Abstract
Split-thickness pig skin was transplanted on severe combined immunodeficient mice so that pig dermal microvessels spontaneously inosculated with mouse microvessels and functioned to perfuse the grafts. Pig endothelial cells in the healed grafts constitutively expressed class I and class II major histocompatibility complex molecules. Major histocompatibility complex molecule expression could be further increased by intradermal injection of pig interferon-γ (IFN-γ) but not human IFN-γ or tumor necrosis factor. Grafts injected with pig IFN-γ also developed a sparse infiltrate of mouse neutrophils and eosinophils without evidence of injury. Introduction of human peripheral blood mononuclear cells into the animals by intraperitoneal inoculation resulted in sparse perivascular mononuclear cell infiltrates in the grafts confined to the pig dermis. Injection of pig skin grafts on mice that received human peripheral blood mononuclear cells with pig IFN-γ (but not human IFN-γ or heat-inactivated pig IFN-γ) induced human CD4+ and CD8+ T cells and macrophages to more extensivley infiltrate the pig skin grafts and injure pig dermal microvessels. These findings suggest that human T cell-mediated rejection of xenotransplanted pig organs may be prevented if cellular sources of pig interferon (e.g., passenger lymphocytes) are eliminated from the graft.
Xenotransplantation of pig organs is being actively investigated as a solution to the increasing shortages of human organs available for clinical transplantation (1). Most studies have focused on strategies to overcome hyperacute rejection reactions mediated by preformed “natural” antibodies, largely because these reaction are so rapid and destructive that they are sufficient by themselves to prevent successful xenotransplantation (2). However, once hyperacute rejection has been controlled, xenografts will likely be subjected to more conventional rejection processes. It has been suggested that such reactions may be less severe than responses to allogeneic tissues because cellular interactions may be compromised by incompatibilities across the species barrier at multiple levels (3). For example, there could be inefficient interactions between T cell receptors for antigen and xenogeneic major histocompatibility complex (MHC) molecules, between T cell receptors such as CD2 or CD28 and costimulator molecules on xenogeneic antigen presenting cells, between leukocyte adhesion molecules and counter receptors on xenogeneic endothelial cells, or between cytokines and xenogeneic cytokine receptors. Unfortunately, in vitro studies have indicated that human T cells can directly recognize pig MHC molecules (4–6) and be stimulated by pig ligands for CD2 and CD28 (4, 5, 7). On the other hand, human interferon-γ (IFN-γ) does not act on pig cells and vice versa (4). However, such in vitro studies, although informative, cannot accurately predict the strength of the in vivo human anti-pig response.
Recently, we developed a small animal model for studying human T cell-mediated allogeneic responses (8). In brief, we established conditions for transplanting split-thickness adult human skin on to severe combined immunodeficient (SCID) mice such that the grafts become largely perfused through retained human microvessels. The introduction of human peripheral blood mononuclear cells (PBMCs) allogeneic to the skin donor into these animals resulted in a stereotypic pattern of human skin infiltration and microvessel injury that recapitulates the histological features of human first set graft rejection (9). In the present study, we have modified this model to use pig skin, providing a system for studying human anti-pig xenogeneic interactions.
METHODS
Animals, Cells, and Reagents.
C.B-17 SCID and SCID beige female mice were purchased from Taconic (Germantown, NY) and used at 5–8 weeks of age. Animals with circulating mouse IgG levels above 1 μg/ml were judged leaky and excluded from use. Pig skin was obtained from freshly slaughtered outbred Yorkshire pigs from a local abattoire. Human PBMCs were collected by leukophoresis from adult volunteer donors. Recombinant pig IFN-γ was obtained from VIDO (Saskatoon, Canada) and recombinant human IFN-γ was a gift from Biogen. Primary monoclonal antibodies for immunohistochemistry were mouse anti-human von Willebrand factor (vWF; Accurate Chemicals), rat anti-mouse CD31 and CD 45 (PharMingen), mouse anti-SLA-I and II (VMRD, Pullman, WA), and mouse anti-human CD3, CD4, CD8, CD19, and CD68 (Dako). Secondary antibodies were from Vector Laboratories or Jackson ImmunoResearch, and kits for detection of enzyme were from Vector Laboratories. Antibodies for analysis of mouse blood for circulating leukocytes by flow cytometry were fluorescein isothiocyanate-conjugated mouse anti-human CD3 (Exalpha, Boston) or quantum red 613-conjugated rat anti-mouse CD45 (Sigma), and antibodies for circulating IgG measured by ELISA were from Cappel. Finally, rabbit anti-asialo-GM1 antibody was from Wako Chemicals (Richmond, VA).
Experimental Protocols.
Mice were transplanted with skin as described (8) under a protocol approved by the Yale Animal Care and Use Committee except that abdominal pig skin, sequentially swabbed with betadine and ethanol, was substituted for human skin. Each animal received two grafts, which completely healed in 14–21 days; the success rate for graft take was more than 95%. Where indicated, animals bearing skin grafts were inoculated i.p. with 3 × 108 human PBMCs as described (8); SCID mice were primed for inoculation by pretreatment with rabbit anti-asialo GM1 antibody to minimize NK cell function, a step omitted in experiments using SCID/beige mice. No differences were noted between these protocols and data were pooled for analysis. Intradermal injections were initiated on day 3 after inoculation with PBMCs and repeated three times per week until the end of the experiment (day 21). Injections used 25 μl of pyrogen-free saline or 200 ng of recombinant pig IFN-γ (native or heat inactivated) or 2,000 ng of recombinant human IFN-γ in pyrogen-free saline. Mice were euthanized on day 21 after PBMC inoculation by cervical dislocation, and one half of each skin graft was processed for routine histology and the other half was snap frozen for immunostaining as described elsewhere (8).
Data Analysis.
Hematoxylin and eosin-stained paraffin-embedded sections of each skin specimen were examined and graded for extent of infiltration by one observer (J.M.M., blinded to the treatment protocol) by using the following scale: 0, leukocytes equal or less than in normal skin (i.e., only rare perivascular inflammatory cells); 1, sparse perivascular infiltrates above baseline; 2, pronounced perivascular infiltrate around most vessels or patchy perivascular inflammation with some involvement of interstitium; 3, dense perivascular infiltrates with some interstitial dermal inflammation; 4, sheets of inflammatory cells filling and obscuring the dermis. Dermal microvascular injury, consisting of endothelial cell loss, fibrin deposition in the vessel wall, and/or intravascular thrombosis, was scored on the same sections by using the following scale: 0, all vessels patent and univolved; 1, fewer than 25% of vessels show injury; 2, at least 50% of vessels show injury; 3, 75% of vessels show injury; 4, all discernable vessels show injury. The mean and SEM of infiltration and of injury were calculated for each group of animals, and comparisons among groups were made by ANOVA, applying the Scheffe test for significance using software program spssg.1 (SPSS, Chicago). Immunostaining was performed on select specimens and results were evaluated by two independent observers (P.S. and J.S.P.) who agreed by consensus on the interpretation.
RESULTS
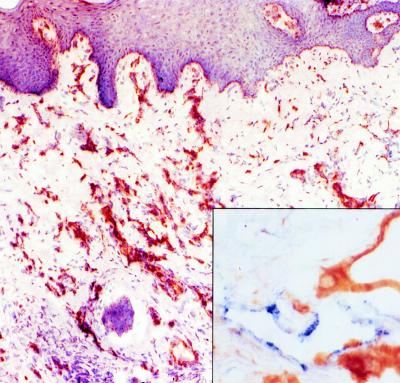
Split-thickness skin grafts, 500 μm thick, harvested from the abdomen of outbred Yorkshire pigs, were reproducibly engrafted on the backs of C.B-17 SCID or SCID/beige mice. As with human skin grafts studied (8), healed pig skin grafts appeared histologically healthy with evidence of normal keratinocyte maturation. Immunohistochemical staining with mouse anti-human vWF antibody, which cross-reacts with pig but not mouse vWF, confirmed that microvessels lined by pig endothelial cells were retained in the graft dermis and had become perfused with mouse blood (Fig. 1). Mouse microvessels, identified by staining with rat anti-mouse CD31 antibody, invaded the pig dermis, intermingling and inosculating with the pig microvessels (Fig. 1 Inset). No significant leukocytic infiltrates were noted in dermis or epidermis of the pig skin grafts (Fig. 2A).
Figure 1.
Light micrograph of an immunostained pig skin graft illustrating the retention of pig endothelial cell-lined microvessels (stained brown with mouse anti-vWF antibody) in the dermis. (Low magnification, counterstained with hematoxylin.) (Inset) Intermingling of pig (stained brown) and mouse (stained blue with rat anti-mouse CD31 antibody) endothelial cells at the borders of the graft. (High magnification, no counterstain.)
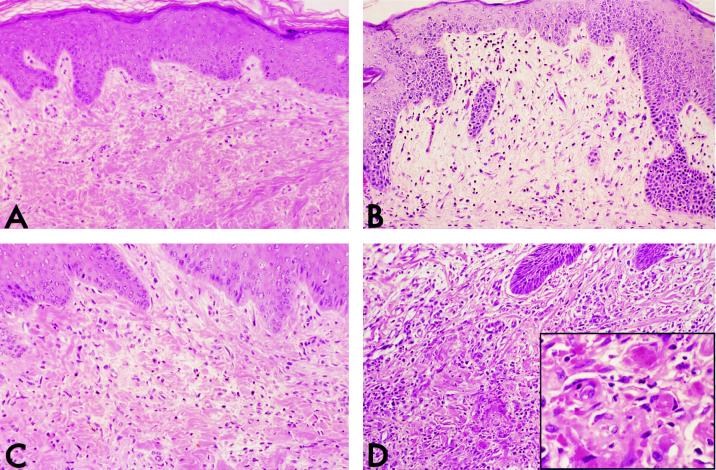
Figure 2.
Light micrographs (low magnification) of hematoxylin and eosin-stained pig skin grafts. The grafts in A and B were harvested from control animals, whereas those in C and D were from animals that had been inoculated with human PBMCs 21 days prior to harvest. Grafts in A and C received repeated injections of saline, whereas grafts in B and D were injected with pig IFN-γ. The graft in A shows a paucity of leukocytes, whereas grafts in B and C show sparse infiltrates of mouse and human leukocytes, respectively. Only the graft in D contains a significant infiltate. (Inset) This reaction is accompanied by microvessel injury and thrombosis (high magnification).
Further analysis of these skin grafts by immunohistochemical staining revealed that the pig endothelial cells constitutively expressed swine class I and II MHC molecules (Fig. 3). Injection of the skin with pig IFN-γ increased the expression of these molecules on the endothelial cells and induced expression on some other cell types as well, notably keratinocytes (Fig. 3). Neither human IFN-γ nor human tumor necrosis factor influenced MHC molecule expression on pig skin cells. Thus, the grafts remained functionally responsive to certain cytokine signals. Repeated local injection of the grafts with pig IFN-γ produced a sparse infiltrate, consisting principally of neutrophils and eosinophils (Fig. 2B). This response was not associated with morphological evidence of injury to the grafts.
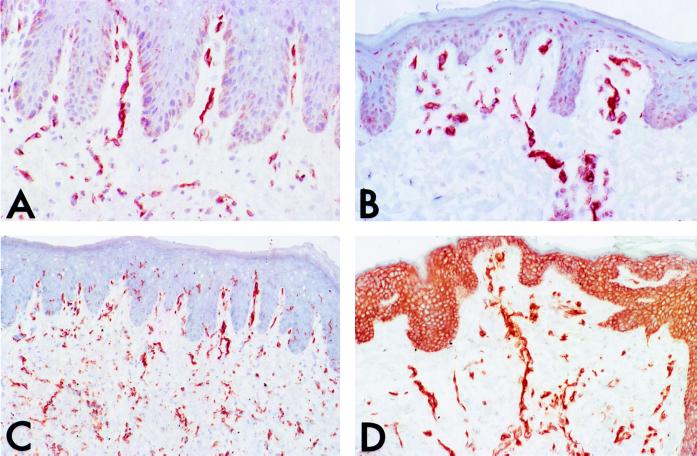
Figure 3.
Light micrographs (low magnification) of pig skin grafts immunostained for pig class I (A and B) or class II (C and D) MHC molecules (stained brown, hematoxylin counterstain). The grafts depicted in A and C had been injected with saline, whereas those in B and D had been injected with pig IFN-γ. Note that pig IFN-γ increases the level of expression of both class I and II MHC molecules on endothelial cells and also spreads expression (especially of class II molecules) to keratinocytes.
Intraperitoneal injection of human PBMCs into mice previously engrafted with pig skins resulted in the appearance of a discrete population of circulating human CD3+ T cells in more than 95% of the animals, within 3–9 days after inoculation (range, 1–65% of the mouse CD45+ leukocytes); the few animals injected with PBMCs that failed to develop circulating human T cells were excluded from the analysis. In agreement with previous experience, these circulating T cells do not infiltrate into mouse skin (data not shown). However, by 21 days after PBMC inoculation, about one half of the pig skin xenografts developed sparse perivascular infiltrates (Fig. 2C). This result differs strikingly from our previous studies with allogeneic (to the PBMCs) human skin in which essentially all of the grafts developed intense dermal infiltrates consisting of human CD3+ T cells. In a few pilot experiments, it was observed that in animals bearing one xenogeneic pig skin graft and one allogeneic human skin graft, the human graft was preferentially infiltrated by human T cells. Also in contrast to previous experience with human allogeneic skin grafts, few of the pig skin grafts showed evidence of vascular injury.
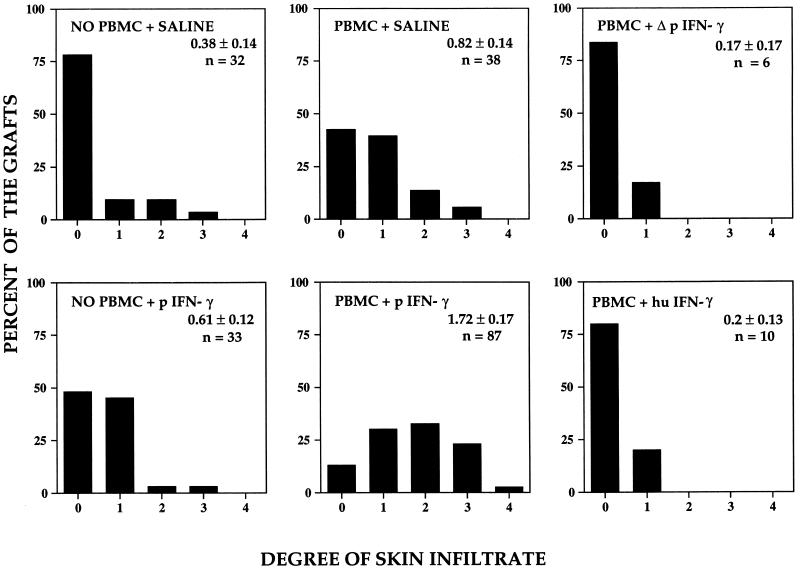
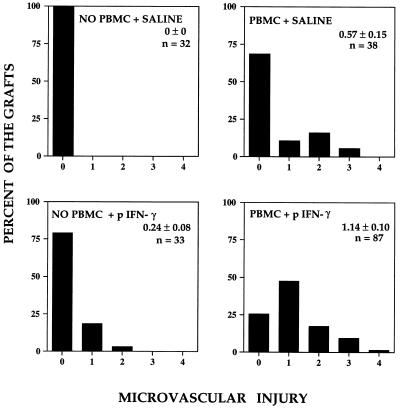
In a final series of experiments, pig IFN-γ was used to determine whether activation of pig skin cells could enhance the response of circulating human T cells to the pig skin grafts. Pilot experiments had suggested that a single injection was not sufficient to produce a consistent response, and thus pig IFN-γ was administered repetitively three times per week starting on day 3 after PBMC inoculation. Control animals were injected in parallel with saline, heat-inactivated pig IFN-γ, or human IFN-γ. As shown in Fig. 2D, injection with pig IFN-γ led to pronounced mononuclear cell infiltrate by day 21 after PBMCs were inoculated. No infiltrates were induced if the pig IFN-γ was injected into mouse skin, if the pig IFN-γ was inactivated by heating prior to injection, or if human IFN-γ was used instead of pig IFN-γ (data not shown). The extent of infiltration was quantified by using a set scoring system (see Methods) by a single observer blinded to the experimental treatments and data were pooled from five separate experiments. As summarized in Fig. 4, the elicitation of infiltrates by pig IFN-γ injection was statistically significant compared with all other experimental groups. The pig IFN-γ-elicited infiltrates in the pig skin were accompanied by microvessel injury, assessed as by histological evidence of endothelial cell death, fibrin deposition, or microvascular lumenal thrombosis. These changes, summarized in Fig. 5, also showed statistically significant differences from all other groups.
Figure 4.
Bar graphs displaying the distribution of intensity of leukocytic infiltrates observed in pig skin grafts as a consequence of human PBMC inoculation and/or injection of IFN-γ. Only the combination of inoculation of human PBMCs followed by intragraft injection of native pig IFN-γ led to marked leukocytic infiltrates that are statistically significantly greater than all other groups (P < 0.05). n, Number of specimens examined.
Figure 5.
Bar graphs displaying the distribution of the extent of microvessel injury observed in pig skin grafts treated as in Fig. 4. The group receiving human PBMCs showed slight injury that was statistically significantly greater than control grafts. More frequent and pronounced injury was observed when grafts from animals receiving human PBMCs were injected with pig IFN-γ; injury in this group was statistically significantly greater than in all other groups (P < 0.05). n, Number of specimens examined.
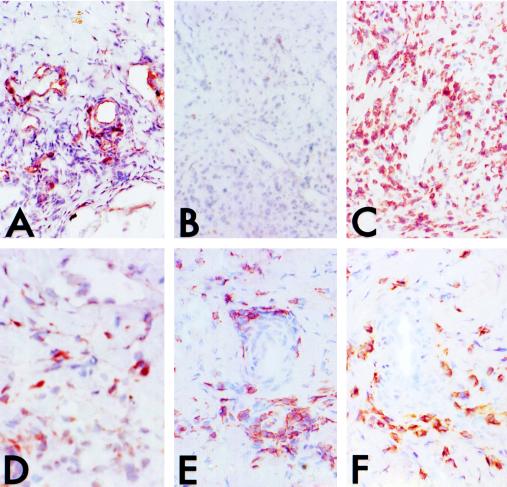
Immunohistochemistry was used to characterize the inflammatory infiltrates elicited by pig IFN-γ. Such infiltrates appeared to be associated with pig microvessels (Fig. 6A). The infiltrates were largely devoid of leukocytes expressing mouse CD45+ (Fig. 6B) and they consisted primarily of human CD3+ T cells (Fig. 6C). Some human CD68+ macrophages were also present (Fig. 6D). The T cells appeared evenly divided between CD4+ (Fig. 6E) and CD8+ (Fig. 6F) subpopulations. No human CD19+ B cells were identified (data not shown). Thus the human mononuclear inflammatory cells accumulating in the grafts resembled the infiltrates associated with allograft injury in this model (8).
Figure 6.
Light micrographs (low magnification, hematoxylin counterstain) of pig skin grafts infiltrated after inoculation of the animals with human PBMCs and intragraft injections of pig IFN-γ, immunostained for the following cells: A, pig endothelial cells (anti-vWF); B, mouse leukocytes (anti-CD45); C, human T cells (anti-CD3); D, human macrophages (anti-CD68); E and F, human T cell subpopulations (CD4 and CD8, respectively). Note that the perivascular infiltrates are composed of about 80% human CD3+ T cells with equal numbers of CD4 and CD8 T cells, the remainder being largely human macrophages, with few mouse leukocytes.
DISCUSSION
The results described in this study identify the actions IFN-γ on graft tissues as an initiator of antixenograft injury and suggest that human–pig species incompatibility in IFN-γ interactions with its receptor, as noted in vitro (4), may serve as a potential barrier to strong human anti-pig T cell-mediated responses in vivo. If true, then elimination of IFN-γ-producing cells from pig xenografts could improve graft survival. It should be noted that mechanisms of allograft injury independent of IFN-γ-producing T cells have been described (10). However, the effector mechanism(s) of graft injury in such settings either may not have been reconstituted in our chimeric human PBMC–SCID mice or is unable to function across the human–pig xenogeneic barrier.
We are not yet certain which actions of pig IFN-γ are important in our system. The inability of human IFN-γ to produce this effect and the lack of effect of pig IFN-γ when injected into mouse skin establish that the relevant target of pig IFN-γ is a cell of pig origin resident in the pig graft. Since human T cell receptors have been shown to directly recognize pig MHC molecules (4, 5), the well known effects of IFN-γ on MHC molecule expression (11), documented in our model, must be considered as a potentially relevant action. However, it is clear that MHC molecules are expressed at readily detectable levels prior to pig IFN-γ injection, at least on graft endothelial cells, yet infiltrates do not develop. Nevertheless, it is possible that newly induced MHC molecules, because they are expressed at higher cell surface densities, because they are on different cell types, or because they contain different peptides from those expressed constitutively, are required for initiating the reaction. Alternatively, pig IFN-γ could act by inducing expression of a costimulator molecule on porcine antigen-presenting cells, by inducing expression of a leukocyte adhesion molecule on porcine graft microvessel cells, and/or by inducing secretion of a pig-cell-derived chemokine critical for human leukocyte recruitment. Such hypothetical possibilities will require further studies.
A key issue raised by our experiments is how useful might our model prove to be for preclinical xenotransplantation research. Previous experiments examining the reaction of human T cells to allogeneic skin in immunodeficient mice have revealed that this destructive reaction could be inhibited by conventional immunosuppressive agents (A.G.M. and J.S.P., unpublished observations), suggesting that it shares some clinically relevant features with human allograft rejection. Even if our xenogeneic injury model recapitulates only part of the human anti-pig response, it still may provide valuable information about the use of immunosuppressive agents prior to testing in pig-into-primate transplant models.
Acknowledgments
We thank Louise Benson for help with screening mice, Mary Lou Perez with assistance in preparation of the manuscript, the Yale Blood Bank for help in leukophoresis, and the SCID mouse/xenograft core of the Yale Skin Diseases Research Center (Grant AR-041942). This work was supported by grants from the National Institutes of Health (HL-51014 and -51448). P.S. was supported by fellowships from the Sarnoff Foundation and Yale University and A.G.M. was supported by the Kidney Foundation of Canada.
ABBREVIATIONS
- MHC
major histocompatibility complex
- IFN-γ
interferon γ
- PBMC
peripheral blood mononuclear cells
- SCID
severe combined immunodeficient
- vWF
von Willebrand factor
References
- 1.Bach F H. Transplant Proc. 1993;25:25–29. [PubMed] [Google Scholar]
- 2.Parker W, Saadi S, Lin S, Holzknbecht Z E, Bustos M, Platt J L. Immunol Today. 1996;17:373–378. doi: 10.1016/0167-5699(96)10028-1. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman C L, Gaines B A, Ildstad S T. Annu Rev Immunol. 1995;13:339–367. doi: 10.1146/annurev.iy.13.040195.002011. [DOI] [PubMed] [Google Scholar]
- 4.Murray A G, Khodadoust M M, Pober J S, Bothwell A L M. Immunity. 1994;1:57–63. doi: 10.1016/1074-7613(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 5.Rollins S A, Kennedy S P, Chodera A J, Elliott E A, Zavoico G B, Matis L A. Transplantation. 1994;57:1709–1716. [PubMed] [Google Scholar]
- 6.Bravery C A, Batten P, Yacomb M H, Rose M L. Transplantation. 1995;60:1024–1033. [PubMed] [Google Scholar]
- 7.Maher S E, Karmann K, Min, Wang, Hughes C C W, Pober J S, Bothwell A L M. J Immunol. 1996;157:3838–3844. [PubMed] [Google Scholar]
- 8.Murray A G, Petzelbauer P, Hughes C C W, Costa J, Askenase P, Pober J S. Proc Natl Acad Sci USA. 1994;91:9146–9150. doi: 10.1073/pnas.91.19.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dvorak H F, Mihm M C, Dvorak A M, Barnes B A, Manseau E J, Galli S. J Exp Med. 1979;150:322–337. doi: 10.1084/jem.150.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saleem S, Konieczny B T, Lowry R P, Baddoura F K, Lak F G. Transplantation. 1996;62:1908–1911. doi: 10.1097/00007890-199612270-00039. [DOI] [PubMed] [Google Scholar]
- 11.Pober J S, Gimbrone M A, Jr, Cotran R S, Reiss C S, Burakoff S J, Fiers W, Ault K A. J Exp Med. 1983;157:1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]