stage, 18 of the 22 hips with Stage II disease did not undergo further surgery. Stage III hips were less successful with only eight of 17 hips surviving. There were similar results when analyzing location of lesion, with more lateral lesions faring more poorly than centrally located lesions (Table 1). Failures (n = 13) had a mean time to femoral head collapse of 13 months (range, 2–34 months) (Table 2).
Table 1.
Correlation between lesion size, location, Ficat and Arlet stage, and incidence of collapse
| Number of hips | Number collapsed | Incidence of collapse | |
|---|---|---|---|
| Lesion Size | |||
| Small | 7 | 1 | 13% |
| Medium | 23 | 5 | 17% |
| Large | 9 | 7 | 78% |
| Location of Lesion | |||
| A | 8 | 1 | 13% |
| B | 12 | 5 | 42% |
| C1 | 12 | 2 | 17% |
| C2 | 7 | 5 | 71% |
| Ficat and Arlet Stage | |||
| Stage I | 0 | NA | NA |
| Stage II | 22 | 4 | 18% |
| Stage III | 17 | 9 | 53% |
| Stage IV | 0 | NA | NA |
NA = not applicable.
Table 2.
Characteristics of clinical failures
| t | Age | Gender | Risk factors | Ficat and Arlet stage | Lesion size (Kerboul) | Location of lesion | Time to failure (months) |
|---|---|---|---|---|---|---|---|
| 1 | 36 | Male | hyperlipidemia, smoking | II | Medium | B | 16 |
| 2 | 22 | Male | alcohol | III | Large | B | 31 |
| 3 | 51 | Female | SLE, corticosteroids | III | Large | C1 | 12 |
| 4 | 27 | Female | SLE, corticosteroids | II | Medium | B | 8 |
| 5 | 37 | Male | HIV, hepatitis C | III | Large | C2 | 6 |
| 6 | 30 | Female | SLE, corticosteroids | II | Small | B | 34 |
| 7 | 36 | Male | alcohol, smoking | III | Large | C2 | 2 |
| 8 | 44 | Female | SLE, corticosteroids | III | Large | B | 16 |
| 9 | 52 | Male | HIV, hepatitis C | III | Large | C2 | 8 |
| 10 | 31 | Female | SLE, corticosteroids | II | Medium | A | 24 |
| 11 | 55 | Female | SLE, corticosteroids | II | Medium | C2 | 10 |
| 12 | 29 | Female | alcohol, smoking | III | Large | C1 | 7 |
| 13 | 41 | Male | corticosteroids | II | Medium | C2 | 9 |
SLE = systemic lupus erythematosus.
The mean preoperative Harris hip scores for all patients in this series was 50 points (range, 28–76 points). The preoperative scores for the hips that subsequently failed (mean, 47 points; range, 28–72 points) were similar (p = 0.175) to those of the survival group (mean, 52 points; range, 28–76 points). At a mean followup of 35 months, the mean postoperative score for the entire series improved to 75 points (range, 27–100 points) (p < 0.001). There were no perioperative complications documented.
Medically, one patient had a urinary tract infection which resolved without any sequelae. There were no other medical complications.
The overall early clinical success (defined as not later undergoing total hip arthroplasty) rate of 67% (26 of 39 hips) for this procedure as well as the 80% (24 of 30 hips) success rate for small and medium sized lesions compared similarly to other nonvascularized procedures performed at similar mean followup (range, 28–144 months) (Table 3). We have also provided results of nonoperative studies for comparison (Table 4).
Table 3.
Literature review of nonvascularized bone grafting techniques
| Study | Year | Hips | Followup (months) | Clinical success (%) | Radiographic success (%) |
|---|---|---|---|---|---|
| Trapdoor technique | |||||
| Meyers et al. [29] | 1983 | 21 | NA | 71 | NA |
| Meyers et al. [29] | 1991 | 9 | 35 (12–107) | 89 | NA |
| Ko et al.* [21] | 1995 | 14 | 53 (24–108) | 85 | 70 |
| Mont et al. [33] | 1998 | 30 | 56 (30–60) | 73 | 73 |
| Phemister technique | |||||
| Bonfiglio and Voke [4] | 1968 | 116 | 67 (24–204) | 78 | NA |
| Boettcher et al. [2] | 1970 | 38 | 72 (24–204) | 79 | 76 |
| Marcus et al. [24] | 1973 | 11 | N/A (24–48) | 90 | 91 |
| Dunn and Grow [9] | 1977 | 23 | 40 (27–98) | 74 | 30 |
| McBeath and Oeljen [26] | 1977 | 6 | NA | 83 | 0 |
| Smith et al. [55] | 1980 | 56 | 144 (24–332) | 57 | NA |
| Steinberg et al. [57] | 1984 | 19 | > 6 | 82 | 36 |
| Buckley et al. [6] | 1991 | 20 | 96 (24–228) | 90 | 90 |
| Nelson and Clark [39] | 1993 | 52 | NA (24–144) | 77 | 13 |
| Steinberg et al. [58] | 2001 | 312 | 63 (23–146) | 64 | 61 |
| Mont et al. [31] | 2003 | 21 | 48 (36–55) | 86 | 76 |
| Plakseychuk et al. [45] | 2003 | 50 | 60 (36–96) | 36 | 28 |
| Rijnen et al. [46] | 2003 | 28 | 50 (24–119) | 71 | 57 |
| Lieberman et al. [23] | 2004 | 17 | 53 (26–94) | 82 | 82 |
| Kim et al. [20] | 2005 | 30 | 50 (36–67) | 78 | 80 |
| Israelite et al. [16] | 2005 | 276 | N/A (24–145) | 62 | NA |
| Wang et al. [61] | 2005 | 28 | 26 (24–39) | 68 | 64 |
| Keizer et al. [18] | 2006 | 80 | 84 (36–NA) | 46 | 43 |
| Lightbulb technique | |||||
| Saito et al. [51] | 1988 | 18 | 48 (24–168) | 72 | 61 |
| Scher and Jakim* [53] | 1993 | 45 | 65 (36–126) | 87 | 71 |
| Rosenwasser et al. [48] | 1994 | 15 | 138 (108–180) | 86 | 86 |
| Mont et al. [31] | 2003 | 21 | 48 (36–55) | 86 | NA |
| Our study | 2007 | 47 | 28 (12–50) | 68 | 64 |
NA = data not available; *combined with osteotomy.
Table 4.
Literature review of nonoperative treatment outcomes
| Musso et al./1986 [38] | 50 | 30 | 32 |
|---|---|---|---|
| Steinberg et al./1989 [56] | 55 | 21 (6–120) | 16 |
| Churchill and Spencer/1991 [8] | 18 | 60 | 50 |
| Stulberg et al./1991 [59] | 22 | 27 | 9 |
| Robinson and Springer/1992 [47] | 16 | 39 (24–61) | 56 |
| Bradway and Morrey/1993 [5] | 15 | 23 (3–66) | 13 |
| Jergesen and Khan/1997 [17] | 19 | 111 (51–81) | 42 |
| Lai et al./2005 [22] | 25 | 24 | 32 |
| Hernigou et al./2006 [15] | 121 | 168 (120–240) | 25 |
| Neumayr et al./2006 [40] | 21 | 36 | 86 |
| Morse et al./2007 [37] | 67 | 23 (17–31) | 70 |
*Defined as not requiring conversion to total hip arthroplasty by final followup.
Discussion
Nonvascularized bone grafting techniques for the treatment of osteonecrosis of the femoral head were popularized in the 1950s and 1960s [3, 4, 43]. The literature reports a wide range of success rates with these techniques and this may be a result of the various surgical techniques and/or reflect the problem of choosing the appropriate treatment modality for the various disease stages. We evaluated our recent experience with nonvascularized bone grafting. The primary questions were whether this technique effectively deferred further surgical treatment when compared to those reported in studies using nonoperative treatment. In addition, we questioned whether the outcomes in this study were comparable to other studies of nonvascularized bone grafts.
Our study has several shortcomings including the small number of patients and the short-term followup. Nevertheless, the early results encourage the continued use and further study of this procedure. A larger series with longer followup will further help assess positive and negative predictors of outcome.
Several authors have described results comparable to ours using variations of these nonvascularized bone grafting procedures. Saito et al. [51] reported various treatment modalities for idiopathic necrosis of the femoral head. Their series included 18 hips with Ficat and Arlet Stage II osteonecrosis treated with a similar technique of nonvascularized bone grafting using cancellous bone obtained from the ipsilateral iliac crest. At a minimum followup of 24 months (mean, 48 months; range, 24–144 months), the clinical evaluation revealed Merle D’Aubigné [27] scores of 15 or more points in 13 of 18 hips. However, radiographic results demonstrated less favorable results, with seven of the 18 hips showing progressive femoral head collapse. We included both Ficat and Arlet Stage II and III hips, which may have contributed to the slightly lower chance of having a Harris hip score above 70.
The percentage of hips in our cohort of patients with nonvascularized bone grafting patients whom we considered had success treatment (67%) was similar to that in other reports in the literature (Table 3). The clinical success of the lightbulb technique ranged from 68% to 87% compared to a range of 36% to 90% reports for the Phemister technique. Similarly, the clinical success of the trapdoor technique ranged from 71% to 89%.
The proportion of nonvascularized bone grafting patients in our cohort who underwent total hip replacement (67%) was lower than six of the 11 studies that reported the outcomes of patients who were treated nonoperatively. The success (defined as not having total hip replacement by final followup) in studies from 1986 to 2007 of nonoperative treatment ranged from 9% to 86% (Table 4).
Other authors combined this technique with intertrochanteric osteotomy, use of growth factors, or gluteus medius muscle pedicle bone graft [31, 48, 52]. Scher and Jakim [52] prospectively studied 45 hips with Ficat and Arlet Stage III osteonecrosis treated with intertrochanteric osteotomy and nonvascularized bone grafting through a window in the femoral neck. The 5-year survival rate was 87%. This encouraging survival rate, however, should be critically evaluated because of the stringent inclusion criteria that were employed. The study included only patients younger than 45 years of age, with Ficat and Arlet Stage III of the anterosuperior part of the femoral head, with no underlying metabolic bone disease or systemic condition treated with chemotherapy or corticosteroids, and with no extensive involvement of the posterior part of the femoral head. Rosenwasser et al. [48] reported the long-term results of their series using the lightbulb technique. At a minimum followup of 120 months (mean, 144 months; range, 120–180 months), the survival rate was 87% with minimal disease progression. In three patients, the authors used a gluteus medius muscle pedicle graft to augment blood supply to the femoral neck. Mont et al. [31] reported on a series of 19 patients (21 hips) treated with bone morphogenetic protein-enriched allograft to avoid donor site morbidity. At a minimum followup of 36 months (mean, 48 months; range, 36–55 months), three hips had failed the bone grafting procedure. Interestingly, all failures occurred in hips with large-sized lesions, suggesting lesion size was associated with failure.
Despite the limitations of the study, we are encouraged by these early results using cancellous bone chips, bone marrow, and bone morphogenetic protein-7 as a nonvascularized bone grafting technique for the treatment of Stage II and III osteonecrosis of the femoral head. The decreased progression of symptoms at a mean of 36 months suggests the natural progression of the disease and subsequent hip arthroplasty surgery has been delayed. This technique is straightforward, has low donor site morbidity, and demonstrates a high degree of efficacy for Stage II and small to medium sized lesions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
The Phemister technique is illustrated with its three key steps. (TIF 699 kb)
The Trapdoor approach is illustrated with its five key steps. (TIF 479 kb)
Acknowledgments
We thank Colleen Kazmarek for her assistance in the preparation of this manuscript.
Footnotes
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-008-0211-x) contains supplementary material, which is available to authorized users.
References
- 1.Australian Orthopaedic Association Web site. The National Joint Replacement Registry: Annual Report. Available at: http://www.dmac.adelaide.edu.au/aoanjrr/documents/aoanjrr_2006_supplementary.pdf. Accessed March 15, 2007.
- 2.Boettcher WG, Bonfiglio M, Smith K. Non-traumatic necrosis of the femoral head. II. Experiences in treatment. J Bone Joint Surg Am. 1970;52:322–329. [PubMed]
- 3.Bonfiglio M, Bardenstein MB. Treatment by bone-grafting of aseptic necrosis of the femoral head and non-union of the femoral neck (Phemister technique). J Bone Joint Surg Am. 1958;40:1329–1346. [PubMed]
- 4.Bonfiglio M, Voke EM. Aseptic necrosis of the femoral head and non-union of the femoral neck. Effect of treatment by drilling and bone-grafting (Phemister technique). J Bone Joint Surg Am. 1968;50:48–66. [PubMed]
- 5.Bradway JK, Morrey BF. The natural history of the silent hip in bilateral atraumatic osteonecrosis. J Arthroplasty. 1993;8:383–387. [DOI] [PubMed]
- 6.Buckley PD, Gearen PF, Petty RW. Structural bone-grafting for early atraumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1991;73:1357–1364. [PubMed]
- 7.Canadian Institute for Health Information Web site. Canadian Joint Replacement Registry: Annual Report. Available at: http://secure.cihi.ca/cihiweb/dispPage.jsp?cw_page = services_cjrr_e#publications. Accessed April 1, 2007.
- 8.Churchill MA, Spencer JD. End-stage avascular necrosis of bone in renal transplant patients. The natural history. J Bone Joint Surg Br. 1991;73:618–620. [DOI] [PubMed]
- 9.Dunn AW, Grow T. Aseptic necrosis of the femoral head. Treatment with bone grafts of doubtful value. Clin Orthop Relat Res. 1977;249–254. [PubMed]
- 10.Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. [DOI] [PubMed]
- 11.Ganz R, Buchler U. Overview of attempts to revitalize the dead head in aseptic necrosis of the femoral head–osteotomy and revascularization. Hip. 1983;296–305. [PubMed]
- 12.Gibson A. Posterior exposure of the hip joint. J Bone Joint Surg Br. 1950;32:183–186. [DOI] [PubMed]
- 13.Hardinge K. The direct lateral approach to the hip. J Bone Joint Surg Br. 1982;64:17–19. [DOI] [PubMed]
- 14.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed]
- 15.Hernigou P, Habibi A, Bachir D, Galacteros F. The natural history of asymptomatic osteonecrosis of the femoral head in adults with sickle cell disease. J Bone Joint Surg Am. 2006;88:2565–2572. [DOI] [PubMed]
- 16.Israelite C, Nelson CL, Ziarani CF, Abboud JA, Landa J, Steinberg ME. Bilateral core decompression for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2005;441:285–290. [DOI] [PubMed]
- 17.Jergesen HE, Khan AS. The natural history of untreated asymptomatic hips in patients who have non-traumatic osteonecrosis. J Bone Joint Surg Am. 1997;79:359–363. [DOI] [PubMed]
- 18.Keizer SB, Kock NB, Dijkstra PD, Taminiau AH, Nelissen RG. Treatment of avascular necrosis of the hip by a non-vascularised cortical graft. J Bone Joint Surg Br. 2006;88:460–466. [DOI] [PubMed]
- 19.Kerboul M, Thomine J, Postel M, Merle d’Aubigne R. The conservative surgical treatment of idiopathic aseptic necrosis of the femoral head. J Bone Joint Surg Br. 1974;56:291–296. [PubMed]
- 20.Kim SY, Kim YG, Kim PT, Ihn JC, Cho BC, Koo KH. Vascularized compared with nonvascularized fibular grafts for large osteonecrotic lesions of the femoral head. J Bone Joint Surg Am. 2005;87:2012–2018. [DOI] [PubMed]
- 21.Ko JY, Meyers MH, Wenger DR. “Trapdoor” procedure for osteonecrosis with segmental collapse of the femoral head in teenagers. J Pediatr Orthop. 1995;15:7–15. [DOI] [PubMed]
- 22.Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am. 2005;87:2155–2159. [DOI] [PubMed]
- 23.Lieberman JR, Conduah A, Urist MR. Treatment of osteonecrosis of the femoral head with core decompression and human bone morphogenetic protein. Clin Orthop Relat Res. 2004;429:139–145. [DOI] [PubMed]
- 24.Marcus ND, Enneking WF, Massam RA. The silent hip in idiopathic aseptic necrosis. Treatment by bone-grafting. J Bone Joint Surg Am. 1973;55:1351–1366. [PubMed]
- 25.Matsuo K, Hirohata T, Sugioka Y, Ikeda M, Fukuda A. Influence of alcohol intake, cigarette smoking, and occupational status on idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 1988;234:115–123. [PubMed]
- 26.McBeath AA, Oeljen CG. Phemister bone graft for osteonecrosis post renal transplant. Clin Orthop Relat Res. 1977:164–168. [PubMed]
- 27.Merle D’Aubigne R. [Numerical classification of the function of the hip. 1970]. Rev Chir Orthop Reparatrice Appar Mot. 1990;76:371–374. [PubMed]
- 28.Merle D’Aubigne R, Postel M, Mazabraud A, Massias P, Gueguen J, France P. Idiopathic necrosis of the femoral head in adults. Journal of Bone and Joint Surgery Br. 1965;47:612–633. [PubMed]
- 29.Meyers MH, Jones RE, Bucholz RW, Wenger DR. Fresh autogenous grafts and osteochondral allografts for the treatment of segmental collapse in osteonecrosis of the hip. Clin Orthop Relat Res. 1983;174:107–112. [PubMed]
- 30.Mont MA, Einhorn TA, Sponseller PD, Hungerford DS. The trapdoor procedure using autogenous cortical and cancellous bone grafts for osteonecrosis of the femoral head. J Bone Joint Surg Br. 1998;80:56–62. [DOI] [PubMed]
- 31.Mont MA, Etienne G, Ragland PS. Outcome of nonvascularized bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2003;417:84–92. [DOI] [PubMed]
- 32.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–474. [DOI] [PubMed]
- 33.Mont MA, Jones LC, Einhorn TA, Hungerford DS, Reddi AH. Osteonecrosis of the femoral head. Potential treatment with growth and differentiation factors. Clin Orthop Relat Res. 1998;355(Suppl):S314–335. [PubMed]
- 34.Mont MA, Jones LC, Elias JJ, Inoue N, Yoon TR, Chao EY, Hungerford DS. Strut-autografting with and without osteogenic protein-1 : a preliminary study of a canine femoral head defect model. J Bone Joint Surg Am. 2001;83:1013–1022. [PubMed]
- 35.Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am. 2006;88:1117–1132. [DOI] [PubMed]
- 36.Mont MA, Ragland PS, Biggins B, Friedlaender G, Patel T, Cook S, Etienne G, Shimmin A, Kildey R, Rueger DC, Einhorn TA. Use of bone morphogenetic proteins for musculoskeletal applications. An overview. J Bone Joint Surg Am. 2004;86(Suppl 2):41–55. [DOI] [PubMed]
- 37.Morse CG, Mican JM, Jones EC, Joe GO, Rick ME, Formentini E, Kovacs JA. The incidence and natural history of osteonecrosis in HIV-infected adults. Clin Infect Dis. 2007;44:739–748. [DOI] [PubMed]
- 38.Musso ES, Mitchell SN, Schink-Ascani M, Bassett CA. Results of conservative management of osteonecrosis of the femoral head. A retrospective review. Clin Orthop Relat Res. 1986;207:209–215. [PubMed]
- 39.Nelson LM, Clark CR. Efficacy of phemister bone grafting in nontraumatic aseptic necrosis of the femoral head. J Arthroplasty. 1993;8:253–258. [DOI] [PubMed]
- 40.Neumayr LD, Aguilar C, Earles AN, Jergesen HE, Haberkern CM, Kammen BF, Nancarrow PA, Padua E, Milet M, Stulberg BN, Williams RA, Orringer EP, Graber N, Robertson SM, Vichinsky EP. Physical therapy alone compared with core decompression and physical therapy for femoral head osteonecrosis in sickle cell disease. Results of a multicenter study at a mean of three years after treatment. J Bone Joint Surg Am. 2006;88:2573–2582. [DOI] [PubMed]
- 41.Ohzono K, Saito M, Takaoka K, Ono K, Saito S, Nishina T, Kadowaki T. Natural history of nontraumatic avascular necrosis of the femoral head. J Bone Joint Surg Br. 1991;73:68–72. [DOI] [PubMed]
- 42.Ono K, Tohjima T, Komazawa T. Risk factors of avascular necrosis of the femoral head in patients with systemic lupus erythematosus under high-dose corticosteroid therapy. Clin Orthop Relat Res. 1992;277:89–97. [PubMed]
- 43.Phemister DB. Treatment of the necrotic head of the femur in adults. J Bone Joint Surg Am. 1949;31:55–66. [PubMed]
- 44.Phemister DB. Repair of bone in the presence of aseptic necrosis resulting from fractures, transplantations, and vascular obstruction. 1930. J Bone Joint Surg Am. 2005;87:672. [DOI] [PubMed]
- 45.Plakseychuk AY, Kim SY, Park BC, Varitimidis SE, Rubash HE, Sotereanos DG. Vascularized compared with nonvascularized fibular grafting for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2003;85:589–596. [DOI] [PubMed]
- 46.Rijnen WH, Gardeniers JW, Buma P, Yamano K, Slooff TJ, Schreurs BW. Treatment of femoral head osteonecrosis using bone impaction grafting. Clin Orthop Relat Res. 2003;417:74–83. [DOI] [PubMed]
- 47.Robinson Jr. HJ, Springer JA. Success of core decompression in the management of early stages of avascular necrosis: A four year prospective study. Orthop Trans. 1993;16:707.
- 48.Rosenwasser MP, Garino JP, Kiernan HA, Michelsen CB. Long term followup of thorough debridement and cancellous bone grafting of the femoral head for avascular necrosis. Clin Orthop Relat Res. 1994;306:17–27. [PubMed]
- 49.Rosenwasser MP, Michelsen CB, Kiernan HA. Treatment of avascular necrosis of the femoral head with curettage, iliac cacellous bone grafting, and revascularization with gluteus muscle pedicle flap. Orthop Trans. 1983;7:396.
- 50.Rue JP, Inoue N, Mont MA. Current overview of neurovascular structures in hip arthroplasty: anatomy, preoperative evaluation, approaches, and operative techniques to avoid complications. Orthopedics. 2004;27:73–81; quiz 82–73. [DOI] [PubMed]
- 51.Saito S, Ohzono K, Ono K. Joint-preserving operations for idiopathic avascular necrosis of the femoral head. Results of core decompression, grafting and osteotomy. J Bone Joint Surg Br. 1988;70:78–84. [DOI] [PubMed]
- 52.Scher MA, Jakim I. Intertrochanteric osteotomy and autogenous bone-grafting for avascular necrosis of the femoral head. J Bone Joint Surg Am. 1993;75:1119–1133. [DOI] [PubMed]
- 53.Scher MA, Jakim I. Late follow-up of femoral head avascular necrosis managed by intertrochanteric osteotomy & bone grafting. Acta Orthop Belg. 1999;65(Suppl 1):73–77. [PubMed]
- 54.Smith-Petersen MN. Evolution of mould arthroplasty of the hip joint. J Bone Joint Surg Br. 1948;30:59–75. [PubMed]
- 55.Smith KR, Bonfiglio M, Montgomery WJ. Non-traumatic necrosis of the femoral head treated with tibial bone-grafting. A follow-up note. J Bone Joint Surg Am. 1980;62:845–847. [PubMed]
- 56.Steinberg ME, Brighton CT, Corces A, Hayken GD, Steinberg DR, Strafford B, Tooze SE, Fallon M. Osteonecrosis of the femoral head. Results of core decompression and grafting with and without electrical stimulation. Clin Orthop Relat Res. 1989;249:199–208. [PubMed]
- 57.Steinberg ME, Brighton CT, Steinberg DR, Tooze SE, Hayken GD. Treatment of avascular necrosis of the femoral head by a combination of bone grafting, decompression, and electrical stimulation. Clin Orthop Relat Res. 1984:137–153. [PubMed]
- 58.Steinberg ME, Larcom PG, Strafford B, Hosick WB, Corces A, Bands RE, Hartman KE. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001;386:71–78. [DOI] [PubMed]
- 59.Stulberg BN, Davis AW, Bauer TW, Levine M, Easley K. Osteonecrosis of the femoral head. A prospective randomized treatment protocol. Clin Orthop Relat Res. 1991;268:140–151. [PubMed]
- 60.Swedish Orthopaedic Association Web site.The Swedish Hip Arthroplasty Register: Annual Report. Available at: http://www.jru.orthop.gu.se/. Accessed March 15, 2007.
- 61.Wang CJ, Wang FS, Huang CC, Yang KD, Weng LH, Huang HY. Treatment for osteonecrosis of the femoral head: comparison of extracorporeal shock waves with core decompression and bone-grafting. J Bone Joint Surg Am. 2005;87:2380–2387. [DOI] [PubMed]
- 62.Watson-Jones R. Fractures of the neck of the femur. Br J Surg. 1935;23:787–808. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
The Phemister technique is illustrated with its three key steps. (TIF 699 kb)
The Trapdoor approach is illustrated with its five key steps. (TIF 479 kb)