Abstract
Alumina-on-alumina bearings for THA have markedly improved in mechanical properties through advances in technology; however, alumina fracture is still a concern. We retrospectively reviewed 77 patients (82 hips) with cemented alumina-on-alumina THAs to identify factors relating to alumina failure. The mean age of the patients at surgery was 63 years. The prostheses had a cemented polyethylene-backed acetabular component with an alumina inlay and a 28-mm alumina head. Revision surgery was performed because of alumina inlay failure in four hips (three fractures and one dissociation; 5.6%), deep infection in two, and recurrent dislocation in one. The 8-year survival rate was 90.7% with revision for any reason and 94.4% with revision for alumina failure as the end point. There were no differences in age, body mass index, gender, mobility, function, abduction angle, or size of component among the four hips with alumina failure and the remaining 68 hips without it; however, radiolucent lines in the sockets were more apparent in four cases with alumina inlay failure. This alumina-on-alumina THA thus yielded unsatisfactory medium-term results because we observed a high rate of catastrophic alumina inlay failure.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
The alumina-on-alumina articulation in THA was introduced in the 1970s to reduce wear and its consequences [19, 26]. Alumina particles induced less macrophage reaction and cytokine secretion than polyethylene particles [8], and THA using alumina-on-alumina articulation induced little periprosthetic osteolysis [13, 25, 31]. However, early alumina prostheses, eg, with conically threaded monoblocks or spherical press-fit acetabular components, generally were found to have insufficient fixation of the acetabular component and the risk of fracture of the alumina component seemed to be a problem [3, 13]. During the last decade, the quality of materials has improved considerably and the risk of fracture has decreased [13, 27]. Subsequently, good clinical results have been obtained with a cementless, press-fit, metal-backed acetabular component with an alumina insert [3, 31]. However, concerns still remain regarding problems with the alumina component.
A unique polyethylene-backed acetabular component with an alumina insert intended for fixation with bone cement was developed in 1998. Recently, there have been reports regarding problems with the alumina inlay in this design [1, 12, 14, 18, 22, 24, 25, 28, 30, 31], but what factors might relate to the socket or insert failure are unclear as there have been no reports regarding the clinical results of this cemented polyethylene-backed alumina-on-alumina THA.
We asked whether age, body mass index (BMI), gender, mobility, function, abduction angle, size of component, the existence of radiolucent lines, or component type with or without a flange were associated with alumina failure.
Materials and Methods
We retrospectively reviewed 77 consecutive patients (82 hips) who underwent THA between February 1998 and July 2000. In all 82 hips, cemented THA was performed using a polyethylene-backed acetabular component with an alumina inlay (ABS Cup; Kyocera, Kyoto, Japan) (Fig. 1), KC stem (Kyocera), and 28-mm alumina femoral head. Four patients (four hips) died within 27 months after the operation without complications from surgery. Two patients (two hips) were excluded because of recurrent dislocation and one patient (one hip) was excluded because of deep infection; these patients underwent revision surgery within 24 months. Three patients (three hips) were lost to followup. The remaining 67 patients (72 hips) were followed for a minimum of 5 years. There were 60 women and seven men. The mean age at the index operation was 63 years (range, 41–87 years), mean weight 53.9 kg (range, 38–85 kg), mean height 152 cm (range, 138–172 cm), and mean BMI 23.2 kg/m2 (range, 16.2–34.7 kg/m2). The primary diagnosis was osteoarthritis in 61 hips, osteonecrosis in five, rheumatoid arthritis in four, and sequelae of pyogenic infection in two. The minimum duration of followup was 5 years (mean, 6.7 years; range, 5–8.3 years) (Table 1). The study was approved by the Institutional Review Board of the hospital, and all patients provided informed consent.
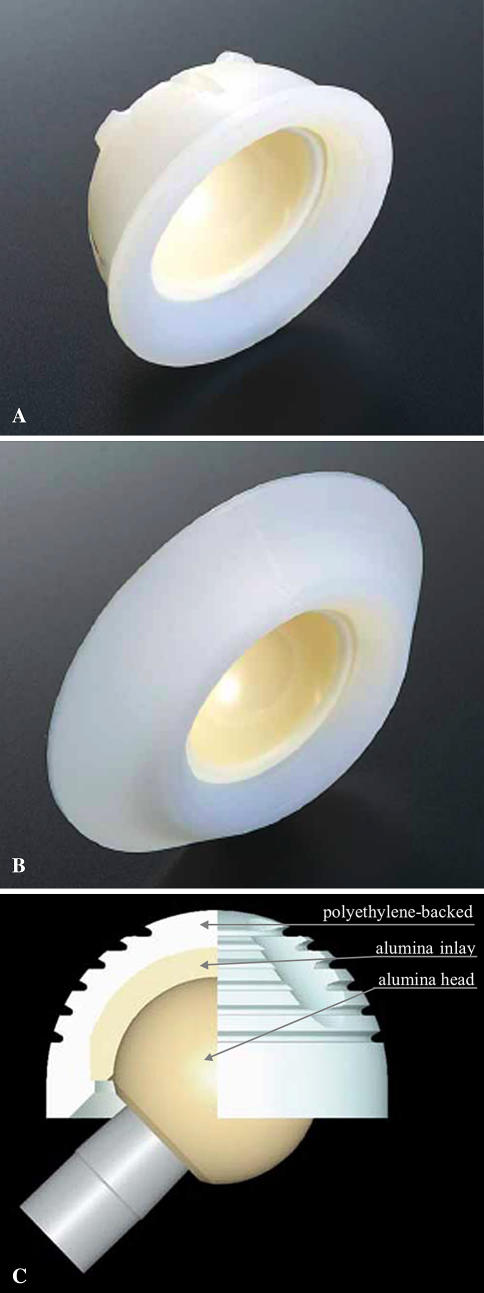
Fig. 1A–C.
A spherical cemented polyethylene-alumina composite cup, ABS cup (Kyocera, Kyoto, Japan), was developed to obtain stability between the alumina cup and bone cement. There were two designs for this cup: (A) one is the ABS cup without a flange and (B) the other is the ABS cup with a flange. (C) A cross section of the ABS cup is shown. The thickness of the alumina inlay is fixed at 4 mm in any size of acetabular component.
Table 1.
Demographics of the 67 patients
| Variable | Value |
|---|---|
| Age at surgery (years)* | 63 (41–87) |
| Gender (male/female) | 7/60 |
| Body mass index (kg/m2)* | |
| Male patients | 22.5 (16.4–30.5) |
| Female patients | 23.3 (16.2–34.7) |
| Preoperative diagnosis (number of hips) | |
| Osteoarthritis resulting from developmental dislocation of the hip | 61 (91%) |
| Osteonecrosis | 5 (7%) |
| Rheumatoid arthritis | 4 (6%) |
| Sequelae of pyogenic infection | 2 (3%) |
*Values expressed as mean, with range in parentheses.
The acetabular components consisted of a spherical cemented polyethylene socket with an alumina inlay (ABS; Kyocera) without a flange (Fig. 1A) in 20 hips and the same socket with a flange (Fig. 1B) in 52 hips. The outer diameter of the acetabular component was 42 mm in 14 hips, 44 mm in 42, 46 mm in eight, 48 mm in seven, and 50 mm in one. The femoral component was a tapered collarless titanium stem (KC stem; Kyocera). The stem was tapered only in the anteroposterior projection. The proximal end of the stem was coated with macrotexture. The neck-shaft angle of the stem was 130°. The standard offset was 40 mm in 42 hips, and the offset of the narrow stem was 35 mm in 30 hips. The femoral component was fixed with Simplex P® cement (Stryker Howmedica Osteonics, Allendale, NJ). A 28-mm alumina femoral head (Kyocera) was used. A short neck-head component was used in 18 hips, a medium neck in 50, and a long neck in four. All operations were performed by one surgeon (HO) through an anterolateral approach in which an attempt was made to place the acetabular component in an anatomic position.
All patients received intravenous antibiotic prophylaxis preoperatively and for 3 days after surgery. The patients received mechanical prophylaxis for thromboembolism by intermittent pneumatic compression with the A-V Impulse System® (Novamedix, Andover, UK) for 2 days, but no pharmacologic prophylaxis using warfarin, heparin, or aspirin was administered. Patients were encouraged to walk with full weightbearing as tolerated without the aid of crutches 4 weeks after surgery. Routine followups were scheduled for 3, 6, 9, and 12 months and yearly thereafter.
We (HI) performed clinical evaluation using the Merle d’Aubigné and Postel score [16] that allocates up to 6 points for each category of pain, mobility, and function with a total of 18 points given to a normal hip. Patients were routinely asked at followup whether they had experienced audible hip noise because we were concerned about separation of alumina-on-alumina bearings and alumina fractures [18, 28].
The radiographic evaluation was performed by one observer (KI) who did not participate in the index operations. The 6-month anteroposterior and lateral radiographs were used for assessment of the abduction angle of the acetabular socket [17]. On the radiographs at the final examination, radiolucency and osteolysis were evaluated around the acetabular component using the zone classification of DeLee and Charnley [9] and around the femoral component using the criteria of Hodgkinson et al. [15] and Gruen et al. [11]. Migration of the acetabular and femoral component center was evaluated by comparing the horizontal and vertical distance from the inferior points of the teardrops and the center of the lesser trochanter, respectively, on the immediate postoperative and final radiographs [20]. Loosening of each component was considered to have occurred when migration of the component was greater than 2 mm. Alumina component failure was checked. Heterotopic ossification was defined according to Brooker et al. [7].
The mean Merle d’Aubigné and Postel hip score of the remaining 68 hips improved from 10.1 (pain 2.4, mobility 4.2, function 3.5) before the operation to 16.3 (pain 5.8, mobility 5.6, function 4.9) at final followup. No patient used any type of walking support. On 6-month anteroposterior radiographs of the 76 hips, the mean abduction angle of the acetabular component was 37.6° (range, 20°–50°). On the final radiographs of the remaining 68 hips, we found radiolucencies around the acetabular component in Zone 1 in 20 hips, in Zone 2 in five, and in Zone 3 in four. However, there was no osteolysis in any zone around the acetabular component and no evidence of migration of the acetabular component. On the femoral side, there was no radiolucency or osteolysis in any of the hips. No patients had heterotopic ossification. During the followup, there were no cases of nerve palsy, deep vein thrombosis, or pulmonary embolism.
Cumulative survival rates were calculated using the Kaplan-Meier method with failure defined as the end point of revision for alumina failure or for any reason. To compare groups with and without alumina failures in age, BMI, gender, mobility, function, abduction angle, size of component, or the existence of radiolucent lines, we used the nonparametric Mann-Whitney U-test. Fisher’s exact probability test was used to compare the alumina failure rate between polyethylene acetabular components with and without flanges. All analyses were performed with SAS® software (Version 9.1; SAS Institute Inc, Cary, NC).
Results
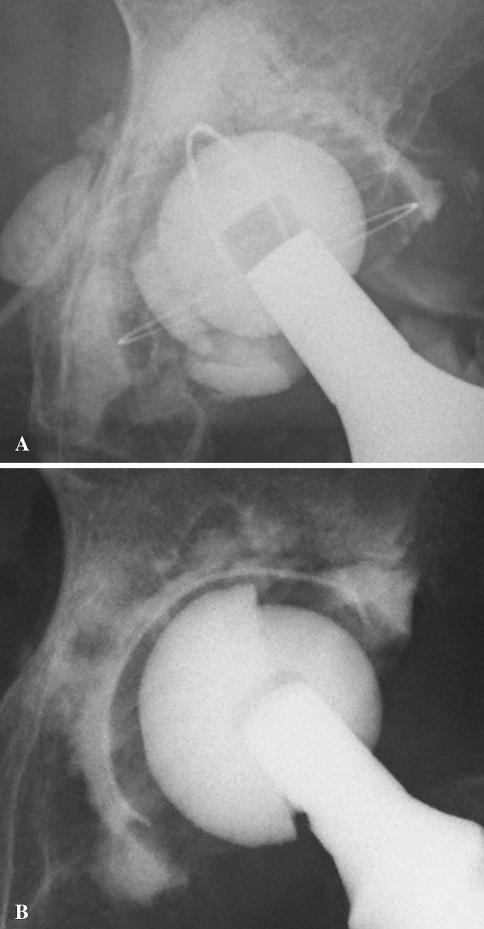
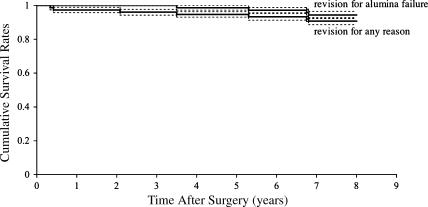
We detected four (5.6%) ceramic failures at a mean of 5.6 years (range, 3.5–6.8 years) after the index operation. Three alumina inlays had fractured and one had dissociated from its polyethylene-backed acetabular component (Fig. 2); three had been revised, whereas for the remaining one, revision surgery was intended. None of the failures occurred during implantation or in association with traumatic episodes. Kaplan-Meier survival analysis revealed a survival rate of 94.4% (95% confidence interval) at 6.8 years with failure defined as revision for alumina failure and 90.7% (95% confidence interval) at 6.8 years with revision for any reason as the end point (Fig. 3).
Fig. 2A–B.
The radiographs show alumina inlay failure: (A) fracture of the alumina inlay occurred in three hips without trauma, and (B) dissociation of the alumina inlay from the polyethylene shell occurred in one hip without trauma.
Fig. 3.
Kaplan-Meier survivorship curves of 72 consecutive polyethylene-backed alumina-on-alumina THAs are shown. The 8-year survival rate was 90.7% with revision for any reason and 94.4% with revision for alumina failure as the end point. Dotted lines indicate the 95% confidence intervals.
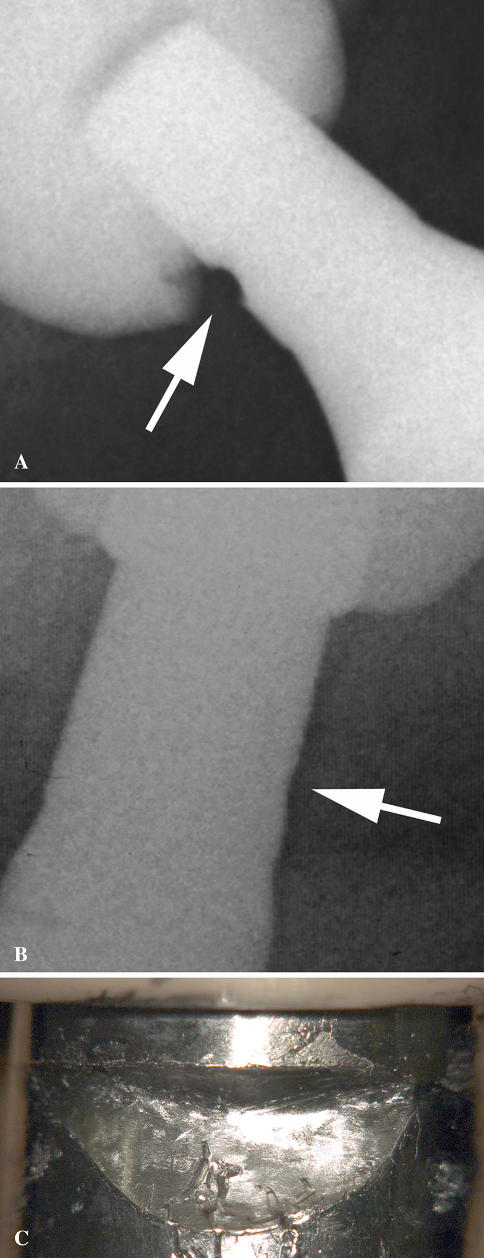
There were no differences in age, BMI, gender, mobility, function, abduction angle, or size of the component between the four hips with alumina failure and the remaining 68 hips without it (p = 0.090, 0.278, 0.512, 0.246, 0.239, 0.607, and 0.912, respectively). However, the four cases of alumina inlay failure had radiolucent lines (especially in Zones 1 and 2) to a greater (p = 0.046) extent than the cases without alumina inlay failure. We observed fretting on the stem neck in one of three fracture cases or a dissociation case (Fig. 4). There was no difference (p = 0.307) in alumina failure rate between polyethylene acetabular components with or without a flange.
Fig. 4A–C.
Fretting by the alumina inlay on the stem neck was observed on the radiographs of (A) a patient with dissociation and (B) a patient with a fracture. (C) Macroscopic fretting on the stem neck is shown.
Discussion
Although evolution in technology has improved the quality of alumina considerably and the risk of alumina fracture has decreased, concerns still remain regarding problems related to alumina. Numerous factors might be related to alumina failure. We explored whether age, BMI, gender, mobility, function, abduction angle, size of component, the existence of radiolucent lines, or component type with or without a flange were associated with alumina failure.
Our study was limited by few patients with failure and therefore low power: we observed four (5.6%) alumina failures at a mean of 5.6 years (range, 3.5–6.8 years) after the index operation.
The first alumina-on-alumina THA was performed in April 1970 by Boutin [6]. During the last two decades, the mechanical strength of alumina has substantially improved. The third generation of alumina, in which the ABS cup is classified, is hot isostatically pressed, laser-marked, and proof-tested [2]. Compared with the first-generation alumina ceramics, grain size has decreased from 4.2 to 1.8 μm and burst strength has improved from 46 to 65 kN [2]. These advantages of alumina materials are related to its distinctive tribologic properties resulting from high scratch resistance and wettability of the material, both of which reduce third-body and adhesive wear. There are three other advantages of alumina: lower linear wear rate than metal-on-polyethylene articulation [10]; lower concentration of wear particles in the periprosthetic tissue around the bearing than metal-on-polyethylene articulation [5]; and less release of TNF-α, which is one of the main factors inducing osteolysis, than by polyethylene particles [23]. These advantages are related to avoidance of the acetabular osteolysis observed with alumina-on-alumina THA [13]. Osteolysis has seldom been reported after alumina-on-alumina THA, and the few cases reported of this usually were associated with use of a Mittelmeier total hip system [29, 32], which is made of first-generation alumina with large grain size, low density, and high porosity. Each of these characteristics of first-generation alumina could have led to the production of a large amount of debris. In addition, this prosthesis had a poor acetabular component design that was responsible for a rate of failure as much as 27% by the 26-month followup [21]. Meanwhile, fracture of the alumina component is one of the disadvantages, and it remains despite the advance in technology [1, 12, 14, 18, 22, 24, 25, 28, 30, 31].
One alumina-on-alumina THA with a cementless, press-fit, metal-backed acetabular component yielded a survival rate of 93.7% at 9 years with revision for any reason as the end point without any alumina fracture [3]. However, results observed with alumina-on-alumina THA with cemented acetabular components rarely have been reported. The survival rate of cemented alumina-on-alumina THA with a monoblock alumina acetabular component for 20 years was reported as 61.2% with failure defined as revision [13]. The main reason for revision was aseptic loosening of the acetabular component. Loosening of the cemented acetabular components was always an acute event related to debonding of the alumina acetabular component from the bone cement caused by a mechanical phenomenon resulting from mismatch of stiffness between the alumina component and either the bone or cement [13].
The unique design of the polyethylene-backed acetabular component with an alumina inlay (ABS cup; Kyocera) was developed in 1998 to ensure sufficient fixation of the alumina surface with bone cement despite mismatch in stiffness [4, 14, 22] by using the stable fixation among bone, cement, and polyethylene; this was used in Japan. Although there were a few reports regarding cementless metal-backed polyethylene-alumina composite liner, none was available for the cemented polyethylene-backed socket while we used this device. Therefore, we continued to implant these components until July 2000.We observed four alumina inlay failures (5.6%), including three fractures and one dissociation, but no alumina head fractures, and the survival rate was 94.4% when failure was revision for alumina failure 7 years postoperatively. Cases with alumina inlay failure had considerably more radiolucent lines (especially in Zones 1 and 2) than cases without failure.
There have been some reports of failures of the polyethylene-alumina composite liner within a cementless titanium alloy shell [1, 12, 14, 22, 24, 30] in which alumina inlay dissociation and fracture were caused by impingement, microseparation, and squeeze force [14, 22, 30]. We thus speculated the following three disadvantageous features in this study led to alumina inlay failure in the unique acetabular component design modification: (1) use of a much thinner, 4-mm alumina inlay despite improved quality of material; (2) the narrow clearance of the alumina inlay and alumina head (5–35 μm), which generated strong squeeze force leading to separation of the alumina inlay from the polyethylene shell; and (3) a relatively narrow oscillation angle, 120°, which readily produced contact force leading to dissociation of the alumina inlay insert from its polyethylene shell or increased the chance of peripheral chip fracture and subsequent crack propagation resulting from the brittle alumina material under conditions of impingement. The radiolucent lines, observed to a considerable extent in the four cases of alumina inlay failure, might have been induced by large numbers of polyethylene particles generated from the interface between the dissociated or fractured alumina inlay and the polyethylene shell or by high shear stress in the bone-cement interface resulting from the strong squeeze force generated on the alumina-on-alumina surface. In July 2000, we discontinued use of this type of THA to avoid alumina failure. We suggest all patients with this type of acetabular component be followed carefully.
Cemented polyethylene-backed alumina-on-alumina THA with a composite of alumina inlay had a relatively high rate of catastrophic alumina inlay failure (5.6%) during a mean of 6.7 years’ followup. We believe the socket fixation still needs to be improved.
Acknowledgments
We thank A. Kobayashi, MD, PhD, M. Ikebuchi, MD, Y. Ohta, MD, and R. Sugama, MD, PhD, for assistance and advice concerning polyethylene-backed, cemented, alumina-on alumina THA.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Akagi M, Nonaka T, Nishisaka F, Mori S, Fukuda K, Hamanishi C. Late dissociation of an alumina-on-alumina bearing modular acetabular component. J Arthroplasty. 2004;19:647–651. [DOI] [PubMed]
- 2.Bierbaum BE, Nairus J, Kuesis D, Morrison JC, Ward D. Ceramic-on-ceramic bearings in total hip arthroplasty. Clin Orthop Relat Res. 2002;405:158–163. [DOI] [PubMed]
- 3.Bizot P, Banallec L, Sedel L, Nizard R. Alumina-on-alumina total hip prostheses in patients 40 years of age or younger. J Bone Joint Surg Br. 2004;86:190–194. [DOI] [PubMed]
- 4.Boehler M, Knahr K, Plenk H Jr, Walter A, Salzer M, Schreiber V. Long-term results of uncemented alumina acetabular implants. J Bone Joint Surg Br. 1994;76:53–59. [PubMed]
- 5.Bohler M, Mochida Y, Bauer TW, Plenk H Jr, Salzer M. Wear debris from two different alumina-on-alumina total hip arthroplasties. J Bone Joint Surg Br. 2000;82:901–909. [DOI] [PubMed]
- 6.Boutin P. [Total arthroplasty of the hip by fritted aluminum prosthesis: experimental study and 1st clinical applications] [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1972;58:229–246. [PubMed]
- 7.Brooker AF, Bowerman JW, Robinson RA, Riley LH Jr. Ectopic ossification following total hip replacement: incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed]
- 8.Catelas I, Petit A, Marchand R, Zukor DJ, Yahia L, Huk OL. Cytotoxicity and macrophage cytokine release induced by ceramic and polyethylene particles in vitro. J Bone Joint Surg Br. 1999;81:516–521. [DOI] [PubMed]
- 9.DeLee J, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed]
- 10.Dorlot JM, Christel P, Meunier A. Wear analysis of retrieved alumina heads and sockets of hip prostheses. J Biomed Mater Res. 1989;23(A3 suppl):299–310. [DOI] [PubMed]
- 11.Gruen T, McNeice G, Amstutz H. “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed]
- 12.Ha YC, Koo KH, Jeong ST, Joon Yoo J, Kim YM, Joong Kim H. Ceramic liner fracture after cementless alumina-on-alumina total hip arthroplasty. Clin Orthop Relat Res. 2007;458:106–110. [DOI] [PubMed]
- 13.Hamadouche M, Boutin P, Daussange J, Bolander ME, Sedel L. Alumina-on-alumina total hip arthroplasty: a minimum 18.5-years follow-up study. J Bone Joint Surg Am. 2002;84:69–77. [PubMed]
- 14.Hasegawa M, Sudo A, Hirata H, Uchida A. Ceramic acetabular liner fracture in total hip arthroplasty with a ceramic sandwich cup. J Arthroplasty. 2003;18:658–661. [DOI] [PubMed]
- 15.Hodgkinson J, Shelley P, Wroblewski B. The correlation between the roentgenographic appearance and operative findings at the bone-cement junction of the socket in Charnley low friction arthroplasties. Clin Orthop Relat Res. 1988;228:105–109. [PubMed]
- 16.Merle d’Aubigné R, Postel M. Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am. 1954;36:451–475. [PubMed]
- 17.Mulliken BD, Nayak N, Bourne RB, Rorabeck CH, Bullas R. Early radiographic results comparing cemented and cementless total hip arthroplasty. J Arthroplasty. 1996;11:24–33. [DOI] [PubMed]
- 18.Nevelos J, Ingham E, Doyle C, Streicher R, Nevelos A, Walter W, Fisher J. Microseparation of the centers of alumina-alumina artificial hip joints during simulator testing produces clinically relevant wear rates and patterns. J Arthroplasty. 2000;15:793–795. [DOI] [PubMed]
- 19.Nizard RS, Sedel L, Christel P, Meunier A, Soudry M, Witvoet J. Ten-year survivorship of cemented ceramic-ceramic total hip prosthesis. Clin Orthop Relat Res. 1992;282:53–63. [PubMed]
- 20.Nunn D, Freeman MA, Hill PF, Evans SJ. The measurement of migration of the acetabular component of hip prostheses. J Bone Joint Surg Br. 1989;71:629–631. [DOI] [PubMed]
- 21.O’Leary JF, Mallory TH, Kraus TJ, Lombardi AV Jr, Lye CL. Mittelmeier ceramic total hip arthroplasty: a retrospective study. J Arthroplasty. 1988;3:87–96. [DOI] [PubMed]
- 22.Park YS, Hwang SK, Choy WS, Kim YS, Moon YW, Lim SJ. Ceramic failure after total hip arthroplasty with an alumina-on-alumina bearing. J Bone Joint Surg Am. 2006;88:780–787. [DOI] [PubMed]
- 23.Petit A, Catelas I, Antoniou J, Zukor DJ, Huk OL. Differential apoptotic response of J774 macrophages to alumina and ultra-high-molecular-weight polyethylene particles. J Orthop Res. 2002;20:9–15. [DOI] [PubMed]
- 24.Ravasi F, Sansone V. Five-year follow-up with a ceramic sandwich cup in total hip replacement. Arch Orthop Trauma Surg. 2002;122:350–353. [DOI] [PubMed]
- 25.Sedel L. Evolution of alumina-on-alumina implants: a review. Clin Orthop Relat Res. 2000;379:48–54. [DOI] [PubMed]
- 26.Sedel L, Nizard R, Kerboull L, Witvoet J. Alumina-alumina hip replacement in patients younger than 50 years old. Clin Orthop Relat Res. 1994;298:175–183. [PubMed]
- 27.Skinner HB. Ceramic bearing surfaces. Clin Orthop Relat Res. 1999;369:83–91. [DOI] [PubMed]
- 28.Toni A, Traina F, Stea S, Sudanese A, Visentin M, Bordini B, Squarzoni S. Early diagnosis of ceramic liner fracture: guidelines based on a twelve-year clinical experience. J Bone Joint Surg Am. 2006;88(suppl 4):55–63. [DOI] [PubMed]
- 29.Wirganowicz PZ, Thomas BJ. Massive osteolysis after ceramic on ceramic total hip arthroplasty: a case report. Clin Orthop Relat Res. 1997;338:100–104. [DOI] [PubMed]
- 30.Yamamoto K, Shishido T, Tateiwa T, Katori Y, Masaoka T, Imakiire A, Clarke IC. Failure of ceramic THR with liner dislocation: a case report. Acta Orthop Scand. 2004;75:500–502. [DOI] [PubMed]
- 31.Yoo JJ, Kim YM, Yoon KS, Koo KH, Song WS, Kim HJ. Alumina-on-alumina total hip arthroplasty: a five-year minimum follow-up study. J Bone Joint Surg Am. 2005;87:530–535. [DOI] [PubMed]
- 32.Yoon TR, Rowe SM, Jung ST, Seon KJ, Maloney WJ. Osteolysis in association with a total hip arthroplasty with ceramic bearing surfaces. J Bone Joint Surg Am. 1998;80:1459–1468. [DOI] [PubMed]