Abstract
We have recently shown that VEGF functions as a survival factor for newly formed vessels during developmental neovascularization, but is not required for maintenance of mature vessels. Reasoning that expanding tumors contain a significant fraction of newly formed and remodeling vessels, we examined whether abrupt withdrawal of VEGF will result in regression of preformed tumor vessels. Using a tetracycline-regulated VEGF expression system in xenografted C6 glioma cells, we showed that shutting off VEGF production leads to detachment of endothelial cells from the walls of preformed vessels and their subsequent death by apoptosis. Vascular collapse then leads to hemorrhages and extensive tumor necrosis. These results suggest that enforced withdrawal of vascular survival factors can be applied to target preformed tumor vasculature in established tumors. The system was also used to examine phenotypes resulting from over-expression of VEGF. When expression of the transfected VEGF cDNA was continuously “on,” tumors became hyper-vascularized with abnormally large vessels, presumably arising from excessive fusions. Tumors were significantly less necrotic, suggesting that necrosis in these tumors is the result of insufficient angiogenesis.
Keywords: neovascularization, vessel regression, hemorrhage, tetracycline-inducible expression
It is well established that a rate-limiting step in solid tumor growth is the ability to recruit blood vessels from the host tissue (for a review, see ref. 1). Angiogenesis has become a major target for antitumor therapy on the premise that limiting angiogenesis will retard tumor growth and will inhibit metastatic spread of tumor cells.
The tumor “angiogenic switch” is determined by the net balance of angiogenic stimulators and natural inhibitors of (2, 3). VEGF is a potent angiogenic factor mediating developmental, physiological, and pathological neovascularization. In the context of tumor angiogenesis, VEGF expression is up-regulated as a consequence of oncogene activation or a loss of tumor suppressor (4–8). In addition to a genetic angiogenic switch, VEGF expression is induced in tumors by hypoxia and/or hypoglycemia generated whenever the angiogenic response is insufficient and vascular growth is lagging behind tumor growth (9–11). Augmented VEGF expression is correlated with increased tumor growth and vascularity (12, 13) and the inhibition of VEGF production or function leads to inhibition of tumor growth (14–17). Thus, it appears that VEGF plays a key role in the promotion of tumor angiogenesis. In the present study we examined whether the level of VEGF produced by tumor cells may determine the size and shapes of tumor vessels. In particular, we examined the thesis that excessive production of VEGF may lead to formation of hyperfused vessels, such as those found in hemangioblastomas.
Growth factors, in general, may also function as survival factors for the respective target cell (18, 19). We have recently shown that VEGF is required for the maintenance of newly formed blood vessels in a natural developmental setting of retina neovascularization (20). Importantly, dependence on VEGF for survival is transient and, upon their maturation, vessels switch to a VEGF-independent state. Tumor expansion is associated with a continuous formation of new vessels and remodeling of existing vessels. We reasoned that these vessels may be sensitive to loss of VEGF resulting in regression of the existing tumor vasculature. To test this hypothesis, we constructed an inducible VEGF expression system in a xenografted C6 glioma tumor. We show that regression of preformed tumor vessels can be induced by VEGF withdrawal. The finding that VEGF is required for the maintenance of immature/remodeling tumor vessels may be exploited to increase the efficiency of anti-angiogenesis tumor therapy.
METHODS
Preparation and Analysis of pTET-VEGF Cell Lines.
pTET-VEGF was constructed using the full-length coding sequence of mouse VEGF165 amplified by PCR from a VEGF cDNA clone with the following primers and cloned as a HindIII–EcoRV fragment into pTET-Splice (BRL): (5′ primer, 5′-CGCGAAGCTTCCACCATGGACTTTCTGCTCTCTTGGGT-3′; 3′ primer, 5′-CGCGGATATCACCGCCTTGGCTTGTCACA-3′). C6 glioma cells were cotransfected with pTET-VEGF, pTET-TAK (BRL), and a plasmid encoding G418 resistance. Following stable selection in G418, colonies were picked, amplified, and tested for a tetracycline-regulated expression of VEGF as follows. Cells were grown for 48 h in the presence or absence of 1 μg/ml tetracycline. Total RNA (2 μg) from these cells was subjected to a reverse transcriptase–PCR analysis using random hexamers as reverse transcriptase primers and the VEGF-specific primers described above for PCR amplification. To serve as internal standard, ribosomal protein L19-specific primers (5′-CTGAAGGTCAAAGGGAATGTG-3′ and 5′-GGACAGAGTCTTGATGATCTC-3′) were included in the PCR. Thirty amplification cycles (94°C for 1 min, 55°C for 1 min, and 72°C for 1 min) were used. The resulting products were visualized by electrophoresis through 2% agarose in the presence of ethidium bromide. Approximately half of the VEGF-expressing colonies showed a tetracycline-regulated expression. Analysis of the particular clone chosen for further study is shown in Fig. 1.
Figure 1.
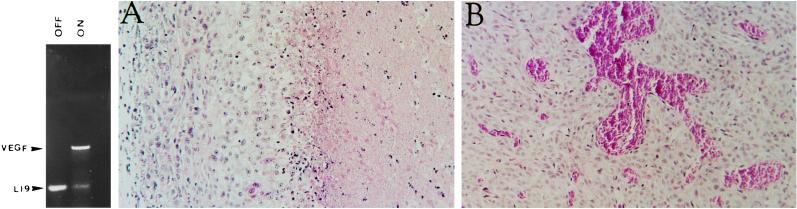
The effect of VEGF165 over-expression on tumor and vessel growth. (Left) Reverse transcriptase–PCR analysis of VEGF165 mRNA in a C6 pTET-VEGF clone grown in the presence (“off”) and absence (“on”) of tetracycline. Arrowheads point at coamplified fragments of VEGF165 and L19 ribosomal protein. (Right) Hematoxylin and eosin staining of sections from C6 tumors grown in Nude mice either in the absence (A) or presence (B) of tetracycline. Note particularly the extensive necrosis in A (to the right of the figure) and the abnormal large vessels in B.
Inoculation of Nude Mice.
Cells (5 × 105) were injected subcutaneously into Nude mice (Harlan). Mice were given tetracycline when tumors reached a size of 0.5–1 cm2. Tetracycline was used at a concentration of 100 μg/ml with 5% sucrose, and the drinking water changed every other day. Tumors were resected into buffered formalin and embedding in paraffin.
Histochemistry.
Sections (4 μm) were cut from paraffin blocks and placed onto precoated slides (Sigma). Slides were deparaffinized in two changes of xylene (10 min) and rehydrated gradually from 100% ethanol to PBS. Hematoxylin and eosin staining was used to examine hemorrhage and necrosis. Blood vessel endothelial cells were visualized either by incubation with the Bandeira simplicifolia isolectin B4 or anti-von Willebrand factor. Lectin staining was done in PBS (biotin-conjugated; Sigma) overnight, followed by incubation with extra-avidin-peroxidase (1:20 dilution; BioMakor, Rehovot, Israel). Anti-von Willebrand factor was used 1:1,000 following 20-min trypsinization (0.1% at 37°C) and blocking with 10% goat serum/1% BSA. The primary antibody was followed by anti-rabbit horseradish peroxidase, and both lectin and antibodies were visualized with 3-amino-9-etylcabazole (AEC; Sigma) in 50 mM Tris (pH 5.0).
RESULTS
A System for Switchable VEGF Expression in Xenografted Tumors.
To determine the effects of VEGF induction and, in turn, withdrawal during tumor neovascularization, a switchable VEGF expression system was created. In the “Tet-off” expression system employed, expression of VEGF by tumor cells is repressed when tetracycline is added to the drinking water of tumor-bearing animals and is induced upon withdrawal of tetracycline.
C6 glioma cells were cotransfected with a VEGF165 encoding cDNA driven by a tetracycline-responsive cytomegalovirus promoter and a vector encoding a transactivator protein that will activate VEGF expression only in the absence of tetracycline. To secure a nonleaky expression of the target gene at the “off” state, the transactivator protein itself is negatively regulated by tetracycline (see Methods for details). Stably transfected colonies in which VEGF expression is tightly regulated by tetracycline were selected. Fig. 1 Left shows an example of a C6 subline that expressed high levels of VEGF in the absence of tetracycline but barely detectable VEGF when tetracycline was included in the culture medium. This particular subline (designated C6 pTET-VEGF) was chosen for inoculation in Nude mice.
Over-Expression of VEGF Results in Tumor Hypervascularity and a Hemangioblastoma-Like Phenotype.
C6 glioma cells produce well-vascularized tumors when inoculated in mice. These tumors express a fairly high level of VEGF in a constitutive manner and production of VEGF is further augmented in hypoxic regions of the tumor (9–11, 21, 22). Despite a strong angiogenic response, the rapid increase in tumor mass is not matched by sufficient vascular growth, and extensive areas of necrosis develop in C6 glioma tumors grown under the skin of nude mice (Fig. 1A).
When C6 pTET-VEGF cells where inoculated subcutaneously and allowed to grow while VEGF expression is switched on, three phenotypic differences were reproducibly detected (compare Fig. 1 A with B): (i) a highly significant increase in vascular density was observed. (ii) tumor necrosis was greatly reduced, and (iii) abnormally large and irregularly shaped tumor vessels and vascular sacs were formed. The first two differences suggested that a suboptimal production of a positive regulator like VEGF might be rate-limiting in neovascularization of gliomas. The results also showed that tumor necrosis in this tumor is a consequence of a insufficient angiogenic response. Of particular interest was the finding that over-production of VEGF leads to exceptionally large vessel diameter and vascular sacs. Examination of serial sections suggested that these structures were sometimes due to fusion of multiple blood vessels (data not shown). The striking resemblance to vascular structures found in hemangioblastoma supports the notion that over-expression of VEGF plays an important role in development of hemangioblastomas (7, 23, 24).
Withdrawal of VEGF Results in Regression of Newly Formed Tumor Vessels.
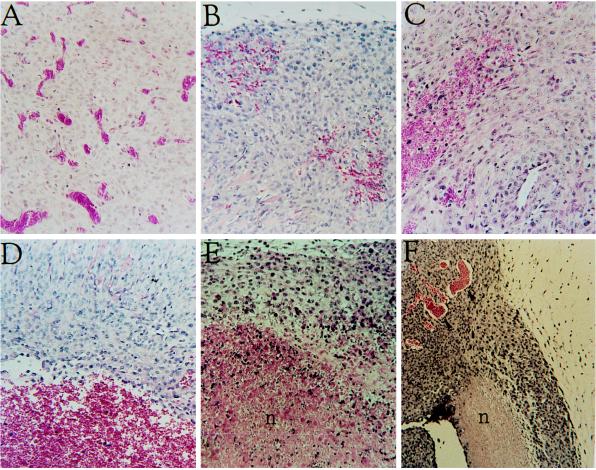
To examine the maintenance requirement for VEGF on tumor vessels, tumors were first allowed to grow in the absence of tetracycline (switch “on”) until reaching a size of 0.5–1 cm2. At this stage of tumor expansion blood vessels are intact (Fig. 2A). Tetracycline was then added to the drinking water to shut off VEGF expression, and tumors were resected from a single tumor-bearing mice at 24-h intervals. Hemorrhaging foci were detected as early as 24 h from VEGF withdrawal (Fig. 2B). The severity of hemorrhaging increased during the next 2 days and was evident as extensive areas occupied by erythrocyte escapees not contained in blood vessels (Fig. 2 C and D). A marked decrease in the number of intact vessels was observed in these regions during the first 3 days from VEGF withdrawal. At later time points, extensive areas of the tumor underwent necrosis (Fig. 2 D and E). This chain of events was highly reproducible and was independent of tumor size at the time of VEGF withdrawal. We conclude that VEGF is required for maintenance of at least a fraction of tumor vessels, and that its abrupt withdrawal during neovascularization results in regression of existing vessels. Interestingly, not all tumor vessels were effected by VEGF withdrawal. This is illustrated in Fig. 2F, which shows a tumor resected 5 days after switching off VEGF expression. A region is highlighted to show side-by-side where vessel regression has led to necrosis yet adjacent tissue contains a healthy tumor vasculature.
Figure 2.
Vessel regression and tumor necrosis following shut off of VEGF expression. Hematoxylin and eosin staining of sections from C6 tumors grown in Nude mice for 2 weeks in the absence of tetracycline and resected 0 h (A), 24 h (B), 48 h (C), 72 h (D), 4 days (E), and 5 days (F) after administration of tetracycline to shut off expression of VEGF from the transfected VEGF165. n, Necrosis.
Regression of Tumor Vessels Involves Endothelial Cell Detachment.
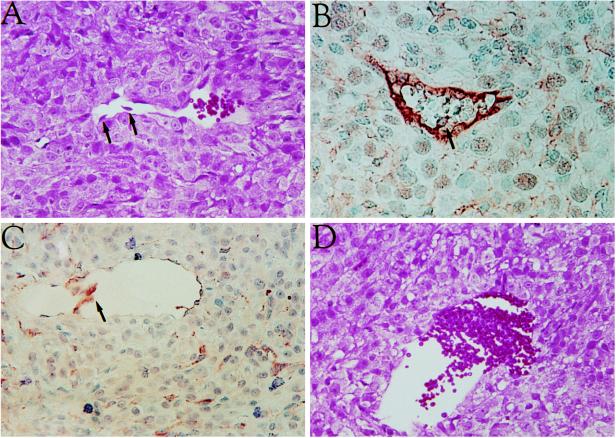
To gain insights into the mechanism of blood vessel regression, endothelial cells were localized by in situ immunostaining during early times following administration of tetracycline. As shown in Fig. 3, endothelial cells were observed in the process of detachment. Progressive stages in this process are shown. Fig. 3A shows endothelial cells separating from the vessel wall (highlighted by arrows). Fig. 3B shows a still-continuous endothelial cell lining (stained with anti-von Willebrand factor antibodies) with only a single endothelial cell on its way to being shed into the lumen. Fig. 3C shows detachment of the few remaining endothelial cells (stained with B. simplicifolia isolectin B4). Fig. 3D shows erythrocytes escaping from the lumen of a vessel devoid of endothelial cell lining.
Figure 3.
Endothelial cell detachment and erythrocyte escape from blood vessels. (A–D) Sections from a tumor resected 24 h after VEGF shut off. A and D were stained with hematoxylin and eosin. Endothelial cells were visualized in B by staining with anti-von Willebrand antibodies and in C by staining with B. simplicifolia isolectin B4. Arrows point at endothelial cells being shed into the lumen.
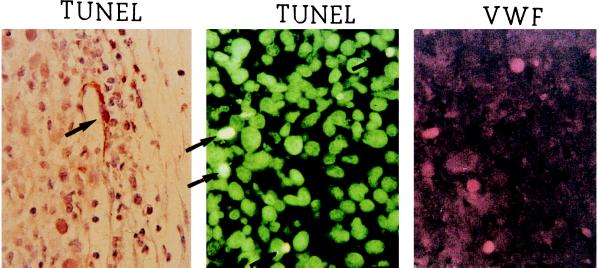
It is well established that cells that lose contact with their extracellular matrix undergo apoptosis. Such a phenomenon has been specifically observed with endothelial cells (25). To observe endothelial cell apoptosis, DNA fragmentation products were stained by in situ 3′ end labeling (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling; TUNEL). Occasionally, TUNEL-positive cells were observed in the endothelial cell lining of blood vessels (Fig. 4 Left). However, the majority of TUNEL-positive cells were seen as isolated cells that could not be identified as endothelial cells simply on a contextual basis. We confirmed that isolated TUNEL-positive cells were in fact endothelial cells using double-labeling with TUNEL and anti-von Willebrand factor antibodies (Fig. 4 Center and Right). These findings supported the hypothesis that detachment of the endothelium was the primary effect of VEGF-loss and preceded endothelial cell apoptosis.
Figure 4.
Extra-vascular TUNEL-positive endothelial cells. (Left) TUNEL-positive endothelial cell (arrow) in the wall of a partially degenerated vessel, 24 h after VEGF shut off (counterstained with hematoxylin and eosin). (Center and Right) Double-labeling of a tumor section with TUNEL (using a fluorescein isothiocyanate-flurophore) (Center) and anti-von Willebrand (VWF) antibody (using a rhodamine flurophore) (Right).
DISCUSSION
We have devised an experimental system that allows to modulate the level of VEGF production during progressive stages of tumor growth and neovascularization. This system enabled us to determine, on the one hand, the effects of VEGF over-production and, on the other hand, the consequences of untimely withdrawal of VEGF. This experimental system is particularly suitable for simulating a situation of a fluctuating VEGF expression, a condition that is likely to develop due to fluctuating tumor oxygenation (26, 27) and a likely cause of vascular injury.
Vascular and Tumor Phenotypes Resulting from Over-Expression of VEGF.
With the contribution of VEGF produced from the transfected VEGF165 expression plasmid, blood vessels grew to a significantly higher density than in control tumors expressing only the endogenous VEGF, suggesting that neovascularization in this system is limited by the availability of angiogenic factors. This result is consistent with previous findings showing that increased expression of VEGF in a xenografted carcinoma tumor also results in increased vascularity (12, 28).
Progression from benign astrocytoma to malignant glioblastoma multiforme is associated with a progressive increase in tumor vascularity. Yet, even the most vascularized glioblastomas are characterized by extensive necrosis. Our finding that boosting VEGF production results in tumor expansion without tumor necrosis again suggests that tumor neovascularization is unable to match the rapid growth of this tumor due to insufficient production of a positive regulator of angiogenesis.
Perhaps the most dramatic phenotype associated with over-expression of VEGF is the formation of abnormally large and malformed vessels. A larger vessel diameter could be, in principle, the result of either dilation, circumferential growth, or vascular fusions. Judging from the course of individual vessels followed in serial sections (data not shown), as well as from their irregular circumference (e.g., Fig. 1B), we favor the third possibility, namely that fusion of vessels occurred at multiple points of contact with neighboring vessels. Thus, it is likely that vascular fusions will be pronounced mostly in cases of an exceedingly high vascular density. VEGF was found to induce a similar hyperfused vessel phenotype when injected during development of quail embryos (29). Thus, in both embryonic and tumor development the level of VEGF produced at the site of neovascularization seems to effect vessel diameter. The vascular pattern shown in Fig. 1, particularly the formation of vascular sacs, resembles vascular structures found in hemangioblastomas. On the basis of findings that hemangioblastomas express relatively high levels of VEGF, it has been suggested that VEGF may mediate neovascularization and cyst formation in capillary hemangioblastoma (23, 24). Findings shown here support this suggestion.
Vascular Regression Resulting from Withdrawal of VEGF.
A major finding of this study is that by switching off expression from the transfected VEGF expression plasmid, preformed tumor vessels regress, suggesting that VEGF functions as a survival factor for tumor vessels. Certain vascular networks regress normally during development (e.g., the hyloid vascular system of the eye regresses upon completion of lens development), and it is likely that a developmentally programmed regression of transient vascular networks is caused by down-regulation of an essential vascular survival factor. A distinction should be made, however, between regression of a fully matured, functional vascular system and vascular obliteration that is coupled to the process of neovascularization or remodeling. An example of the latter is the process of vascular pruning associated with retina neovascularization. We have recently shown that pruning of retina vessels is induced when VEGF is down-regulated to a level lower than the level required to sustain newly formed, immature vessels (20). We argued that vascular pruning is an integral part of a process of adjusting the vascular density to match oxygen requirements of the surrounding tissue. VEGF, by virtue of its up-regulation by hypoxia and down-regulation by hyperoxia, is poised to mediate both vascular expansion as well as vascular regression. We reasoned, therefore, that the potential for regressing blood vessels under conditions of VEGF deprivation will be shared with neovascularizing tumors. The results shown in Figs. 2 and 3 indicate that this is indeed the case. It should be noted that, in the experimental system used, even when VEGF is “switched off” there is still protein produced from the endogenous alleles and yet many vessels regress. This suggests that there is a direct correlation between the amount of available VEGF and the number of vessels it can sustain.
From a mechanistic point of view, the shedding of endothelial cells from the vessel wall appeared to be an early step in vessel regression. Though we could occasionally detect TUNEL-positive endothelial cells in intact vessels, more often the endothelial cells had separated from the vessel wall before evidence of DNA fragmentation could be detected. A similar observation was recently made in the process of vessel regression in the corpus luteum (30). It is well established that cells that lose contact with their extracellular matrix undergo apoptosis (31). Such a phenomenon has been specifically observed with endothelial cells (25, 32). Findings reported here suggest that VEGF may function as a vascular survival factor by mediating interaction of endothelial cells with the underlying matrix. In turn, the transition to a VEGF-independent state may take place concomitantly with establishment of endothelial cell–extracellular matrix interactions that are independent of VEGF.
Clinical Implications of Vascular Regression Caused by VEGF Withdrawal.
Should enforced regression of tumor vessels be considered as a component of anti-angiogenic cancer therapy? At the time of clinical diagnosis solid tumors are already well vascularized and contain vessels at different degrees of maturity. The success of survival-factor loss therapy in tumors might depend on the fraction of immature vascular elements engaged in neovascularization or remodeling and might, therefore, differ from one type of tumor to another (33, 34). Analysis of primary human glioblastomas has shown that the 5-bromodeoxyuridine (BrdUrd) labeling index of tumor endothelial cells is a 4.5%. Moreover, the labeling index of glomerular-shaped vessels, which are formed mostly in response to VEGF induced by environmental stress (9), is as high as 20% (35). Thus, it appears that ongoing neovascularization and remodeling will render a significant fraction of tumor vessels an appropriate target for enforced regression. Also, the collapse of a relatively small fraction of vessels might cause damage to a much larger fraction of tumor tissue. Our observations massive areas of the tumors were effected by hemorrhage and subsequently underwent necrosis (Fig. 2 D and E) is consistent with this suggestion. The appearance of blood vessels and healthy tumor tissue after longer times (Fig. 2F) may be due to revascularization mediated by the endogenous VEGF or by other angiogenic factors, such as fibroblast growth factor produced by C6 cells (36). Alternatively, a fraction of the tumor vessels may be “mature” and refractory to VEGF loss. It is likely that the high efficiency of anti-VEGF treatments to reduce the size of xenografted tumors (14–16) is, at least in part, due to regression of preformed tumor vessels (37).
VEGF is currently being used in clinical trials to stimulate neovascularization (i.e., to induce formation of collateral blood vessels). Our studies suggest that, while VEGF may effectively induce endothelial cell proliferation, its untimely discontinuation might lead to subsequent regression of the new endothelium. For example, findings that a therapeutic neovascularization following VEGF gene transfer was temporary and failed to sustain circulation in a gangrenous leg after 2 months (38) could be explained by a requirement for a sustained VEGF production to maintain the newly formed vessels. We feel, therefore, that our finding that newly formed or remodeling blood vessels require sustained VEGF levels will be critical in the success of many angiogenic and anti-angiogenic therapies.
Acknowledgments
We thank Ahuva Itin and Hadassah Gnessin for excellent technical assistance. This research was supported by the Israel Science Foundation (administered by the Israel Academy of Sciences) and by the Mireille and James I. Levy Foundation. L.E.B. was funded by the Valazzi–Pikovsky Fellowship Fund.
ABBREVIATIONS
- VEGF
vascular endothelial growth factor
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
References
- 1.Hanahan D, Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 2.Holmgren L, O’Reilly M S, Folkman J. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 3.O’Reilly M S, Holmgren L, Shing Y, Chen C, Rosenthal R A, Cao Y, Moses M, Lane W S, Sage E H, Folkman J. Cold Spring Harbor Symp Quant Biol. 1994;59:471–482. doi: 10.1101/sqb.1994.059.01.052. [DOI] [PubMed] [Google Scholar]
- 4.Mukhopadhyay D, Tsiokas L, Sukhatme V P. Cancer Res. 1995;55:6161–6165. [PubMed] [Google Scholar]
- 5.Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, Kerbel R S. Cancer Res. 1995;55:4575–4580. [PubMed] [Google Scholar]
- 6.Grugel S, Finkenzeller G, Weindel K, Barleon B, Marme D. J Biol Chem. 1995;270:25915–25919. doi: 10.1074/jbc.270.43.25915. [DOI] [PubMed] [Google Scholar]
- 7.Wizigmann Voos S, Breier G, Risau W, Plate K H. Cancer Res. 1995;55:1358–1364. [PubMed] [Google Scholar]
- 8.Kieser A, Weich H A, Brandner G, Marme D, Kolch W. Oncogene. 1994;9:963–969. [PubMed] [Google Scholar]
- 9.Shweiki D, Itin A, Soffer D, Keshet E. Nature (London) 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 10.Plate K H, Breier G, Weich H A, Risau W. Nature (London) 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 11.Shweiki D, Neeman M, Itin A, Keshet E. Proc Natl Acad Sci USA. 1995;92:768–772. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H T, Craft P, Scott P A, Ziche M, Weich H A, Harris A L, Bicknell R. J Natl Cancer Inst. 1995;87:213–219. doi: 10.1093/jnci/87.3.213. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi Y, Kitadai Y, Bucana C D, Cleary K R, Ellis L M. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- 14.Kim K J, Li B, Winer J, Armanini M, Gillett N, Phillips H S, Ferrara N. Nature (London) 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 15.Millauer B, Shawver L K, Plate K H, Risau W, Ullrich A. Nature (London) 1994;367:576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- 16.Millauer B, Longhi M P, Plate K H, Shawver L K, Risau W, Ullrich A, Strawn L M. Cancer Res. 1996;56:1615–1620. [PubMed] [Google Scholar]
- 17.Borgstrom P, Hillan K J, Sriramarao P, Ferrara N. Cancer Res. 1996;56:4032–4039. [PubMed] [Google Scholar]
- 18.Raff M C, Barres B A, Burne J F, Coles H S, Ishizaki Y, Jacobson M D. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- 19.Raff M C, Barres B A, Burne J F, Coles H S, Ishizaki Y, Jacobson M D. Philos Trans R Soc London B. 1994;345:265–268. doi: 10.1098/rstb.1994.0104. [DOI] [PubMed] [Google Scholar]
- 20.Alon, T., Hemo, I., Itin, A., Pe’er, J., Stone, J. & Keshet, E. (1995) Nat. Med. 1024–1028. [DOI] [PubMed]
- 21.Plate K H, Breier G, Millauer B, Ullrich A, Risau W. Cancer Res. 1993;53:5822–5827. [PubMed] [Google Scholar]
- 22.Stein I, Neeman M, Shweiki D, Itin A, Keshet E. Mol Cell Biol. 1995;15:5363–5368. doi: 10.1128/mcb.15.10.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morii K, Tanaka R, Washiyama K, Kumanishi T, Kuwano R. Biochem Biophys Res Commun. 1993;194:749–755. doi: 10.1006/bbrc.1993.1885. [DOI] [PubMed] [Google Scholar]
- 24.Bohling T, Hatva E, Kujala M, Claesson Welsh L, Alitalo K, Haltia M. J Neuropathol Exp Neurol. 1996;55:522–527. doi: 10.1097/00005072-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Re F, Zanetti A, Sironi M, Polentarutti N, Lanfrancone L, Dejana E, Colotta F. J Cell Biol. 1994;127:537–546. doi: 10.1083/jcb.127.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trotter M J, Chaplin D J, Durand R E, Olive P L. Int J Radiat Oncol Biol Phys. 1989;16:931–945. doi: 10.1016/0360-3016(89)90889-4. [DOI] [PubMed] [Google Scholar]
- 27.Chaplin D J, Olive P L, Durand R E. Cancer Res. 1987;47:597–601. [PubMed] [Google Scholar]
- 28.Fox S B, Gatter K C, Bicknell R, Going J J, Stanton P, Cooke T G, Harris A L. Cancer Res. 1993;53:4161–4163. [PubMed] [Google Scholar]
- 29.Drake C J, Little C D. Proc Natl Acad Sci USA. 1995;92:7657–7661. doi: 10.1073/pnas.92.17.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modlich U, Kaup F-J, Augustin H G. Lab Invest. 1996;74:771–780. [PubMed] [Google Scholar]
- 31.Merdith J E, Schwarz M A. Trends Cell Biol. 1997;7:146–150. doi: 10.1016/S0962-8924(97)01002-7. [DOI] [PubMed] [Google Scholar]
- 32.Merdith J E, Fazeli B, Schwartz M A. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rak J, Kerbel R S. Cancer Metastasis Rev. 1996;15:231–236. doi: 10.1007/BF00437476. [DOI] [PubMed] [Google Scholar]
- 34.Barinaga M. Science. 1997;275:482–484. doi: 10.1126/science.275.5299.482. [DOI] [PubMed] [Google Scholar]
- 35.Nagashima T, Hoshino T, Cho K G. Acta Neuropathol. 1987;73:301–305. doi: 10.1007/BF00686626. [DOI] [PubMed] [Google Scholar]
- 36.Powell P P, Klagsbrun M. Exp Cell Res. 1993;209:224–230. doi: 10.1006/excr.1993.1305. [DOI] [PubMed] [Google Scholar]
- 37.Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain R. Proc Natl Acad Sci USA. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isner J M, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, Rosenfield K, Razvi S, Walsh K, Symes J F. Lancet. 1996;348:370–374. doi: 10.1016/s0140-6736(96)03361-2. [DOI] [PubMed] [Google Scholar]