Abstract
Enterotoxigenic Escherichia coli associated with human diarrheal disease utilize any of a limited group of serologically distinguishable pili for attachment to intestinal cells. These include CS1 and CFA/I pili. We show here that chemical modification of arginyl residues in CS1 pili abolishes CS1-mediated agglutination of bovine erythrocytes, which serves as a model system for attachment. Alanine substitution of the single arginyl residue in CooA, the major pilin, had no effect on the assembly of pili or on hemagglutination. In contrast, substitution of alanine for R181 in CooD, the minor pilin associated with the pilus tip, abolished hemagglutination, and substitution of R20 reduced hemagglutination. Neither of these substitutions affected CS1 pilus assembly. This shows that CooD is essential for CS1-mediated attachment and identifies specific residues that are involved in receptor binding but not in pilus assembly. In addition to mediating agglutination of bovine erythrocytes, CFA/I also mediates agglutination of human erythrocytes. Substitution of R181 by alanine in the CooD homolog, CfaE, abolished both of these reactions. We conclude that the same region of the pilus tip protein is involved in adherence of CS1 and CFA/I pili, although their receptor specificities differ. This suggests that the region of the pilus tip adhesin protein that includes R181 might be an appropriate target for therapeutic intervention or for a vaccine to protect against human diarrhea caused by enterotoxigenic E. coli strains that have serologically different pili.
Enterotoxigenic Escherichia coli (ETEC) is a major cause of diarrheal disease affecting millions of people every year (1). ETEC enter the host via ingestion of contaminated water or food and proceed to colonize the small intestine, where they secrete the heat-labile and/or heat-stable enterotoxins that cause diarrhea. This disease, although self-limiting in adults, causes significant mortality in infants and very young children, particularly those in developing countries. Colonization of the small intestine, the first step in the establishment of diarrheal disease, is mediated by pili that specifically recognize and bind to surface receptors on intestinal cells. Such interactions are critical in ETEC virulence and are potential points of intervention at which bacterial colonization and subsequent disease may be blocked. A pilus-based vaccine is effective in preventing animal disease caused by ETEC strains (2, 3). However, for ETEC infections of humans, development of a vaccine based on the whole pilus is complicated by the serological diversity of the pili associated with the different human ETEC strains. Therefore, identification of a conserved epitope essential for attachment of all these serologically different pili would be an important step toward a preventive therapy.
Although ETEC strains associated with human disease produce several serologically distinct types of pili (4), many of these pili are closely related in terms of the sequence of the pilin proteins and the proteins needed for their assembly (5). CS1 pili represent one family of related but serologically distinct pili, which includes CS2, CS4, CS14, CS17, CS19, and CFA/I (5). These pili have no significant sequence homology with Pap-related pili and they do not conserve the peptide motifs that are essential for morphogenesis of Pap-related pili (5–7). Furthermore, in contrast to some Pap-related pili that require up to 9 structural genes and Type IV pili, which require up to 14 structural genes for pilus assembly, the assembly of CS1 pili requires only 4 genes (5). However, both CS1- and Pap-related pili require chaperone and usher proteins for the transport and polymerization of pilin subunits into the pilus.
CS1 pili are composed almost entirely of the major pilin, CooA. A minor pilin, CooD, is found only at the pilus tip and is estimated to contribute only one subunit per pilus (8). Although CooD is a structural component of CS1 pili, it is also essential for their assembly (9). CooD is needed for the transport of CooA across the outer membrane, and the level of CooD expression determines the number of pili assembled on the cell surface, indicating that CooD is needed for the initiation of CS1 pilus assembly (10).
CooA and CooD are assembled into structures on the cell surface by two other proteins, CooB and CooC. CooB, a periplasmic chaperone-like protein, forms intermolecular complexes with, and stabilizes, the major and minor pilins as well as an outer membrane protein, CooC (11). Like other chaperones, CooB may prevent the misfolding of the proteins to which it binds and possibly the premature polymerization of pilins in the periplasm (11). Because of its subcellular location, CooC probably has a role in the secretion of the pilins from the periplasm across the outer membrane (11).
As well as mediating the adhesion of ETEC to intestinal cells, CS1 pili mediate the attachment of bacteria to bovine erythrocytes in vitro, an interaction that causes hemagglutination. Because this is likely to be due to structural similarities in the receptors on the surfaces of enterocytes and bovine erythrocytes, the hemagglutination reaction is used as a model system to study attachment of these pili. Similarly, CFA/I pili mediate the agglutination of bovine erythrocytes and, additionally, the agglutination of human erythrocytes (12). In this study, we characterized further the CS1 and CFA/I adhesins by identifying specific amino acid residues involved in binding. In contrast to previous reports suggesting that the major pilins are the adhesive subunits (13, 14), we found that the minor tip-located pilins, CooD and CfaE, from CS1 and CFA/I pili, respectively, are essential for pilus-mediated hemagglutination. In addition, despite differences in receptor-binding specificity, the same residues on the tip-located minor pilin proteins of CS1 and CFA/I are needed for receptor binding.
Materials and Methods
Bacterial Strains and Culture Conditions.
K12 strains MC4100 (15) and LMG194 (16) are deleted for lac, as is LMC10, an ETEC derivative (17). Plasmids used in this study are listed in Tables 1 and 2.
Table 1.
Plasmids used in this study
| Plasmid | Characteristics | Copy no. | Ref. |
|---|---|---|---|
| pEU478 | Plac-cooCD | Low | 9 |
| pEU493 | Plac-cooD | Low | 9 |
| pEU605 | Plac-cooBA | High | 18 |
| pEU1208 | Plac-cooBA3* | High | This study |
| pEU1290 | Ptrc-cooBAC | High | 10 |
| pEU2124 | Ptet-cfaABCE1† | High | This study |
| pEU2030 | rns | High | 17 |
| pEU2040 | rns | Low | 18 |
| pFDX500 | lacIq | Low | 19 |
| pHSG576 | Plac | Low | 20 |
| pJGX15W | Ptet-cfaABCE | High | 21 |
| pUC19 | Plac | High | 22 |
R148A mutation in cooA.
R181A mutation in cfaE.
Table 2.
Hemagglutination (HA) and assembly of CS1 pili in LMG194/pEU1290 (cooBAC) complemented with wild-type and mutant alleles of cooD
| Allele | Mutation | Plasmid | Pili* | HA |
|---|---|---|---|---|
| — | — | pHSG576 | − | − |
| cooD | — | pEU493 | + | + |
| cooD1 | R181A | pEU1227 | + | − |
| cooD2 | R219A | pEU1222 | + | + |
| cooD4 | R314A | pEU1224 | + | + |
| cooD5 | R105A | pEU1225 | + | + |
| cooD6 | R110A | pEU1226 | + | + |
| cooD7 | R20A | pEU1228 | + | +/− |
| cooD8 | R37AR40A | pEU1229 | +/− | − |
Indicated by slide agglutination test.
Tests for Hemagglutination and Piliation.
For slide hemagglutination, overnight cultures were resuspended in PBS (20 mM sodium phosphate/150 mM sodium chloride), pH 7.4, to give an OD600 of 10. In glass depression slides, 20 μl of bacterial suspension was mixed with 20 μl of PBS containing 0.1 M d-mannose and 20 μl of washed erythrocyte suspension. The minimal bacterial density resulting in clearly visible hemagglutination was used for hemagglutination inhibition assays in microtiter plates (13). Erythrocytes were preincubated with CS1 subunits, CFA/I subunits, or BSA for 20 min at room temperature, mixed with bacterial suspensions, and incubated at 4°C for 90 min.
For detection of pili by slide agglutination, 50 μl of bacterial suspension in PBS (adjusted to give an OD600 of 5) was incubated for 1 min. at room temperature with 10 μl of anti-CS1 or CFA/I serum in glass depression slides. Electron microscopy of negatively stained cells was performed as described previously (8).
Protein Methods and Site-Directed Mutagenesis.
CS1 pili were purified from overnight LB cultures of MC4100/pFDX500/pEU605/pEU478 and CFA/I pili were purified from MC4100/pJGX15W. Cells were resuspended in 0.9% (wt/vol) saline and subjected to mechanical shearing in a blender (Waring VB100 model 15BL21) for 4 min at medium setting, low-speed range. Whole cells and debris were removed by two centrifugations at 10,000 × g for 20 min, and the supernatants were used for comparison of amounts of pili in different strains. Pili were purified further by sedimentation by overnight centrifugation at 125,000 × g at 4°C, precipitation with ammonium sulfate at 10% saturation, followed by sedimentation at 12,000 × g for 30 min at 4°C and dialysis against distilled water. Pilin subunits were generated by boiling for 20 min (13).
Site-directed mutagenesis of cooA and cooD was performed with the template plasmids pEU605 and pEU493, respectively, and mutagenic primers by using the QuickChange mutagenesis kit (Stratagene). Plasmid pJGX15W was digested with SphI to remove a 5-kb fragment carrying cfaABC and the amino terminus of cfaE. The remaining 3.2 kb (vector and codons 84–361 of cfaE) then was self-ligated to produce plasmid pEU2113. A mutation changing Arg-181 of CfaE to Ala was introduced into the truncated cfaE gene of pEU2113 by divergent PCR with the mutagenic primers, followed by self-ligation of the PCR product to produce pEU2114. Cloning of a 5-kb SphI fragment from pJGX15W into the SphI site of pEU2114 yielded the plasmid pEU2124, which carries cfaABC and the mutant cfaE1 allele under Ptet. The presence of each directed mutation was confirmed by nucleotide sequencing of the entire cooD gene for alleles carrying R20A, R181A, and R37A R40A mutations and of the region surrounding the directed mutations for all other alleles.
Chemical Modification of Bacteria.
Biotinylation of whole bacteria was performed at room temperature for 3 hr by using sulfo-NHS-LC-biotin (Pierce) at 40 mg/ml. Whole bacteria were modified with 2,3-butanedione in borate-buffered saline [BBS; 50 mM borate/0.9% (wt/vol) NaCl, pH 7.5] for 2 hr at room temperature.
Results
Arginyl Residues Play a Critical Role in CS1-Mediated Adhesion to Erythrocytes.
During the development of assays to measure binding of bacteria to erythrocytes, we labeled the CS1-piliated bacterium LMC10/pEU2030 with the biotinylation reagent sulfo-NHS-LC-biotin. Unexpectedly, we found that biotinylation prevented whole piliated bacteria from agglutinating bovine erythrocytes. Sulfo-NHS-LC-biotin was required for this inhibitory effect because piliated bacteria from a control reaction that lacked the biotinylation reagent retained hemagglutination activity. This result suggests that primary amines are essential for CS1-mediated adhesion to erythrocytes because sulfo-NHS-LC-biotin selectively modifies primary amines.
Of the amino acids with primary amines in their side chains, we chose arginyl residues for further study because arginines play an essential role in the adhesion of K99 pili to erythrocytes (23). Whole CS1-piliated bacteria were chemically modified with 2,3-butanedione, which specifically modifies arginyl residues by covalently bonding to both amines within each arginyl side chain. This addition also neutralizes the positive charge on the amino acid (24). The hemagglutinating activity of the CS1-piliated E. coli strain LMC10/pEU2040 was abolished by exposure to 2,3-butanedione in BBS, whereas BBS alone had no effect on hemagglutination by this strain. This suggested that one or more arginyl residues within the CS1 pilus are required for hemagglutination.
The Single Arginyl Residue in CooA Is Not Involved in CS1-Mediated Hemagglutination.
We used site-specific mutagenesis to identify the specific arginyl residues essential for adhesion. Because it was suggested that the major pilin subunit, CooA, is the adhesin for CS1-mediated agglutination of bovine erythrocytes (14), and our results suggest that hemagglutination requires at least one arginyl, we constructed cooA3 (see Materials and Methods) in which the single arginyl residue of cooA, at position 148, is substituted by alanine. This mutation did not affect CS1 pilus assembly because both the mutant strain MC4100/pFDX500/pEU1208/pEU478 (cooBA3, cooCD) and the positive control strain MC4100/pFDX500/pEU605/pEU478 (cooBA, cooCD) were agglutinated in the presence of anti-CS1 serum and both strains appeared to be similarly piliated when examined by electron microscopy (data not shown). Both strains also agglutinated bovine erythrocytes. The negative control strain MC4100/pFDX500/pHSG576/pUC19 did not produce pili as determined by the same assays (data not shown) and was negative for hemagglutination. These results indicate that the single arginyl in CooA is not essential for the assembly of CS1 pili and, surprisingly, it is not involved in the agglutination of bovine erythrocytes.
Pilin Subunits Prepared by Boiling Intact Pili Do Not Inhibit Hemagglutination.
Because the R148A mutation in CooA did not abolish hemagglutination by the mutant strain, we sought independent evidence to determine whether CooA was the CS1 pilus adhesin. For the closely related pilus CFA/I, preincubation of erythrocytes with the major pilus subunit was reported to inhibit hemagglutination, suggesting that the major pilin was the adhesive subunit (13). Therefore, we tried the same strategy with CS1 and CFA/I.
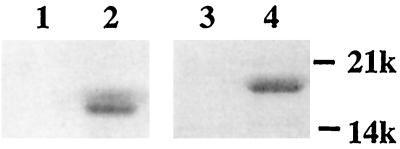
Subunits were dissociated by boiling purified pili as described previously for CFA/I (13) and visualized by SDS/PAGE. As expected because of their high-molecular-weight polymeric nature, the intact pili were excluded from the gel (Fig. 1, lanes 1 and 3). However, for the subunit preparations, proteins of the molecular weights expected for the CFA/I and CS1 major pilins were observed (Fig. 1, lanes 2 and 4, respectively), indicating that these proteins were monomeric.
Figure 1.
CFA/I and CS1 subunits generated from whole pili. Purified pili or subunits (2 μg) were fractionated by SDS/PAGE and stained with Coomassie blue. Protein samples were not heated before electrophoresis. Lanes 1 and 2, CFA/I pili and subunits, respectively; lanes 3 and 4, CS1 pili and subunits, respectively.
To test whether CS1 subunits prepared by boiling block hemagglutination, subunits at concentrations of 0.1 mg/ml and 0.5 mg/ml were reacted with bovine erythrocytes before addition of suspensions of MC4100 and MC4100/pEU605/pEU478 (CS1). As a negative control, the same concentration of BSA was preincubated with erythrocytes. In this experiment, CS1 subunits showed no inhibition of CS1-mediated agglutination of bovine erythrocytes; the reaction was as strong as that to which BSA had been added. CFA/I subunits also failed to inhibit the CFA/I-producing strain MC4100/pJGX15W from agglutinating human type A or bovine erythrocytes. Thus, for both CS1 and CFA/I, we found that the major pilin subunit does not inhibit pilus-mediated hemagglutination.
Arginyl Residues at Positions 20 and 181 of CooD Are Required for CS1-Mediated Hemagglutination.
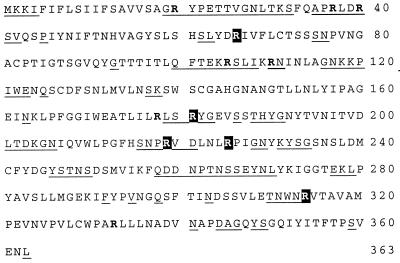
Although arginyl residues were essential for CS1-mediated hemagglutination, the single arginyl residue in CooA is not required for this process. This suggests that arginyl residues in the minor, tip-associated pilin, CooD (8), may be critical for hemagglutination. On the assumption that residues involved in receptor binding would be surface-exposed, arginyl residues were selected for mutagenesis by using the formula of Emini et al. (25) to predict surface exposure. This restriction limited the 12 arginyls of CooD to 7 (Fig. 2). Additionally, R314 was mutated because of its proximity to a predicted surface region and its conservation in the CooD homologs CfaE and CotD from the closely related CFA/I and CS2 pili.
Figure 2.
Predicted surface distribution and conservation of arginyl residues in CooD. Arginyl residues in the CooD sequence are indicated as bold letters; those conserved in CfaE and CotD are shaded. Residues predicted to be surface-exposed are underlined.
Each of the selected arginines was replaced with alanine by oligonucleotide-directed mutagenesis of plasmid pEU493 (cooD). The E. coli strain LMG194/pEU1290 (cooBAC) was transformed with the mutant plasmids, the cloning vector pHSG576, or the positive control plasmid pEU493 (cooD) (see Table 2). To test for the production of CS1 pili, bacterial suspensions were reacted with anti-CS1 serum in slide agglutination tests. The negative control strain LMG194/pEU1290/pHSG576 was not agglutinated by the anti-CS1 serum, whereas the positive control strain LMG194/pEU1290/pEU493 (cooBAC, cooD) was agglutinated. In comparison with the positive control, the double-mutant strain LMG194/pEU1290/pEU1229 (cooBAC, cooD8), in which R37 and R40 were substituted with alanine residues, was agglutinated very poorly by the antiserum, indicating that pilus assembly was greatly reduced by the substitutions (Table 2). Consistent with this, the double mutation also abolished hemagglutination (Table 2). In contrast, alanine substitutions at R105, R110, R219, and R314 did not affect CS1 pilus assembly or CS1-mediated hemagglutination (Table 2).
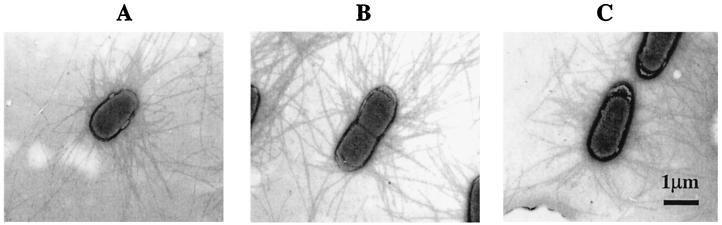
The strains carrying R181A, LMG194/pEU1290/pEU1227 (cooBAC, cooD1), and R20A, LMG194/pEU1290/pEU1228 (cooBAC, cooD7), were strongly agglutinated by anti-CS1 serum, indicating that pilus assembly was unaffected in either. However, the R181A mutation abolished hemagglutination and the R20A mutation greatly reduced hemagglutination (Table 2). To confirm that they were piliated, we examined these strains by electron microscopy. Although pili were not visible on the negative control strain, LMG194/pEU1290/pHSG576 (data not shown), they were readily seen on the positive control strain (Fig. 3A) and on both mutants (Fig. 3 B and C).
Figure 3.
Piliation in strains carrying the cooD1 and cooD7 mutant alleles. Electron micrographs of LMG194/pEU1290 (cooBAC) carrying pEU493 (cooD) (A), pEU1227 (cooD1) (B), and pEU1228 (cooD7) (C).
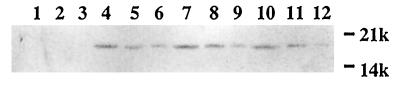
Although both the R181A and R20A mutant strains were piliated and no difference from the positive control strain was apparent by electron microscopy, we investigated whether their decreased hemagglutination activity could be a result of a decrease in the number of pili assembled. Pili extracted by mechanical shearing from broth cultures standardized for cell density were assayed by immunoblotting with antiserum to CooA (Fig. 4). Comparison of the extracts from the positive control strain LMG194/pEU1290/pEU493 (cooBAC, cooD) (Fig. 4, lanes 4–6) and the two cooD mutant strains (Fig. 4, lanes 7–12) by serial 2-fold dilutions revealed no significant difference in the amounts of CooA.
Figure 4.
Immunoblot analysis of CS1 pili produced in wild-type and cooD mutant strains. CS1 pili recovered from the cell surfaces of strains LMG194/pEU1290/pHSG576 (cooBAC) (lanes 1–3), LMG194/pEU1290/pEU493 (cooBAC cooD) (lanes 4–6), LMG194/pEU1290/pEU1227 (cooBAC cooD1) (lanes 7–9), and LMG194/pEU1290 (cooBAC cooD7) (lanes 10–12) were detected by immunoblot analysis with anti-CooA antiserum. Lanes 2, 5, 8, and 11 contain 2-fold-diluted samples. Lanes 3, 6, 9, and 12 contain 4-fold-diluted samples.
CS1 and CFA/I Pili Share a Similar Mechanism for Binding to Erythrocyte Receptors.
Because there is an arginyl residue at position 181 in the CooD homolog, CfaE, from CFA/I pili, it seemed possible that this arginine was required for binding of CFA/I pili to erythrocytes. To test this, the cfaE1 allele (R181A) was constructed. The mutant strain MC4100/pEU2124 (cfaABCE1) produced pili, as demonstrated by its agglutination with anti-CFA/I serum. Strain MC4100/pJGX15W (cfaABCE) served as a positive control and strain MC4100/pEU2113 (cfa deletion) served as a negative control. However, although MC4100/pJGX15W agglutinated bovine erythrocytes, MC4100/pEU2124 (which has the R181A mutation) was unable to do so. This showed that the involvement of R181 in binding to bovine erythrocytes is a conserved feature in CS1 and CFA/I pili.
CFA/I pili also mediate agglutination of human type A erythrocytes, so we tested whether the R181A mutation affected this reaction as well. Using the strains described above, we found that the R181 residue is also essential for the agglutination of human erythrocytes. This indicates that a single adhesin, rather than two distinct adhesins, specifies binding to both bovine and human erythrocytes in CFA/I pili.
Discussion
Adhesin Location and Presentation in Pili.
The adherence of bacterial pili to eukaryotic cells is mediated by adhesive pilus subunits that recognize specific receptors on the surface of their target cells. In a few cases, e.g., K88 and K99 pili from animal ETEC strains, it is the major pilus subunit, which constitutes the pilus shaft, that is responsible for pilus-mediated adherence to erythrocytes from a variety of animal species (23, 26). However, for most types of pili, including Pap, type 1, and 987P, adherence to a receptor is mediated by a minor pilus subunit located at the pilus tip (27–29).
Role of the Major and Minor Subunits in CS1 and CFA/I-Mediated Adherence.
In a previous study it was suggested that the adherence of CS1 pili of human ETEC strains to erythrocytes is mediated by the major pilin subunit, CooA. However, the role of CooA in adherence was not tested directly and CooD was not recognized at that time as a minor subunit of CS1 pili (14). Instead, the conclusion that CooA is an adhesive subunit was based on a prior report that the major pilin, CfaB, is responsible for CFA/I-mediated hemagglutination (13).
An adhesive function was proposed for CfaB for two reasons. First, antibodies specific for CfaB inhibited the adherence of CFA/I pili to erythrocytes (13). However, this evidence is not conclusive because the large Ig molecules bound to CfaB may have masked the minor pilin subunit, CfaE, thereby indirectly preventing adherence. Second, isolated CfaB subunits inhibited the binding of CFA/I pili to erythrocytes, suggesting that the subunits competed with whole pili for binding to erythrocyte receptors (13). We were unable to reproduce this result. Even at a subunit concentration 5-fold greater than that used in the original study we found no inhibition of CFA/I-mediated hemagglutination. Furthermore, the boiling method Buhler et al. (13) used to dissociate pilus subunits might be expected to inactivate the adhesin because we have found that temperatures as low as 65°C inactivate binding activity of the related CS1 pili (data not shown).
Instead of relying on inhibition experiments, our current study used a direct approach to identify the CS1 pilus adhesin. After learning that chemical modification of arginyl residues in pili prevents receptor binding, we used site-specific mutagenesis to determine that the important arginyl residues are in CooD, the minor pilus subunit, and not in CooA, the major pilin protein. Site-specific mutagenesis of the cooD homolog cfaE established that R181 is also critical for binding of CFA/I pili. This demonstrates that the minor pilins, CooD and CfaE, are essential for pilus-mediated adhesion to erythrocytes for CS1 and CFA/I, respectively. Therefore, the pili from human ETEC strains appear to follow the paradigm of Pap, type 1, and 987P rather than that of K88 and K99.
Observations on hemagglutination by isolated pili further support the conclusion that the adhesin is located at the CS1 pilus tip. Although isolated pili with tip-located adhesins, such as Pap and type 1, are unable to agglutinate erythrocytes (23), those whose major shaft protein contains the adhesin, like K88 and K99, can do so, presumably because adherence to the receptor along the entire pilus length provides opportunities for interaction with more than one erythrocyte (23). Isolated CS1 and CFA/I pili behave like pili of the former group because they do not agglutinate erythrocytes (H.S., unpublished data) unless pilus aggregation is promoted (12). Therefore, CS1 and CFA/I pili appear to be monovalent, as expected for a pilus whose adhesin is located at the tip of the structure.
Although the adhesive subunit of CS1-type pili is located at the pilus tip, these pili do not appear to have the complex tip fibrillar structures of Pap and type 1 pili, which are composed of multiple minor subunits (28, 30). However, it is still possible that the CS1 adhesin may involve participation of more than one protein because residues from the major CS1 pilin may contribute to the reaction with host receptors required for adherence. We have shown that in both CooD of CS1 and CfaE of CFA/I, arginyl residues play a critical role in the interaction of pili with erythrocyte receptors. For K99 pili, it is thought that the side chains of arginyl and other positively charged residues interact with the negatively charged sugar, sialic acid, present in receptors (23). Although little is known about receptors to which CS1 and CFA/I bind, some studies have shown that the latter bind to sialic acid-containing glyconjugates (12, 31, 32), suggesting that R181 of the tip adhesin protein may play a role similar to that proposed for the arginines in K99.
Receptor Binding and Its Specificity.
Although CFA/I pili agglutinate both bovine and human erythrocytes, CS1 pili agglutinate only the former. Thus, these two types of ETEC pili have overlapping but different specificities. It seemed possible that this results from the presence of a single adhesin in CS1 but that CFA/I contains, in addition to this adhesin, one that recognizes human erythrocytes. We have shown here that this is not the case. Alanine substitution for R181 in the CS1 adhesin CooD prevented agglutination of bovine erythrocytes, indicating the importance of this residue in binding to these cells. Because substitution of R181 in CfaE, the adhesin of CFA/I, abolishes agglutination of human as well as bovine erythrocytes, this residue is essential for both reactions. We conclude that there is a conserved domain of the adhesin needed for receptor binding in CS1 and CFA/I pili, even though the latter pili have a broader binding specificity. In addition, we predict that the mechanism of binding is also conserved in the closely related CS2 pili, because R181 is conserved in CotD, the CooD homolog in CS2 pili. We are studying currently whether such interactions also reflect the binding of pili to intestinal cell receptors in the human host.
We found that substituting A for R20 in the CS1 tip adhesin CooD reduced hemagglutination, indicating that this residue is also involved in adherence. We suggest that the positively charged residues (K25 and H22) near the N termini of CfaE and CotD, respectively, may serve a similar role. It is possible that the exact location of positively charged residues near the N terminus of the adhesin protein may be important in determining receptor specificity in the different pili. In addition, other residue types, not identified in this study, may be involved in determining receptor specificity.
Vaccine Prospects.
In conclusion, we have found that the minor CS1 and CFA/I pilins located at the distal tip of these flexible structures are essential for adherence to erythrocytes. We also have identified a conserved amino acid residue that is required for adherence by these serologically distinct pili even though their receptor specificities differ. It seems likely that targeting the essential region of the adhesin, which is universally present in the members of this pilus family, will be an effective approach to the prevention of ETEC infections.
Acknowledgments
We thank Mike Levine for supplying CFA/I antiserum and pJGX15W. This work was supported by National Institutes of Health Grant AI24870, and G.P.M. was supported, in part, by National Research Service Award AI10145-01 from the National Institutes of Health.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Black R E. Pediatr Infect Dis J. 1993;12:751–761. doi: 10.1097/00006454-199309000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Nagy B. Infect Immunol. 1980;27:21–24. doi: 10.1128/iai.27.1.21-24.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan R L, Isaacson R E, Moon H W, Brinton C C, To C C. Infect Immunol. 1978;22:771–777. doi: 10.1128/iai.22.3.771-777.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaastra W, Svennerholm A-M. Trends Microbiol. 1996;4:444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 5.Sakellaris H, Scott J R. Mol Microbiol. 1998;30:681–687. doi: 10.1046/j.1365-2958.1998.01088.x. [DOI] [PubMed] [Google Scholar]
- 6.Hultgren S J, Jacob-Dubuisson F, Jones C H, Branden C I. Adv Protein Chem. 1993;44:99–123. doi: 10.1016/s0065-3233(08)60565-3. [DOI] [PubMed] [Google Scholar]
- 7.Lindberg F, Lund B, Johansson L, Normark S. Nature (London) 1987;328:84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- 8.Sakellaris H, Balding D P, Scott J R. Mol Microbiol. 1996;21:529–541. doi: 10.1111/j.1365-2958.1996.tb02562.x. [DOI] [PubMed] [Google Scholar]
- 9.Froehlich B J, Karakashian A, Melsen L R, Wakefield J C, Scott J R. Mol Microbiol. 1994;12:387–401. doi: 10.1111/j.1365-2958.1994.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 10.Sakellaris H, Penumalli V R, Scott J R. J Bacteriol. 1999;181:1694–1697. doi: 10.1128/jb.181.5.1694-1697.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voegele K, Sakellaris H, Scott J R. Proc Natl Acad Sci USA. 1997;94:13257–13261. doi: 10.1073/pnas.94.24.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans D G, Evans D J, Clegg S, Pauley J A. Infect Immunol. 1979;25:738–748. doi: 10.1128/iai.25.2.738-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buhler T, Hoschutzky H, Jann K. Infect Immunol. 1991;59:3876–3882. doi: 10.1128/iai.59.11.3876-3882.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marron M B, Smyth C J. Microbiol. 1995;141:2849–2859. doi: 10.1099/13500872-141-11-2849. [DOI] [PubMed] [Google Scholar]
- 15.Casabadan M. J Mol Biol. 1976;104:557–566. [Google Scholar]
- 16.Guzman L M, Belin D, Carson M J, Beckwith J. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caron J, Coffield L, Scott J. Proc Natl Acad Sci USA. 1989;86:963–967. doi: 10.1073/pnas.86.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Casal J, Swartley J S, Scott J R. Infect Immunol. 1990;58:3594–3600. doi: 10.1128/iai.58.11.3594-3600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnetz K, Sutrina S L, Saier M H J, Rak B. J Biol Chem. 1990;265:13464–13471. [PubMed] [Google Scholar]
- 20.Takeshita S, Sato M, Toba M, Masahashi W, Hashimoto-Gotoh T. Gene. 1987;61:63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- 21.Giron J A, Xu J G, Gonzalez C R, Hone D, Kaper J B, Levine M M. Vaccine. 1995;13:939–946. doi: 10.1016/0264-410x(95)00003-j. [DOI] [PubMed] [Google Scholar]
- 22.Yanisich-Perron C, Vieira J, Messing J. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs A A C, Simons B H, de Graaf F K E J. EMBO J. 1987;6:1805–1808. doi: 10.1002/j.1460-2075.1987.tb02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riordan J F. Mol Cell Biochem. 1979;26:71–92. doi: 10.1007/BF00232886. [DOI] [PubMed] [Google Scholar]
- 25.Emini E A, Hughes J V, Perlow D S, Boyer J. J Virol. 1985;55:836–839. doi: 10.1128/jvi.55.3.836-839.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakker D, Willemsen P T J, Simons L H, van Zijderveld F K, de Graaf F K. Mol Microbiol. 1992;6:247–255. doi: 10.1111/j.1365-2958.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 27.Lindberg F, Lund B, Normark S. Proc Natl Acad Sci USA. 1986;83:1891–1895. doi: 10.1073/pnas.83.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones H J, Pinkner J S, Roth R, Heuser J, Nicholes A V, Abraham S N, Hultgren S J. Proc Natl Acad Sci USA. 1994;92:2081–2085. doi: 10.1073/pnas.92.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao J, Salam Khan A, Bayer M E, Schifferli D M. J Bacteriol. 1995;177:3704–3713. doi: 10.1128/jb.177.13.3704-3713.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuehn M J, Heuser J, Normark S, Hultgren S. Nature (London) 1992;356:252–255. doi: 10.1038/356252a0. [DOI] [PubMed] [Google Scholar]
- 31.Pieroni P, Worobec E A, Paranchych W, Armstrong G D. Infect Immunol. 1988;56:1334–1340. doi: 10.1128/iai.56.5.1334-1340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wenneras C, Holmgren J, Svennerholm A-M. FEMS Microbiol Lett. 1990;66:107–112. doi: 10.1016/0378-1097(90)90266-s. [DOI] [PubMed] [Google Scholar]