Abstract
Core decompression procedures have been used in osteonecrosis of the femoral head to attempt to delay the joint destruction that may necessitate hip arthroplasty. The efficacy of core decompressions has been variable with many variations of technique described. To determine whether the efficacy of this procedure has improved during the last 15 years using modern techniques, we compared recently reported radiographic and clinical success rates to results of surgeries performed before 1992. Additionally, we evaluated the outcomes of our cohort of 52 patients (79 hips) who were treated with multiple small-diameter drillings. There was a decrease in the proportion of patients undergoing additional surgeries and an increase in radiographic success when comparing pre-1992 results to patients treated in the last 15 years. However, there were fewer Stage III hips in the more recent reports, suggesting that patient selection was an important reason for this improvement. The results of the small-diameter drilling cohort were similar to other recent reports. Patients who had small lesions and were Ficat Stage I had the best results with 79% showing no radiographic progression. Our study confirms core decompression is a safe and effective procedure for treating early stage femoral head osteonecrosis.
Level of Evidence: Level IV, therapeutic study (see the Guidelines for Authors for a complete description of levels of evidence).
Introduction
Various techniques for performing core decompression have been used to save the osteonecrotic femoral head. There is also considerable disagreement as to the degree of efficacy of this procedure, how it might help, and the level of influence of various patient factors (such as a history of alcohol abuse or smoking, corticosteroid use, as well as underlying diagnoses such as systemic lupus erythematosus or sickle cell anemia) and radiographic lesion characterizations (such as presence or degree of collapse, lesion size or location).
The technique of performing core decompression has varied in terms of surgical approaches, number of drillings, and the diameter of the trephines. A number of authors have advocated the use of small-diameter percutaneous drilling and believe that it as effective as large-diameter core decompression procedures [56, 73, 95]. Some authors have supplemented core decompression with electrical stimulation [79] or growth and differentiation factors [19, 24, 82]. Other studies have reported adjunctive vascularized [96] and/or nonvascularized bone grafting [35, 63]. Vascularized fibular grafting is essentially a large core decompression procedure with the introduction of a vascularized fibula, ilium, or trochanteric bone on a more local pedicle. While vascularized and nonvascularized long cortical strut bone grafting approaches could be considered variations of core decompression procedures, we believe these procedures are sufficiently different that they should be considered as alternate approaches, rather than variations of core decompression and will not be considered in this study.
The primary question we asked was whether the efficacy of core decompression, measured in terms of decreased proportion of patients having additional surgeries or showing radiographic progression to collapse, has improved during the last 15 years using modern techniques. Using these same measures of efficacy, we also asked whether modern core decompression techniques provide better outcomes than those reported in studies using non-operative treatment. Secondary questions were: (1) whether the clinical and radiographic outcomes of hip osteonecrosis patients who were treated using a recently developed small-diameter drilling core decompression technique were similar to other modern studies; and (2) whether patients who had less radiographic progression and smaller lesion sizes at the time of treatment using small-diameter drilling would be less likely to have poor outcomes with subsequent collapse and the need for additional more invasive surgeries.
Materials and Methods
We systematically reviewed the literature on the Medline and EMBASE bibliographic databases that were related to core decompression and osteonecrosis of the hip. The initial search parameters used to identify potentially relevant articles were “necrosis and hip and decompression.” We then searched bibliographies of review articles for any additional relevant studies. Two of us (DRM, TMS) screened all articles according to a previously defined protocol [94]. The following inclusion/exclusion criteria were used: (1) The report provided radiographic outcomes and/or indicated whether patients underwent additional surgeries following an initial core decompression for the treatment of osteonecrosis of the hip; (2) We excluded reports that did not provide sufficient data to analyze outcomes or involved fewer than 10 patients, for example a report of a single patient treated with a powered core decompression [50]; (3) Only the most recent studies were included for patient cohorts reported at multiple times at different followups; (4) Although some reports included patients who were younger than 18 years old, we excluded studies that focused only on adolescent patients [84]; (5) We did not include reports that used long cortical strut bone grafting or vascularized bone grafting. We did include studies that reported the use of ancillary cancellous bone grafting such as the technique reported by Steinberg et al. [82]; (6) Studies with a mean followup of less than 18 months were excluded (see below for this exclusion rationale) [10, 40, 44, 65, 91]; (7) We also included the previously unpublished results of patients at our institution that were treated using a small-diameter drilling technique.
The criteria, which required a minimum mean 18-month followup for study inclusion, were used because it was believed unreasonable to consider shorter term followups when trying to assess efficacy and “failure” of these procedures. Eighteen months was utilized as approximately one standard deviation above the mean time to collapse of multiple studies (11 months). It can be difficult to determine the exact time to femoral head collapse, which may predict when a patient needs a hip replacement. This could occur fairly soon or months after head collapse when the patients’ hips become more symptomatic. An example of a study with data for mean time to collapse was from our patients who had percutaneous drilling. In this study patients had a mean time to detected femoral head collapse of 11 months which led to needing a total hip replacement at a mean of 14 months. For the purpose of this report, we used the mean of 11 months plus one standard deviation (6.9 months) to determine the previously noted minimum mean followup of 18 months for the studies in our literature review.
We made an attempt to stratify all studies that met our inclusion/exclusion criteria into two groups according to when the reported procedures were performed: before 1992, and from 1992 to 2007. When the dates of surgery were not specifically noted in the study, the followup and year the study was published were used to estimate the period in which the surgeries were performed. Some studies reported procedures both before and after 1992. For these studies, attempts were made to subgroup each patient according to when the procedure was performed. However, because it was impossible to stratify the patients for some reports, we categorized these studies by when the majority of the patients were treated. There were five studies classified as pre-1992 based on these criteria [7, 52, 54, 70, 82].
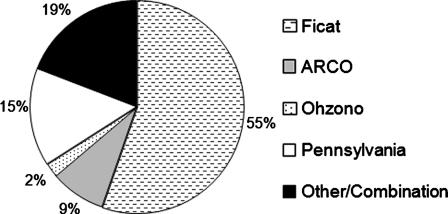
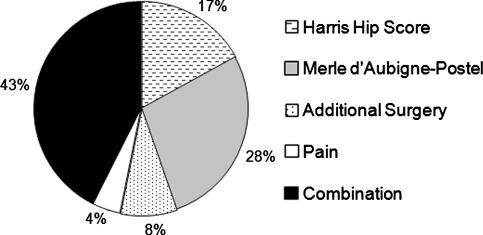
For each report included in this study, the level of evidence was determined using the Clinical Orthopaedics and Related Research guidelines [14]. The demographic data fields analyzed included: etiology/associated risk factors, age, followup, and preoperative stage of the disease as defined by Ficat [18]. The outcome parameters collected for each report were the number and percentage of additional surgeries and radiographic failures. Additional surgeries were only included if they were directly related to progression of the osteonecrosis. For example, if a patient had an evacuation of a hematoma it would not have been included as a case that underwent additional surgery. Due to the variability in the modalities used in the studies to assess radiographic outcomes (Fig. 1), progression to collapse or advancement after collapse was defined as radiographic failure for this study (Table 1). Radiographic outcomes were excluded for studies that did not indicate whether radiographic progression was to collapse [15, 41, 42, 70, 71] or if success was defined only in terms of a combination of radiographic and clinical failure without stratification [88, 97]. An attempt was made to also compare reported clinical outcomes. However, it was determined that the question of whether there were any differences was unanswerable using the literature given the variability and the inconsistency in clinical evaluation criteria used by the studies (Fig. 2).
Fig. 1.
The Ficat and Arlet system [18] has historically been the most frequently used staging modality. However, as noted in this graph, a large percentage of recent core decompression studies have reported using various other radiographic staging systems such as the Pennsylvania [81], ARCO [55], and Ohzono classifications [60].
Table 1.
Criteria for assessing effectiveness of core decompressions
| Measure | Inclusion/exclusion criteria | Examples |
|---|---|---|
| Additional surgery | 1. Include additional surgeries associated with progression of osteonecrosis. | Total hip arthroplasty, vascularized bone grafting, osteotomy |
| 2. Exclude surgeries not directly related to long-term failure of core decompression. | Evacuation of a hematoma | |
| Radiographic failure | 1. Include progression to collapse. | Progression from Ficat II to III. |
| 2. Include progression from collapse to further stage of degeneration. | Progression from Steinberg IV to V. | |
| 3. Exclude progression without collapse.† | Progression from ARCO I to II. |
†Studies that only indicated “progression” in stage without indicating whether the progression was to collapse were excluded from our analysis.
Fig. 2.
This figure provides the percentage of studies that used various clinical assessment modalities. The Harris hip score [22] and the Merle d’Aubigné-Postel scale [49] were the two most common evaluation methods used to assess clinical outcomes.
We identified 47 studies that reported on the outcome of core decompression in hip osteonecrosis and met our inclusion criteria. Approximately half (25 of 47, 53%) of these reports were Level of Evidence IV, and 6% (n = 3) were conducted at Level I (Fig. 3). Alcohol abuse and corticosteroid usage were the most frequently cited risk factors (Fig. 4). Overall, there were 2,605 hips treated with core decompression. From studies reporting relevant demographic data, the mean age for patients was 39 years (range, 12–83 years), and the minimum followup was 1 month (mean, 64 months; range, 1–216 months).
Fig. 3.
The studies reviewed in our meta-analysis were grouped according to their levels of evidence [14], and the proportion of studies for each level is presented in this chart. There have been relatively few randomized, prospective studies concerning osteonecrosis of the hip, and the majority of the reports have been level of evidence IV.
Fig. 4.
The most frequently reported etiology/risk factors are listed and the number of studies in our meta-analysis that reported the outcomes of patients who were diagnosed with each of these factors is noted.
While we do not consider withholding surgery an appropriate option based on previous studies showing outcomes that are less efficacious than interventional procedures used at our institution [51], we recognize that some physicians continue to utilize nonoperative treatment methods. To compare the results of core decompression to a baseline of natural progression, we conducted a separate literature search using the same criteria to identify a group of patients who were treated by nonoperative measures. Because the purpose of this review was to assess natural progression, we excluded nonoperative treatment modalities using external electrical therapy, ultrasound therapy, or pharmacological agents [39, 78, 90]. The mean age for these studies was 38 years (range, 13–79 years) and the minimum followup was 3 months (mean, 54 months; range, 3–240 months). The same outcome data was collected for these studies as for the review of core decompression reports.
From our institution, we identified 52 consecutive patients (79 hips) who had a core decompression utilizing a multiple small-diameter drilling (3.2–3.4 mm) technique with a minimum followup of 36 months (mean, 65 months; range, 36–81 months). The surgical technique used for these patients and the initial short-term followup of the first 45 hips was previously reported [56]. The most common risk factors in this cohort of patients were corticosteroids (n = 47 hips), tobacco abuse (n = 26 hips), and systemic lupus erythematosus (n = 20 hips) with some hips having multiple risk factors. Patients were assessed preoperatively and at final followup using the Harris hip score [22] and the Ficat and Arlet staging system [18] for clinical and radiographic evaluations, respectively. Additionally, lesion size was measured using the combined necrotic angle as described by Kerboul et al. [34]. For Stage I hips or patients in whom the lesion was not seen on radiographs, magnetic resonance imaging was used to determine the lesion size. Patients with collapse (Ficat Stage III or greater) were not candidates for this procedure. The radiographic evaluations were conducted by two of the authors (TMS, SDU). We evaluated the overall effectiveness of the small-diameter core decompression technique by combining the results of our study with those of a previously published small-diameter drilling study by Song et al. [73] and compared the proportions of patients who had radiographic failures or underwent additional surgeries to the outcomes of the other modern studies published since 1992.
To address the specific questions asked in this study, we compared the following groups: (1) procedures before 1992; (2) procedures from 1992 forward; (3) reports of nonoperative treatment; and (4) reports using the multiple small-diameter drilling technique. The number and percentage of additional surgeries and radiographic failures were stratified by Ficat stage when possible. For our percutaneous multiple small-diameter drilling cohort we also stratified the results by lesion size. A chi-square analysis was used to compare the differences in outcomes for all the groups that were evaluated. The key variable used for the power analysis was the difference in proportions of patients who underwent additional surgery in the pre-1992 studies compared to the studies from 1992 to 2007. A power analysis was conducted to ensure the comparison of failure rates was sufficiently powered (p < 0.05; power: 80%) to reveal the p values necessary to answer the primary research questions in this study. Prior studies that reported on comparisons of core decompression techniques were assessed to determine a clinically justifiable and appropriate effect size [1, 20]. Based on these studies and the success rates of core decompression that we have seen at our institution, we determined that we would need a minimum proportions sample size of 186 hips to identify an improvement from 60 percent to 45 percent of patients undergoing additional surgery. All comparisons were conducted using 95% confidence intervals where a p value of less than 0.05 was considered significant. We used SPSS version 13.0 software (SPSS Inc, Chicago, IL) for all analyses.
Results
Overall, the success rates were higher for the studies that reported core decompressions performed during the last 15 years (Table 2) compared to procedures performed before 1992 (Table 3). From these reports, there were 1337 hips treated before 1992 and 1268 hips since 1992. The proportion of patients surviving without additional surgery increased (p < 0.001) from 59% (range, 29%–85%) in the earlier studies to 70% (range, 39%–100%) in the more recent reports. Similarly, the radiographic success also increased (p = 0.027) from 56% (range, 0–94%) for the pre-1992 cohort to 63% (range, 22%–90%). Stratification by Ficat stage (Table 4) showed there were fewer (p < 0.001) patients who were Ficat Stage III after 1992.
Table 2.
Literature review of core decompression outcomes for 1992 to 2007 patient cohort studies
| Author/Year | Number of hips | Months followup (range) | Additional surgery (%) | Radiographic failure (%) |
|---|---|---|---|---|
| Kane et al./1996 [33] | 19 | (24–60) | 11 (58) | 11 (58) |
| Markel et al./1996 [47] | 54 | (2–53) | 26 (48) | – |
| Chang et al./1997 [11] | 84 | 57 (24 to 165) | 22 (26) | 59 (70) |
| Mazieres et al./1997 [48] | 20 | 24 | 9 (45) | 9 (45) |
| Powell et al./1997 [64] | 34 | 48 | 9 (26) | – |
| Iorio et al./1998 [30] | 33 | 64 (24–120) | 11 (33) | 18 (55) |
| Scully et al./1998 [68] | 98 | (21–50) | 52 (53) | – |
| Chen et al./2000 [12] | 27 | 28 (12–128) | – | 10 (37) |
| Lavernia and Sierra/2000 [41] | 67 | 41 | 16 (24) | – |
| Maniwa et al./2000 [46] | 26 | 94 (53–164) | 8 (31) | – |
| Specchiulli et al./2000 [74] | 20 | 67 | 4 (20) | 4 (20) |
| Piperkovski/2001 [62] | 39 | 48 | 4 (10) | – |
| Yoon et al./2001 [97] | 39 | 61 (24–118) | 19 (49) | – |
| Aigner et al./2002 [2] | 45 | 69 (31–120) | 7 (16) | 12 (27) |
| Hernigou et al./2003a [23] | 189 | 84 (60–132) | 34 (18) | 39 (21) |
| Wirtz et al./2003† [93] | 51 | (36–132) | 18 (35) | – |
| Gangji et al./2004a [20] | 10 | 24 | 0 (0) | 1 (10) |
| Gangji et al./2004 [20] | 8 | 24 | 2 (25) | 5 (63) |
| Lieberman et al./2004a [45] | 17 | 53 (26–94) | 3 (18) | 3 (18) |
| Bellot et al./2005 [4] | 31 | (1–176) | 19 (61) | 19 (61) |
| Ha et al./2006 [21] | 18 | (50–96) | – | 14 (78) |
| Neumayr et al./2006 [59] | 17 | 36 | 3 (18) | – |
| Veillette et al./2006c [89] | 58 | 24 (6–52) | 9 (16) | 16 (28) |
| Marker et al./2007b, †† | 79 | 24 (20–39) | 27 (34) | 27 (34) |
| Shuler et al./2007c [69] | 22 | 39 (27–59) | 3 (14) | 3 (14) |
| Song et al./2007b [73] | 163 | 87 (60–134) | 50 (31) | – |
| Total | 1268 | 63 (1–176)‡ | 366 (30)‡‡ | 250 (37)¥ |
†Previous study not listed includes Wirtz et al. [92]; †† Results of the present study. Previous study not listed includes Mont et al. [56]; ‡ Weighted average follow-up; ‡‡ Data for total of 1223 hips; ¥ Data for total of 680 hips; a biologics; b multiple small diameter drilling; c tantalum; – = Data meeting our definition of additional surgery or radiographic failure was not available.
Table 3.
Literature review of core decompression outcomes for pre-1992 patient cohort studies
| Author/Year | Number of hips | Months follow-up (range) | Additional surgery (%) | Radiographic failure (%) |
|---|---|---|---|---|
| Solomon/1981 [72] | 22 | 24 (6–48) | 5 (23) | – |
| Ficat/1985 [18] | 133 | 114 (60–204) | – | 28 (21) |
| Camp and Colwell/1986 [9] | 40 | 18 (3–40) | 6 (15) | 8 (20) |
| Hopson and Siverhus/1988 [28] | 20 | 39 (12–78) | 12 (57) | – |
| Saito et al./1988 [67] | 17 | 48 (24–168) | – | 9 (53) |
| Tooke et al./1988 [86] | 45 | 36 (12–84) | 16 (36) | 16 (36) |
| Aaron et al./1989 [1] | 50 | 26 | 28 (56) | 32 (64) |
| Aaron et al./1989a [1] | 56 | 27 | 18 (32) | 22 (39) |
| Beltran et al./1990 [5] | 34 | 23 (11–47) | – | 16 (47) |
| Trancik et al./1990a [87] | 11 | 45 (24–60) | 5 (45) | 11 (100) |
| Kristensen et al./1991 [37] | 18 | 39 (12–60) | – | 3 (17) |
| Stulberg et al./1991 [83] | 28 | 27 | 8 (29) | 21 (75) |
| Robinson and Springer/1993 [66] | 19 | 48 | 3 (16) | 4 (21) |
| Lafforgue et al./1993 [38] | 27 | 46 | – | 17 (63) |
| Leder and Knahr/1993 [43] | 47 | 44 (24–100) | 9 (19) | 11 (23) |
| Holman et al./1995 [27] | 31 | (18–67) | 14 (45) | 8 (40)* |
| Koo et al./1995 [36] | 18 | (minimum 24) | – | 14 (78) |
| Smith et al./1995 [71] | 114 | 40 (24–78) | 64 (56) | – |
| Mont et al./1997† [52] | 79 | 144 (48–216) | 37 (47) | – |
| Mont et al./1998 [53] | 68 | 144 (48–216) | 48 (71) | 48 (71) |
| Bozic et al./1999 [7] | 54 | 120 (24–196) | 28 (52) | 34 (62) |
| Simank et al./1999 [70] | 94 | 72 (18–180) | 32 (34) | – |
| Steinberg et al./2001††,a [82] | 312 | 48 (3–155) | 113 (36) | – |
| Total | 1337 | 65 (3–216)‡ | 446 (41)‡‡ | 302 (44)¥ |
* Radiographic outcomes were only provided for 20 hips; † Previous studies not listed include Hungerford and Zizic [29] and Fairbank et al. [17]; †† Other studies not listed include Steinberg et al. [75, 77–80] and Israelite et al. [31]; ‡ Weighted average follow-up; ‡‡ Data for total of 1090 hips; ¥ Data for total of 685 hips; a core decompression combined with electrical stimulation; – = Data meeting our definition of additional surgery or radiographic failure was not available.
Table 4.
Comparison of historical and modern core decompression studies
| Data* | Studies prior to 1992 | Studies from 1992 to 2007 | p-Value |
|---|---|---|---|
| Demographic variables | |||
| Mean age (range) | 39 (15–83) years | 39 (13–72) years | – |
| Mean followup (range) | 65 (3–216) months | 63 (1–176) months | – |
| Preoperative ficat stage | |||
| Ficat Stage I | 32% | 29% | 0.302 |
| Ficat Stage II | 42% | 52% | < 0.001† |
| Ficat Stage III | 27% | 19% | < 0.001† |
| Outcomes | |||
| Additional surgery | |||
| Overall | 41% | 30% | < 0.001† |
| Ficat Stage I | 15% | 20% | 0.413 |
| Ficat Stage II | 44% | 35% | 0.056 |
| Ficat Stage III | 67% | 66% | 0.939 |
| Radiographic failure | |||
| Overall | 44% | 37% | < 0.001† |
| Ficat Stage I | 22% | 21% | 0.919 |
| Ficat Stage II | 47% | 48% | 0.887 |
| Ficat Stage III | 66% | 50% | 0.708 |
* Some studies did not stratify by Ficat stage and/or report both outcome measures.
†Values were statistically significant.
The reports of nonoperative treatment (Table 5) had higher proportions of failures compared to the core decompression studies from 1992 to 2007. There were 791 hips in 18 studies between 1960 and 2007. In the studies that reported relevant data, the proportion of patients who underwent surgery by final followup at a mean of 67% (range, 14% to 91%) was statistically higher than the modern reports (p < 0.001). Similarly, the mean reported radiographic failure rates at 72% (range, 41% to 100%) were considerably higher (p < 0.001). Only 164 natural history patients were reported between 1992 and 2007, although the clinical and radiographic failure rates were similar between this group of patients and those evaluated before 1992.
Table 5.
Literature review of nonoperative treatment outcomes
| Author/Year | Number of hips | Months followup (range) | Additional surgery (%) | Radiographic failure (%) |
|---|---|---|---|---|
| Coste et al./1965 [16] | 100 | 24 | – | 73 (73) |
| Merle d’Aubigne et al./1965 [49] | 90 | 36 (12–48) | – | 61 (68) |
| Boettcher et al./1970 [6] | 5 | (minimum 24) | – | 4 (80) |
| Zizic and Hungerford/1985 [98] | 15 | 44 | – | 13 (87) |
| Musso et al./1986† [58] | 50 | 30 | 34 (68) | 41 (82) |
| Steinberg et al./1989 [79] | 55 | 21 (6–120) | 46 (84) | – |
| Churchill and Spencer/1991 [13] | 18 | 60 | 9 (50) | 8 (44) |
| Ohzono et al./1991 [61] | 115 | 63 | – | 78 (68) |
| Stulberg et al./1991 [83] | 22 | 27 | 20 (91) | 11 (50) |
| Robinson and Springer/1993 [66] | 16 | 39 (24–61) | 7 (44) | 9 (56) |
| Bradway and Morrey/1993 [8] | 15 | 23 (3–66) | 13 (87) | 15 (100) |
| Koo et al./1995 [36] | 19 | (minimum 24) | – | 15 (79) |
| Jergesen et al./1997 [32] | 19 | 111 (51–81) | 11 (58) | 7 (41)* |
| Lai et al./2005 [39] | 25 | 24 | 17 (68) | 19 (76) |
| Ha et al./2006 [21] | 19 | (50–96) | – | 15 (79) |
| Hernigou et al./2006†† [26] | 121 | 168 (120–240) | 91 (75) | 93 (77) |
| Neumayr et al./2006 [59] | 21 | 36 | 3 (14) | – |
| Morse et al./2007 [57] | 67 | 23 (17–31) | 20 (30) | – |
| Total | 792 | 53 (3–240)‡ | 271 (63)‡‡ | 455 (72)¥ |
* Radiographs were only available for 17 patients; † Previous study not listed includes Bassett et al. [3]; †† Previous studies not listed include Hernigou et al. [23, 25]; ‡ Weighted average follow-up; ‡‡ Data for total of 429 hips; ¥ Data for total of 630 hips; – = Data meeting our definition of additional surgery or radiographic failure was not available.
The results using the small-diameter drilling technique at our institution combined with those reported by Song et al. [73] were similar to other studies of the last 15 years (Table 6). At our institution, there were 21 patients (27 hips, 34%) who underwent additional surgery. The distribution of Harris hip scores by number of hips were: 25 (90 points or greater), 24 (80–89 points), seven (70–79 points), and 23 (less than 70 points). Excluding the patients who underwent additional surgery, the mean Harris hip score was 89 points (range, 72–100 points). Two patients (three hips) both had scores of 72 points but did not receive additional treatment. The patient who had bilateral osteonecrosis reported moderate pain in both hips. The other patient progressed from Ficat stage I to Ficat Stage II and his reported pain scores increased from mild (30 points) preoperatively to moderate (20 points) at final followup. There were 27 hips (34%) that showed radiographic progression of the disease to collapse following core decompression.
Table 6.
Multiple small-diameter drilling compared to other modern studies
| Data | Small-diameter technique | Other 1992–2007 studies | p Value |
|---|---|---|---|
| Demographics | |||
| Number of hips | 242 | 1026 | – |
| Mean age (range) | 39 (18–72) | 39 (12–71) | – |
| Mean followup (range) | 80 (36–134) | 58 (1–176) | – |
| Outcomes | |||
| Additional surgery | 32% | 29% | 0.520 |
| Radiographic failure | 34% | 37% | 0.437 |
Patients in our small-diameter drilling cohort with higher Ficat stages and larger lesion sizes had increased failure rates. The proportion of hips (n = 13, 59%) with a large lesion (combined necrotic angle ≥ 200°) that underwent additional surgery was greater (p = 0.008) than the proportion of hips (n = 14, 25%) that had small lesions (a combined necrotic angle < 200°) and underwent additional surgery. Similarly, the rate of additional surgery was higher (p = 0.044) for hips that were Ficat Stage II (52%) preoperatively, compared to Ficat Stage I (26%). The best results were seen in patients who had small lesions and Ficat Stage I prior to treatment with 79% of these hips showing no radiographic stage progression.
Discussion
While core decompression is relatively commonly performed for ON of the femoral head, the variations in reported techniques and drilling procedures make it difficult to interpret the efficacy of these procedures. Some recent reports using innovative techniques such as growth and differentiation factors to fill the core decompression tract suggest excellent results, although the literature contains a wide variety of results. Because of the relatively small number of procedures reported for each of these studies reporting on varied techniques, we analyzed recent techniques by comparing studies that reported procedures that were performed before 1992 to reports that had procedures between 1992 and 2007. The primary question of our study was whether the outcomes reported in the recent studies were better than those prior to 1992 in terms of reduced proportions of patients having additional surgeries and/or showing radiographic signs of femoral head collapse. Additionally, using these same measures, we asked whether modern core decompression techniques provided better outcomes than non-operative treatment.
One of the limitations of this study was the small numbers of patients in many of the reports reviewed. Another limitation was that in some cases it was difficult to determine when the core decompressions were performed in order to stratify the study as pre-1992 or 1992 to 2007. However, we believe our approach of using the publication date and the mean followup to estimate when procedures were performed would correctly stratify the majority of the studies that were close to our 1992 cutoff. In addition, there were only midterm mean followups (range, 18 months to 144 months) for many studies, and the long-term outcome of core decompression is unclear. Another limitation was the level of evidence for the scientific literature reviewed. As previously noted, most of the studies were Level IV and there were few Level I studies. There is a need for more prospective randomized multicenter studies that further analyze some of these newer techniques which will need longer followup and larger patient numbers in the future. Additionally, if standardized clinical and radiographic evaluation criteria were adopted, future meta-analyses could provide more valid comparisons across studies. The limitations of our assessment of the percutaneous multiple small-diameter drilling technique were similar to those of other studies: a limited number of patients from a single center, no long-term followup, and lack of a randomized control group. Nevertheless, we do not believe these limitations detract from the overall results of the present study, as in general, the results of all of the different techniques were somewhat comparable and appear better than the natural history.
The meta-analysis and our cohort of multiple small-diameter drilling patients suggest that core decompression provides fewer treatment failures than nonoperative treatment. Although there are improvements in overall success rates for the procedures performed from 1992 to present, the stratification of the meta-analysis data by Ficat stage suggests that patient selection may have been the primary reason for this gain as there were fewer Ficat Stage III patients in the later studies. However, based on the improvements in clinical outcomes for Ficat Stage II hips, it appears that modern core decompression techniques did provide improved outcomes for some subsets of patients.
The literature review (Table 2) suggests patients who have hips with Ficat Stage III disease are more likely to have radiographic progression, clinical failure, and have additional surgeries, suggesting these patients may not be appropriate candidates for this procedure. Although there appears to have been increased patient selectivity in the past 15 years in terms of fewer Ficat Stage III hips being treated with core decompressions, a number of surgeons continue to use this procedure. Based on the literature review, there were 132 patients (18% of all patients in studies after 1992 that stratified hips by Ficat stage) who were Stage III and treated using core decompression. These patients were included in 9 of the 35 studies (26%) after 1992. A recent study by Tingart et al. [85] reported similar results. They reported 11% of surgeons they surveyed used core decompression for patients who were Ficat Stage III or IV. While some surgeons may be using core decompression only as a pain-relieving procedure or assessing the potential efficacy of modern techniques in Stage III hips, we continue to recommend that other treatment options such as total hip arthroplasty or resurfacing be used for these difficult to treat patients.
Our own data from patients in whom we used small-diameter multiple drilling also confirms that the prognosis is influenced by the extent of the lesion size (Table 3). These results are similar to a prospective study of 73 hips by Steinberg et al. [76] which evaluated the effect of lesion size on the outcome of core decompression. They defined three groups based on lesion size: small, less than 15% of femoral head involvement; medium, 15% to 30%; and large, greater than 30%. The difference between the percentage of patients who had small lesions and later underwent total hip arthroplasty (7%) was lower than patients with large lesions (33%) who received a total hip arthroplasty.
The overall success rate of our cohort of small-diameter multiple drilling patients was similar to two other recent studies that used a similar technique. In one of these studies, Yan et al. [95] reported an improvement in Harris hip score from a mean of 58 points (range, 46–89 points) preoperatively to a mean of 86 points (range, 70–94 points) at a minimum 2-year followup. In the other study by Song et al. [73], 79% of patients who had Ficat Stage I disease had no additional surgery at a minimum 5-year followup. The rationale and advantages for the small-diameter drilling presented in these prior studies were that: (1) the small diameter drill can more easily reach the anterior portion of the femoral head, an area frequently involved in osteonecrosis; (2) there is minimal morbidity; (3) the risk of weakening or penetrating the femoral head and injuring the articular cartilage when using a large-diameter trephine for multiple drillings is potentially reduced; and (4) the risk of stress risers that can lead to a subtrochanteric fracture is also reduced.
The literature review and our data suggest recent techniques provide better clinical scores or radiographic outcomes than pre-1992 studies of core decompression. However, it is unclear whether this improvement is due to improved patient selection or surgical technique. At a minimum, the additional accumulation of successful reports in the last decade confirms that core decompression is a safe and effective procedure for the treatment of early stages of osteonecrosis of the femoral head. Based on the results of our experience as well as other studies, we will use core decompression to treat patients who have early small- and medium-sized lesions and are Ficat Stage I or II. Additionally, the midterm followup of the multiple small-diameter core decompression patients at our institution was longer than most studies, and had a success rate similar to or higher than other reports, which makes this technique the authors’ preferred modality. However, prospective, randomized studies are recommended to verify these observations before this technique can be recommended as a standard for practicing surgeons.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aaron RK, Lennox D, Bunce GE, Ebert T. The conservative treatment of osteonecrosis of the femoral head. A comparison of core decompression and pulsing electromagnetic fields. Clin Orthop Relat Res. 1989;249:209–218. [PubMed]
- 2.Aigner N, Schneider W, Eberl V, Knahr K. Core decompression in early stages of femoral head osteonecrosis–an MRI-controlled study. Int Orthop. 2002;26:31–35. [DOI] [PMC free article] [PubMed]
- 3.Bassett CA, Schink MM, Mitchell SN. Treatment of osteonecrosis of the hip with specific, pulsed electromagnetic fields (PEMFs): a preliminary clinical report. In: Arlet J, Ficat RP, Hungerford DS (eds). Bone Circulation. Baltimore, MD: William and Wilkins; 1984:343–354.
- 4.Bellot F, Havet E, Gabrion A, Meunier W, Mertl P, de Lestang M. Core decompression of the femoral head for avascular necrosis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2005;91:114–123. [DOI] [PubMed]
- 5.Beltran J, Knight CT, Zuelzer WA, Morgan JP, Shwendeman LJ, Chandnani VP, Mosure JC, Shaffer PB. Core decompression for avascular necrosis of the femoral head: correlation between long-term results and preoperative MR staging. Radiology. 1990;175:533–536. [DOI] [PubMed]
- 6.Boettcher WG, Bonfiglio M, Smith K. Non-traumatic necrosis of the femoral head. II. Experiences in treatment. J Bone Joint Surg Am. 1970;52:322–329. [PubMed]
- 7.Bozic KJ, Zurakowski D, Thornhill TS. Survivorship analysis of hips treated with core decompression for nontraumatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 1999;81:200–209. [DOI] [PubMed]
- 8.Bradway JK, Morrey BF. The natural history of the silent hip in bilateral atraumatic osteonecrosis. J Arthroplasty. 1993;8:383–387. [DOI] [PubMed]
- 9.Camp JF, Colwell CW Jr. Core decompression of the femoral head for osteonecrosis. J Bone Joint Surg Am. 1986;68:1313–1319. [PubMed]
- 10.Chan TW, Dalinka MK, Steinberg ME, Kressel HY. MRI appearance of femoral head osteonecrosis following core decompression and bone grafting. Skeletal Radiol. 1991;20:103–107. [DOI] [PubMed]
- 11.Chang MC, Chen TH, Lo WH. Core decompression in treating ischemic necrosis of the femoral head. Zhonghua Yi Xue Za Zhi (Taipei). 1997;60:130–136. [PubMed]
- 12.Chen CH, Chang JK, Huang KY, Hung SH, Lin GT, Lin SY. Core decompression for osteonecrosis of the femoral head at pre-collapse stage. Kaohsiung J Med Sci. 2000;16:76–82. [PubMed]
- 13.Churchill MA, Spencer JD. End-stage avascular necrosis of bone in renal transplant patients. The natural history. J Bone Joint Surg Br. 1991;73:618–620. [DOI] [PubMed]
- 14.Clinical Orthopaedics and Related Research. Author Resources. Available at: http://edmgr.ovid.com/corr/accounts/ifauth.htm. Accessed April 1, 2007.
- 15.Coleman BG, Kressel HY, Dalinka MK, Scheibler ML, Burk DL, Cohen EK. Radiographically negative avascular necrosis: detection with MR imaging. Radiology. 1988;168:525–528. [DOI] [PubMed]
- 16.Coste F, Merle DAR, Postel M, Massias P, Gueguen J, Grellat P. Course of primary osteonecrosis of the femoral head (pon) and therapeutic prospects [in French]. Presse Med. 1965;73:263–267. [PubMed]
- 17.Fairbank AC, Bhatia D, Jinnah RH, Hungerford DS. Long-term results of core decompression for ischaemic necrosis of the femoral head. J Bone Joint Surg Br. 1995;77:42–49. [PubMed]
- 18.Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. [DOI] [PubMed]
- 19.Gangji V, Hauzeur JP. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. Surgical technique. J Bone Joint Surg Am. 2005;87 Suppl 1:106–112. [DOI] [PubMed]
- 20.Gangji V, Hauzeur JP, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86:1153–1160. [DOI] [PubMed]
- 21.Ha YC, Jung WH, Kim JR, Seong NH, Kim SY, Koo KH. Prediction of collapse in femoral head osteonecrosis: a modified Kerboul method with use of magnetic resonance images. J Bone Joint Surg Am. 2006;88 Suppl 3:35–40. [DOI] [PubMed]
- 22.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed]
- 23.Hernigou P, Bachir D, Galacteros F. The natural history of symptomatic osteonecrosis in adults with sickle-cell disease. J Bone Joint Surg Am. 2003;85:500–504. [DOI] [PubMed]
- 24.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14–23. [DOI] [PubMed]
- 25.Hernigou P, Galacteros F, Bachir D, Goutallier D. Deformities of the hip in adults who have sickle-cell disease and had avascular necrosis in childhood. A natural history of fifty-two patients. J Bone Joint Surg Am. 1991;73:81–92. [PubMed]
- 26.Hernigou P, Habibi A, Bachir D, Galacteros F. The natural history of asymptomatic osteonecrosis of the femoral head in adults with sickle cell disease. J Bone Joint Surg Am. 2006;88:2565–2572. [DOI] [PubMed]
- 27.Holman AJ, Gardner GC, Richardson ML, Simkin PA. Quantitative magnetic resonance imaging predicts clinical outcome of core decompression for osteonecrosis of the femoral head. J Rheumatol. 1995;22:1929–1933. [PubMed]
- 28.Hopson CN, Siverhus SW. Ischemic necrosis of the femoral head. Treatment by core decompression. J Bone Joint Surg Am. 1988;70:1048–1051. [PubMed]
- 29.Hungerford DS, Zizic TM. Alcoholism associated ischemic necrosis of the femoral head. Early diagnosis and treatment. Clin Orthop Relat Res. 1978;130:144–153. [PubMed]
- 30.Iorio R, Healy WL, Abramowitz AJ, Pfeifer BA. Clinical outcome and survivorship analysis of core decompression for early osteonecrosis of the femoral head. J Arthroplasty. 1998;13:34–41. [DOI] [PubMed]
- 31.Israelite C, Nelson CL, Ziarani CF, Abboud JA, Landa J, Steinberg ME. Bilateral core decompression for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2005;441:285–290. [DOI] [PubMed]
- 32.Jergesen HE, Khan AS. The natural history of untreated asymptomatic hips in patients who have non-traumatic osteonecrosis. J Bone Joint Surg Am. 1997;79:359–363. [DOI] [PubMed]
- 33.Kane SM, Ward WA, Jordan LC, Guilford WB, Hanley EN Jr. Vascularized fibular grafting compared with core decompression in the treatment of femoral head osteonecrosis. Orthopedics. 1996;19:869–872. [DOI] [PubMed]
- 34.Kerboul M, Thomine J, Postel M, Merle d’Aubigne R. The conservative surgical treatment of idiopathic aseptic necrosis of the femoral head. J Bone Joint Surg Br. 1974;56:291–296. [PubMed]
- 35.Kim SY, Kim YG, Kim PT, Ihn JC, Cho BC, Koo KH. Vascularized compared with nonvascularized fibular grafts for large osteonecrotic lesions of the femoral head. J Bone Joint Surg Am. 2005;87:2012–2018. [DOI] [PubMed]
- 36.Koo KH, Kim R, Ko GH, Song HR, Jeong ST, Cho SH. Preventing collapse in early osteonecrosis of the femoral head. A randomised clinical trial of core decompression. J Bone Joint Surg Br. 1995;77:870–874. [PubMed]
- 37.Kristensen KD, Pedersen NW, Kiaer T, Starklint H. Core decompression in femoral head osteonecrosis. 18 Stage I hips followed up for 1–5 years. Acta Orthop Scand. 1991;62:113–114. [DOI] [PubMed]
- 38.Lafforgue P, Dahan E, Chagnaud C, Schiano A, Kasbarian M, Acquaviva PC. Early-stage avascular necrosis of the femoral head: MR imaging for prognosis in 31 cases with at least 2 years of followup. Radiology. 1993;187:199–204. [DOI] [PubMed]
- 39.Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am. 2005;87:2155–2159. [DOI] [PubMed]
- 40.Lausten GS, Mathiesen B. Core decompression for femoral head necrosis. Prospective study of 28 patients. Acta Orthop Scand. 1990;61:507–511. [DOI] [PubMed]
- 41.Lavernia CJ, Sierra RJ. Core decompression in atraumatic osteonecrosis of the hip. J Arthroplasty. 2000;15:171–178. [DOI] [PubMed]
- 42.Learmonth ID, Maloon S, Dall G. Core decompression for early atraumatic osteonecrosis of the femoral head. J Bone Joint Surg Br. 1990;72:387–390. [DOI] [PubMed]
- 43.Leder K, Knahr K. Results of medullary space decompression in the early stage of so-called idiopathic femur head necrosis [in German]. Z Orthop Ihre Grenzgeb. 1993;131:113–119. [DOI] [PubMed]
- 44.Li YZ, Yue T. Diagnosis and treatment of idiopathic necrosis of the femoral head. Proc Chin Acad Med Sci Peking Union Med Coll. 1990;5:88–92. [PubMed]
- 45.Lieberman JR, Conduah A, Urist MR. Treatment of osteonecrosis of the femoral head with core decompression and human bone morphogenetic protein. Clin Orthop Relat Res. 2004;429:139–145. [DOI] [PubMed]
- 46.Maniwa S, Nishikori T, Furukawa S, Kajitani K, Iwata A, Nishikawa U, Ochi M. Evaluation of core decompression for early osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2000;120:241–244. [DOI] [PubMed]
- 47.Markel DC, Miskovsky C, Sculco TP, Pellicci PM, Salvati EA. Core decompression for osteonecrosis of the femoral head. Clin Orthop Relat Res. 1996;323:226–233. [DOI] [PubMed]
- 48.Mazieres B, Marin F, Chiron P, Moulinier L, Amigues JM, Laroche M, Cantagrel A. Influence of the volume of osteonecrosis on the outcome of core decompression of the femoral head. Ann Rheum Dis. 1997;56:747–750. [DOI] [PMC free article] [PubMed]
- 49.Merle D’Aubigne R, Postel M, Mazabraud A, Massias P, Gueguen J, France P. Idiopathic necrosis of the femoral head in adults. J Bone Joint Surg Br. 1965;47:612–633. [PubMed]
- 50.Mihalko WM, Balos L, Santilli M, Mindell ER. Osteonecrosis after powered core decompression. Clin Orthop Relat Res. 2003;412:77–83. [DOI] [PubMed]
- 51.Mont MA, Carbone JJ, Fairbank AC. Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res. 1996;324:169–178. [DOI] [PubMed]
- 52.Mont MA, Fairbank AC, Petri M, Hungerford DS. Core decompression for osteonecrosis of the femoral head in systemic lupus erythematosus. Clin Orthop Relat Res. 1997;334:91–97. [PubMed]
- 53.Mont MA, Jones LC, Einhorn TA, Hungerford DS, Reddi AH. Osteonecrosis of the femoral head. Potential treatment with growth and differentiation factors. Clin Orthop Relat Res. 1998;355 Suppl:S314–335. [DOI] [PubMed]
- 54.Mont MA, Jones LC, Pacheco I, Hungerford DS. Radiographic predictors of outcome of core decompression for hips with osteonecrosis stage III. Clin Orthop Relat Res. 1998;354:159–168. [DOI] [PubMed]
- 55.Mont MA, Marulanda GA, Jones LC, Saleh KJ, Gordon N, Hungerford DS, Steinberg ME. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88 Suppl 3:16–26. [DOI] [PubMed]
- 56.Mont MA, Ragland PS, Etienne G. Core decompression of the femoral head for osteonecrosis using percutaneous multiple small-diameter drilling. Clin Orthop Relat Res. 2004;429:131–138. [DOI] [PubMed]
- 57.Morse CG, Mican JM, Jones EC, Joe GO, Rick ME, Formentini E, Kovacs JA. The incidence and natural history of osteonecrosis in HIV-infected adults. Clin Infect Dis. 2007;44:739–748. [DOI] [PubMed]
- 58.Musso ES, Mitchell SN, Schink-Ascani M, Bassett CA. Results of conservative management of osteonecrosis of the femoral head. A retrospective review. Clin Orthop Relat Res. 1986;207:209–215. [PubMed]
- 59.Neumayr LD, Aguilar C, Earles AN, Jergesen HE, Haberkern CM, Kammen BF, Nancarrow PA, Padua E, Milet M, Stulberg BN, Williams RA, Orringer EP, Graber N, Robertson SM, Vichinsky EP. Physical therapy alone compared with core decompression and physical therapy for femoral head osteonecrosis in sickle cell disease. Results of a multicenter study at a mean of three years after treatment. J Bone Joint Surg Am. 2006;88:2573–2582. [DOI] [PubMed]
- 60.Ohzono K, Saito M, Sugano N, Takaoka K, Ono K. The fate of nontraumatic avascular necrosis of the femoral head. A radiologic classification to formulate prognosis. Clin Orthop Relat Res. 1992:73–78. [PubMed]
- 61.Ohzono K, Saito M, Takaoka K, Ono K, Saito S, Nishina T, Kadowaki T. Natural history of nontraumatic avascular necrosis of the femoral head. J Bone Joint Surg Br. 1991;73:68–72. [DOI] [PubMed]
- 62.Piperkovski T. Results of treatment in patients with nontraumatic avascular necrosis of the femoral head by monitor assisted core decompression. Rentgenol. Radiol. 2001;40:281–284.
- 63.Plakseychuk AY, Kim SY, Park BC, Varitimidis SE, Rubash HE, Sotereanos DG. Vascularized compared with nonvascularized fibular grafting for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2003;85:589–596. [DOI] [PubMed]
- 64.Powell ET, Lanzer WL, Mankey MG. Core decompression for early osteonecrosis of the hip in high risk patients. Clin Orthop Relat Res. 1997;335:181–189. [PubMed]
- 65.Radke S, Kirschner S, Seipel V, Rader C, Eulert J. Treatment of transient bone marrow oedema of the hip–a comparative study. Int Orthop. 2003;27:149–152. [DOI] [PMC free article] [PubMed]
- 66.Robinson Jr. HJ, Springer JA. Success of core decompression in the management of early stages of avascular necrosis: A four year prospective study. Orthop Trans. 1993;16:707.
- 67.Saito S, Ohzono K, Ono K. Joint-preserving operations for idiopathic avascular necrosis of the femoral head. Results of core decompression, grafting and osteotomy. J Bone Joint Surg Br. 1988;70:78–84. [DOI] [PubMed]
- 68.Scully SP, Aaron RK, Urbaniak JR. Survival analysis of hips treated with core decompression or vascularized fibular grafting because of avascular necrosis. J Bone Joint Surg Am. 1998;80:1270–1275. [DOI] [PubMed]
- 69.Shuler MS, Rooks MD, Roberson JR. Porous tantalum implant in early osteonecrosis of the hip: preliminary report on operative, survival, and outcomes results. J Arthroplasty. 2007;22:26–31. [DOI] [PubMed]
- 70.Simank HG, Brocai DR, Strauch K, Lukoschek M. Core decompression in osteonecrosis of the femoral head: risk-factor-dependent outcome evaluation using survivorship analysis. Int Orthop. 1999;23:154–159. [DOI] [PMC free article] [PubMed]
- 71.Smith SW, Fehring TK, Griffin WL, Beaver WB. Core decompression of the osteonecrotic femoral head. J Bone Joint Surg Am. 1995;77:674–680. [DOI] [PubMed]
- 72.Solomon L. Idiopathic necrosis of the femoral head: pathogenesis and treatment. Can J Surg. 1981;24:573–578. [PubMed]
- 73.Song WS, Yoo JJ, Kim YM, Kim HJ. Results of multiple drilling compared with those of conventional methods of core decompression. Clin Orthop Relat Res. 2007;454:139–146. [DOI] [PubMed]
- 74.Specchiulli F. Core decompression in the treatment of necrosis of the femoral head. Long-term results. Chir Organi Mov. 2000;85:395–402. [PubMed]
- 75.Steinberg ME. Core decompression of the femoral head for avascular necrosis: indications and results. Can J Surg. 1995;38 Suppl 1:S18–24. [PubMed]
- 76.Steinberg ME, Bands RE, Parry S, Hoffman E, Chan T, Hartman KM. Does lesion size affect the outcome in avascular necrosis? Clin Orthop Relat Res. 1999;367:262–271. [DOI] [PubMed]
- 77.Steinberg ME, Belmar CJ. Role of core decompression in the treatment of avascular necrosis of the femoral head. Current Orthopaedics. 1997;11:173–178. [DOI]
- 78.Steinberg ME, Brighton CT, Bands RE, Hartman KM. Capacitive coupling as an adjunctive treatment for avascular necrosis. Clin Orthop Relat Res. 1990;261:11–18. [PubMed]
- 79.Steinberg ME, Brighton CT, Corces A, Hayken GD, Steinberg DR, Strafford B, Tooze SE, Fallon M. Osteonecrosis of the femoral head. Results of core decompression and grafting with and without electrical stimulation. Clin Orthop Relat Res. 1989;249:199–208. [PubMed]
- 80.Steinberg ME, Brighton CT, Steinberg DR, Tooze SE, Hayken GD. Treatment of avascular necrosis of the femoral head by a combination of bone grafting, decompression, and electrical stimulation. Clin Orthop Relat Res. 1984;186:137–153. [PubMed]
- 81.Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed]
- 82.Steinberg ME, Larcom PG, Strafford B, Hosick WB, Corces A, Bands RE, Hartman KE. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001;386:71–78. [DOI] [PubMed]
- 83.Stulberg BN, Davis AW, Bauer TW, Levine M, Easley K. Osteonecrosis of the femoral head. A prospective randomized treatment protocol. Clin Orthop Relat Res. 1991;268:140–151. [PubMed]
- 84.Styles LA, Vichinsky EP. Core decompression in avascular necrosis of the hip in sickle-cell disease. Am J Hematol. 1996;52:103–107. [DOI] [PubMed]
- 85.Tingart M, Bathis H, Perlick L, Lerch K, Luring C, Grifka J. Therapy of femoral head osteonecrosis: results of a national survey [in German]. Z Orthop Ihre Grenzgeb. 2004;142:553–558. [DOI] [PubMed]
- 86.Tooke SM, Nugent PJ, Bassett LW, Nottingham P, Mirra J, Jinnah R. Results of core decompression for femoral head osteonecrosis. Clin Orthop Relat Res. 1988;228:99–104. [PubMed]
- 87.Trancik T, Lunceford E, Strum D. The effect of electrical stimulation on osteonecrosis of the femoral head. Clin Orthop Relat Res. 1990;256:120–124. [PubMed]
- 88.Van Laere C, Mulier M, Simon JP, Stuyck J, Fabry G. Core decompression for avascular necrosis of the femoral head. Acta Orthop Belg. 1998;64:269–272. [PubMed]
- 89.Veillette CJ, Mehdian H, Schemitsch EH, McKee MD. Survivorship analysis and radiographic outcome following tantalum rod insertion for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88 Suppl 3:48–55. [DOI] [PubMed]
- 90.Wang CJ, Wang FS, Huang CC, Yang KD, Weng LH, Huang HY. Treatment for osteonecrosis of the femoral head: comparison of extracorporeal shock waves with core decompression and bone-grafting. J Bone Joint Surg Am. 2005;87:2380–2387. [DOI] [PubMed]
- 91.Warner JJ, Philip JH, Brodsky GL, Thornhill TS. Studies of nontraumatic osteonecrosis. The role of core decompression in the treatment of nontraumatic osteonecrosis of the femoral head. Clin Orthop Relat Res. 1987;225:104–127. [PubMed]
- 92.Wirtz C, Zilkens KW, Adam G, Niethard FU. MRI-controlled outcome after core decompression of the femur head in aseptic osteonecrosis and transient bone marrow edema [in German]. Z Orthop Ihre Grenzgeb. 1998;136:138–146. [DOI] [PubMed]
- 93.Wirtz DC, Rohrig H, Neuss M. Core decompression for avascular necrosis of the femoral head. Oper Orthop Traumatol. 2003;15:288–303. [DOI]
- 94.Wright RW, Brand RA, Dunn W, Spindler KP. How to write a systematic review. Clin Orthop Relat Res. 2007;455:23–29. [DOI] [PubMed]
- 95.Yan ZQ, Chen YS, Li WJ, Yang Y, Huo JZ, Chen ZR, Shi JH, Ge JB. Treatment of osteonecrosis of the femoral head by percutaneous decompression and autologous bone marrow mononuclear cell infusion. Chin J Traumatol. 2006;9:3–7. [PubMed]
- 96.Yoo MC, Chung DW, Hahn CS. Free vascularized fibula grafting for the treatment of osteonecrosis of the femoral head. Clin Orthop Relat Res. 1992;277:128–138. [PubMed]
- 97.Yoon TR, Song EK, Rowe SM, Park CH. Failure after core decompression in osteonecrosis of the femoral head. Int Orthop. 2001;24:316–318. [DOI] [PMC free article] [PubMed]
- 98.Zizic TM, Hungerford DS. Avascular necrosis of bone. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. Ed 2. Philadelphia, PA: WB Saunders Co; 1985:1689–1710.