Abstract
The maintenance of meiotic prophase arrest in fully grown vertebrate oocytes depends on the activity of a Gs G-protein that activates adenylyl cyclase and elevates cAMP, and in the mouse oocyte, Gs is activated by a constitutively active orphan receptor, GPR3. To determine whether the action of luteinizing hormone (LH) on the mouse ovarian follicle causes meiotic resumption by inhibiting GPR3-Gs signaling, we examined the effect of LH on the localization of Gαs. Gs activation in response to stimulation of an exogenously expressed β2-adrenergic receptor causes Gαs to move from the oocyte plasma membrane into the cytoplasm, whereas Gs inactivation in response to inhibition of the β2-adrenergic receptor causes Gαs to move back to the plasma membrane. However, LH does not cause a change in Gαs localization, indicating that LH does not act by terminating receptor-Gs signaling.
Keywords: Meiotic resumption, Oocyte maturation, Luteinizing hormone, Heterotrimeric G proteins, Follicle-enclosed oocytes, Mouse
Introduction
Fully grown vertebrate oocytes are stored in the ovary for prolonged periods, arrested at meiotic prophase by a pathway requiring the activity of a Gs G-protein (Gallo et al., 1995; Mehlmann et al., 2002; Kalinowski et al., 2004). In mouse oocytes, Gs is activated by the receptor GPR3 (Mehlmann et al., 2004; Ledent et al., 2005; Hinckley et al., 2005; Mehlmann 2005a), and in rat oocytes, this function is served by the related receptor GPR12 (Hinckley et al., 2005). Ligands for GPR3 and GPR12 have not been definitively identified, although some effects of sphingolipids have been reported (Uhlenbrock et al., 2002; Ignatov et al., 2003; Hinckley et al., 2005). These receptors show constitutive activity when expressed in a variety of cell lines (Eggerickx et al., 1995; Tanaka et al., 2007), so if an agonist is required for their activity, it would appear to be produced by the same cells that express the receptor. Likewise, GPR3 activates Gs even in isolated oocytes, and the level of Gs activity is independent of the presence of the surrounding somatic cells (Freudzon et al., 2005).
The primary adenylyl cyclase activated by Gs in mouse and rat oocytes is AC3 (Horner et al., 2003), and the resulting elevation of cAMP is essential for maintaining meiotic arrest (Eppig, 1991). This pathway involves cAMP-dependent protein kinase-mediated phosphorylation of the phosphatase CDC25B and the kinase WEE1B, which in turn keeps CDC2A, the kinase that directly controls the prophase-to-metaphase transition, in the phosphorylated and inactive state (Ferrell, 1999; Duckworth et al., 2002; Lincoln et al., 2002; Han et al., 2005).
Luteinizing hormone (LH) from the pituitary acts on the ovarian follicle to cause the resumption of meiosis in the oocyte, as well as ovulation (Eppig et al., 2004; Mehlmann, 2005b). Within mouse and rat follicles, LH receptors are located in the mural granulosa cells, and not in the cumulus cells that directly surround the oocyte, or in the oocyte itself (see Eppig et al., 1997). Current evidence indicates that LH causes the synthesis in the somatic cells of EGF-like proteins (Espey and Richards, 2002; Park et al., 2004; Ashkenazi et al., 2005; Shimada et al., 2006), which by activating EGF receptors in the somatic cells, leads to meiotic resumption (Dekel and Sherizly, 1985; Downs et al., 1988; Park et al., 2004; Ashkenazi et al., 2005; Hsieh et al., 2007). However, the links between EGF receptor activation in the somatic cells and meiotic progression in the oocyte are incompletely understood. In some way, LH signaling leads to a decrease in cAMP in mouse and rat oocytes (Schultz et al., 1983; Sela-Abramovich et al., 2006), and this cAMP decrease appears to be required for the resumption of meiosis; mice lacking the primary cAMP phosphodiesterase found in the oocyte, PDE3A, produce oocytes with elevated cAMP, which are ovulated in response to LH, but remain arrested in prophase (Masciarelli et al., 2004).
Possible pathways by which LH action on the somatic cells might decrease oocyte cAMP include decreasing the production of cAMP by inhibition of GPR3-Gs-AC3 signaling, increasing the degradation of cAMP by stimulation of cAMP phosphodiesterase, or modulating transport of cAMP between the cumulus cells and oocyte (Fig. 1). Here we examine the first of these possibilities.
Figure one.
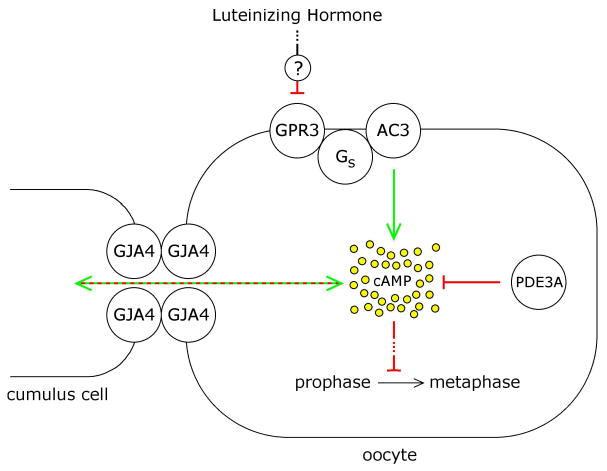
Pathways by which luteinizing hormone action on the somatic cells of the ovarian follicle might lead to decreased cAMP in the oocyte, thus initiating the prophase-to-metaphase transition. This paper tests the hypothesis that LH signaling could inhibit cAMP production in the oocyte by inhibiting the receptor-Gs-adenylyl cyclase system in the oocyte (GPR3-Gs-AC3). Alternatively, LH signaling could stimulate cAMP degradation by stimulating cAMP phosphodiesterase (PDE3A), or could modulate transport of cAMP between the cumulus cells and oocyte through gap junctions, which are comprised at least mostly of connexin 37 subunits (GJA4) (Simon et al., 1997; Kidder and Mhawi, 2002; Veitch et al., 2004).
In Gpr3 knockout mice, ∼80-90% of oocytes in antral follicles from prepubertal ovaries resume meiosis spontaneously, with only ∼10-20% remaining arrested in prophase (Mehlmann et al., 2004; Ledent et al., 2005). The concept that almost all oocytes in antral follicles would show meiotic resumption if GPR3 signaling was turned off by LH action is further supported by the finding that 93% of oocytes that were isolated from such follicles and maintained in prophase arrest by the addition of the phosphodiesterase inhibitor hypoxanthine resumed meiosis when injected with a morpholino oligonucleotide targeting Gpr3 (Hinkley et al., 2005). Injection of follicle-enclosed oocytes with siRNA targeting Gpr3 also causes meiotic resumption in the majority of oocytes (Mehlmann et al., 2005a). When Gs is turned off experimentally, by injecting follicle-enclosed mouse oocytes with an inhibitory antibody or a dominant negative form of Gαs, nuclear envelope breakdown and polar body formation occur (Mehlmann et al., 2002; Kalinowski et al., 2004), and likewise mice lacking Adcy3 show spontaneous resumption of meiosis (Horner et al. 2003). All of these observations indicate that inhibition of the GPR3-Gs-AC3 pathway is a plausible mechanism by which LH might cause meiotic resumption. Specifically, LH-induced signaling from the mural granulosa cells could cause the cumulus cells to release a GPR3 antagonist onto the oocyte, or in some way could reduce the production of a GPR3 agonist in the oocyte.
Testing the hypothesis that LH signaling in the somatic cells results in a decrease in Gs activity in the oocyte presents the challenge of measuring Gs activity while the oocyte remains enclosed within the multilayered complex of somatic cells. If the somatic cells are removed from the oocyte, cAMP decreases (Törnell et al., 1990), and meiosis resumes spontaneously (Pincus and Enzmann, 1935). In order to avoid this problem, we used an optical method.
In various cell types including mouse oocytes (Freudzon et al., 2005), receptor-mediated activation of Gs causes its α subunit to translocate from the plasma membrane to the cytoplasm (Marrari et al., 2007), which is due at least in part to depalmitoylation of Gαs (Wedegaertner and Bourne, 1994; Iiri et al., 1996; Thiyagarajan et al., 2002). The translocation of Gαs has been detected by subcellular fractionation (Rasenick et al., 1984; Levis and Bourne, 1992), immunostaining (Wedegaertner et al., 1996; Thiyagarajan et al., 2002; Freudzon et al., 2005), and most recently by use of fluorescent fusion proteins (Yu and Rasenick, 2002; Hynes et al., 2004; Allen et al., 2005; Freudzon et al., 2005). The Gαs-GFP constructs used to monitor Gαs internalization in response to adrenergic receptor agonists also activate adenylyl cyclase in response to these agonists (Yu and Rasenick, 2002; Hynes et al., 2004). Gαs is then repalmitoylated (Jones et al., 1997), associates with intracellular vesicles (Hynes et al., 2004), and is transported back to the plasma membrane (Wedegaertner et al., 1996). Thus, if receptor stimulation is terminated, Gαs reaccumulates on the plasma membrane (Wedegaertner et al., 1996). In the experiments to be described, we examine whether LH application to follicle-enclosed mouse oocytes causes Gαs to accumulate in the oocyte plasma membrane.
Materials and methods
Follicle and oocyte isolation and culture
Ovaries were dissected from 22-27 day old C57BL/6 X SJL F1 hybrid mice (The Jackson Laboratory, Bar Harbor, ME), and preantral follicles (140-180 μm diameter) or antral follicles (320-360 μm) were isolated using fine forceps or 30 gauge needles. All manipulations were performed in MEMα medium (#12000-022, Invitrogen, Carlsbad, CA) with 25 mM NaHCO3, 75 μg/ml penicillin G, 50 μg/ml streptomycin sulfate, and 5-10% FBS (#16000-044, Invitrogen). For experiments in which follicles were cultured for 24 hours, the medium also contained 10 ng/ml ovine FSH (National Hormone and Peptide Program) and insulin-transferrin-sodium selenite medium supplement (Sigma Chemical Co., St. Louis, MO). Follicles were cultured on Millicell culture plate inserts (PICMORG50, Millipore Corp., Bedford, MA) in 35 mm dishes of medium, at 37°C, with 5% CO2 in air. For experiments involving LH, the mice were injected with 3-5 I.U. of equine chorionic gonadotropin (eCG) 42-44 hours before ovary isolation, to stimulate the expression of LH receptors (eCG and ovine LH were obtained from the National Hormone and Peptide Program, Torrance, CA). The antral follicles dissected from eCG-primed mice were 360-570 μm in diameter.
Where indicated, oocytes were isolated from preantral follicles using 30 gauge needles and a mouth-controlled pipet with an opening slightly smaller than the oocyte diameter. The oocytes were cultured in 200 μl drops of MEMα + 5% FBS under light mineral oil (Fisher Scientific, Pittsburgh, PA).
Microinjection
Microinjections were performed using a chamber in which the oocyte or follicle was held between 2 coverslips separated by double stick tape (1 layer for oocytes and preantral follicles, 2 layers for antral follicles, resulting in spacers of 100 μm and 200 μm, respectively) (see Jaffe and Terasaki, 2004; Jaffe et al., 2007; video1.mov in online supplement). The injection volumes were quantitated as previously described (Jaffe and Terasaki, 2004); the volume of the oocyte was considered to be 200 pl.
GαsGFP localization
RNAs encoding GαsGFP and the rat β2-adrenergic receptor were transcribed in vitro and post-translationally polyadenylated (see Aida et al., 2001; Freudzon et al., 2005). The amounts injected per oocyte were 3 pg and 30 fg, respectively. After injection, the oocytes were cultured for ∼24 hours, to allow GαsGFP expression. Under these conditions, the amount of GαsGFP protein in the oocytes was comparable to the amount of endogenous Gαs (Freudzon et al., 2005). For imaging, isolated oocytes were placed in 10 μl droplets of medium on a coverslip-bottomed dish (P35G-0-20-C-INV, MatTek Corp., Ashland, MA) under oil, and transferred to droplets containing 10 μM isoproterenol or 10 μM propranolol (both from Sigma Chemical Co.), as indicated.
GαsGFP was imaged using a Zeiss 510 confocal microscope with a 40X/0.8 NA water immersion lens contacting the bottom surface of the dish (Freudzon et al., 2005). The dish was perfused with water-saturated 5% CO2/air, and was maintained at ∼30°C by use of a heated stage; it was impractical to keep the dish at 37°C, due to the contact between the dish and the immersion lens. Thus, it is possible that the return of Gαs to the plasma membrane at 37°C is somewhat more rapid than determined here. However, such a temperature effect would, if anything, strengthen the conclusion (see Results) that an LH-induced change in Gαs distribution would have occurred sufficiently rapidly to be detectable at 2-3 hours after LH application. Plasma membrane:cytoplasm ratios were determined using Metamorph software (Freudzon et al., 2005).
Gαs immunofluorescence
For experiments involving expression of β2 adrenergic receptors, antral follicles were dissected from unprimed mice, and injected with 30 fg of RNA encoding the rat β2 adrenergic receptor (see above). After RNA injection, the follicles were cultured for 24 hours on Millicell membranes. For experiments involving LH, antral follicles were dissected from ovaries of eCG-primed mice, and placed on Millicell membranes. Isoproterenol, propranolol, or LH were applied by exchanging the medium underlying the Millicell membranes, and after the indicated times, the follicles were collected from the membranes and placed in 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) in PBS at 4°C.
After 2 hours, the follicles were rinsed in PBS and embedded in 3% low gelling temperature agarose (SeaPlaque #50102; American Bioanalytical, Natick, MA). This was accomplished by placing them in 2.0 ml polypropylene tubes with conical bottoms (#72.693.005, Sarstedt Inc., Newton, NC), adding 1 ml of melted agarose at 50°C, and stirring slightly with a toothpick. After solidifying the agarose by placing the tubes on ice for 1 hour, the tips of the tubes were cut off with a razor blade, and the agarose blocks containing ∼6-10 follicles were trimmed and placed in 30% sucrose at 4°C. After 1-2 h, the blocks were transferred to freezing medium (#H-TFM, Triangle Biomedical Sciences, Inc., Durham, NC) in 6 mm diameter gelatin capsules (#5214, Ernest F. Fullam, Inc., Latham, NY). The samples were frozen in isopentane cooled with dry ice, and 8-10 μm sections were cut using a cryostat, and collected on SuperFrost Plus glass slides (Fisher Scientific).
The sections were stained with an affinity purified antibody made against the C-terminal decapeptide of Gαs, which recognizes Gαs in oocytes with high specificity (RM, 3-6 μg/ml; Simonds et al., 1989; Freudzon et al., 2005). The RM antibody was provided by T.L.Z. Jones (National Institutes of Health, Bethesda MD). The sections were imaged with a Pascal confocal microscope, with a 40X/1.2 NA objective (Carl Zeiss, Inc., Thornwood, NY). Other conditions were as previously described (Freudzon et al., 2005). Plasma membrane and cytoplasmic fluorescence intensities were measured using MetaMorph software (Molecular Devices Corp., Downingtown, PA), and ratios were calculated after subtraction of background values obtained from sections stained with nonimmune IgG and processed in parallel (see Freudzon et al., 2005).
Evaluation of the kinetics of nuclear envelope breakdown in follicle-enclosed oocytes stimulated with LH or Gs inhibitory antibody
To determine the time course of nuclear envelope breakdown following LH application, antral follicles from eCG-primed mice were placed on Millicell culture plates, and exposed to 1 μg/ml LH. At various times after LH application, cumulus-oocyte complexes were dissected from the follicles, and cumulus cells were stripped from the oocytes using a mouth-controlled pipet, to score for the presence or absence of a prophase nucleus. Only oocytes from compact cumulus-oocyte complexes were included.
To determine the time course of nuclear envelope breakdown following injection of the Gs inhibitory antibody (RM), follicle-enclosed oocytes from unprimed mice were first placed in a microinjection chamber and injected with 10 pl of a 10 mg/ml stock of the antibody in PBS. This resulted in a final concentration of 500 μg/ml = 3.3 μM of IgG in the oocyte. The follicles were then placed on Millicell culture plates held in a 35 mm dish, with a volume of 1.2-1.6 ml of medium beneath the membrane. This volume resulted in only a thin film of medium on top of each follicle, thus slightly flattening the follicle, and allowing the nucleus and nucleoli to be clearly visualized using an upright microscope with a long working distance 20X/0.4 NA objective. (It should be noted that this simple and physiologically unperturbing observation method cannot be used with follicles from eCG-primed mice, because such follicles are not as optically clear.) The dishes were maintained at 34-36°C in an environment of water-saturated 5% CO2/air, except during brief (∼1 min) observation periods. Only one injected follicle and one uninjected control follicle were placed together on an individual Millicell membrane, to minimize the time outside of the incubator needed for observation. The time of nuclear envelope breakdown was considered to be halfway between the last time point at which the nuclear envelope and nucleoli were visible, and the first time point at which they had disappeared. The time between these 2 observation points was 12-25 min.
Results
Kinetics of Gαs movement between the oocyte plasma membrane and cytoplasm in response to receptor activation and inactivation
In a previous study (Freudzon et al., 2005), we established that localization of Gαs can be used as an indicator of receptor activation of Gs in follicle-enclosed mouse oocytes. In the absence of the Gs-activating receptor, GPR3, Gαs is primarily associated with the oocyte plasma membrane, and GPR3 expression causes Gαs to move into the oocyte cytoplasm. This change in Gαs localization was seen both by immunofluorescence and by expression of GαsGFP in live oocytes. However, these observations, made by comparing wildtype or Gpr3-/- oocytes, or Gpr3-/- oocytes after injection of Gpr3 RNA, did not provide information about the kinetics of Gαs movement between the plasma membrane and cytoplasm in response to receptor activation and inactivation. Such kinetic information is needed in order to use Gαs localization to monitor the possible effect of LH action on Gs activity.
To examine these kinetics, we would have ideally liked to investigate the effect of agonists and antagonists of GPR3 on the time course of Gαs relocalization. However, ligands for GPR3 have not been clearly identified, and the only proposed candidates, sphingosine 1-phosphate and dihydrosphingosine 1-phosphate, have small effects (Uhlenbrock et al., 2002). As an alternative, we used an exogenously expressed Gs-activating receptor, the β2-adrenergic receptor, to change Gαs localization in response to receptor activation and inactivation. β2-adrenergic receptor activation caused Gαs to move into the oocyte cytoplasm, as monitored with GαsGFP (see Freudzon et al., 2005, supplementary material, and Fig. 2A of the present paper). β2-adrenergic receptor inactivation caused GαsGFP to move back to the plasma membrane (Fig. 2B). For these experiments, we used prophase-arrested oocytes that had been isolated from preantral follicles, rather than those from antral follicles, to prevent spontaneous resumption of meiosis in culture. We injected RNA encoding the β2-adrenergic receptor, along with RNA encoding GαsGFP; ∼24 hours later, we imaged GαsGFP as a function of time after applying a β2-adrenergic receptor agonist (isoproterenol), followed by an antagonist (propranolol) (Fig. 2A,B).
Figure two.
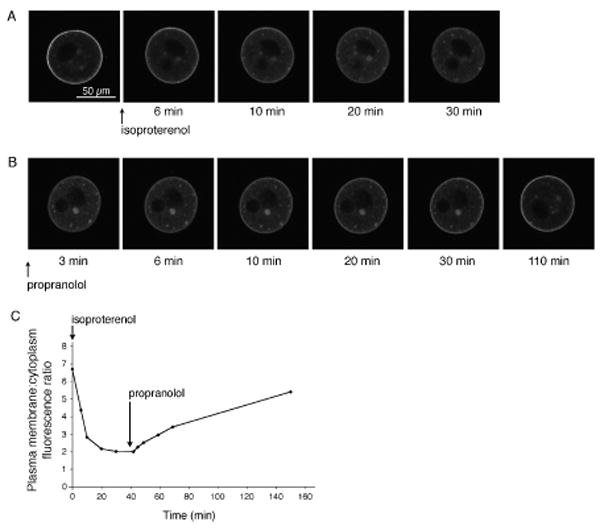
Translocation of GαsGFP in oocytes in response to activation and inactivation of an exogenously expressed β2 adrenergic receptor. Oocytes were isolated from preantral follicles, and injected with RNAs encoding GαsGFP and the rat β2-adrenergic receptor. A. GαsGFP movement from the plasma membrane to cytoplasm in an oocyte in which the β2-adrenergic receptor was activated with 10 μM isoproterenol. B. GαsGFP movement back to the plasma membrane when isoproterenol was washed out and replaced with 10 μM propranolol. In A and B, the black areas within the oocyte are the nucleus and an oil drop introduced by the microinjection; one or both are visible, depending on the optical section. Because the oocytes used for these experiments were obtained from preantral follicles, they remained prophase arrested throughout the entire period. GαsGFP was expressed at a level comparable to that of endogenous Gαs (see Freudzon et al., 2005). There was no significant autofluorescence in oocytes without GαsGFP and imaged under identical conditions (see Freudzon et al., 2005). C. Plasma membrane:cytoplasm GαsGFP fluorescence ratios as a function of time, for the oocyte illustrated in A and B. The mean ratio for GαsGFP in isolated oocytes expressing the β2-adrenergic receptor, but without agonist stimulation, was 6.1 ± 0.5 (mean ± SEM, n = 6), which is slightly smaller than that reported previously for isolated oocytes without exogenous receptor expression (8.8 ± 0.8, n = 19) (Freudzon et al., 2005), perhaps due to some degree of constitutive activity of the β2-adrenergic receptor (Chidiac et al., 1994). At 30 min after isoproterenol application, the mean plasma membrane:cytoplasm fluorescence ratio was 2.1 ± 0.1 (n = 6).
In response to 10 μM isoproterenol, GαsGFP moved into the cytoplasm with a half time of 6 ± 1 min (mean ± SEM, n = 6) (Fig. 2C). After exchanging the medium to wash out isoproterenol and to add 10 μM propranolol, the time for the plasma membrane:cytoplasm fluorescence ratio to return to a value halfway between those before and after applying isoproterenol was 67 ± 3 min (n = 4) (Fig. 2C). From this we concluded that if LH caused inactivation of the receptor (GPR3) that keeps Gs active in the plasma membrane of antral follicle-enclosed oocytes, this would result in an easily detectable change in Gαs distribution within less than one hour.
We also examined whether activation of the β2-adrenergic receptor with isoproterenol would cause endogenous Gαs to move into the oocyte cytoplasm, and whether subsequent inactivation of this receptor with propranolol would cause a return of Gαs to the plasma membrane. Antral follicle-enclosed oocytes were injected with RNA encoding the β2-adrenergic receptor, then incubated for 24 hours to allow protein expression. They were then treated with isoproterenol, or with isoproterenol followed by propranolol, and then fixed, frozen, sectioned, and stained for Gαs immunofluorescence imaging (Fig. 3).
Figure three.
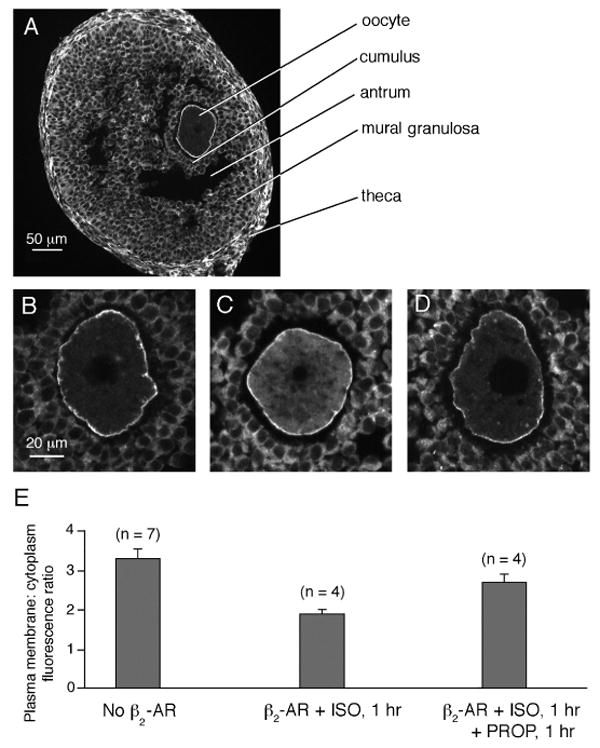
Translocation of endogenous Gαs in antral follicle-enclosed oocytes in response to activation and inactivation of an exogenously expressed β2 adrenergic receptor. A. Immunofluorescence image of a cryosection of an antral follicle labeled with an antibody against Gαs. Gαs is present in the oocyte, as well as the cumulus cells, mural granulosa cells, and theca cells. The black space within the oocyte cytoplasm is the prophase nucleus. The black space between the mural granulosa cells and the cumulus cells is the fluid-filled antrum. For immunoblots demonstrating antibody specificity, and for images illustrating the absence of significant fluorescence in sections stained with nonimmune IgG, see Freudzon et al. (2005). B. A magnified view of the oocyte from another follicle like that shown in A. C. An antral follicle-enclosed oocyte expressing the β2-adrenergic receptor and treated for 1 hour with 10 μM isoproterenol, then fixed and cryosectioned for Gαs immunofluorescence imaging. D. An antral follicle-enclosed oocyte expressing the β2-adrenergic receptor and treated for 1 hour with 10 μM isoproterenol, followed by 1 hour with 10 μM propranolol, then fixed and cryosectioned for Gαs immunofluorescence imaging. E. Plasma membrane:cytoplasm Gαs immunofluorescence ratios in antral follicle-enclosed oocytes for each of the conditions shown in the images above the graph (mean ± SEM, n = number of oocytes): no β2-AR (3.3 ± 0.24); β2-AR + ISO (1.9 ± 0.11); β2-AR + ISO + PROP (2.7 ± 0.21). The value for β2-AR + ISO is statistically different from both of the other values (unpaired t-test, p < 0.04), but the value for β2-AR + ISO + PROP is not different from the value for no β2-AR (p = 0.13). (Note that the absolute values of the ratios seen in the optical sections of isolated oocytes in Fig. 2 are artifactually higher than those seen in the physically sectioned specimens in Fig. 3, due to an optical effect as previously described (Freudzon et al., 2005)).
The application of isoproterenol to follicle-enclosed oocytes expressing the β2-adrenergic receptor caused the ratio of Gαs immunofluorescence in the plasma membrane vs cytoplasm to decrease to 58% of that in the control oocytes without β2-adrenergic receptor (Fig. 3). 60 min after washout of the isoproterenol and addition of the propranolol, the ratio had returned to 82% of that in the control. Although it was impractical to use these methods to obtain a time course of the return of Gαs to the plasma membrane following application of propranolol, as we did for GαsGFP in live oocytes (Fig. 2), these results showed that at 1 hour, a statistically significant relocation of Gαs was detectable (see Fig. 3 legend).
LH application to antral follicles does not cause a change in Gαs localization
To determine if LH action inhibited Gs in antral follicle-enclosed oocytes, we examined whether LH application to isolated follicles caused Gαs to redistribute from the oocyte cytoplasm to the plasma membrane, as seen with propranolol. Follicles were cultured for 1-3 hours with or without 1 μg/ml LH, and then fixed and prepared for Gαs immunofluorescence. These times for LH treatment were chosen because after 3 hours almost all oocytes have undergone nuclear envelope breakdown (Fig. 4A).
Figure four.
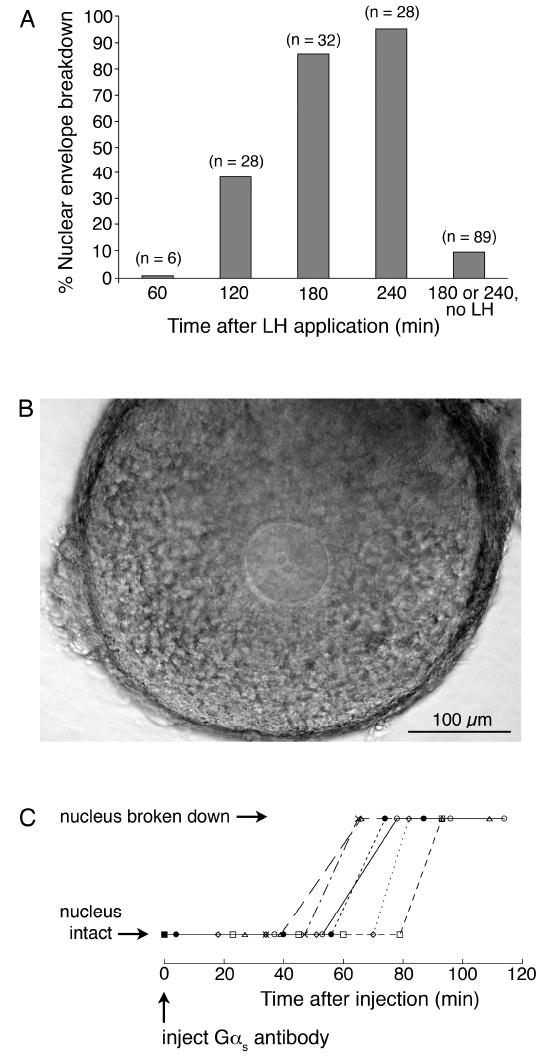
Determination of the time of nuclear envelope breakdown following LH application to isolated antral follicles, or injection of a Gs inhibitory antibody into follicle-enclosed oocytes. A. Percentage of oocytes that have undergone nuclear envelope breakdown at various times after treatment of antral follicles with 1 μg/ml LH. Numbers in parentheses indicate the number of oocytes scored for each point; results were obtained from 13 mice. B. Transmitted light image of an antral follicle-enclosed oocyte on a Millicell membrane, as used to determine the time to nuclear envelope breakdown following injection of the Gs-inhibitory antibody. C. Nuclear envelope breakdown as a function of time after injection of the Gs-inhibitory antibody. Each symbol represents an individual oocyte.
Immunostaining for Gαs showed a plasma membrane:cytoplasm fluorescence ratio in the oocyte that was not significantly different for samples with or without LH treatment for 1-3 hours (Fig. 5). The lack of effect of LH on Gαs localization is in contrast to the 3.7X increase in the ratio of Gαs immunofluorescence in the plasma membrane:cytoplasm, comparing oocytes with and without the GPR3 receptor (Freudzon et al., 2005; see Fig. 5E). Thus, these results indicated that LH action did not terminate the GPR3-mediated activation of oocyte Gs, since this would have been expected to cause Gαs to accumulate in the plasma membrane, if sufficient time had elapsed between Gs inactivation and fixation such that Gαs could have relocated (see Figs 2 and 3).
Figure five.
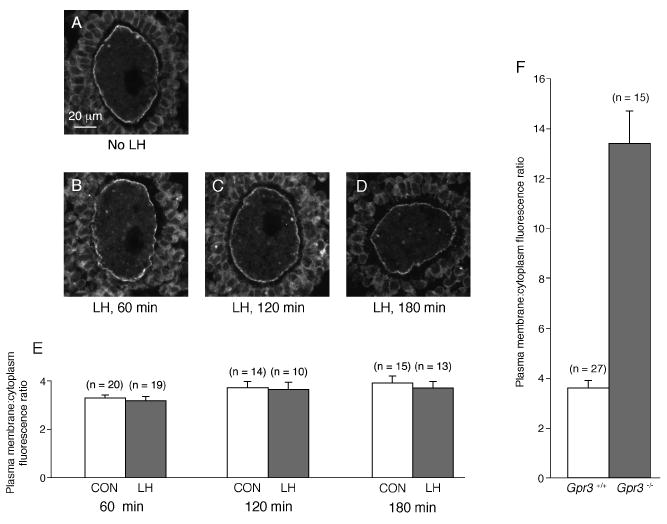
Lack of translocation of Gαs in antral follicle-enclosed oocytes in response to LH. A-D show immunofluorescence images of cryosections of antral follicle-enclosed oocytes, labeled with an antibody against Gαs. A. Oocyte in a follicle fixed without LH exposure. B, C, D. Oocytes in follicles fixed after a 1, 2, or 3 hour incubation with 1 μg/ml LH. Gαs is present in the oocyte plasma membrane as well as the cytoplasm, with a similar distribution with or without LH. E. Plasma membrane:cytoplasm Gαs immunofluorescence ratios in antral follicle-enclosed oocytes at 1-3 hours after LH application, and in samples cultured in parallel without LH (mean ± SEM, n = number of oocytes). The results of 2-3 independent experiments for each time point were combined; in each experiment, we fixed follicles at a single time point ± LH. The plasma membrane: cytoplasm fluorescence ratios, without or with LH, were as follows: 1 hr (3.29 ± 0.14 vs 3.18 ± 0.17), 2 hrs (3.72 ± 0.26 vs 3.65 ± 0.30), 3 hrs (3.92 ± 0.29 vs 3.71 ± 0.27). The values without or with LH were not significantly different for any of the time points (unpaired t-tests, p > 0.5). F. For comparison, the plasma membrane: cytoplasm fluorescence ratios for Gαs immunofluorescence in preantral follicle-enclosed oocytes from Gpr3+/+ and Gpr3-/- mice (from Freudzon et al., 2005). For Gpr3+/+, the ratio was 3.6 ± 0.3; for Gpr3-/-, the ratio was 13.4 ± 1.3; these values are significantly different (p = 0.0001).
Time between Gs inactivation and nuclear envelope breakdown
In order to interpret the results described above, we next determined how much time would be required to cause nuclear envelope breakdown, if LH acted by turning off Gs signaling. To do this, we injected antral follicle-enclosed oocytes with an affinity-purified antibody against the C-terminal 10 amino acids of the α subunit of Gs that specifically recognizes the 52 and 45 kDa forms of Gαs present in mouse oocytes (Mehlmann et al., 2002). This antibody inhibits the stimulation of adenylyl cyclase activity in membranes from frog oocytes (Gallo et al., 1995) as well as other cells (Simonds et al., 1989; Nair et al., 1990), in response to hydrolysis-resistant GTP analogs or β-adrenergic receptor stimulation. These studies have shown that binding of the antibody to the C-terminus of the GTP-bound form of Gαs reduces its ability to stimulate adenylyl cyclase, and have indicated that it may also interfere with the interaction of Gαs with receptors. When injected into follicle-enclosed mouse oocytes, the Gαs antibody decreases cAMP as measured with a fluorescent indicator (unpublished results), and causes meiotic resumption (Mehlmann et al., 2002).
After injection of the Gαs antibody, follicle-enclosed oocytes were placed on optically clear Millicell membranes, such that they were slightly flattened by surface tension (see Materials and methods). Under these conditions, the nuclear envelope and 1-2 nucleoli within the nucleus could be visualized (Fig. 4B) without the use of glass coverslips, which can inhibit gas exchange. Of 8 injected oocytes, 6 underwent nuclear envelope breakdown at 67 ± 5 min (mean ± SEM) (Fig. 4C). (2 other oocytes did not undergo nuclear envelope breakdown within a 3 hour period of observation.) 8/9 uninjected oocytes were prophase-arrested at 3 hours, and a previous study has shown that injection of nonimmune IgG does not cause nuclear envelope breakdown (Mehlmann et al., 2002). From these observations, we determined that the time between Gs inactivation and nuclear envelope breakdown is ∼1 hour. Thus, if LH action had caused nuclear envelope breakdown by terminating Gs activation, a change in Gαs distribution should have been visible by the time of nuclear envelope breakdown, based on the kinetics of the recycling of Gαs to the plasma membrane shown in Figs 2 and 3.
Discussion
The maintenance of meiotic prophase arrest in follicle-enclosed mouse oocytes depends on the constitutive activity of the Gs-linked receptor GPR3 (Mehlmann et al., 2004; Ledent et al., 2005; Hinckley et al., 2005; Mehlmann 2005a), suggesting that LH action on the mouse ovarian follicle could cause meiotic resumption by way of terminating receptor-Gs signaling in the oocyte. The experiments presented here argue against this hypothesis. Gs activity was monitored by measuring the relative distribution of Gαs in the oocyte plasma membrane and cytoplasm. LH did not cause a change in this distribution, under conditions where it would have been detected if GPR3-Gs signaling had been turned off. One caveat is that the method we used to monitor Gs activity would not have detected a transient decrease, due to the delay in the response of the indicator, and thus we can only conclude that LH does not cause the GPR3-Gs pathway to inactivate and remain inactivated during the 3 hour duration of the experiment.
The kinetics of nuclear envelope breakdown following experimental inhibition of Gs (Fig. 4C) show that if LH acted by terminating GPR3-Gs signaling, the time of inhibition would precede nuclear envelope breakdown by only ∼1 hour. Nuclear envelope breakdown occurs at ∼2-3 hours after LH application (Fig. 4A), indicating that if termination of GPR3-Gs signaling was the cause of nuclear envelope breakdown, the inactivation would have to occur at ∼1-2 hours after LH application. However, since the method we used to analyze Gs activity imposes a delay of ∼1 hour between the inhibition of Gs and the detectability of a change in Gαs localization (Figs 2 and 3), such a change would not be expected to be detectable before 2-3 hours after LH application.
The only established means by which cellular Gs activity can be regulated by extracellular signals is by way of regulation of a Gs-linked receptor. Another class of proteins that regulate heterotrimeric G-proteins, the cytoplasmic “RGS” proteins, have not as yet been demonstrated to have any effect on Gs activity (see Wieland et al., 2007), but in principle, such a receptor-independent means of inhibiting Gs activity might exist, and might not result in Gαs movement to the plasma membrane. Our findings cannot exclude this possibility. Nevertheless, the concept that LH causes the termination of an ongoing supply of a GPR3 agonist, or causes the release of a GPR3 antagonist, does not appear to account for the signal that causes meiotic resumption in mouse oocytes.
Several other optical methods for measuring Gs activity are available, but none of these appear to be applicable to follicle-enclosed mouse oocytes. One such approach is based on the change in localization of G protein coupled receptors from plasma membrane to cytoplasm, in response to activation (Hynes et al., 2004). Another involves fluorescence resonance energy transfer (FRET) between a YFP tag on a Gs-linked receptor, and a CFP tag on a G protein γ subunit (Hein et al., 2006). However, endogenous expression levels of the GPR3 receptor are ∼10X lower than can be detected by fluorescent probes (Freudzon et al., 2005), and as a consequence of the low expression level, specific antibodies for detecting native amounts of GPR3 have not been found (unpublished results). Thus neither of these methods could be used to study the effect of LH on GPR3-Gs signaling at endogenous GPR3 expression levels. Gs activity can also be monitored by measuring FRET between its α and γ subunits (Hein et al., 2006), but the FRET changes that have been detected with currently available probes are too small for studying the LH response in the oocyte, due to its slow time course. Thus further studies of possible regulation of receptor-Gs signaling in the follicle-enclosed mouse oocyte are likely to require the development of new optical techniques.
In a previous study, we established that LH does not cause meiotic resumption in the mouse oocyte by activating a G-protein of the Gi family, which inhibits adenylyl cyclase (Mehlmann et al., 2006). LH action also does not require an increase in oocyte Ca2+, which could in principle act to cause meiotic resumption by inhibiting the Ca2+-sensitive enzyme AC3 (Mehlmann et al., 2006). As illustrated in Fig. 1, two other possibilities for how LH signaling might lower oocyte cAMP and stimulate meiotic resumption in mouse oocytes are stimulation of cAMP phosphodiesterase in the oocyte (Eppig et al., 1985; Richard et al., 2001; Han et al., 2006), or regulation of cAMP flux through gap junctions with the somatic cells (see Sela-Abramovich et al., 2006).
Although elevated cAMP is required to maintain meiotic arrest (see Introduction), another unidentified factor from the follicle cells is also required, since removal of oocytes from their follicles causes meiotic resumption, even though GPR3/Gs activity is not reduced by oocyte isolation (see Freudzon et al., 2005). This unidentified factor could be cAMP, a regulator of cAMP such as an inhibitor of PDE3A, or a molecule unrelated to cAMP. Thus, it is also possible that LH acts by reducing the activity of this unidentified follicle cell factor.
While a decrease in oocyte cAMP has been reported to occur in response to LH receptor stimulation, prior to nuclear envelope breakdown in both mouse and rat (Schultz et al., 1983; Sela-Abramovich et al., 2006), such a decrease has not been seen in hamster (Hubbard, 1986), and all of these measurements are complicated by the issue that the oocytes had to be removed from their follicles in order to carry out radioimmunassays. Thus, optical methods for measurement of cAMP (see Nikolaev and Lohse, 2006) in live oocytes within intact follicles would be very useful. The strongest evidence that a decrease in cAMP is required for LH action is that mice lacking the primary cAMP phosphodiesterase found in the oocyte, PDE3A, produce oocytes with elevated cAMP, which are ovulated in response to LH, but remain arrested in prophase (Masciarelli et al., 2004). However, since basal cAMP is elevated above the normal level in such oocytes, the lack of LH-stimulated meiotic resumption in oocytes from Pde3a knockout mice does not definitively show that a decrease in cAMP from its normal basal level is a required step in LH signaling. The possibility needs to be considered that LH in some way bypasses the cAMP requirement for maintenance of meiotic arrest, acting at a level closer to the CDC2A kinase that regulates the prophase-to-metaphase transition.
Acknowledgments
We dedicate this paper to the late Richard D. Berlin, a pioneer in the use of fluorescence microscopy to study biological membranes, whose support was critical for these studies. We thank Mark Rasenick for suggesting the idea of using Gαs localization to investigate its activity in the oocyte, and Cathy Berlot, Teresa Jones, Mark Terasaki, and John Eppig for their interest and advice. We also thank Cathy Berlot for the GαsGFP plasmid, Nick Ancellin for the β2-adrenergic receptor plasmid, Teresa Jones for the Gαs antibody, A.F. Parlow (National Hormone and Peptide Program, Torrance, CA) for eCG, oLH, and oFSH, Mark Terasaki for his help in making the movie of microinjection, and William Ratzan for technical assistance. This work was supported by grants from the National Institutes of Health to L.A. Jaffe (HD014939 and DK073499), and to the Richard D. Berlin Center for Cell Analysis and Modeling (RR022232).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at… (video1.mov; legend for video1.mov).
References
- Aida T, Oda S, Awaji T, Yoshida K, Miyazaki S. Expression of a green fluorescent protein variant in mouse oocytes by injection of RNA with an added long poly(A) tail. Mol Human Reprod. 2001;7:1039–1046. doi: 10.1093/molehr/7.11.1039. [DOI] [PubMed] [Google Scholar]
- Allen JA, Yu JZ, Donati RJ, Rasenick MM. β-adrenergic receptor stimulation promotes Gαs internalization through lipid rafts: a study in living cells. Mol Pharmacol. 2005;67:1493–1504. doi: 10.1124/mol.104.008342. [DOI] [PubMed] [Google Scholar]
- Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146:77–84. doi: 10.1210/en.2004-0588. [DOI] [PubMed] [Google Scholar]
- Chidiac P, Hebert TE, Valiquette M, Dennis M, Bouvier M. Inverse agonist activity of β-adrenergic antagonists. Mol Pharmacol. 1994;45:490–499. [PubMed] [Google Scholar]
- Dekel N, Sherizly I. Epidermal growth factor induces maturation of rat follicle-enclosed oocytes. Endocrinology. 1985;116:406–409. doi: 10.1210/endo-116-1-406. [DOI] [PubMed] [Google Scholar]
- Downs SM, Daniel SAJ, Eppig JJ. Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool. 1988;245:86–96. doi: 10.1002/jez.1402450113. [DOI] [PubMed] [Google Scholar]
- Duckworth BC, Weaver JS, Ruderman JV. G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase. A Proc Natl Acad Sci USA. 2002;99:16794–16799. doi: 10.1073/pnas.222661299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggerickx D, Denef JF, Labbe O, Hayashi Y, Refetoff S, Vassart G, Parmentier M, Libert F. Molecular cloning of an orphan G-protein-coupled receptor that constitutively activates adenylate cyclase. Biochem J. 1995;309:837–843. doi: 10.1042/bj3090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ. Maintenance of meiotic arrest and the induction of oocyte maturation in mouse oocyte-granulosa cell complexes developed in vitro from preantral follicles. Biol Reprod. 1991;45:824–830. doi: 10.1095/biolreprod45.6.824. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod. 1997;56:976–984. doi: 10.1095/biolreprod56.4.976. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Viveiros MM, Marin-Bivens C, De La Fuente R. Regulation of mammalian oocyte maturation. In: Leung PCK, Adashi EY, editors. The Ovary. second. Elsevier/Academic Press; San Diego: 2004. pp. 113–129. [Google Scholar]
- Espey LL, Richards JS. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol Reprod. 2002;67:1662–1670. doi: 10.1095/biolreprod.102.005173. [DOI] [PubMed] [Google Scholar]
- Ferrell JE. Xenopus oocyte maturation: new lessons from a good egg. BioEssays. 1999;21:833–842. doi: 10.1002/(SICI)1521-1878(199910)21:10<833::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Freudzon L, Norris RP, Hand AR, Tanaka S, Saeki Y, Jones TLZ, Rasenick MM, Berlot CH, Mehlmann LM, Jaffe LA. Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J Cell Biol. 2005;171:255–265. doi: 10.1083/jcb.200506194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo CJ, Hand AR, Jones TLZ, Jaffe LA. Stimulation of Xenopus oocyte maturation by inhibition of the G-protein αs subunit, a component of the plasma membrane and yolk platelet membranes. J Cell Biol. 1995;130:275–284. doi: 10.1083/jcb.130.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Chen R, Paronetto MP, Conti M. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol. 2005;15:1670–1676. doi: 10.1016/j.cub.2005.07.056. [DOI] [PubMed] [Google Scholar]
- Han SJ, Vaccari S, Nedachi T, Andersen CB, Kovacina KS, Roth RA, Conti M. Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J. 2006;25:5716–5725. doi: 10.1038/sj.emboj.7601431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein P, Rochais R, Hoffmann C, Dorsch S, Nikolaev VO, Engelhardt S, Berlot CH, Lohse MJ, Bünemann M. Gs activation is time-limiting in initiating receptor-mediated signaling. J Biol Chem. 2006;281:33345–33351. doi: 10.1074/jbc.M606713200. [DOI] [PubMed] [Google Scholar]
- Hinckley M, Vaccari S, Horner K, Chen R, Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol. 2005;287:249–261. doi: 10.1016/j.ydbio.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol. 2003;258:385–396. doi: 10.1016/s0012-1606(03)00134-9. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–1924. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard CJ. Cyclic AMP changes in the component cells of Graafian follicles: possible influences on maturation in the follicle-enclosed oocytes of hamsters. Dev Biol. 1986;118:343–351. doi: 10.1016/0012-1606(86)90003-5. [DOI] [PubMed] [Google Scholar]
- Hynes TR, Mervine SM, Yost EA, Sabo JL, Berlot CH. Live cell imaging of Gs and the β2-adrenergic receptor demonstrates that both αs and β1γ7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the β2-adrenergic receptor. J Biol Chem. 2004;279:44101–44112. doi: 10.1074/jbc.M405151200. [DOI] [PubMed] [Google Scholar]
- Ignatov A, Lintzel J, Hermans-Borgmeyer I, Kreienkamp H-J, Joost P, Thomsen S, Methner A, Schaller HC. Role of the G-protein-coupled receptor GPR12 as high-affinity receptor for sphingosylphosphorylcholine and its expression and function in brain development. J Neurosci. 2003;23:907–914. doi: 10.1523/JNEUROSCI.23-03-00907.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iiri T, Backlund PS, Jones TLZ, Wedegaertner PB, Bourne HR. Reciprocal regulation of Gsα by palmitate and the βγ subunit. Proc Natl Acad Sci USA. 1996;93:14592–14597. doi: 10.1073/pnas.93.25.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe LA, Terasaki M. Quantitative microinjection of oocytes, eggs, and embryos. Meth Cell Biol. 2004;74:219–242. doi: 10.1016/s0091-679x(04)74010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe LA, Norris R, Freudzon M, Ratzan WJ, Mehlmann LM. Microinjection of follicle-enclosed mouse oocytes. Meth Mol Biol. 2007 doi: 10.1007/978-1-59745-202-1_12. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TLZ, Degtyarev MY, Backlund PS. The stoichiometry of Gαs palmitoylation in its basal and activated states. Biochemistry. 1997;36:7185–7191. doi: 10.1021/bi9628376. [DOI] [PubMed] [Google Scholar]
- Kalinowski RR, Berlot CH, Jones TLZ, Ross LF, Jaffe LA, Mehlmann LM. Maintenance of meiotic prophase arrest in vertebrate oocytes by a Gs protein-mediated pathway. Dev Biol. 2004;267:1–13. doi: 10.1016/j.ydbio.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Kidder GM, Mhawi AA. Gap junctions and ovarian folliculogenesis. Reproduction. 2002;123:613–620. doi: 10.1530/rep.0.1230613. [DOI] [PubMed] [Google Scholar]
- Ledent C, Demeestere I, Blum D, Petermans J, Hamalainen T, Smits G, Vassart G. Premature ovarian aging in mice deficient for Gpr3. Proc Natl Acad Sci USA. 2005;102:8922–8926. doi: 10.1073/pnas.0503840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis MJ, Bourne HR. Activation of the α subunit of Gs in intact cells alters its abundance, rate of degradation, and membrane avidity. J Cell Biol. 1992;119:1297–1307. doi: 10.1083/jcb.119.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln AJ, Wickramasinghe D, Stein P, Schultz RM, Palko ME, De Miguel MP, Tessarollo L, Donovan PJ. Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nature Gen. 2002;30:446–449. doi: 10.1038/ng856. [DOI] [PubMed] [Google Scholar]
- Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB. Assembly and trafficking of heterotrimeric G Proteins. Biochemistry. 2007;46:7665–7677. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, Nedachi T, Jin C, Conti M, Manganiello V. Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest. 2004;114:196–205. doi: 10.1172/JCI21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM. Oocyte-specific expression of Gpr3 is required for the maintenance of meiotic arrest in mouse oocytes. Dev Biol. 2005a;288:397–404. doi: 10.1016/j.ydbio.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005b;130:791–799. doi: 10.1530/rep.1.00793. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Jones TLZ, Jaffe LA. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science. 2002;297:1343–1345. doi: 10.1126/science.1073978. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Kalinowski RR, Ross LF, Parlow AF, Hewlett EL, Jaffe LA. Meiotic resumption in response to luteinizing hormone is independent of a Gi family G protein or calcium in the mouse oocyte. Dev Biol. 2006;299:345–355. doi: 10.1016/j.ydbio.2006.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair BG, Parikh B, Milligan G, Patel TB. Gsα mediates epidermal growth factor-elicited stimulation of rat cardiac adenylate cyclase. J Biol Chem. 1990;265:21317–21322. [PubMed] [Google Scholar]
- Nikolaev VO, Lohse MJ. Monitoring of cAMP synthesis and degradation in living cells. Physiology. 2006;21:86–92. doi: 10.1152/physiol.00057.2005. [DOI] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SLC, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Pincus G, Enzmann EV. The comparative behavior of mammalian eggs in vivo and in vitro. J Exp Med. 1935;62:665–675. doi: 10.1084/jem.62.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasenick MM, Wheeler GL, Bitensky MW, Kosack CM, Malina RL, Stein PJ. Photoaffinity identification of colchicine-solubilized regulatory subunit from rat brain adenylate cyclase. J Neurochem. 1984;43:1447–1454. doi: 10.1111/j.1471-4159.1984.tb05407.x. [DOI] [PubMed] [Google Scholar]
- Richard FJ, Tsafriri A, Conti M. Role of phosphodiesterase type 3A in rat oocyte maturation. Biol Reprod. 2001;65:1444–1451. doi: 10.1095/biolreprod65.5.1444. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte meiotic maturation: Implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983;97:264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology. 2006;147:2280–2286. doi: 10.1210/en.2005-1011. [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzales I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385:525–529. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- Simonds WF, Goldsmith PK, Woodard CJ, Unson CG, Spiegel AM. Receptor and effector interactions of Gs. Functional studies with antibodies to the αs carboxyl-terminal decapeptide. FEBS Lett. 1989;249:189–194. doi: 10.1016/0014-5793(89)80622-2. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Ishii K, Kasai K, Yoon SO, Saeki Y. Neural expression of G protein-coupled receptors GPR3, GPR6, and GPR12 up-regulates cyclic AMP levels and promotes neurite outgrowth. J Biol Chem. 2007;282:10506–10515. doi: 10.1074/jbc.M700911200. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan MM, Bigras E, Van Tol E, Hebert TE, Evanko DS, Wedegaertner PB. Activation-induced subcellular redistribution of Gαs is dependent upon its unique N-terminus. Biochemistry. 2002;41:9470–9484. doi: 10.1021/bi025533u. [DOI] [PubMed] [Google Scholar]
- Törnell J, Billig H, Hillensjö T. Resumption of rat oocyte meiosis is paralleled by a decrease in guanosine 3′, 5′-cyclic monophosphate (cGMP) and is inhibited by microinjection of cGMP. Acta Physiol Scand. 1990;139:511–517. doi: 10.1111/j.1748-1716.1990.tb08953.x. [DOI] [PubMed] [Google Scholar]
- Uhlenbrock K, Gassenhuber H, Kostenis E. Sphingosine 1-phosphate is a ligand of the human gpr3, gpr6 and gpr12 family of constitutively active G protein-coupled receptors. Cell Signal. 2002;14:941–953. doi: 10.1016/s0898-6568(02)00041-4. [DOI] [PubMed] [Google Scholar]
- Veitch GI, Gittens JEI, Shao Q, Laird DW, Kidder GM. Selective assembly of connexin37 into heterocellular gap junctions at the oocyte/granulosa cell interface. J Cell Sci. 2004;117:2699–2707. doi: 10.1242/jcs.01124. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Bourne HR. Activation and depalmitoylation of Gsα. Cell. 1994;77:1063–1070. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Bourne HR, von Zastrow M. Activation-induced subcellular redistribution of Gsα. Mol Biol Cell. 1996;7:1225–1233. doi: 10.1091/mbc.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland T, Lutz S, Chidiac P. Regulators of G protein signalling: a spotlight on emerging functions in the cardiovascular system. Curr Opin Pharmacol. 2007;7:1–7. doi: 10.1016/j.coph.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Yu J-Z, Rasenick MM. Real-time visualization of a fluorescent Gαs: dissociation of the activated G protein from plasma membrane. Mol Pharmacol. 2002;61:352–359. doi: 10.1124/mol.61.2.352. [DOI] [PubMed] [Google Scholar]


