Abstract
Prolonged immobilization reduces passive range of motion of joints creating joint contractures. Whether and to what extent these iatrogenic contractures can be reduced is unknown. We raised three questions using an animal model: What degree of contracture remains at the end of a defined remobilization period? Do contractures in sham-operated and immobilized joints differ? What is the contribution of the posterior knee capsule in limiting knee extension? We immobilized one knee of 11 adult male rats in flexion to induce a joint contracture; 10 control animals underwent a sham operation. After 8 weeks, the internal fixation device was removed, and the animals were allowed to resume unrestricted activity for 4 weeks at the end of which the knee range of motion was measured with standardized torques. The mean flexion contracture was higher in the immobilized group (51.9° ± 2.8°) than in the sham-operated group (18.9° ± 2.1°). Eighty-eight percent of the contractures remained in the immobilized group after dividing skin and muscle, suggesting an important contribution of the posterior knee capsule in limiting knee mobility. Based on our preliminary study the range of motion of rat knees immobilized for 8 weeks remained substantially reduced after a 4-week period of unassisted remobilization.
Introduction
Motion of synovial joints is essential to maintain mobility in their full anatomic range of motion (ROM). Immobility can reduce passive ROM of joints [1, 6, 32], creating a joint contracture [2, 22, 28]. Contractures diminish joint function and lead to a reduction in patient autonomy [17, 20]. The important role and frequent use of immobilizing procedures to treat musculoskeletal disorders put joints at risk for having contractures develop [1, 37]. Patients with limited mobility and those confined to bed have multiple immobility-induced joint contractures develop [7, 9, 15, 25].
A few experimental studies on immobility-induced joint contractures suggest the timing of development of contractures. In several studies an internal fixation device to immobilize one hind limb of rats duplicated joint contractures in humans, with substantial reductions in the passive ROM [8, 12]. More recently, a time course study over 32 weeks in rat knees immobilized at 135° flexion caused a gradual decrease of the ROM in extension that reached 69° after the first 8 weeks and then leveled off [32]. The posterior capsular structures contributed to the restriction [31, 32]. The process by which immobility changes the mechanical properties of various articular structures and limits joint movement is not fully understood, but shortening, atrophy, and fibrosis of the posterior capsule have been reported [1, 18, 27, 30, 33].
Reversibility is clinically and functionally important for patients. Michelsson [19] used a plastic splint to immobilize 11 rabbit knees in extension and reported a reduction in mobility after 5 weeks that disappeared spontaneously in most cases after the splint was removed. But, should the immobilization last 6 to 7 weeks or longer, there was gradual but incomplete recovery after 8 weeks of remobilization, with the contracture being severe and permanent. Akeson et al. [4] investigated the reversibility of immobility-induced joint contractures using a model of rabbit knees fixed in a flexed position using transarticular wires. Knees immobilized for 9 weeks recovered completely 3 weeks after the fixation was removed. We immobilized canine shoulders for 12 weeks with a cast to create a contracture. Four weeks of remobilization did not reverse the limited motion but the limitation began to reverse after 8 weeks of remobilization and returned to normal after 12 weeks [24]. Van Harreveld et al. [35] enforced 8 weeks of exercise after 7 weeks of immobilization of the horse metacarpophalangeal joint but could not restore joint motion. Finally, immobilization of the rat ankle for 2 weeks revealed that treadmill running improved ROM compared with no running [23]. The literature on reversibility of joint contractures in animals therefore seems contradictory. The differences likely stem in part from the variety of joints, times and methods of immobilization, and animal species. The development of joint contractures secondary to immobility is gradual, and reversibility is not well documented.
We therefore raised three questions using an animal model: (1) What degree of contracture remains at the end of a 4-week remobilization period? (2) Do contractures in sham-operated and immobilized joints differ? (3) What is the contribution of the posterior knee capsule in limiting knee extension?
Materials and Methods
We immobilized the knees of 11 adult Sprague Dawley rats (300–350 g) in 135° flexion for 8 weeks then remobilized the knees for 4 weeks. Ten similar rats with sham surgery but no immobilization served as controls. All rats were obtained from one source (Charles River Laboratories, St Constant, Quebec, Canada) and housed individually in standard cages. At the end of 8 weeks we removed the fixation devices (experimental animals) and sham screws (control animals) and allowed free activity (unassisted remobilization) for 4 weeks. We then sacrificed the animals and measured knee motion. The contribution of the posterior knee capsule in limiting joint motion was determined by sectioning all skin and posterior muscles.
We immobilized the knees of the 11 experimental rats in 135° flexion using an internal system consisting of a Delrin® plate (DuPont Engineering Polymers, Wilmington, DE) secured with one screw to the proximal femur and one screw to the distal tibia. The course of the plate was such that it passed laterally between the peronei and lateral gastrocnemius muscles at the leg and medial to the tensor fasciae lata at the thigh without violating the knee. Details of the surgical protocol and a description of the immobilization system were published previously [32]. The knees of 10 rats (control group) underwent similar surgery with the insertion of two screws but not the plate.
Postoperatively the rats were allowed free activity in individual cages for 8 weeks. At the end of the 8-week period, the immobilized and control groups underwent limited surgery to remove the plate and/or screws and then were returned to their cages for a 4-week period of unrestricted activity in their cages. We defined remobilization as this 4-week period of spontaneous active mobilization after discontinuation of immobilization.
At the end of the 4-week remobilization period, the animals were euthanized with carbon dioxide exposure using a protocol approved by our animal ethics committee. Joint ROM was measured immediately with a graded spring-loaded goniometer (arthrometer) adapted to the rat knee [29]. The arthrometer measured the joint angles reached at two torque values: 667 g/cm (Torque 1) and 1060 g/cm (Torque 2). The motion in extension was measured on the operated knee and the contralateral knee. The angular displacement of the operated leg, immobilized or sham, was reported as the mean lack of ROM in extension (ie, an flexion contracture). The mean joint contracture was calculated by subtracting the ROM missing to reach full extension of the operated leg from the measurement taken from the contralateral leg (Fig. 1). To elucidate the contribution of the posterior knee capsule in limiting knee ROM, all skin and muscles crossing the knee were divided with a scalpel and the ROM measurement repeated. The contribution of the posterior knee capsule in limiting knee extension was calculated by the lack of ROM after division of skin and transarticular muscles.
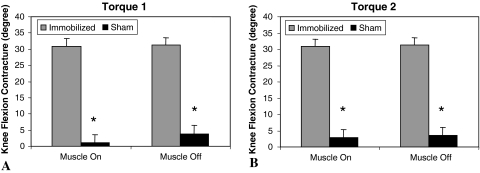
Fig. 1A–B.
Knee flexion contractures caused by 8 weeks of immobilization were not reversed by 4 weeks of remobilization. Contractures are calculated as the ROM lacking in extension of the surgically treated knee (sham or immobilized group) minus the ROM lacking in extension of the contralateral knee (sham or immobilized group) (data presented in Table 1) at two torque values: (A) Torque 1 = 667 g/cm and (B) Torque 2 = 1060 g/cm. Muscle on refers to results calculated from data on the intact knee; muscle off refers to results calculated from data on the knee after the skin and transarticular muscles were removed. Error bars represent standard error of the mean. * = p ≤ 0.05 between immobilized and sham-operated groups.
In humans, the knee extends to 0°. A knee flexion contracture is defined by the number of degrees missing to full extension. In normal Sprague Dawley rats, the knee does not reach full extension, possibly owing to the habitual knee flexion posture. Therefore, to report knee flexion contracture, we subtracted the lack of extension of the contralateral knee from the lack of extension of the surgically treated knee for the immobilized and the control animals.
All data were expressed as mean ± standard error of the mean. Lack of extension was compared between the immobilized and sham-operated groups using an unpaired t test. In both groups, the ROM was compared with that of the contralateral knee using paired t tests. For contracture, an unpaired t test was used to compare the means of the immobilized and control animals. We used a post hoc Bonferroni correction for multiple comparisons. The data were stored and analyzed using SPSS 15.0 (SPSS Inc, Chicago, IL).
Results
The mean flexion contracture after remobilization was 51.9° ± 2.8° in the immobilized knees and 18.9° ± 2.1° in the sham-operated knees at Torque 1 (Table 1, muscle on). The immobilized group lacked 41.9° ± 3.0° extension while the sham-operated group lacked 9.8° ± 2.4° at Torque 2 (Table 1, muscle on).
Table 1.
Range of motion in extension of rat knees*
| Group | Number of joints | Mean lack of knee extension ± SEM (°) | |||
|---|---|---|---|---|---|
| Torque 1 | Torque 2 | ||||
| Muscle on | Muscle off† | Muscle on | Muscle off | ||
| Experimental | |||||
| Immobilized | 11 | 51.9 ± 2.8 | 45.6 ± 2.7 | 41.9 ± 3.0 | 34.7 ± 2.9 |
| Contralateral | 11 | 21.1 ± 2.9‡ | 15.2 ± 2.5‡ | 10.3 ± 2.3‡ | 6.5 ± 2.3‡ |
| Control | |||||
| Sham-operated | 8 | 18.9 ± 2.1‡ | 15.4 ± 1.4‡ | 9.8 ± 2.4‡ | 6.3 ± 2.0‡ |
| Contralateral | 8 | 17.6 ± 2.7‡ | 11.5 ± 2.4‡ | 6.9 ± 2.0‡ | 2.8 ± 2.5‡ |
* Range of motion measurements correspond to the angular displacement missing to reach complete extension (0°) at the knee; Torque 1 = 667 g/cm; Torque 2 = 1060 g/cm; †the muscle off procedure isolated the posterior joint capsule as the tissue limiting knee ROM; ‡p < 0.05; SEM = standard error of the mean.
The contracture was greater (p < 0.05) in the immobilized group (51.9° ± 2.8°) than in the sham-operated group (18.9° ± 2.1°) (Table 1). The contracture also was greater (p < 0.05) in the immobilized legs than in their contralateral legs (21.1° ± 2.9°) at both torques (Table 1). Immobilized legs also lacked more (p < 0.05) ROM in extension than contralateral legs at both torques (Torque 1: 21.1° ± 2.9°; Torque 2: 10.3° ± 2.3°). Range of motion measurements of the surgically treated and contralateral legs in the sham-operated group were similar (Table 1).
Most (88% of the limitation at Torque 1 and 83% of the limitation at Torque 2) of the contracture remained after dividing the skin and transarticular muscles (Table 1). Similarly, the knee flexion contracture remained in the immobilized group after division of skin and muscle at both torque values (Fig. 1).
Discussion
Prolonged immobilization reduces passive ROM of joints creating joint contractures. Whether and to what extent these iatrogenic contractures can be reduced is not clearly known from the few published studies. We therefore raised three questions using an animal model: (1) What degree of contracture remains at the end of a defined remobilization period? (2) Do contractures in sham-operated and immobilized joints differ? (3) What is the contribution of the posterior knee capsule in limiting knee extension?
Given the single time point and number of animals, we considered this a preliminary study and therefore did not perform a power analysis. Additional studies would be required to fully characterize the response to remobilization, and we cannot say whether the contractures are permanent. We first tested animals for combined arthrogenic and myogenic restriction (muscle on) at both torque values and then for arthrogenic restriction only (muscle off) at both torque values. In the first series of testing, the posterior capsule and other arthrogenic structures may have been damaged beyond their elastic properties. This limitation would underestimate the restriction imposed by the capsular component of the knee.
Using the model of knee immobility, we previously documented a contracture after 8 weeks of immobility with a ROM of 69° before sectioning skin and transarticular muscles [32]. The current data show 4 weeks of remobilization did not restore normal joint mobility and there was a residual 42° extension contracture at Torque 1. Most of the limitation in knee ROM remained after division of skin and muscles. Sectioning of the posterior knee capsule eliminated all resistance to knee extension [31]. Therefore we concluded the posterior capsule of the knee caused the major limitation to knee extension after immobilization and remobilization.
In previous reports on the reversibility of immobility-induced joint contractures (Table 2), five studies used a protocol of remobilization not complemented with exercise or stretching and resulted in a lack of complete reversibility [10, 14, 19, 34, 35]. Of those five studies of unassisted remobilization, only one study reported both measurements of angular displacement and statistical analysis [14]. Similar to ours, that study reported incomplete reversibility of joint contractures secondary to immobility [14]. However, the duration of the remobilization period influenced the reversibility according to two studies [4, 24]: joint contractures were reversible after long periods of remobilization but irreversible after short periods although ROM measurements [24] and statistical analysis [4, 24] were not reported in those studies. Our data on remobilization after 8 weeks of immobility provide evidence for the lack of reversibility at 4 weeks.
Table 2.
Literature on reversibility of immobility-induced joint contractures
| Study | Animal joint | Immobilization system | Remobilization | Range of motion (measured at the end of remobilization) | Number | Statistics | Reversible |
|---|---|---|---|---|---|---|---|
| Michelsson [19] | Rabbit knee | Extension plaster, 5 weeks | Not assisted 8 weeks | No | 11 | No | No |
| Akeson et al. [4] | Rabbit knee | Flexion internal, 9 weeks | Not assisted 1,2,3,6,9 weeks | Yes | 25 | No | No: 1, 2, 3 weeks Yes: 6, 9 weeks |
| Finsterbush and Friedman [10] | Rabbit knee | Flexion cast, 2 weeks 6 weeks | Not assisted 6, 8 months | No | 22 | No | No |
| Hildebrand et al. [14] | Rabbit knee | Flexion 8 weeks | Not assisted 8, 16, 32 weeks | Yes | 28 | Yes | No |
| Usuba et al. [34] | Rat knee | Flexion internal, 40 days | Stretching 4 weeks | Yes | 53 | Yes | No |
| Sakakima et al. [23] | Rat ankle | Flexion cast, 2 weeks | Exercise 6 weeks | Yes | 39 | Yes | No: without exercise Yes: with exercise |
| Schollmeier et al. [24] | Canine shoulder | Cast, 12 weeks | Not assisted 4, 8, 12 weeks | No | 10 | No | No: 4, 8 weeks Yes: 12 weeks |
| Van Harreveld et al. [35] | Horse metacarpo- phalangeal | Cast, 7 weeks | Exercise 2 weeks | Yes | 5 | No | No |
Many anatomic structures potentially limit mobility of the knee. They include skin, bone, muscle, tendons, ligaments, and capsule. Published studies on the response of those structures to immobility is available except for the capsule. Although muscle fibers have plasticity for adaptive lengthening [26, 37], intrafascicular endomysium and intramuscular septa and perimysium, composed of dense connective fascia, may be more resistant to reestablishment of their previous length [1]. This also applies to tendons and ligaments [3, 5, 11, 13, 16, 21, 36, 38, 39]. Our data suggest a possible role of the capsule and intraarticular ligaments of the knee in the contracture. The capsular changes, synovial villi adhesion, synovial shortening, and atrophy were not amenable to spontaneous recovery after 4 weeks of remobilization [27, 30]. A temporal study to evaluate the duration of the immobilization period leading to irreversible joint contractures would provide the rationale to develop a protocol for timely clinical intervention. The incomplete reversibility of joint contractures underscores the need for active preventive action on immobile joints.
Range of motion of rat knees immobilized for 8 weeks remains substantially reduced after a 4-week period of unassisted remobilization. The posterior knee capsule was the primary structure restricting ROM.
Acknowledgments
We thank Julie Courchesne for assistance with these experiments.
Footnotes
One or more of the authors have received funding from the Canadian Institutes of Health Research (GT, HKU, OL) and the Canadian Institute of Musculoskeletal Health and Arthritis (JZ).
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Akeson WH, Amiel D, Abel MF, Garfin SR, Woo SL. Effects of immobilization on joints. Clin Orthop Relat Res. 1987;219:28–37. [PubMed]
- 2.Akeson WH, Amiel D, Woo SL. Immobility effects on synovial joints: the pathomechanics of joint contracture. Biorheology. 1980;17:95–110. [DOI] [PubMed]
- 3.Akeson WH, Woo SL, Amiel D, Coutts RD, Daniel D. The connective tissue response to immobility: biochemical changes in periarticular connective tissue of the immobilized rabbit knee. Clin Orthop Relat Res. 1973;93:356–362. [DOI] [PubMed]
- 4.Akeson WH, Woo SL, Amiel D, Doty DH. Rapid recovery from contractures in rabbit hindlimbs: a correlative biomechanical and biochemical study. Clin Orthop Relat Res. 1977;122:359–365. [PubMed]
- 5.Amiel D, Woo SL, Harwood FL, Akeson WH. The effect of immobilization on collagen turnover in connective tissue: a biochemical-biomechanical correlation. Acta Orthop Scand. 1982;53:325–332. [DOI] [PubMed]
- 6.Brandt KD. Response of joint structures to inactivity and to reloading after immobilization. Arthritis Rheum. 2003;49:267–271. [DOI] [PubMed]
- 7.Clavet H, Hebert P, Fergusson D, Doucette S, Trudel G. Joint contractures following prolonged stays in the intensive care unit. CMAJ. 2008, DOI: 10.1503/cmaj.071054. [DOI] [PMC free article] [PubMed]
- 8.Evans EB, Eggers GW, Butler JK, Blumel J. Experimental immobilization and remobilization of rat knee joints. J Bone Joint Surg Am.1960;42:737–758.
- 9.Fergusson D, Hutton B, Drodge A. The epidemiology of major joint contractures: a systematic review of the literature. Clin Orthop Relat Res. 2007;456:22–29. [DOI] [PubMed]
- 10.Finsterbush A, Friedman B. Reversibility of joint changes produced by immobilization in rabbits. Clin Orthop Relat Res. 1975;111:290–298. [DOI] [PubMed]
- 11.Frank C, MacFarlane B, Edwards P, Rangayyan R, Liu ZQ, Walsh S, Bray R. A quantitative analysis of matrix alignment in ligament scars: a comparison of movement versus immobilization in an immature rabbit model. J Orthop Res. 1991;9:219–227. [DOI] [PubMed]
- 12.Furlow LT Jr, Peacock EE Jr. Effect of beta-aminopropionitrile on joint stiffness in the rat. Ann Surg. 1967;165:442–447. [DOI] [PMC free article] [PubMed]
- 13.Harper J, Amiel D, Harper E. Collagenases from periarticular ligaments and tendons: enzyme levels during the development of joint contractures. Matrix. 1989;9:200–205. [DOI] [PubMed]
- 14.Hildebrand KA, Sutherland C, Zhang M. Rabbit knee model of post-traumatic joint contractures: the long-term natural history of motion loss and myofibroblasts. J Orthop Res. 2004;22:313–320. [DOI] [PubMed]
- 15.Kagaya H, Sharma M, Kobetic R, Marsolais EB. Ankle, knee, and hip moments during standing with and without joint contractures: simulation study for functional electrical stimulation. Am J Phys Med Rehabil. 1998;77:49–54. [DOI] [PubMed]
- 16.Karpakka J, Vaananen K, Virtanen P, Savolainen J, Orava S, Takala TE. The effects of remobilization and exercise on collagen biosynthesis in rat tendon. Acta Physiol Scand. 1990;139:139–145. [DOI] [PubMed]
- 17.Leblebici B, Adam M, Bagis S, Tarim AM, Noyan T, Akman MN, Haberal MA. Quality of life after burn injury: the impact of joint contracture. J Burn Care Res. 2006;27:864–868. [DOI] [PubMed]
- 18.Matsumoto F, Trudel G, Uhthoff H. High collagen type I and low collagen type III levels in knee joint contracture: an immunohistochemical study with histological correlate. Acta Orthop Scand. 2002;73:335–343. [DOI] [PubMed]
- 19.Michelsson JE. Thickness of the rabbit knee during and after immobilization. IRCS Med Sci Connect Tissue Skin Bone Pathol Surg Transplant. 1979;7:36.
- 20.Morris PE. Moving our critically ill patients: mobility barriers and benefits. Crit Care Clin. 2007;23:1–20. [DOI] [PubMed]
- 21.Noyes FR. Functional properties of knee ligaments and alterations induced by immobilization. Clin Orthop Relat Res. 1977;123:210–242. [PubMed]
- 22.Perry J. Contractures: a historical perspective. Clin Orthop Relat Res. 1987;219:8–14. [PubMed]
- 23.Sakakima H, Yoshida Y, Sakae K, Morimoto N. Different frequency treadmill running in immobilization-induced muscle atrophy and joint contracture of rats. Scand J Med Sci Sports. 2004;14:186–192. [DOI] [PubMed]
- 24.Schollmeier G, Sarker K, Fukuhara K, Uhthoff HK. Structural and functional changes in the canine shoulder after cessation of immobilization. Clin Orthop Relat Res. 1996;323:310–315. [DOI] [PubMed]
- 25.Souren LE, Franssen EH, Reisberg B. Contractures and loss of function in patients with Alzheimer’s disease. J Am Geriatr Soc. 1995;43:650–655. [DOI] [PubMed]
- 26.Tabary JC, Tabary C, Tardieu C, Tardieu G, Goldspink G. Physiological and structural changes in the cat’s soleus muscle due to immobilization at different lengths by plaster casts. J Physiol. 1972;224:231–244. [DOI] [PMC free article] [PubMed]
- 27.Trudel G, Jabi M, Uhthoff H. Localized and adaptive synoviocyte proliferation characteristics in rat knee joint contractures secondary to immobility. Arch Phys Med Rehabil. 2003;84:1350–1356. [DOI] [PubMed]
- 28.Trudel G, Laneuville O, Uhthoff HK. Joint contractures: editorial comment. Clin Orthop Relat Res. 2007;456:2. [DOI] [PubMed]
- 29.Trudel G, O’Neill PA, Goudreau LA. A mechanical arthrometer to measure knee joint contracture in rats. IEEE Trans Rehab Eng. 2000;8:149–155. [DOI] [PubMed]
- 30.Trudel G, Seki M, Uhthoff HK. Synovial adhesions are more important than pannus proliferation in the pathogenesis of knee joint contracture after immobilization: an experimental investigation in the rat. J Rheumatol. 2000;27:351–357. [PubMed]
- 31.Trudel G, Uhthoff HK. Contractures secondary to immobility: is the restriction articular or muscular? An experimental longitudinal study in the rat knee. Arch Phys Med Rehabil. 2000;81:6–13. [DOI] [PubMed]
- 32.Trudel G, Uhthoff HK, Brown M. Extent and direction of joint motion limitation after prolonged immobility: an experimental study in the rat. Arch Phys Med Rehabil. 1999;80:1542–1547. [DOI] [PubMed]
- 33.Trudel G, Uhthoff HK, Laneuville O. Prothrombin gene expression in articular cartilage with a putative role in cartilage degeneration secondary to joint immobility. J Rheumatol. 2005;32:1547–1555. [PubMed]
- 34.Usuba M, Akai M, Shirasaki BS, Miyakawa S. Experimental joint contracture correction with low torque: long duration repeated stretching. Clin Orthop Relat Res. 2007;456:70–78. [DOI] [PubMed]
- 35.Van Harreveld PD, Lillich JD, Kawcak CE, Gaughan EM, Mclaughlin RM, Debowes RM. Clinical evaluation of the effects of immobilization followed by remobilization and exercise on the metacarpophalangeal joint in horse. Am J Vet Res. 2002;63:282–288. [DOI] [PubMed]
- 36.Walsh S, Frank C, Hart D. Immobilization alters cell metabolism in an immature ligament. Clin Orthop Relat Res. 1992;277:277–288. [PubMed]
- 37.Winkelman C. Inactivity and inflammation in the critically ill patients. Crit Care Clin. 2007;23:21–34. [DOI] [PubMed]
- 38.Woo SL, Gomez MA, Inoue M, Akeson WH. New experimental procedures to evaluate the biomechanical properties of healing canine medial collateral ligaments. J Orthop Res. 1987;5:425–432. [DOI] [PubMed]
- 39.Woo SL, Gomez MA, Sites TJ, Newton PO, Orlando CA, Akeson WH. The biomechanical and morphological changes in the medial collateral ligament of rabbit after immobilization and remobilization. J Bone Joint Surg Am. 1987;69:1200–1211. [PubMed]