Abstract
Low socioeconomic status (SES) increases the risk for developing psychiatric and chronic medical disorders. A stress-related pathway by which low SES may affect mental and physical health is through the perception of holding a low social standing, termed low subjective social status. This proposal implicates overlapping brain regions mediating stress reactivity and socioemotional behaviors as neuroanatomical substrates that could plausibly link subjective social status to health-related outcomes. In a test of this proposal, we used a computational structural neuroimaging method (voxel-based morphometry) in a healthy community sample to examine the relationships between reports of subjective social status and regional gray matter volume. Results showed that after accounting for potential demographic confounds, subclinical depressive symptoms, dispositional forms of negative emotionality and conventional indicators of SES, self-reports of low subjective social status uniquely covaried with reduced gray matter volume in the perigenual area of the anterior cingulate cortex (pACC)—a brain region involved in experiencing emotions and regulating behavioral and physiological reactivity to psychosocial stress. The pACC may represent a neuroanatomical substrate by which perceived social standing relates to mental and physical health.
Keywords: anterior cingulate cortex, gray matter volume, socioeconomic status, subjective social status, stress
Health and longevity track a socioeconomic gradient. Low socioeconomic status (SES)—defined as having fewer resources or holding a lesser social standing than others—increases the risk for most major medical diseases (Adler et al., 1993, 1994, 1999) and prevalent psychiatric disorders, particularly psychopathologies of mood and substance abuse (Kessler et al., 1994; Lorant et al., 2003). Low SES has long been thought to affect mental and physical health, in part, through stress-related psychosocial and biobehavioral pathways (Dohrenwend, 2000; Marmot, 2004). Hence, low SES has been associated with symptoms of psychological distress and negative emotionality (Kessler and Cleary, 1980; Mirowsky and Ross, 1986; Gallo and Matthews, 2003), with maladaptive behavioral coping responses to stressful life circumstances (Kessler et al., 1985) and with putatively pathogenic endpoints of the neuroendocrine and autonomic stress-response axes (Steptoe et al., 2003; Cohen et al., 2006). Despite these largely epidemiological associations, however, the neuroanatomical substrates linking SES to health outcomes via stress-related pathways remain speculative.
As a multidimensional and multilevel construct, SES is conventionally measured by a number of different indicator variables, which can be referenced to a single person, to a person's family, or to members of a person's residential community. Importantly, conventional indicators of SES, which include educational, monetary and occupational characteristics, are often only modestly correlated with one another, and they may reflect relatively distinct pathways by which different dimensions of SES can affect health over the lifespan (Braveman et al., 2005). Moreover, conventional SES indicators are not thought to fully characterize an individual's perception of her or his relative standing or ranking in a social hierarchy, formally termed subjective social status (Cohen et al., 1997; Kaplan and Manuck, 1999; Wilkinson, 1999; Adler et al., 2000; McEwen, 2000a; Steptoe and Marmot, 2002; Sapolsky, 2005b). Consequently, conventional SES indicators may not fully capture the presumptive stress-related dimension of low socioeconomic position; namely, the subjective perception of holding a low social status.
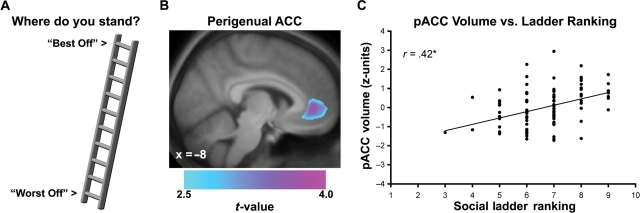
To this end, Adler and coworkers (2000) have recently developed and validated a single-item, self-anchoring scale to reliably assess subjective ratings of perceived social status. This scale is presented to respondents as a pictorial ‘social ladder’ of where people rank in a social hierarchy (Figure 1A; appendix). On the ladder, respondents mark the rung corresponding to their perceived standing or ranking within a particular social group, such as other individuals residing in a respondent's country. In line with prior speculations on perceived social standing and health, lower social ladder rankings have been associated with adverse physical and mental health outcomes in both cross-sectional (Adler et al., 2000; Ostrove et al., 2000; Singh-Manoux et al., 2003; Kopp et al., 2004; Operario et al., 2004; Hu et al., 2005) and prospective (Singh-Manoux et al., 2005) studies. Lower social ladder rankings have also been associated with autonomic and metabolic risk factors for all-cause mortality, including a higher basal heart rate and a greater central body fat distribution (Adler et al., 2000). Finally, lower social ladder rankings have been associated with plausible neuroendocrine mediators of disease risk, including an exaggerated rise in the stress-hormone, cortisol, on awakening from sleep (Wright and Steptoe, 2005) and a non-habituating cortisol response to recurrent psychological stress (Adler et al., 2000). Critically, social ladder rankings in the forgoing studies have predicted both health outcomes and related risk factors not only after accounting for correlated variation in conventionally-defined levels of SES (e.g. education and income levels), but also after accounting for potential reporting biases attributable to state and trait forms of negative emotionality and social hostility—supporting theoretical considerations that emphasize the relative importance of perceived social standing in association with health-related outcomes (Adler et al., 1994; Marmot, 2004).
Fig. 1.
Lower subjective social status, as reflected by a lower self-reported ranking on a ‘social ladder’, was associated with reduced gray matter volume in the perigenual area of the anterior cingulate cortex (pACC). (A) Illustration of 10-point social ladder scale used to assess subjective social status (instructions provided in the appendix). (B) Overlaid on a sagittal view of an anatomical template generated from the present sample is a statistical parametric map of color-scaled t-values, which illustrate the pACC area where lower subjective social status was associated with reduced gray matter volume in a multiple regression analysis. The regression analysis controlled for conventional indicators of personal socioeconomic status (assessed by family income and education) and community socioeconomic status (assessed by census tract information reflecting social advantage), as well as age, sex and total gray matter volume. (C) Plotted along the y-axis is the standardized (z-score) gray matter volume from the peak pACC voxel within the cluster of voxels profiled in B. Plotted along the x-axis are social ladder rankings from the scale illustrated in A (1 = ‘Worst Off’, 10 = ‘Best Off’). *P < 0.001.
On the view that the perception of holding a low standing in a human social hierarchy is a source of psychosocial stress associated with low socioeconomic position, negative emotionality and maladaptive changes in behavior and physiology, McEwen (McEwen and Seeman, 1999; McEwen, 2000a) and others (Blanchard, McKittrick and Blanchard, 2001; Sapolsky, 2005a, 2005b) have speculated that the neuroanatomical substrates linking perceived social standing to health-related risk factors likely involve paralimbic brain areas that jointly (i) support social and emotional information processing; (ii) regulate neuroendocrine and autonomic nervous system activity; and (iii) express well-characterized morphological (structural) changes in association with conditions of chronic stress in non-human animal models. Although several paralimbic brain areas likely meet one or more of these criteria, there is cumulative evidence from non-human animal studies of social hierarchies and chronic stress to implicate three in particular: the anterior cingulate cortex (ACC), the hippocampus and the amygdala (for reviews, see McEwen, 2000a; Blanchard et al., 2001; Fuchs and Flugge, 2003; Sapolsky, 2003; Radley and Morrison, 2005).
Together, the ACC, hippocampus and amygdala are viewed to represent networked components of a distributed corticolimbic circuitry that coordinates behavior with neuroendocrine [hypothalamic-pituitary-adrenal (HPA)] and autonomic (sympathoadrenal) function in the service of adaptively coping with emotionally salient environmental and psychosocial challenges (Fuchs and Flugge, 2003; Phillips et al., 2003). Non-human animal work further indicates that chronic and social stressors are associated with unique structural changes in the ACC, hippocampus and amygdala. For example, chronic stressors, such as prolonged immobilization, simplify the branching complexity and shorten the length of apical dendrites of pyramidal neurons in the rat ACC (Cook and Wellman, 2004; Radley et al., 2004; Radley and Morrison, 2005; Radley et al., 2006). In both rodent and non-rodent animal models, housing in dominance hierarchies also remodels pyramidal neurons in the CA3 region of the hippocampus, and it arrests the proliferation of new neurons in the dentate gyrus, two types of cellular changes that may partly contribute to a decrease in hippocampal volume (Magarinos et al., 1996; McKittrick et al., 2000; Blanchard et al., 2001; McEwen, 2001; Fuchs and Flugge, 2003; Sapolsky, 2003). In contrast to these atrophy-like changes that occur in the ACC and hippocampus, chronic stressors, such as prolonged immobilization, result in some forms of hypertrophy in the rat amygdala, mediated in part by an increase in the dendritic length and branching complexity of neurons in the basolateral complex (Vyas et al., 2002; Vyas et al., 2003; Vyas et al., 2004). Such chronic stress-related changes in the cellular and macroscopic morphology of the ACC, hippocampus and amygdala can result from alterations in central glucocorticoid levels and receptor densities, acting in conjunction with alterations in the transmission and expression of excitatory amino acids and neurotrophic factors that regulate cellular plasticity and neurogenesis (McEwen, 2000a, 2000b; Fuchs and Flugge, 2003; Sapolsky, 2005b). Thus, from a psychosocial stress perspective developed within the context of the above animal findings, low subjective social status (a putative stress-related dimension of low socioeconomic position) could plausibly covary with changes in the morphology of the ACC, hippocampus or amygdala.
Here, we thus employed a computational neuroanatomical strategy in a cross-sectional neuroimaging study to examine the association between individual differences in subjective social status—as reflected by social ladder rankings—and an in vivo marker of regional brain morphology: gray matter volume. Specifically, we used optimized voxel-based morphometry (Ashburner and Friston, 2000; Good et al., 2001) in a healthy community sample to question (i) whether subjective social status covaries with regional gray matter volume in the ACC, hippocampus or amygdala, and (ii) whether subjective social status uniquely covaries with regional gray matter volume in these targeted regions above-and-beyond potentially confounding demographic and psychological factors as well as conventional indicators of personal and community SES.
METHOD
Participants
Participants were 44 men (mean age = 45.5; range = 31–54 years) and 56 women (mean age = 44.0; range = 31–53 years) who were recruited from a parent study, the Adult Health and Behavior (AHAB) project. AHAB is a community-based registry of 1379 non-patient, middle-aged adults residing in Southwestern Pennsylvania (primarily Allegheny County), USA who were recruited by mass mail solicitations. Participants were ineligible for the neuroimaging protocol described as follows if they had (i) any history of cardiovascular disease or surgery, including a prior myocardial infarction or coronary revascularization; (ii) a prior stroke or cerebrovascular disease; (iii) any neurological disorder, prior convulsions, or a concussion in the year prior to testing; (iv) chronic kidney or liver disease; (v) cancer; (vi) insulin-dependent diabetes; (vii) resting systolic/diastolic blood pressure ≥180/110 mm Hg; (vii) current use of psychotropic, hyperlipidemic, or cardiovascular medications; and (ix) a lifetime history of Bipolar I or psychotic symptoms, or any current DSM-IV Axis I diagnoses, as assessed by the Structured Clinical Interview for DSM-IV, non-patient edition (First et al., 1996). Women who were pregnant or lactating were also ineligible, as were individuals taking psychotropic, glucocorticoid, or weight-loss medications. Participants provided informed consent before completing study protocols, which were approved by the University of Pittsburgh Institutional Review Board.
Of the 100 participants, 88 identified their race as Caucasian, seven as being of African decent, one as Asian decent and four as ‘other’ or ‘multi-racial’. At testing, 39 men and 39 women reported being employed full- or part-time; remaining individuals reported that they were (i) seeking employment (four men, one woman), (ii) currently a homemaker (15 women) or (iii) retired (one man, one woman). Also at testing, 32 men and 42 women were married or living with a partner; others were separated (two women), divorced (three men, four women) or single (nine men, eight women). Participants’ general intellectual ability, as estimated by combined performance on the vocabulary and matrix reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1997), was 116.5 (s.d. = 10.3; range = 83–139). Other sample characteristics are provided in Table 1.
Table 1.
Descriptive statistics for demographic, psychological and socioeconomic variables, and their univariate correlations with subjective social status, personal SES and community SES (N = 100)
| Variable | Descriptive statistics |
Univariate correlations with subjective social status and conventional SES indicators |
|||
|---|---|---|---|---|---|
| Subjective statusa | Personal SESb | Community SESc | |||
| M | s.d. | r | r | r | |
| Demographic variables | |||||
| Age (years) | 44.6 | 6.8 | 0.12 | 0.17† | 0.11 |
| Intellectual ability (WASI) | 116.5 | 10.3 | 0.04 | 0.24* | 0.10 |
| Psychological variables | |||||
| Depressive symptoms (CES-D)d | 5.2 | 5.9 | –0.17† | –0.22* | –0.14 |
| Recent life stress (PSS) | 11.6 | 5.9 | –0.33** | –0.24* | –0.24* |
| Pessimism (LOT-R) | 9.1 | 2.7 | 0.18† | 0.16 | 0.09 |
| Negative affect (PANAS)d | 13.3 | 4.3 | –0.27** | –0.14 | –0.03 |
| Cynicism (CMHS) | 5.6 | 3.2 | –0.05 | –0.18† | –0.16 |
| Hostile affect (CMHS) | 1.9 | 1.2 | –0.19† | –0.15* | –0.16 |
| Aggressive responding (CMHS) | 3.2 | 1.7 | 0.03 | –0.21* | –0.16 |
| Hostile attributions (CMHS) | 3.1 | 2.4 | –0.19† | –0.20* | –0.16 |
| Social avoidance (CMHS) | 1.6 | 1.2 | –0.15 | 0.02 | 0.05 |
| Socioeconomic variables | |||||
| Educational attainmente | 5.4 | 1.7 | 0.27** | 0.68** | 0.22* |
| Family incomef | 6.0 | 1.8 | 0.35** | 0.68** | 0.30** |
| Personal SESb | 0.01 | 0.7 | 0.46** | 0.38** | |
| Community SESc | 0.07 | 1.0 | 0.31** | ||
| Subjective social status | |||||
| Subjective status | 6.6 | 1.3 | |||
WASI = Wechsler Abbreviated Scale of Intelligence; CES-D = Center for Epidemiologic Studies Depression Scale; PSS = Perceived Stress Scale; LOT-R = Revised Life Orientation Test; PANAS = Positive and Negative Affect Schedule; CMHS = Cook–Medley Scale; SES = socioeconomic status.
aDerived from social ladder ranking.
bComputed as the standardized average of educational attainment and family income.
cComputed as the standardized average of census-tract variables.
dVariable was natural-log transformed prior to statistical analysis.
eCoded according to six categories (see Method).
fCoded according to eight categories (see Method).
†P < 0.10. *P < 0.05. **P < 0.01.
Study measures
Subjective social status
Ratings of subjective social status were derived from the MacArthur Scale of Subjective Social Status (Adler et al., 2000; available at: http://www.macses.ucsf.edu/). For this 10-point scale, participants were provided with an image of a ‘social ladder’, and they were asked to place an ‘X’ on the rung corresponding to their standing or ranking in relation to other individuals in the United States according to income, education and occupational prestige (see Figure 1A for illustration and the appendix for complete instructions).
Conventional indicators of personal and community SES
Personal SES was assessed from a composite measure of educational attainment and pretax family (household) income. Participants’ educational attainment, coded according to six categories, was distributed as follows: no high school diploma (n = 1); high school diploma or some technical training (n = 16); an associate's degree or some college without a bachelor's degree (n = 16); bachelor's degree (n = 47); master's degree (n = 15); and doctoral degree (n = 5). Participants’ family income in US dollars, coded according to eight categories, was distributed as follows: <$10 K (n = 3); $10–14.999 K (n = 3); $15–24.999 K (n = 4); $25–34.999K (n = 9); $35–49.999 K (n = 14); 50–64.999 K (n = 25); 65–79.999 K (n = 17); and ≥$80 K (n = 25). As in our prior reports (Matthews et al., 2000; Manuck et al., 2005), we computed a composite indicator of personal SES by averaging the standardized (z-score) values of the two index variables—educational attainment and family income—for each individual.
Community SES was assessed from US Census Bureau variables measured at the level of census tracts, which geographically subsume blocks and groups of blocks comprising residential areas of ∼4000 individuals. By zip code, participants resided in 82 different tracts. For all tracts, the following variables were extracted from the Year 2000 US Census Report (available at: http://factfinder.census.gov/): (i) median household income (in 1999 US dollars); (ii) percentage of adults >25 years of age holding a bachelor's degree or higher; (iii) proportion of households with incomes falling beneath the federally designated poverty line; and (iv) percentage of households with a female head of household, with no spouse present and one or more children <18 years of age (i.e. single mother households, as a proportion of all households with two or more residents). Distributions of the latter two census variables were normalized by natural log-transformation prior to further data reduction. To compute a composite indicator of community SES following our prior reports (Manuck et al., 2005; Petersen et al., 2006), census variables were submitted to a principal components analysis, from which we retained any factor with an eigenvalue >1. As expected, census variables loaded onto a single factor (median factor loading = 0.86; range = –0.76 to 0.92), which accounted for 73.4% of the total variance in the four variables. A composite indicator of community SES was then calculated for each participant by averaging the standardized (z-score) values for the four census variables (after adjusting variables for loading direction), whereby higher community SES values were taken to represent relative socioeconomic advantage at the tract level. One participant did not provide a zip code, and was assigned the mean community SES value (z score = 0).
Depressive symptoms, recent life stress and psychological characteristics related to negative emotionality and hostility
In addition to depressive symptoms, our analyses described later accounted for recent levels of life stress and psychological characteristics that could plausibly covary with ratings of subjective social status or that have been treated as covariates in prior studies of subjective social status (e.g. Adler et al., 2000). Depressive symptoms were assessed using the 20-item version of the Center for Epidemiologic Studies Depression Scale (CES-D; Weissman et al., 1977). Recent levels of life stress, defined as the extent to which an individual appraises life situations over the last month as unpredictable, uncontrollable and overloading, were assessed using the Perceived Stress Scale (PSS; Cohen et al., 1983). Dispositional pessimism, which reflects negative as opposed to positive (optimistic) generalized outcome expectancies, was assessed from the pessimism scale of the Revised Life Orientation Test (LOT-R; Scheier et al., 1994). Dispositional negative emotionality was assessed using the trait negative affect scale of the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988). Forms of dispositional hostility were assessed with the Cook–Medley Hostility Scale (CMHS), a 50-item inventory that provides index scores for five principal dimensions of hostility: cynicism, hostile affect, hostile attributions to the behavior of others, aggressive social response tendencies and social avoidance (Cook and Medley, 1954; Barefoot et al., 1989). Because of their positive distributional skew, scores on the CES-D and PANAS negative affect subscale were natural-log transformed prior to analysis. Summary statistics for all scales are provided in Table 1.
Procedures for the assessment of regional gray matter volume
Image acquisition
Structural neuroimaging data were collected from the participants described earlier as part of a protocol that was nested within the AHAB project. This neuroimaging protocol was designed specifically to identify neural correlates of individual differences in risk factors for neuropsychiatric and cardiovascular disease. As part of the protocol, high-resolution structural brain images were acquired on a 3-Tesla Siemens Allegra scanner, equipped with a standard birdcage radiofrequency head coil. Total and regional gray matter volumes were assessed from T1-weighted 3D fast-gradient magnetization prepared rapid gradient-echo (MPRAGE) structural images (TR/TE = 1540/3.0 ms; flip angle = 8°; NEX = 1; bandwidth = 170 Hz/pixel; echo spacing = 7.7 ms), which encompassed the whole brain and consisted of 192 sagittal slices (1 mm thick; 0 mm spacing between slices; matrix size = 256 × 256 pixels; FOV = 256 mm). Prior to the assessment of gray matter volume, individual images were inspected for image distortions and artifacts. After clearing all images for such problems, raw images were realigned to the axial plane of the anterior and posterior commissures.
Image processing
Optimized voxel-based morphometry was used to quantify regional (voxel-wise) and total gray matter volume from MPRAGE images (Ashburner and Friston, 2000; Good et al., 2001). All voxel-based morphometry procedures were executed using statistical parametric mapping software (SPM2; Wellcome Department of Imaging Neuroscience; available at: http://www.fil.ion.ucl.ac.uk/spm/) and MATLAB (The MathWorks, Inc., Natick, MA) scripts co-authored by John Ashburner and Christian Gaser (available at: http://dbm.neuro.uni-jena.de/vbm.html).
To determine regional and total gray matter volumes, MPRAGE images from the present sample were first used to create a study-specific (customized) T1-weighted template along with tissue-specific (segmented) templates for gray matter, white matter and cerebrospinal fluid. All templates were normalized to the coordinate space of the International Consortium for Brain Mapping (CBM) 152 template (Montreal Neurological Institute; MNI), and they were smoothed with an 8 mm FWHM Gaussian spatial filter prior to subsequent processing. After template creation, each participant's T1-weighted MPRAGE image was segmented into gray matter, white matter and cerebrospinal fluid images with a mixture model cluster analysis that employed the study-specific tissue templates as Bayesian prior probability maps of tissue intensity. During segmentation, image intensities were bias-corrected for the influence of inhomogeneities in the magnetic field of the MR scanner. After segmentation, individual gray matter images were spatially normalized to the study-specific gray matter template using affine transformations and smooth basis functions that minimized global (and non-linear) squared differences between each gray matter image and the template. Voxel values in the resulting normalized gray matter images were then multiplied (modulated) by the Jacobian matrix parameters that were derived from spatial normalization. Thus, the volume change introduced by deformation (expansion and contraction) during spatial normalization was incorporated into each voxel value, providing for each participant a modulated image of voxel-wise gray matter volume in milliliters. Prior to analysis, modulated (volumetric) gray matter images were smoothed with a 10-mm FWHM Gaussian spatial filter to (i) accommodate individual differences in sulcal and gyral anatomy, and to (ii) meet the distributional assumptions of the general linear models that were used to examine voxel-wise gray matter volume in relation to measures of subjective social status and conventionally defined levels of personal and community SES.
Data reduction and analysis by research question
Does subjective social status covary with regional gray matter volume in the ACC, hippocampus or amygdala?
We addressed this question by conducting a multiple regression analysis in SPM2, employing the framework of the general linear model (Friston et al., 1995). In the model, we tested whether subjective social status scores (derived from social ladder rankings), personal SES (derived from the composite of educational attainment and income levels) and community SES (derived from the composite of census-level variables) predicted gray matter volume in our regions-of-interest. Each of these three primary explanatory factors was treated as a continuous variable, and age, sex and total gray matter volume were entered simultaneously as confounding covariates. After the model's regression parameters were estimated, we tested for associations between subjective social status, personal SES and community SES and voxel-wise gray matter volume within each region-of-interest (bilateral anterior cingulate cortex, amygdala and hippocampus). For this analytic region-of-interest approach, we used anatomical masks defined by the Automated Anatomical Labeling system available in the Wake-Forest University Pick-Atlas (Maldjian et al., 2003). To correct for multiple testing across all regions-of-interest, we employed a voxel-wise statistical significance threshold of P < 0.05 using the family-wise error rate (FWE) correction procedure.
Does subjective social status uniquely covary with regional gray matter volume in regions of interest, above-and-beyond demographic and psychological factors and of conventional indicators of personal and community SES?
We addressed this question by conducting a hierarchical multiple regression analysis. For the regression, we first extracted the unadjusted volume value from the voxel coordinates localizing the peak association between subjective social status and gray matter volume within a region of interest at PFWE-corrected < 0.05. This gray matter volume value was then imported into Statistical Package for the Social Sciences 11.0 (SPSS, Chicago, IL), and it was used as a dependent variable in a four-step hierarchical regression model. In step 1, we entered age, sex and total gray matter volume as standard covariates. In step 2, we entered a set of psychological factors that included depressive symptoms (assessed by the CES-D), recent levels of life stress (assessed by the PSS) and—to constrain the total number of relevant variables in the model—any additional psychological factor that correlated with subjective social status ladder rankings at P ≤ 0.10 in ancillary univariate correlation analyses summarized in Table 1 (note that by this criterion, pessimism, negative emotionality, hostile affect and a tendency to ascribe hostile attributions to the behavior of others were included in this step of the model). In step 3, we entered the conventional indicators of personal and community SES. In step 4, we entered subjective social status scores derived from social ladder rankings. This hierarchical regression approach allowed us to examine the unique percentage of variance in gray matter volume explained by the set of variables entered in each step, after accounting for the influence of variables already entered in the model.
RESULTS
Relationships between subjective social status and regional gray matter volume
ACC
Lower subjective social status, as indexed by a lower social ladder ranking, was associated with reduced gray matter volume in the perigenual area of the anterior cingulate cortex (pACC), spatially encompassing Brodmann areas 24 and 32 (Figure 1). This finding was revealed by a multiple regression analysis of voxel-wise gray matter volume in which conventional indicators of personal and community SES were entered simultaneously as additional explanatory factors (age, sex and total gray matter volume were also entered as confounding covariates). The MNI coordinates for the voxel of peak association between lower subjective social status and reduced pACC gray matter volume were x = –8, y = 45 and z = 1, t(1, 93) = 3.8, PFWE-corrected = 0.01, Puncorrected < 0.001, spatial extent = 3711 voxels. As shown in Figure 1C, subjective social status scores accounted for ∼18% of the variance in pACC volume centered at these coordinates, R = 0.42, P < 0.001.
In contrast to the above findings, subjective social status did not show a significant ‘negative’ association with gray matter volume in any area of the ACC at a corrected or at a more lenient uncorrected voxel-wise statistical significance threshold (P < 0.001). Furthermore, neither of the conventional indicators of personal or community SES showed a positive or a negative association with gray matter volume in the ACC at corrected or at uncorrected (P < 0.001) statistical significance thresholds.
Hippocampus
Subjective social status ladder rankings and conventional indicators of personal or community SES did not show significant positive or negative associations with regional gray matter volume in the hippocampus at corrected or at uncorrected (P < 0.001) voxel-wise statistical thresholds.
Amygdala
On initial analysis, subjective social status showed a trend toward a negative association with gray matter volume in the right amygdala (MNI coordinates for peak association: x = 19, y = 0, z = –19, t(1, 93) = 2.3, PFWE-corrected = 0.10, Puncorrected = 0.01, spatial extent = 787 voxels). However, a closer inspection of this association, reflecting increased amygdala gray matter volume as a function of lower subjective social status, revealed that an outlier (defined as expressing a right amygdala volume at x = 19, y = 0, z = –19 more than 3 s.d. above the sample mean) likely drove the association. In further analyses that included and excluded the data for this outlying case, neither subjective social status ladder rankings nor conventional indicators of personal or community SES showed significant positive or negative associations with amygdala gray matter volume at corrected or at uncorrected (P < 0.001) voxel-wise statistical thresholds.
Unique relationships between subjective social status and gray matter volume in pACC
As expected, subjective social status scores correlated significantly and at trend levels (P's ≤ 0.10) with depressive symptoms, recent levels of life stress, dispositional negative emotionality, pessimism, hostility and with conventional indicators of personal and community SES (Table 1). To determine whether subjective social status scores covaried with gray matter volume in the pACC (identified by the region-of-interest analysis previously described), above-and-beyond demographic, psychological and conventional SES variables, we conducted a four-step hierarchical multiple regression analysis. First, as summarized in Table 2, social ladder rankings continued to account for an appreciable percentage of the unique variance in pACC gray matter volume after hierarchical control for all sets of variables entered in the preceding three steps of the model, step 4 ΔR2 = 0.13, F(1,87) = 14.4, P < 0.001. Second, a noteworthy finding was that none of the first three sets of variables in the model accounted for a significant percentage of the variance in pACC gray matter volume prior to entering subjective social status on the final step (ΔR2 for all three steps ≤ 0.06, P's > 0.26; Table 2)—suggesting that it is unlikely that these sets of variables accounted for (or mediated) the association between subjective social status and pACC volume. Third, in an exploratory and ancillary five-step hierarchical regression model predicting pACC volume using the same initial four sets of variables shown in Table 2, we tested for a possible interaction between subjective social status and sex in the final step; however, the sex-by-subjective social status interaction term did not approach statistical significance, step 5 F(1, 86) = 1.3, ΔR2 = 0.01, P's = 0.26). In line with this finding, after covariate control for total gray matter volume, we found that men and women did not differ in pACC volume at x = –8, y = 45, and z = 1, F(1, 97) = 0.66, P = 0.42 by ANCOVA.
Table 2.
Summary of hierarchical regression analysis for variables predicting perigenual anterior cingulate gray matter volume (N = 100)
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard covariates |
Life stress, depressive symptoms, and psychological variables |
Conventional indicators of personal and community SES |
Subjective social status |
|||||||||
| Variable | β | pr | sr | β | pr | sr | β | pr | sr | β | pr | sr |
| Age | 0.04 | 0.04 | 0.04 | –0.02 | –0.02 | –0.02 | –0.04 | –0.04 | –0.04 | –0.02 | –0.03 | –0.02 |
| Sex (1 = male, 2 = female) | –0.10 | –0.07 | –0.07 | –0.10 | –0.08 | –0.07 | –0.07 | –0.06 | –0.05 | 0.01 | 0.01 | 0.01 |
| Total gray matter volume | –0.02 | –0.01 | –0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.03 | 0.03 | 0.02 |
| Depressive symptoms | –0.12 | –0.09 | –0.09 | –0.10 | –0.08 | –0.08 | –0.16 | –0.13 | –0.11 | |||
| Recent life stress | 0.14 | 0.10 | 0.09 | 0.19 | 0.13 | 0.12 | 0.28 | 0.20 | 0.18 | |||
| Negative affect | –0.12 | –0.08 | –0.08 | –0.16 | –0.11 | –0.10 | –0.11 | –0.08 | –0.07 | |||
| Pessimism | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Hostile affect | –0.03 | –0.03 | –0.03 | –0.02 | –0.02 | –0.02 | –0.01 | –0.01 | –0.01 | |||
| Hostile attributions | –0.13 | –0.10 | –0.10 | –0.10 | –0.08 | –0.08 | –0.09 | –0.08 | –0.07 | |||
| Personal SES | 0.14 | 0.13 | 0.12 | –0.02 | –0.02 | –0.02 | ||||||
| Community SES | 0.08 | 0.07 | 0.07 | 0.03 | 0.03 | 0.03 | ||||||
| Subjective social status | 0.43* | 0.38 | 0.36 | |||||||||
| Model Adjusted R2 | –0.02 | –0.03 | –0.02 | 0.11 | ||||||||
| Step ΔR2 | 0.01 | 0.06 | 0.03 | 0.13 | ||||||||
| F for Step ΔR2 | 0.34 | 0.88 | 1.36 | 14.38* | ||||||||
*P < 0.001.
Supplementary (exploratory) whole-brain analyses of voxel-wise gray matter volume
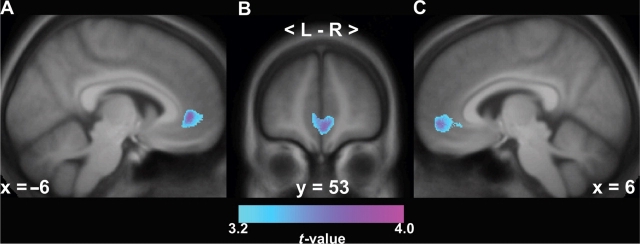
We executed a whole-brain multiple regression analysis to explore whether gray matter volume in areas outside our targeted regions-of-interest covaried with subjective social status, personal SES or community SES, after employing appropriate statistical control for inflated levels of Type I error. Specifically, we employed an uncorrected voxel-wise threshold of P < 0.001 (t ≥ 3.18) in conjunction with an extent threshold of 25 voxels for display and exploration; however, any detected associations were considered significant at P < 0.05 after cluster-level correction for multiple statistical testing across the whole-brain volume. Again, age, sex and total gray matter volume were treated as covariates in the regression. We found that reports of lower subjective social status, indexed by lower social ladder rankings, were associated with reduced gray matter volume in only one cluster of voxels, which encompassed three peak coordinates that bilaterally bounded the coordinates for the pACC area highlighted in Figure 1 (MNI coordinates for peak 1: x = 1, y = 57, z = –4; peak 2: –8, 45, 1; peak 3: 4, 50, –7, t's (1, 93)>3.53, Pwhole-brain, cluster-level corrected = 0.026, spatial extent = 3538 voxels (Figure 2). In parallel to the above region-of-interest analyses, subjective social status did not show a significant negative association with gray matter volume in any brain region. Finally, neither of the conventional indicators of personal or community SES showed a positive or negative association with gray matter volume in any brain area (all corrected P's > 0.20).
Fig. 2.
A whole-brain exploratory analysis demonstrated that lower subjective social status was associated with reduced gray matter volume only in the bilateral perigenual anterior cingulate cortex (pACC), after cluster-level correction for multiple statistical testing across the entire brain volume (P < 0.05). Illustrating this pACC area is a statistical parametric map of color-scaled t-values overlaid onto left (A) and right (C) sagittal sections and a coronal section (B) of an anatomical template derived from the present sample. Montreal Neurological Institute coordinates in A–C refer to the distance in mm from the midline for sagittal sections (+ = right; – = left) and from the anterior commissure for the coronal section.
Supplementary analyses of total gray matter volume
We found no evidence to suggest that subjective social status, personal SES or community SES were significantly associated with global cerebral integrity, as indirectly indicated by ‘total’ gray matter volume in the full sample (mean total gray matter volume = 678.8, s.d. = 63.3 ml), or in men (mean = 726.4, s.d. = 47.3 ml) and women (mean = 641.3, s.d. = 47.3 ml) considered separately, all r's ≤ 0.12, P's ≥ 0.25.
DISCUSSION
Individuals who reported holding a low social standing in the United States—as reflected by low subjective social status ladder rankings—showed a reduced gray matter volume in the pACC—a paralimbic brain region implicated in adaptive emotional, behavioral and physiological responding to environmental and psychosocial stressors (Vogt et al., 1992; Diorio et al., 1993; Devinsky et al., 1995; Vogt et al., 1995; Bush et al., 2000; Vogt, 2005). Furthermore, an appreciable relationship between low subjective social status and reduced pACC gray matter volume persisted after accounting for the potential influence of several demographic and psychological factors and conventionally defined levels of personal and community SES. However, in apparent contrast to theoretical expectations derived from animal models of psychosocial stress and structural remodeling in paralimbic brain systems, individual differences in subjective social status were not systematically associated with gray matter volume in the hippocampus or the amygdala. While the present findings do not establish a causal direction of association, they do implicate reduced pACC gray matter volume as a structural neural correlate of low subjective social status, a presumptive stress-related dimension of socioeconomic position that has been linked to dysregulated neuroendocrine activity and to adverse mental and physical health outcomes.
When subjectively judging one's social status, an individual may consider several facets of social advantage and disadvantage that accompany socioeconomic position, including prior and anticipated life opportunities, access to material resources and educational, monetary and occupational trajectories that unfold over the lifespan (Jackman and Jackman, 1973; Singh-Manoux et al., 2003, 2005). According to a life-history perspective, ratings of subjective social status, such as those reflected by scores on the social ladder scale used here, are thought to result from a so-called ‘cognitive averaging’ process, wherein individuals draw on accumulated and anticipated life experiences related to socioeconomic position in order to form a judgment regarding their relative social standing (Singh-Manoux et al., 2003). As such, subjective social status ratings are not fully redundant with conventional indicators of SES (e.g. absolute levels of income and education), which themselves may not fully capture an individual's perceived social standing. In accordance with this perspective and with prior findings (Adler et al., 2000; Goodman et al., 2003; Singh-Manoux et al., 2003; Kopp et al., 2004; Operario et al., 2004; Hu et al., 2005; Singh-Manoux et al., 2005; Wright and Steptoe, 2005), social ladder rankings in the present sample showed moderate correlations with conventional indicators of both personal and community SES (Table 1). Hence, individuals who earned a higher income, attained a higher level of education, and resided in a more socially advantaged community tended to rank themselves as higher in their social standing—as reflected by social ladder rankings that were anchored to the criteria of income, education and occupational prestige in relation to other individuals living in the United States. Taken together, these associations agree with the view that individual differences in subjective social status are closely related to, but not redundant with, conventionally defined levels of socioeconomic position. In this regard, is interesting to note that individual differences in subjective social status, but not personal or community SES, covaried with pACC gray matter volume in the present sample. If replicated, these particular findings could build on prior research to suggest a dissociation between the neural correlates of subjective and conventional dimensions of socioeconomic position at the level of brain morphology.
Also agreeing with the view that subjective social status partly reflects a dimension of socioeconomic position associated with psychosocial stress and negative emotionality, lower social ladder rankings showed modest to moderate correlations with higher levels of recent life stress, subclinical depressive symptoms, negative affect, pessimism and hostility (Table 1). These particular findings accord with those from prior reports linking low subjective social status with indicators of psychosocial stress and forms of negative emotionality in the context of dysregulated neuroendocrine activity (Adler et al., 2000; Wright and Steptoe, 2005) and prospective risk for adverse self-reported mental and physical health outcomes (Singh-Manoux et al., 2005). We note, however, that the present cross-sectional correlations cannot disambiguate the causal directions of association between social ladder rankings, reports of life stress and other psychological factors related to negative emotionality. Indeed, it is both plausible and likely that the perception of holding a low social standing is bi-directionally related to such factors (Adler et al., 2000; Marmot, 2004). Given our cross-sectional study design, we thus modeled reports of life stress, indicators of emotionality, demographic characteristics and conventionally defined levels of SES as potential confounders and possible sources of reporting bias. Notably, after hierarchical statistical control for these factors, social ladder rankings continued to account for an appreciable percentage of the unique inter-individual variability in pACC gray matter volume (Table 2). As such, it is unlikely that confounding demographic factors or that state- or trait-related individual differences in psychological characteristics potentially contributing to reporting biases accounted for the association between low subjective social status and reduced pACC gray matter volume. As elaborated below, the present findings could thus implicate the pACC as a candidate neural substrate that could link subjective social status to previously documented forms of dysregulated neuroendocrine activity and health-related outcomes.
The ACC occupies much of the medial wall of the prefrontal cortex, and it is an evolutionally old cortical system common to mammals (Allman et al., 2001). Within the anterior cingulate, there are regional differences in cellular architecture and efferent and afferent projections that largely correspond to functionally distinct subregions, which have been nominally described in terms of a supragenual cognitive-motor division, a subgenual visceral-motor division, and an intermediate perigenual affective division (Vogt et al., 1992; Devinsky et al., 1995; Vogt et al., 1995; Bush et al., 2000; Vogt, 2005). The ACC subregion identified in association with individual differences in subjective social status in the present study largely overlaps with the perigenual affective division, but extended somewhat into the more ventral subgenual visceral-motor division (Figures 1 and 2). This pACC region has been broadly implicated by human functional neuroimaging studies and patient lesion studies as playing a critical role in emotional experience and in regulating neuroendocrine and autonomic reactivity to psychological stress (Bush et al., 2000; Critchley, 2003, 2005; Vogt, 2005). Furthermore, reciprocal projections between the pACC and other networked paralimbic brain circuits, including the adjacent orbitofrontal cortex, the amygdala and the hippocampus, are held to support such functions as meeting the demands of real or perceived environmental challenges, mobilizing the neuroendocrine and autonomic stress-response axes (via projections to cell groups in the hypothalamus, midbrain and brainstem), and adaptively reorienting behavior to shifting social and environmental contingencies (Vogt et al., 1992; Devinsky et al., 1995; Vogt et al., 1995; Bush et al., 2000; Critchley, 2005; Vogt, 2005). In this framework, changes in the morphology or structural integrity of the pACC in association with individual differences in perceived social standing may thus impact a range of functions supported by the pACC and networked subregions of the cingulate and other paralimbic areas.
A unique relationship between low subjective social status and reduced pACC gray matter volume could be speculatively interpreted from at least two perspectives. First, according to a chronic social stress perspective posited by McEwen (McEwen and Seeman, 1999; McEwen, 2000a) and others (Blanchard et al., 2001; Sapolsky, 2005a, 2005b), the emotional stress accompanying an enduring experience of low social standing could remodel the pACC by cellular mechanisms that have been detailed in prior rodent studies of prolonged immobilization and dendritic atrophy of the prelimbic and infralimbic areas of the ACC, anatomical homologues of the human perigenual and subgenual ACC, respectively (Ongur et al., 2003). Such a chronic stress perspective has been described previously with respect to the volumetric changes of the ACC and other paralimbic brain areas, including the hippocampus and amygdala, that have been documented in stress-related psychiatric disorders, such as major depressive disorder and post-traumatic stress disorder (Sheline, 2000; McEwen, 2000a, 2003, 2005; Fuchs and Flugge, 2003; Yamasue et al., 2003; Campbell, Marriott, Nahmias and MacQueen, 2004; Fuchs et al., 2004; Videbech and Ravnkilde, 2004; Sapolsky, 2005b; Karl et al., 2006; Woodward et al., 2006). In extension, though, a chronic stress perspective would also have predicted an association between low subjective social status volumetric changes in the hippocampus and amygdala, which were not observed in the present study. We note that these null findings could be accounted for, in part, by several factors. For example, it is possible that low subjective social status relates to changes in the cellular architecture, dynamic proliferation of new neurons, or to changes in the volume of subnuclei within the hippocampus or amygdala that are not detectable by the voxel-based morphometry method used herein. It is also possible that longitudinal, as opposed to cross-sectional, assessments of subjective social status may better capture the cumulative or chronic-stress related aspects of perceived social standing over the lifespan, and may thus better predict volumetric changes in the hippocampus and amygdala. In point, we have recently demonstrated that prospective reports of chronic life stress, which were measured over an ∼20-year period of life, predicted decreased hippocampal volume in a healthy sample of older women (Gianaros et al., 2007). Notwithstanding these possibilities and study design limitations, a relationship between low subjective social status and reduced pACC volume could also be interpreted from a vulnerability perspective.
More precisely, it is possible that a reduced gray matter volume or an impaired structural integrity of the pACC could predispose individuals toward viewing themselves as holding a lower social standing than others. Such predisposing individual differences in pACC volume could result from several potentially interacting developmental and genetic factors. For example, there is evidence that exposure to early childhood stressors—particularly witnessing domestic violence or experiencing the death of a parent or primary family member—predicts a decreased volume of the ACC, but not hippocampus or amygdala, in adulthood (Cohen et al., 2006)1. In addition, it has recently been found that carriers of the short allelic variant of a functional 5' promoter polymorphism of the serotonin (5-HT) transporter gene show an ∼25% reduction in pACC gray matter volume in comparison with carriers of the long allelic variant (Pezawas et al., 2005). These particular findings underscore the important role of genetically mediated 5-HT influence on ACC morphology, which has been established in rodent models of the regulation of synaptic plasticity and synaptogenesis by 5-HT during early cortical development (Gaspar et al., 2003). In light of these two perspectives, which respectively emphasize the roles of chronic social stress and vulnerability, it will be important for future longitudinal studies to account for the possible influence of early, perhaps stress-related, developmental and genetic factors on normative variation in pACC gray matter volume in association with subjective social status over the lifespan—as such studies may help to determine whether low pACC volume results from or contributes to individual differences in perceived social standing.
It will also be important for future research to determine whether there is a functional relationship between individual differences in subjective social status, pACC gray matter volume and pACC-dependent functions related to potential biomediators of disease risk. Here, it is noteworthy that rodent models have established an important role of the ACC in regulating HPA activity under both basal and stressful conditions (e.g. Diorio et al., 1993). Furthermore, recent rodent models demonstrate that the ACC is a target site for the negative-feedback effects of glucocorticoids on stress-induced HPA activity, which likely contribute to volumetric changes in the prelimbic and infralimbic ACC subregions (Cerqueira et al., 2005a, 2005b). Finally, there is recent in vivo imaging evidence in humans that reduced ACC volume is associated with HPA axis dysregulation, as indicated by a non-suppressed cortisol response to a dexamethasone challenge (MacLullich et al., 2006). Thus, it is plausible that volumetric or other morphological changes in the pACC could account in part for the dysregulated forms of neuroendocrine—particularly cortisol—reactivity that have been found among individuals reporting a low subjective social status (Adler et al., 2000; Wright and Steptoe, 2005).
While the present novel findings implicate reduced pACC gray matter volume as a structural neural correlate of low subjective social status, several study limitations should be noted. First, we tested a modally Caucasian, well-educated, higher-income and healthy sample of middle-aged men and women without a psychiatric, neurological, cerebrovascular or cardiovascular disease. Therefore, the racial composition, socioeconomic distribution and general health of our sample constrain extrapolations to the general population. Furthermore, it is possible that by studying otherwise healthy individuals at the upper end of the socioeconomic distribution, we were unable to detect relationships between individual differences in subjective social status and volumetric changes in putatively stress-sensitive paralimbic brain areas, such as the hippocampus and amygdala. Finally, as noted earlier, because the present study was cross-sectional, we cannot discount the notable possibility that reduced pACC gray matter volume predisposes individuals toward perceiving themselves as holding a low social standing.
To build on the present findings, an important next step will to determine the social, environmental and possibly genetic factors that characterize individuals who perceive themselves as holding a low social standing and who exhibit structural changes in the pACC that may reciprocally relate to maladaptive forms of behavioral and physiological reactivity to psychosocial stress. More broadly, we hold that in the context of vulnerability and resilience to psychiatric and other medical disorders, it is unlikely that the pACC functions independently of other networked corticolimbic areas, such as the amygdala and hippocampus, whose net activity mediates complex neurobehavioral processes. Notably, such processes include stress-related reactivity and coping behaviors, which are likely to be impacted by individual differences in perceived social standing. In this regard, it is noteworthy that several studies have begun to delineate how reciprocal circuitry between the pACC and other corticolimbic areas facilitates the integration and regulation of emotional information in the service of adaptive behavioral responding to environmental challenge (Phillips et al., 2003; Pezawas et al., 2005; Etkin et al., 2006; Hariri and Holmes, 2006). Importantly, increasing evidence further indicates that a compromised structural or functional coupling between the pACC and networked corticolimbic areas—particularly in the context of environmental adversity and genetic risk—may increase vulnerability to psychiatric and medical syndromes characterized by dysregulated emotion-related behaviors and physiology (Hariri and Holmes, 2006). Based on the present findings, it is thus reasonable to speculate that the dynamic interplay between the pACC and anatomically networked corticolimbic areas, which are critical for stress-related coping behaviors and emotion regulatory processes, may be compromised among individuals of low perceived social standing.
Acknowledgments
We thank Dr Rebecca C. Thurston, Lei K. Sheu and two anonymous reviewers for their constructive comments. We also thank Scott Kurdilla for his assistance in data acquisition. This research was supported by National Institutes of Health (NIH) Grant K01 MH070616 to P.J.G.; by NIH Grants P01 HL040962 and RO1 HL065137 to S.B.M.; by NIH Grant K01 MH072837 to A.R.H.; and by the John D. and Catherine T. MacArthur Foundation Network on Socioeconomic Status and Health.
APPENDIX: INSTRUCTIONS FOR SOCIAL LADDER SCALE OF SUBJECTIVE SOCIAL STATUS
‘Think of this ladder as representing where people stand in the United States. At the top of the ladder are the people who have the most money, most education, and most respected jobs. At the bottom are the people who have the least money, least education, and least respected jobs or no job. The higher up you are on this ladder, the closer you are to the people at the very top, and the lower you are, the closer you are to the people at the very bottom. Where would you place yourself on this ladder? Please, place an “X” on the rung where you think you stand at this time in your life, relative to other people in the United States.’
Footnotes
Conflict of Interest
None declared.
1 In the context of the present findings, however, we note that the putative and parallel effects of early childhood trauma and other potentially adverse early developmental experiences on perceived social standing and pACC gray matter would, at least conceptually, be traumatic events and experiences that are not strongly linked to individual differences in subclinical depression, negative affect, dispositional pessimism and other related factors, because such factors were accounted for in our hierarchical regression models (Table 2).
REFERENCES
- Adler NE, Boyce T, Chesney MA, et al. Socioeconomic status and health. The challenge of the gradient. American Psychologist. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health. No easy solution. Journal of the American Medical Association. 1993;269:3140–5. [PubMed] [Google Scholar]
- Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy white women. Health Psychology. 2000;19:586–92. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Adler NE, Marmot M, McEwen B, Stewart J, editors. Socioeconomic status and health in industrialized nations: Social, psychological, and biological pathways. Vol. 896. New York: Academy of Sciences; 1999. [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Annals of the New York Academy of Sciences. 2001;935:107–17. [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. NeuroImage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB., Jr The Cook-Medley hostility scale: Item content and ability to predict survival. Psychosomatic Medicine. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: Effects on behavior and brain neurochemical systems. Physiology and Behavior. 2001;73:261–71. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: One size does not fit all. Journal of the American Medical Association. 2005;294:2879–88. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: A meta-analysis. American Journal of Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Catania C, Sotiropoulos I, et al. Corticosteroid status influences the volume of the rat cingulate cortex - a magnetic resonance imaging study. Journal of Psychiatric Research. 2005a;39:451–460. doi: 10.1016/j.jpsychires.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. Journal of Neuroscience. 2005b;25:7792–8000. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry. 2006;59:975–82. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosomatic Medicine. 2006;68:414–20. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–96. [PubMed] [Google Scholar]
- Cohen S, Line S, Manuck SB, Rabin BS, Heise ER, Kaplan JR. Chronic social stress, social status, and susceptibility to upper respiratory infections in nonhuman primates. Psychosomatic Medicine. 1997;59:213–21. doi: 10.1097/00006842-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of Neurobiology. 2004;60:236–48. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. Journal of Applied Psychology. 1954;38:414–18. [Google Scholar]
- Critchley HD. Emotion and its disorders. British Medical Bulletin. 2003;65:35–47. doi: 10.1093/bmb/65.1.35. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of Neuroscience. 1993;13:3839–47. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BP. The role of adversity and stress in psychopathology: Some evidence and its implications for theory and research. Journal of Health and Social Behavior. 2000;41:1–19. [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: New York State Psychiatric Institute, Biometrics Research Department; 1996. research version, non-patient. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Fuchs E, Czeh B, Kole MH, Michaelis T, Lucassen PJ. Alterations of neuroplasticity in depression: The hippocampus and beyond. European Journal of Neuropsychopharmacology. 2004;14:481–90. doi: 10.1016/j.euroneuro.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flugge G. Chronic social stress: Effects on limbic brain structures. Physiology and Behavior. 2003;79:417–27. doi: 10.1016/s0031-9384(03)00161-6. [DOI] [PubMed] [Google Scholar]
- Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: Do negative emotions play a role? Psychological Bulletin. 2003;129:10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: News from mouse molecular genetics. Nature Review Neuroscience. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. NeuroImage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Goodman E, Adler NE, Daniels SR, Morrison JA, Slap GB, Dolan LM. Impact of objective and subjective social status on obesity in a biracial cohort of adolescents. Obesity Research. 2003;11:1018–26. doi: 10.1038/oby.2003.140. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: The role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10:182–91. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hu P, Adler NE, Goldman N, Weinstein M, Seeman TE. Relationship between subjective social status and measures of health in older Taiwanese persons. Journal of the American Geriatric Society. 2005;53:483–8. doi: 10.1111/j.1532-5415.2005.53169.x. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Status, stress, and atherosclerosis: The role of environment and individual behavior. Annals of the New York Academy of Sciences. 1999;896:145–61. doi: 10.1111/j.1749-6632.1999.tb08112.x. [DOI] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neuroscience and Biobehavioral Reviews. 2006;30:1004–31. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Cleary PD. Social class and psychological distress. American Sociological Review. 1980;45:463–78. [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Price RH, Wortman CB. Social factors in psychopathology: Stress, social support, and coping processes. Annual Review of Psychology. 1985;36:531–72. doi: 10.1146/annurev.ps.36.020185.002531. [DOI] [PubMed] [Google Scholar]
- Kopp M, Skrabski A, Rethelyi J, Kawachi I, Adler NE. Self-rated health, subjective social status, and middle-aged mortality in a changing society. Behavioral Medicine. 2004;30:65–70. doi: 10.3200/BMED.30.2.65-72. [DOI] [PubMed] [Google Scholar]
- Lorant V, Deliege D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: A meta-analysis. American Journal of Epidemiology. 2003;157:98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- MacLullich AM, Ferguson KJ, Wardlaw JM, Starr JM, Deary IJ, Seckl JR. Smaller left anterior cingulate cortex volumes are associated with impaired hypothalamic-pituitary-adrenal axis regulation in healthy elderly men. Journal of Clinical Endocrinology and Metabolism. 2006;91:1591–4. doi: 10.1210/jc.2005-2610. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. Journal of Neuroscience. 1996;16:3534–40. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Bleil ME, Petersen KL, et al. The socio-economic status of communities predicts variation in brain serotonergic responsivity. Psychological Medicine. 2005;35:519–28. doi: 10.1017/s0033291704003757. [DOI] [PubMed] [Google Scholar]
- Marmot M. The status syndrome: How social standing affects our health and longevity. New York: Henry Holt and Company; 2004. [Google Scholar]
- Matthews KA, Flory JD, Muldoon MF, Manuck SB. Does socioeconomic status relate to central serotonergic responsivity in healthy adults? Psychosomatic Medicine. 2000;62:231–7. doi: 10.1097/00006842-200003000-00015. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology. 2000a;22:108–24. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Effects of adverse experiences for brain structure and function. Biological Psychiatry. 2000b;48:721–31. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Annals of the New York Academy of Sciences. 2001;933:265–77. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biological Psychiatry. 2003;54:200–7. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism. 2005;54:20–3. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Mirowsky J, Ross CE. Social patterns of distress. Annual Review of Sociology. 1986;12:23–45. [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology. 2003;460:425–49. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Operario D, Adler NE, Williams DR. Subjective social status: Reliability and predictive utility for global health. Psychology and Health. 2004;19:237–46. [Google Scholar]
- Ostrove JM, Adler NE, Kuppermann M, Washington AE. Objective and subjective assessments of socioeconomic status and their relationship to self-rated health in an ethnically diverse sample of pregnant women. Health Psychology. 2000;19:613–8. doi: 10.1037//0278-6133.19.6.613. [DOI] [PubMed] [Google Scholar]
- Petersen KL, Bleil ME, McCaffery J, et al. Community socioeconomic status is associated with carotid artery atherosclerosis in untreated, hypertensive men. American Journal of Hypertension. 2006;19:560–6. doi: 10.1016/j.amjhyper.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Research Reviews. 2005;4:271–87. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral Cortex. 2006;16:313–20. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochemical Research. 2003;28:1735–42. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Sick of poverty. Scientific American. 2005a;293:92–9. doi: 10.1038/scientificamerican1205-92. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005b;308:648–52. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-estee'm): A reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: The role of stress and medical comorbidity. Biological Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Adler NE, Marmot MG. Subjective social status: Its determinants and its association with measures of ill-health in the Whitehall II study. Social Science and Medicine. 2003;56:1321–33. doi: 10.1016/s0277-9536(02)00131-4. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosomatic Medicine. 2005;67:855–61. doi: 10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kunz-Ebrecht S, Owen N, et al. Socioeconomic status and stress-related biological responses over the working day. Psychosomatic Medicine. 2003;65:461–70. doi: 10.1097/01.psy.0000035717.78650.a1. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Marmot M. The role of psychobiological pathways in socio-economic inequalities in cardiovascular disease risk. European Heart Journal. 2002;23:13–25. doi: 10.1053/euhj.2001.2611. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: A meta-analysis of MRI studies. American Journal of Psychiatry. 2004;161:1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Review Neuroscience. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: The anterior executive and posterior evaluative regions. Cerebral Cortex. 1992;2:435–43. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. Journal of Comparative Neurology. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Research. 2003;965:290–4. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–73. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: A validation study. American Journal of Epidemiology. 1977;106:203–14. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- Wilkinson RG. Health, hierarchy, and social anxiety. In: Adler NE, Marmot M, McEwen B, Stewart J, editors. Annals of the New York Academy of Sciences: Socioeconomic status and health in industrialized nations: Social, psychological, and biological pathways. Vol. 896. New York: Academy of Sciences; 1999. pp. 48–63. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S. Decreased anterior cingulate volume in combat-related PTSD. Biological Psychiatry. 2006;59:582–7. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Wright CE, Steptoe A. Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology. 2005;30:582–90. doi: 10.1016/j.psyneuen.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proceedings of the National Academy of Sciences; 2003. pp. 9039–43. [DOI] [PMC free article] [PubMed] [Google Scholar]