Abstract
Socioeconomic disadvantage during childhood and adolescence predicts poor mental and physical health and premature death by major medical diseases in adulthood. However, the neural pathways through which socioeconomic factors may exert a developmental influence on health and longevity remain largely unknown. This fMRI study provides novel evidence of a unique relationship between the perception that one's parents had a relatively low social standing—a putative indicator of early socioeconomic disadvantage—and greater amygdala reactivity to threatening facial expressions. This relationship was not explained by several possible confounders, including sex, ethnicity, dispositional emotionality, symptoms of depression and anxiety, parental education and participants’ perceptions of their own social standing. The amygdala expresses marked developmental plasticity and plays instrumental roles in processing emotional information, regulating emotion-related behaviors and orchestrating biobehavioral stress responses throughout life. Thus, these findings may provide insight into the neurodevelopmental pathways impacting socioeconomic disparities in health.
Keywords: amygdala, developmental stress, perceived social standing, socioeconomic status, threat
Low socioeconomic status (SES) confers disproportional risk for physical and psychiatric illnesses and premature death by major medical diseases (Adler et al., 1994). From a life course perspective, social information processing models postulate that lower SES individuals may develop an early sensitivity to social threats, leading to dysregulated forms of emotional control and recurrent biobehavioral stress responses that increase risk for ill health in later life (Chen and Matthews, 2001; Chen et al., 2002; Taylor et al., 2004). This postulate parallels the notion that risk trajectories for ill health may be developmentally ‘embedded’ in the brain and in biobehavioral stress-response systems by early and unfavorable socioeconomic circumstances (Hertzman, 1999; McEwen, 2000, 2007; Miller and Chen, 2007).
Here, we questioned whether such a putative embedding process may be reflected in the functioning of the amygdala, a neural system that expresses stress-related developmental plasticity and that orchestrates biobehavioral responses to salient and threatening social cues (Whalen, 1998; LeDoux, 2000; Pollak, 2005; McEwen, 2007). To answer this question, we assessed young adults’ retrospective perceptions of their parents’ social standing as an indicator of childhood and adolescent SES (cf., Adler et al., 2000; Goodman et al., 2007). This indicator was selected because growing evidence indicates that perceived social standing may be more closely associated with stress-related mental and physical health outcomes than are so-called ‘objective’ SES indicators (e.g. education, income and occupation; see Discussion section). Using an emotional facial-expression processing task administered during neuroimaging (Hariri et al., 2002), we then tested whether lower perceived parental social standing predicted greater amygdala reactivity to explicitly threatening (angry) faces, in contrast to ambiguous or non-explicitly threatening (surprised and neutral) faces. Finally, we tested whether perceived parental social standing predicted amygdala reactivity to threatening faces after accounting for potential confounders, including sex, ethnicity, dispositional emotionality, recent symptoms of depression and anxiety, parental education and participants’ perceptions of their own social standing.
METHOD
Participants
Participants were 33 consenting right-handed, first- and second-year undergraduates (12 men; M age = 20, s.d. = 1.3; 7 non-Caucasian) who were tested with approval by the University of Pittsburgh Institutional Review Board. None had a lifetime diagnosis or prior or current treatment for any psychiatric disorder or neurological condition.
Study measures
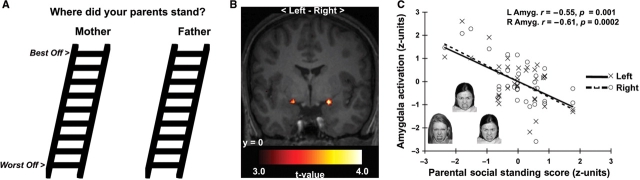
Participants used nine-rung ‘social ladders’ depicted in Figure 1A to rank each parent's SES during the participants’ childhood and adolescence according to income, education and occupational prestige (cf., Adler et al., 2000; Goodman et al., 2007). Maternal (M = 5.94, s.d. = 1.61) and paternal (M = 6.19, s.d. = 1.80) rankings [r(32) = 0.65, P < 0.001] were standardized and averaged to compute aggregate perceived parental social standing scores. Participants also ranked themselves at present by the same SES criteria (M = 5.53, s.d. = 1.90). Participants used 9-point scales indicating the highest education of each parent (1 = no high school diploma, 9 = doctorate). Resulting maternal and paternal education scores were standardized and averaged to compute parental education scores. To assess potentially biasing traits related to dispositional emotionality, participants completed inventories for self-mastery (Pearlin and Schooler, 1978), optimism (Scheier et al., 1994), neuroticism, extraversion and agreeableness (Goldberg, 1992). To assess depressive symptoms experienced in the past week, participants completed the Center for Epidemiological Studies Depression (CES-D) Scale (Radloff, 1977). Finally, because of evidence that individual differences in anxiety are particularly associated with amygdala reactivity to emotional facial expressions (Etkin et al., 2004) and because anxiety could bias perceived social standing ratings, we specifically tested whether perceived parental social standing accounted for inter-individual variability in amygdala reactivity to threatening faces above-and-beyond anxiety subscale scores from the Profile of Mood States (POMS) inventory (Usala and Hertzog, 1989), which assessed anxiety symptoms experienced over the past week. Cronbach's αs in the present sample were ≥0.70 for all inventories.
Fig. 1.
Lower perceived parental social standing predicted greater amygdala reactivity to angry faces. (A) Social ladders used to assess perceived parental social standing (instructions in Appendix). (B) Statistical parametric maps projected onto an averaged structural template derived from study participants. The maps profile amygdala clusters where lower perceived parental social standing predicted greater reactivity to angry faces. (C) Plots depicting standardized perceived parental social standing scores (x-axis) and extracted, mean-centered and standardized reactivity values derived from the Angry>Shape-matching parameter contrast for the peak voxels in the left (L, open circles, dashed line) and right (R, closed circles, solid line) amygdala clusters in B. Inset in C illustrates exemplar face-matching trial.
Amygdala reactivity task
To elicit amygdala reactivity during fMRI, participants performed six blocks of an archival face-matching task interleaved with seven blocks of a shape-matching control task (Hariri et al., 2002). In both the tasks, participants viewed three luminance-controlled gray-scaled faces or simple geometric shapes for 4 s and selected from two options at bottom that matched a center target. Face-matching blocks consisted of six randomized, non-repeating trials (three females, three males), each depicting angry, surprised or neutral expressions (NimStim database; http://www.macbrain.org/resources.htm). Angry, surprised and neutral blocks were pseudorandomly presented twice, with blocks of the same expression never administered sequentially. Task accuracy exceeded 99%.
Data analysis
Brain imaging data were preprocessed and analyzed using statistical parametric mapping software (SPM2; see Supplementary Methods, available at http://scan.oxfordjournals.org). Following preprocessing, general linear models employing canonical hemodynamic response functions estimated blood–oxygen level-dependent activation for each individual. Linear contrasts then determined expression-specific effects (Angry > Shapes, Neutral > Shapes, Surprise > Shapes). Resulting contrast images were next submitted to three regressions in SPM to determine inter-individual relationships between expression-specific amygdala reactivity and perceived parental social standing. The amygdala was targeted as a region-of-interest using a standard bilateral anatomical mask (Maldjian et al., 2003). Type-I error in the amygdala was controlled using a family-wise error-rate (FWE) threshold (P < 0.05). Subsequently, two-step hierarchical regressions tested whether perceived parental social standing scores predicted extracted amygdala reactivity values above-and-beyond potential confounders.
RESULTS
Parental social standing and amygdala reactivity to threat
Lower perceived parental social standing predicted greater amygdala reactivity to angry faces (left amygdala x, y, z Montreal Neurological Institute coordinates: −18, 0, −15, t[31] = 3.70, pFWE = 0.02, Cohen's estimate of effect size d = 0.66, cluster [k] = 27 voxels; right amygdala: 21, 0, −15, t[31] = 4.29, pFWE = 0.005, d = 0.77, k = 35; Figure 1). Perceived parental social standing did not predict amygdala reactivity to surprised or neutral faces, even at a lenient threshold (puncorrected < 0.05, k = 0). It is unlikely that lower perceived parental social standing did not predict amygdala reactivity to surprised or neutral faces because they did not engage the amygdala. Hence, random-effects analyses revealed marked amygdala activation to all faces across individuals (Angry>Shapes: left amygdala: −27, −3, −21; right amygdala: 21, −3, −18; Surprise>Shapes: left amygdala: −21, −6, −18; right amygdala: 21, −3, −18; Neutral > Shapes: left amygdala: −24, −9, −15; right amygdala: 21, −3, −18; ts[32] ≥ 5.83, p'sFWE < 0.05, d's ≥ 1.03, k's ≥ 62; see Supplementary figure, available at http://scan.oxfordjournals.org). Moreover, as a presumptive control for non-emotional facial expression processing, lower perceived parental social standing predicted greater amygdala reactivity to angry than to neutral faces (Angry > Neutral) in an ancillary regression analysis (puncorrected < 0.005, k = 15-voxel extent threshold: left amygdala: −21, 0, −12; right amygdala: 21, 0, −15; ts[31] ≥ 2.93, p'suncorrected ≤ 0.003, d's ≥ 0.53, k's ≥ 23).
Specificity of the association between parental social standing and amygdala reactivity
We assessed the specificity of the relationship between perceived parental social standing and amygdala reactivity in two ways. First, we executed a whole-brain analysis that tested for areas of increased activation to Angry faces (Angry>Shapes) at a whole-brain threshold of pFWE < 0.05 (see Supplementary Table 1, available at http://scan.oxfordjournals.org). We then used the coordinates for the area of peak activation from the Angry > Shapes contrast in the middle occipital gyrus of the visual cortex (24, −96, 0) to define a 6 mm control region-of-interest. We found that perceived parental social standing was not associated with activation in this visual processing region, even at a lenient threshold within the small-volume search area (puncorrected < 0.05, k = 0). Second, we executed another supplementary whole-brain regression analysis that revealed no associations between perceived parental social standing and activation to angry faces outside the amygdala (pFWE-whole brain < 0.05).
Failure of confounders to explain the association between parental social standing and amygdala reactivity
Lower perceived parental social standing continued to predict greater amygdala reactivity to angry faces in two-step hierarchical regressions controlling for sex, ethnicity, self-mastery, optimism, neuroticism, extraversion, agreeableness, depressive symptoms, parental education and participants’ perceptions of their own social standing in step 1 (left amygdala step-1 R2-adj. = 0.132, P = 0.21; ΔR2 = 0.204, F[1,21] = 10.89, P = 0.003; right amygdala step-1 R2-adj. = 0.154, P = 0.18; ΔR2 = 0.152, F[1,21] =7.44, P = 0.01; reactivity values used for regressions shown in Figure 1; see Supplementary Tables 2–4 for univariate correlations and regression summaries, available at http://scan.oxfordjournals.org). Moreover, lower perceived parental social standing predicted greater amygdala reactivity to angry faces in two-step hierarchical regressions that specifically controlled for recent levels of anxiety, as assessed by the POMS [left amygdala step-1 R2-adj. = 0.14, P = 0.02; ΔR2 = 0.19, F(1,30) = 8.50, P = 0.007; right amygdala step-1 R2-adj. = 0.14, P = 0.03; ΔR2 = 0.26, F(1,30) = 12.60, P = .001].
Finally, we found no associations between amygdala reactivity and current employment status (employed, unemployed) or annual income in this sample of full-time college students (see Supplementary Table 2).
DISCUSSION
This study provides novel evidence that a retrospective measure of lower perceived parental social standing, a putative indicator of socioeconomic disadvantage during childhood and adolescence, is uniquely associated with greater amygdala reactivity to threatening (angry) facial expressions. This association was observed among healthy individuals who had not yet reached their adult SES, and it was not explained by several potential confounding factors. As such, this association may provide insight into the possible neurodevelopmental pathways that could plausibly link early life experiences to socioeconomic gradients in mental and physical health.
An individual's socioeconomic position delimits access to material goods and resources and it can define several dimensions of interpersonal relationships throughout the life (Adler et al., 1994; Ben-Shlomo and Kuh, 2002). Longstanding epidemiological evidence further indicates that disparities in income, education, occupation and other conventional socioeconomic indicators account for a substantive proportion of the variance in all-cause and disease-specific morbidity and mortality rates, as well as the prevalence of risk factors for chronic medical conditions and stress-related psychiatric disorders (Adler et al., 1994). That ill health and premature death vary with lower socioeconomic position cannot be entirely attributed to material deprivation, illiteracy or restricted availability of quality health care (Adler et al., 1994; Marmot, 2004; Sapolsky, 2004). Hence, several theoretical perspectives on SES-related health disparities posit that subjective experiences inherent to socioeconomic position could aggregate throughout life to influence well-being and disease risk, particularly through stress-related pathways (Adler et al., 2000; Marmot, 2004). In support of such perspectives, individuals who subjectively rank themselves as occupying a lower socioeconomic position than others on visual ladders depicting hierarchical rungs of SES report poorer health (Adler et al., 2000; Singh-Manoux et al., 2005; Goodman et al., 2007), show dysregulated neuroendocrine stress responses (Adler et al., 2000), and even have a greater susceptibility to the common cold (Cohen et al., 2008). Furthermore, because associations between rank-related indicators of perceived social standing and health outcomes largely persist after accounting for so-called ‘objective’ socioeconomic indicators (e.g. income, education and occupational characteristics), perceived social standing appears to capture unique variability in health status and risk for ill health.
A particularly important facet of low perceived social standing is thought to be the internalized distress that could accompany the recurrent experience of daily financial hardships, a sense of insecurity regarding future prosperity, and the possible demoralizing feelings of marginalization or social exclusion attributable to a person's self-judgment of comparative social, occupational or material disadvantage (Adler et al., 2000; Marmot, 2004). In addition, subjective rankings of social standing are thought to capture finer gradations of relative weightings of individual SES components than do objective indicators themselves. For example, consider that the objective SES indicator variables ‘years-of-education’ and ‘educational attainment’ do not take into account the prestige of different educational institutions (e.g. community colleges vs Ivy League universities). But, respondents may assign differential weightings to ‘education’ when judging their own or their family members’ social standing—depending on the institutions attended (Cohen et al., 2008). Also, different individuals may place a greater emphasis on different dimensions of SES (e.g. wealth over education) when judging their own social standing. As such, a differential weighting of SES components may not only track finer gradations of perceived social standing, but also contribute systematically to inter-individual variability in subjective social ladder rankings in the context of relative homogeneity in so-called ‘objective’ levels of SES (e.g. as reflected in this young sample of first and second year college students).
In parallel to adult epidemiological evidence on SES and health, emerging evidence indicates that individuals who develop in lower SES environments are at risk for poorer health when they reach adulthood, regardless of the SES they achieve as adults (Poulton et al., 2002; Cohen et al., 2004; Galobardes et al., 2006; Evans and Kim, 2007; Melchior et al., 2007; Kroenke, 2008). This evidence supports the notion that stressful early life experiences, perhaps occasioned by lower SES environments, may negatively influence later health by altering developmental risk trajectories (Repetti et al., 2002; Taylor et al., 2004; Lupien et al., 2005; McEwen, 2007), a process recently referred to as psychobiological ‘embedding’ (Hertzman, 1999; Miller and Chen, 2007). Indeed, children and adolescents from lower SES backgrounds are more likely to be exposed to violence, residential crowding, environmental noise and toxins, unfavorable housing conditions and conflict-laden family environments than those from higher SES backgrounds (Repetti et al., 2002; Taylor et al., 2004; Evans and Kim, 2007). In view of such exposures, a postulate is that maturing in unsafe, unpredictable or otherwise stressful conditions inherent to lower SES environments may consequently impair the development of stress regulatory systems and bias an individual's processing of social and emotional information by increasing vigilance and sensitivity to potential social threats in early and later life (Chen and Matthews, 2001; Repetti et al., 2002; Taylor et al., 2004; Evans and Kim, 2007). In line with this postulate, the current findings suggest that increased sensitivity to threatening information among individuals who report themselves to be from lower SES backgrounds may be expressed as greater amygdala reactivity to explicitly threatening (angry) facial expressions.
It is well established that the amygdala is an instrumental component of the neural circuitry that gauges the emotional salience of social and environmental information (LeDoux, 2000). Hence, the amygdala is especially sensitive to social cues, such as facial expressions, that vary in their depicted emotionality (Whalen, 1998). In addition, the amygdala plays a critical role in regulating the neuroendocrine and autonomic stress-response axes (McEwen, 2007). Particularly noteworthy in the present context, cell groups in the amygdala show marked neural plasticity as a function of early life stress, which may influence adult sensitivity, resiliency and reactivity to life stressors and associated risk for ill health (Gunnar and Quevedo, 2007; McEwen, 2007). Therefore, it is possible that increased amygdala reactivity to angry or otherwise threat-related facial expressions could represent a neural correlate of a so-called developmental ‘embedding’ of early SES-related experiences that influence sensitivity to perceived social threats.
On balance, however, our inferences regarding parental social standing and amygdala reactivity to threatening facial expressions are restricted by several study limitations. First, the relationship between retrospective reports of parental social standing and amygdala reactivity might be confounded by dispositional biases in emotionality. However, we controlled for several potential confounders, including dispositional traits related to self-mastery, optimism, neuroticism, extraversion and agreeableness. We also controlled for symptoms of depression and anxiety and the participants’ ratings of their own social standing. Second, we did not formally or prospectively assess early maltreatment, neglect or other adverse early life experiences at the level of the family, which could be prevalent among lower SES households and plausibly influence amygdala reactivity (Pollak, 2005; Teicher et al., 2003; Taylor et al., 2006). However, if such adverse early experiences per se were to explain the association between parental social standing and amygdala reactivity, then these experiences would arguably seem to operate via pathways that do not involve influencing individual differences in dispositional emotionality and symptoms of depression and anxiety, because these individual differences were accounted for in our analyses. Third, our participants were all university students, mostly ethnically homogenous, and had parents who were relatively well-educated. These participant characteristics necessarily constrain extrapolations to more representative populations with greater ethnic and socioeconomic diversity. Fourth, due to the cross-sectional nature of our study and the retrospective assessments of parental social standing, which encompassed a diffuse developmental period (‘childhood and adolescence’), the causal directions of association are uncertain, as are the possible effects of upward and downward shifts in familial social mobility during critical or sensitive developmental periods. Finally, it could be argued that there is an empirical basis for expecting a possible association between perceived parental social standing and amygdala reactivity to surprised and neutral facial expressions. This expectation is based on prior work showing that objective SES indicators are associated with perceptions of social threat in situations with ambiguous outcomes, but not in situations with explicitly threatening or negative outcomes (Chen and Matthews, 2001; Chen et al., 2004). To the extent that surprised and neutral faces are conceptually similar to ambiguous information (Whalen, 1998), our null findings could seem unexpected. In view of these limitations and interpretive caveats, it will be important to determine the multilevel and likely multidimensional factors that proximally link early life SES with amygdala processing of and reactivity to social and emotional information that range in threat-related ambiguity, particularly in the developmental context of psychological and physical well-being.
To conclude, we note that lower adult perceived social standing has recently been associated with reduced gray matter volume in the perigenual anterior cingulate cortex (pACC), a region densely networked with the amygdala and involved in regulating emotional behavior and physiological reactivity to stress (Gianaros et al., 2007). As speculated previously, it is possible that dynamic regulatory functions supported by pACC–amygdala circuitry may be altered by developing in lower SES environments (Eisenberger, 2007; Gianaros et al., 2007). To test this speculation, future work should use explicit emotion and stress regulation paradigms, as opposed to the passive emotional information processing paradigm employed here, in conjunction with computational connectivity methods to examine developmental socioeconomic-related variations in pACC–amygdala dynamics in association with possible risk for ill health.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This work was supported by NIH grants MH-K01-070616 (PJG) and HL-076852/076858 (KAM). We thank Leslie Mitrik for her assistance in data collection and Dr Brooks B. Gump for his constructive comments on a draft of this report.
APPENDIX
Think of this ladder as representing where people stand in the United States. At the top of the ladder are the people who have the most money, most education and most respected jobs. At the bottom are the people who have the least money, least education and least respected jobs or no job. The higher up you are on this ladder, the closer you are to the people at the very top, and the lower you are, the closer you are to the people at the very bottom. Where, during your childhood and adolescence, would you have placed each of your parents on this ladder, relative to other people in the United States?
REFERENCES
- Adler NE, Boyce T, Chesney MA, et al. Socioeconomic status and health: the challenge of the gradient. American Psychologist. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychology. 2000;19:586–92. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology. 2002;31:285–93. [PubMed] [Google Scholar]
- Chen E, Langer DA, Raphaelson YE, Matthews KA. Socioeconomic status and health in adolescents: the role of stress interpretations. Child Development. 2004;75:1039–52. doi: 10.1111/j.1467-8624.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews KA. Cognitive appraisal biases: an approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Annals of Behavioral Medicine. 2001;23:101–11. doi: 10.1207/S15324796ABM2302_4. [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children's health: how and why do these relationships change with age? Psychological Bulletin. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- Cohen S, Alper CM, Doyle WJ, Adler N, Treanor JJ, Turner RB. Objective and subjective socioeconomic status and susceptibility to the common cold. Health Psychology. 2008 doi: 10.1037/0278-6133.27.2.268. (in press) [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosomatic Medicine. 2004;66:553–8. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. Using neuroimaging techniques to explore the relationship between social status and health. Social Cognition and Affective Neuroscience. 2007;2:159–60. [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–55. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Evans G, Kim P. Childhood poverty and health: cumulative risk exposure and stress dysregulation. Psychological Sciences. 2007;18:953–7. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Annals of Epidemiology. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Cohen S, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Social Cognition and Affective Neuroscience. 2007;2:161–73. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg L. The development of markers for the Big-Five factor structure. Psychological Assessment. 1992;4:26–42. [Google Scholar]
- Goodman E, Huang B, Schafer-Kalkhoff T, Adler NE. Perceived socioeconomic status: a new type of identity that influences adolescents’ self-rated health. Journal of Adolescence Health. 2007;41:479–87. doi: 10.1016/j.jadohealth.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–73. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage. 2002;17:317–23. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Annals of the New York Academy of Sciences. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Kroenke C. Socioeconomic status and health: youth development and neomaterialist and psychosocial mechanisms. Social Science and Medicine. 2008 doi: 10.1016/j.socscimed.2007.07.018. (in press) [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30:225–42. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marmot M. The Status Syndrome: How Social Standing Affects our Health and Longevity. New York: Henry Holt and Company; 2004. [Google Scholar]
- McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–24. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Melchior M, Moffitt TE, Milne BJ, Poulton R, Caspi A. Why do children from socioeconomically disadvantaged families suffer from poor health when they reach adulthood? A life-course study. American Jornal of Epidemiology. 2007;166:966–74. doi: 10.1093/aje/kwm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosomatic Medicine. 2007;69:402–9. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- Pearlin L, Schooler C. The structure of coping. Journal of Health and Social Behavior. 1978;19:2–21. [PubMed] [Google Scholar]
- Pollak SD. Early adversity and mechanisms of plasticity: integrating affective neuroscience with developmental approaches to psychopathology. Development and Psychopathology. 2005;17:735–52. doi: 10.1017/S0954579405050352. [DOI] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Milne BJ, et al. Association between children's experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360:1640–5. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Repetti R, Taylor S, Seeman T. Risky families: family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–66. [PubMed] [Google Scholar]
- Sapolsky R. Social status and health in humans and other animals. Annual Review of Anthropology. 2004;33:393–418. [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67:1063–78. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosomatic Medicine. 2005;67:855–61. doi: 10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry. 2006;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. Journal of Personality. 2004;72:1376–93. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience and Biobehavioral Reviews. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Usala P, Hertzog C. Measurement of affective states in adults. Evaluation of an adjective rating scale instrument. Research on Aging. 1989;11:403–26. doi: 10.1177/0164027589114001. [DOI] [PubMed] [Google Scholar]
- Whalen P. Fear, vigilance and ambiguity: initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7:177–88. [Google Scholar]