Abstract
Endometriosis, the presence of ectopic endometrial tissue outside the uterine cavity, is a common disease affecting women during their reproductive years. Current therapeutic success is often unsatisfactory because of limited insight into disease mechanisms. Nevertheless, angiogenesis plays an essential role in the pathogenesis of the disease, making it a potential novel target for therapy. In the current study, we demonstrate in an established mouse model of endometriosis that transient hypoxia in transplanted endometriosis-like lesions results in the up-regulation of hypoxia-inducible factor-1α (HIF-1α), leading to the expression of vascular endothelial growth factor (VEGF), a key player in endometriosis-associated angiogenesis. Systemic treatment with the angiogenesis inhibitor 2-methoxyestradiol suppressed HIF-1α expression in vivo, resulting in a decreased downstream expression of HIF-1α target genes, such as for VEGF, phosphoglycerate kinase, and glucose transporter-1. 2-Methoxyestradiol also suppressed VEGF-induced vascular permeability, as demonstrated in a modified Miles assay. Finally, systemic treatment with 2-methoxyestradiol significantly inhibited the growth of endometriosis-like lesions in a dose-dependent manner. In conclusion, hypoxia appears to play an important role in the pathogenesis of endometriosis and endometriosis-associated angiogenesis, and the angiogenesis inhibitor 2-methoxyestradiol may be a potential candidate for systemic treatment in the future.
Endometriosis, defined as the presence of ectopic endometrial tissue, is a disease associated with pelvic pain and reduced fertility. Women are affected almost exclusively during their reproductive years, because the establishment and growth of endometriotic lesions is highly estrogen dependent. It is estimated that 10 to 15% of women in the general population suffer from endometriosis, whereas subgroups, such as women undergoing laparoscopy for fertility investigations or hysterectomy, show a higher prevalence.1 Currently, it is believed that most endometriotic lesions originate from shed endometrium that enters the abdominal cavity through the Fallopian tubes at the time of menstruation.2 In women susceptible to the disease, these fragments or cells attach to the peritoneal wall and are then thought to invade the mesothelial cell layer, the underlying basement membrane, and stroma. The endometriotic lesions generate a blood supply by recruiting new blood vessels from the surrounding vasculature.3 This process known as angiogenesis also plays an essential role during many other disease states, such as tumor growth, rheumatoid arthritis, and diabetic retinopathy.4 However, blood vessel growth is also vital in physiological processes, such as development, wound healing, and reproduction.5
Proliferation of endothelial cells from pre-existing vessels is dependent on an increase in local proangiogenic cytokines and a decrease in inhibitors.6 Vascular endothelial growth factor (VEGF) is one of the best studied proangiogenic factors.7 It was originally identified as vascular permeability factor because of its enhancing effect on blood vessel permeability, but the mitogenic and survival-promoting effect of the molecule on endothelial cells eventually led to its current name.8,9 The VEGF-A gene is alternatively spliced to yield three major isoforms of 189, 165, and 121 amino acids.10,11 The splicing variant VEGF165 is most abundantly expressed and has been repeatedly described as being overexpressed in the peritoneal fluid of women with endometriosis.12,13
VEGF production is regulated by various stimuli. Hypoxia is one of the most potent factors resulting in VEGF expression.14 Hypoxia-inducible factor-1 α (HIF-1α) is believed to play a key role in VEGF-induced angiogenesis.15 HIF-1α is a helix-loop-helix transcription factor that is ubiquitously expressed in mammalian cells. Under normoxic conditions, the protein is hydroxylated at prolyl and asparaginyl residues, binds to the von-Hippel-Lindau protein, and undergoes proteasomal degradation.16,17 HIF-1α degradation is prevented in hypoxic environments, and the molecule translocates into the nucleus where it heterodimerizes with HIF-1β/aryl hydrocarbon nuclear receptor translocator. This heterodimer binds to hypoxia-responsive elements on various genes, such as those encoding glycolytic enzymes, erythropoietin, and VEGF.18,19
In the current study, we hypothesize that endometriosis-like lesions may acquire new blood vessels through hypoxia-induced up-regulation of VEGF. We demonstrate that systemic therapy with the angiogenesis inhibitor 2-methoxyestradiol inhibits the growth of induced endometriosis-like lesions in vivo. We propose that this inhibitory effect may be partially due to the effect of the drug on HIF-1α.
Materials and Methods
Animal Handling
All procedures were performed in the animal facility at Children’s Hospital (Boston, MA) in accordance with federal, local, and institutional guidelines. The Institutional Animal Care and Use Committee of Children’s Hospital approved all animal handling and procedures. Eight-week-old female C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). They were caged in groups of 5 to 10 mice with free access to chow and water and acclimated for 1 week before any experiment. The animal room was kept constantly at 26°C, 38.5% humidity, with a 12-hour light, 12-hour dark cycle (6:30 AM to 6:30 PM). The laboratories were located just a few meters above sea level.
Surgical Procedure and Treatment
All surgical procedures were performed under inhalative anesthesia with isoflurane (Baxter, Deerfield, IL), and mice were observed until fully recovered. Mice were ovarectomized through bilateral paravertebral incisions, and the wound was closed with a 5-0 braided silk suture (Ethicon, Somerville, NJ). Two days later, endometriosis was surgically induced as described previously.20,21,22,23 Briefly, after midline abdominal incision, the uterine horns were removed and opened longitudinally in a Petri dish containing warmed Dulbecco’s modified Eagle’s medium F-12 (Gibco, Grand Island, NY). Seven biopsies (2 mm in diameter) were taken using a dermal biopsy punch (Miltex, Bethpage, NY), and the lesions were autotransplanted to the peritoneal wall (four biopsies) and the mesentery (three biopsies) with a braided silk suture. The wound was closed with a 5-0 suture (all Ethicon). At the end of the procedure, a 17-β estradiol pellet (0.36 mg; 60-day release; Innovative Research of America, Sarasota, FL) was implanted subcutaneously into the interscapular area of all mice.
Treatment was initiated 2 to 3 hours before the procedure. Mice received 200 μl of 2-methoxyestradiol solution (2-ME2) (Panzem NCD; generous gift of EntreMed Inc., Rockville, MD) or vehicle per daily oral gavage. For dose-finding experiments (n = 6/group), mice received 10, 30, 60, and 100 mg/kg drug or vehicle for 4 weeks. All experiments were repeated three times. For all other experiments, 100 mg/kg was given. Control mice received vehicle at the identical volume.
At the end of each experiment, mice were euthanized individually under brief isoflurane anesthesia by cervical dislocation. The abdominal cavity was immediately reopened through the original incision, and the lesions were instantly excised and processed. In the experiments in which tissue was not collected, all seven lesions were measured in two perpendicular diameters (D1 and D2) with a caliper, and cross-sectional lesion area (CSA) was calculated using the formula for an ellipse (D1 × D2 × π/4) as previously described.20,21,24
Green Fluorescent Protein Imaging
In a different set of experiments, uterine tissue from 8-week-old transgenic mice expressing green fluorescent protein25 was removed and prepared as described above. In these animals, green fluorescent protein is expressed in all tissue because the gene is coupled with the ubiquitin promoter. The lesions were then transplanted to the peritoneal wall of wild-type C57BL/6 mice (n = 6). After closing the abdomen, the mice were kept for 4 weeks. Under general anesthesia, the abdominal cavity was then re-opened, and the lesion was visualized using a dissecting microscope with external light source and a green fluorescent protein filter lens. Pictures were captured using a CCD camera.
RNA Extraction and Real-Time PCR
Mice were pretreated with 2-methoxyestradiol or vehicle for 4 days (n = 3 mice per time point). All experiments were repeated three times. Mice were then ovarectomized, and endometriosis was induced as described above. The endometriosis-like lesions were removed at different time points. Tissue was snap frozen in liquid nitrogen and then stored at −80°C until further use. Usually two to three lesions were used per time point to yield enough RNA and real-time PCR was performed at least two times per experiment.
Total RNA was harvested with TRIzol Reagent (Invitrogen, Karlsruhe, Germany) and extracted with chloroform/isopropanol. The RNA was reverse transcribed into cDNA using Omniscript RT kit (Qiagen, Valencia, CA) and random primers (Invitrogen) according to the manufacturer’s instructions. Quantification of mRNA expression was performed using a DNA Engine Opticon 2 System (Bio-Rad, Hercules, CA). TaqMan Universal PCR Master Mix (Roche, Branchbury, NJ) was used for the real-time PCR reaction according to the manufacturer’s protocol. cDNA (50 μg) was used as template to determine the relative amount of mRNA by real-time PCR. Cycle threshold value means the number of PCR cycles required for the detection of fluorescence signal to exceed a fixed threshold. The relative levels of gene expression of target mRNA were normalized against β-actin. The following primers and probes were used: VEGF forward, 5′-AGTCCCATGAAGTGATCAAGTTCA-3′; VEGF reverse, 5′-ATCCGCATGATCTGCATGG-3′; VEGF probe, 5′-(6FAM)TGCCCACGTCAGAGAGCAACATCAC(BHQ∼6FAM)-3′; phosphoglycerate kinase (PGK) forward, 5′-CTGTGGTACTGAGAGCAGCAAGA-3′; PGK reverse, 5′-CAGGACCATTCCAAACAATCTG-3′; PGK probe, 5′-(6FAM)TAGCTCGACCCACAGCCTCGGCATAT(BHQ)∼6FAM-3′; glucose transporter-1 (Glut-1) forward, 5′-GGGCATGTGCTTCCAGTATGT-3′; Glut-1 reverse, ACGAGGAGCACCGTGAAGAT-3′; Glut-1 probe, 5′-(6FAM)CAACTGTGCGGCCCCTACGTCTTC(BHQ∼6FAM)-3′.
Hypoxyprobe Detection
For the immunohistochemical assessment of hypoxia in endometriosis-like lesions, in a separate set of experiments, mice (n = 2/group) were sacrificed at different time points (0 hours, 1 hour, 4 hours, 24 hours, 48 hours, 1 week, and 6 months). The experiment was repeated twice. The hypoxyprobe-1 kit (Chemicon International, Temecula, CA) was used according to the manufacturer’s protocol. At each time point, 30 minutes before euthanasia, mice received an i.p. injection of 60 mg/kg pimonidazole hydrochloride (hypoxyprobe-1), a 2-nitroimidazole, which forms insoluble protein adducts under hypoxic conditions in vitro and in vivo. After euthanization, the collected tissue was immediately fixed in 10% buffered formalin and then paraffin embedded. Five-micrometer-thick sections were subsequently incubated with primary anti-pimonidazole hydrochloride antibody, biotinylated secondary antibody, and streptavidin/peroxidase conjugates (Vector, Burlingame, CA). An isotype-matched irrelevant murine IgG1 monoclonal antibody (Genzyme, Cambridge, MA) directed against Shigela toxin, was used as a control antibody. Counterstaining was performed with hematoxylin.
Immunohistology for HIF-1α and Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling
Paraffin sections (3 μm) were dewaxed in xylene, rehydrated in a series of ethanol washes, and placed in distilled water before staining procedures. A rabbit polyclonal HIF-1α antibody (NB100-449; 1:10,000; Novus Biologicals, Littleton, CO) was used to detect HIF-1α. For signal amplification and visualization of HIF-1α, a catalyzed amplification system (CSA kit; Dako, Hamburg, Germany) based on a streptavidin-biotin-peroxidase reaction was used according to the manufacturer’s instructions. Antigen retrieval was performed for 6 minutes in preheated Dako target retrieval solution, using a pressure cooker. All incubations were performed in a humidified chamber. 3,3′-Diaminobenzidine tetrahydrochloride (Dako) served as chromogen for peroxidase reaction. Substitution of the primary antibody with a nonreactive rabbit IgG control antibody (Invitrogen, Carlsbad, CA) served as a negative control. Between incubations, specimens were washed two to four times in buffer (50 mmol/L Tris-HCl, 300 mmol/L NaCl, and 0.1% Tween 20, pH 7.6). Samples were counterstained with Richardson’s staining solution or hematoxylin for 30 seconds and coverslipped using an aqueous mounting media (Aquatex; Merck KGaA, Darmstadt, Germany). Apoptotic cells were visualized in formalin-fixed, paraffin-embedded tissue sections with the DeadEnd fluorometric terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling system (Promega, Madison, WI) according to the manufacturer’s instructions.
Miles Vascular Permeability Assay
Mice were treated for 5 days with 100 mg/kg 2-methoxyestradiol, vehicle, or tap water (control) (n = 6 mice/group). We then performed a modified Miles assay to test the effect of the drug on vascular permeability.26,27,28,29 In brief, anesthetized mice received an injection of Evans Blue into the retro-orbital venous plexus (100 μl of a 1% solution in 0.9% sterile saline). Mice were kept on a warming blanket set at 37°C throughout the procedure. After 10 minutes, 50 μl of 1 ng/μl human VEGF165 or PBS (control) was injected intradermally. Twenty minutes later, the mice were euthanized, the dorsal skin was removed, and lesions were excised using an 8-mm biopsy punch (Miltex, York, PA). Evans Blue was extracted over 5 days at room temperature in formamide (Sigma Aldrich, St. Louis, MO). The absorption (620 nm) was then measured in a 96-well microplate (Sigma Aldrich). The absorption readings from the PBS-injected skin lesions were then subtracted from the lesions injected with VEGF to receive the final result.
Western Blot
Protein lysates (75 μg) were run on a 12% SDS-polyacrylamide gel along an electric current and then transferred to a nitrocellulose membrane. Western blot analysis was performed using a polyclonal rabbit antibody against murine HIF-1α (1:1000 dilution; NB100-449; Novus Biologicals). To demonstrate equal loading, blots were stripped and reprobed with a specific antibody recognizing β-actin (1:5000 dilution; Sigma Chemicals, Munich, Germany). A mouse anti-rabbit antibody conjugated to horseradish peroxidase (Sigma Chemicals, St. Louis, MO) diluted 1:20,000 was used as the secondary antibody. Enzyme activity was visualized using the enhanced chemiluminescence method according to the instructions of the manufacturer (NEN, Boston, MA). The experiment was repeated twice, and two to three lesions were used per time point to yield enough tissue. Free Image J software (National Institutes for Health, Bethesda, MD) was used to perform semiquantitative analysis of the Western blot.
Statistical Analysis
For statistical analysis, GraphPad InStat and the Sigma Stat statistics software package were used. The mean, SD, and SEM were calculated for the mice in each group as described previously.20,21,24 Repeated-measures analysis did not show any significant difference in lesion size within animals.
The least square means of the CSA for established lesions (lesions >0 mm2) were compared among groups using one-way analysis of variance. When the overall analysis of variance indicated a significant F-test, pairwise comparisons among treatments with control mice were conducted using the post hoc Dunnett test. Two-tailed values of P < 0.05 were considered statistically significant.
Results
Hypoxia Occurs Early in Endometriosis-Like Lesions
Endometriosis-like lesions were removed at different time points after transplantation. The lesions stained positively for a monoclonal antibody against hypoxyprobe-1, thereby indicating that these specific tissues were hypoxic (Figure 1, D–I). No immunoreactivity was seen in the uterine endometrial tissue (Figure 1, A–C) or in the control endometriotic tissue (Figure 1J). Hypoxia emerged as soon as 1 hour after transplantation, and immunohistochemical staining remained strong during the first 48 hours. However, no staining was present in the lesions 1 week and 6 months after transplantation.
Figure 1.
A: Hypoxyprobe staining. Representative murine endometriotic tissue removed at different time points after transplantation. D: 1 hour. E: 4 hours. F: 24 hours. G: 48 hours. H: 1 week. I: 6 months. Each mouse was injected with pimonidazole hydrochloride (hypoxyprobe-1) 30 minutes before removal. Eutopic uterine lesions from the same mice were used as negative controls (A–C). Staining with hypoxyprobe antibody and counterstaining with hematoxylin. Control endometriotic tissue (4 hours; J) stained with an isotype-matched irrelevant murine IgG1 monoclonal antibody directed against Shigela toxin.
HIF-1α Is Up-Regulated in Hypoxic Endometriotic Tissue
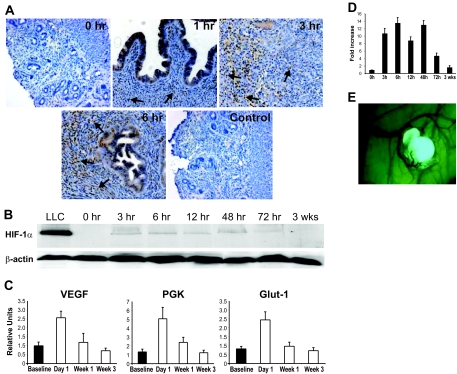
Staining of endometriosis-like lesions with a monoclonal antibody against murine HIF-1α showed distinctive, time-dependent patterns (Figure 2A). The glandular epithelial cells stained positive for HIF-1α independent of the time point. However, in stromal tissue, no immunoreactivity for HIF-1α was found at baseline, but it increased dramatically in tissues removed 1, 3, and 6 hours after transplantation. Western blot analysis of endometriosis-like lesions demonstrated a similar pattern (Figure 2B). Staining with a monoclonal antibody against HIF-1α demonstrated that the protein showed increased expression up to 48 hours after transplantation (P < 0.01). At 72 hours, expression decreased, and none was detected in lesions 3 weeks after surgery (Figure 2D).
Figure 2.
A: Immunohistochemistry staining of endometriosis-like lesions with anti-HIF-1α antibody. Counterstaining with hematoxylin. Increasing immunoreactivity (brown color) in glandular epithelial and stromal cells (arrows) over time (0, 1, 3, and 6 hours). Control (6 hours) stained with nonspecific rabbit IgG antibody. B: Western blot of endometriotic tissue stained with ant-HIF-1α antibody from different time points after transplantation. Loading control of same Western blot stained with anti-β-actin antibody. C: Real-time RT PCR of endometriosis-like lesions at different time points using primers for HIF-1α-dependent genes (VEGF, PGK, and Glut-1). Bars indicate SEM. D: Semiquantitative measurement of Western blot for HIF-1α as seen in B. Error bars indicate SEM. E: Endometriosis-like lesion from a green fluorescent protein+/+ mouse on the peritoneal wall of a wild-type recipient. Typical growth of blood vessels into the endometriosis-like lesion.
Next, we determined HIF-1α activity by measuring mRNA levels of target genes (Figure 2C). Real-time RT PCR revealed that mRNA levels of VEGF and the two glycolytic enzymes PGK and Glut-1 were strongly elevated 24 hours after transplantation (P < 0.001). These levels decreased subsequently over time and were back to baseline levels after 3 weeks.
2-Methoxyestradiol Inhibits the Growth of Endometriosis-Like Lesions
Mice with surgically induced endometriosis were treated for various periods with 2-ME2 or vehicle. Endometriosis-like lesions from vehicle-treated mice decreased in size within 1 week after surgery compared with baseline but increased in size thereafter (Figure 3A). Treatment with 100 mg/kg 2-ME2 suppressed the growth of endometriosis-like lesions after 1, 3, and 5 weeks compared with matched controls, and the inhibitory effect increased with the duration of treatment [37% (P < 0.01), 44% (P < 0.001), and 63% (P < 0.001), respectively].
Figure 3.
Systemic 2-methoxyestradiol inhibits growth of endometriosis-like lesions. Endometriosis-like lesions were measured in two perpendicular diameters (D1 and D2) with a caliper, and CSA was calculated using the formula of an ellipse (D1 × D2 × π/4) as previously described.20,21,24 A: Mean CSA of endometriosis-like lesions (n = 7 lesions) of mice treated with vehicle (▪) or 2-methoxyestradiol (□) (n = 6/group). B: Mean CSA after 4 weeks of oral treatment with different doses of 2-methoxyestradiol. Bars indicate the SEM.
A dose-dependent effect was established when mice were treated for 4 weeks with different doses of 2-ME2. Doses of 10, 30, and 100 mg/kg suppressed the growth of the endometriosis-like lesions by 21% (P < 0.02), 41% (P < 0.001), and 64% (P < 0.001), respectively, compared with vehicle controls (Figure 3B).
Systemic Treatment with 2-Methoxyestradiol Inhibits HIF-1α Expression in Endometriotic Tissue
Endometriosis-like lesions were removed from mice that had been treated systemically with 2-ME2 or vehicle. When the endometriotic tissue was analyzed from the control mice, HIF-1α protein was not detectable at baseline, but strong expression was seen 6 hours after transplantation. At that time point, HIF-1α expression was strongly suppressed in mice treated with 2-ME2 (Figure 5A).
Figure 5.
A: Western blot of endometriotic tissue samples at baseline (0 hours) and 6 hours. Lewis lung carcinoma is used as positive control in equivalent concentration. Staining with anti-HIF-1α antibody and anti-β actin as loading control. B: A modified Miles assay shows that systemic 2-methoxyestradiol treatment inhibits vascular permeability. Mice were pretreated orally with tap water (control), vehicle, and 2-methoxyestradiol for 5 days and then anesthetized and injected with Evans blue (i.v.). After 10 minutes, mice were given intradermal injections of PBS (50 μl; top two spots) and VEGF/vascular permeability factor (50 ng in 50 μl; bottom two spots), and mice were euthanized after 20 minutes. Reduced extravasation of dye in the skin of mice treated with 2-methoxyestradiol. C: Dye from excised skin was extracted with formamide for 5 days, and dye contents were measured at 620 nm. Data are expressed as mean ± SEM.
We then determined mRNA levels of HIF-1α target genes. Real-time PCR analysis of RNA isolated from endometriosis-like lesions showed reduced transcription of HIF-1α target genes. Systemic 2-methoxyestradiol treatment significantly reduced RNA levels for VEGF (P < 0.03 at 24 hours), PGK (P < 0.001 at 3, 24, and 72 hours), and Glut-1 (P < 0.001 at 3 hours; P < 0.01 at 24 hours) (Figure 4 A–C).
Figure 4.
Systemic 2-methoxyestradiol treatment reduces expression of HIF-1α target genes. Results of a representative experiment using real-time RT-PCR of endometriosis-like lesions removed after different time intervals after autotransplantation. Mice had been treated systemically with 2-methoxyestradiol before the induction of endometriosis. Lesions were removed at different time points (baseline, 1 hour, 3 hours, 24 hours, 72 hours, and 1 week) and tested for mRNA expression of VEGF (A), PGK (B), and Glut-1 (C). Bars indicate SEM.
Vascular Permeability Is Reduced by 2-Methoxyestradiol
Mice treated with 2-ME2 showed reduced vascular permeability in a modified Miles assay (Figure 5B). Leakage of Evans Blue into the perivascular space after intradermal VEGF injection was suppressed in mice that had been pretreated with 2-ME2 compared with controls. This was quantified by measuring absorbance, which was significantly lower in the excised tissue areas of mice that received 2-ME2 when compared with mice treated with placebo or tap water [0.020 (SEM 0.005); 0.054 (SEM 0.005); 0.052 (SEM 0.013), respectively; P = 0.0002] (Figure 5C).
Discussion
Endometriosis, despite its relatively high prevalence in the female population, still remains a somewhat enigmatic disorder. Little is known about the underlying mechanisms responsible for this often debilitating disease. Sampson’s theory of retrograde menstruation and transplantation of endometrial tissue in the abdominal cavity is the most widely accepted hypothesis to explain the pathogenesis of endometriosis. However, the fact that retrograde menstruation occurs commonly in women shows that other contributory factors for this disease must exist. It has been suggested that women with endometriosis may have impaired immunity or an altered expression of surface ligands on the endometrium or the mesothelial cell layer facilitating adhesion and survival of endometrial tissue.30,31,32,33 Recently, Donnez et al proposed that endometriotic lesions at various sites may be different,34,35 a concept that has been supported by data demonstrating the differential expression of aromatase.36 Although it has not directly been demonstrated in humans, data from various animal models suggests that single endometrial cells or tissue fragments invade the mesothelial cell layer and basement membrane after attachment to the peritoneal wall.37 Maas et al38 reported in the chick embryo chorioallantoic membrane CAM assay that glandular and stromal cells from endometrial fragments begin to invade the mesothelial cell layer within 48 to 72 hours of transplantation. Similar results were shown in another in vitro study using human mesothelial cell cultures.39 The Dutch group also demonstrated that the presence of intact glandular structures and stromal components are required for the invasive potential of endometrial cells and the formation of endometriosis-like lesions. However, other data suggest that endometriotic lesion evolve from single cells, and it is currently unclear whether endometriosis is a monoclonal disease.40,41,42,43,44
Regardless of the underlying theory, a basic requirement for the establishment and growth of all endometriotic lesions is the acquisition of new blood vessels to ensure sufficient supply with oxygen and nutrients (Figure 2E). Angiogenesis is dependent on the local balance between proangiogenic cytokines and endogenous angiogenesis inhibitors. In tumors, it has been proposed that an equilibrium of these factors leads to a dormant state, in which the number of proliferating cells equals the number of cells undergoing apoptosis.6,45,46 A shift in the balance toward the proangiogenic phenotype leads to tumor growth and metastases.47 Cells undergo apoptosis as soon as the distance between the supplying blood vessel and the growing cells exceeds the maximal distance of oxygen diffusion. Many elegant tumor studies demonstrate that large amounts of VEGF are expressed in these hypoxic areas, which provokes a mitotic stimulus for the endothelial cells to proliferate and to form new blood vessels.48,49,50 The VEGF gene contains a hypoxia-responsive element to which, at the time of oxygen deprivation, the transcription factor HIF-1α binds, resulting in expression of VEGF.51,52,53,54,55
In our mouse model, we have shown that hypoxia is present in surgically induced endometriosis-like lesions during the early stages of transplantation and invasion. We demonstrated that hypoxyprobe-1 (pimonidazole hydrochloride, a 2-nitroimidazole that forms a protein adduct to hypoxic cells in vitro and in vivo56,57,58) accumulated in the lesions immediately after transplantation, and this immunoreactivity increased during the first 24 to 48 hours. During the same period, uterine endometrial tissue was negative for hypoxyprobe-1 staining. Although it is not surprising that transplanted tissue is hypoxic, we also found that the lesions were hypoxyprobe-1-negative 1 week after transplantation, suggesting supply of oxygen to the transplanted tissue.
These results correlate well with our findings from a recent study.24 Using in vivo bioluminescence imaging of transgenic luciferase-expressing endometriosis-like lesions in wild-type mice, we demonstrated that transplanted lesions emit light after systemic i.v. injection of the substrate luciferin 3 to 4 days after surgery. No signal was seen beforehand suggesting that connection to the circulation was not established yet. Others have also reported, using immunohistochemistry, that new blood vessels could first be detected in endometriosis-like lesions 3 days after transplantation.59,60,61,62 These results and the findings of the current study support the concept that hypoxia is an early phenomenon in the development of transplanted lesions, which diminishes once new blood vessels start to invade into the tissue. Assuming that Sampson’s theory of retrograde menstruation and transplantation/invasion is correct, one can propose that the small tissue fragments are similarly hypoxic until they achieve a connection to the systemic circulation. Hypoxia is a strong stimulus for the growth of new blood vessels under the regulation of the HIF-1α-VEGF pathway.14,51,53 Generally, under low oxygen levels, the transcription factor HIF-1α is not hydroxylated, avoiding binding to the von-Hippel-Lindau protein and thereby inhibiting its ubiquitination and degradation. HIF-1α then translocates into the nucleus, forms a complex with HIF-1β/aryl hydrocarbon nuclear receptor translocator, and binds to hypoxia-responsive elements on various genes.63,64 The VEGF gene contains a hypoxia-responsive element, and the HIF-1α/HIF-1β/aryl hydrocarbon nuclear receptor translocator complex is known to induce the expression of VEGF strongly.14
In this study, we demonstrate for the first time that HIF-1α is expressed in surgically induced endometriosis-like lesions. Western blot analysis of endometriotic tissue from this mouse model shows that the protein is expressed early and that levels decline after approximately 72 hours. Interestingly, immunohistochemical studies reveal that HIF-1α is expressed mainly in the epithelial layer 1 hour after transplantation, whereas expression is increased in the stromal cell compartment at later time points. Because HIF-1α undergoes posttranscriptional modifications, we decided to test for mRNA levels of downstream genes, such as VEGF, Glut-1, and PGK. Using real-time PCR, we confirmed our findings by showing strong up-regulation of the hypoxia-inducible genes during the 1st day after transplantation and a subsequent decrease in mRNA levels over time.
It has recently been proposed that the suppression of angiogenesis may be an attractive approach for the treatment of endometriosis.3 Our group and others have been able to show that the use of angiogenesis inhibitors is effective in suppressing endometriotic growth in various murine models. Systemic therapy with either the endogenous angiogenesis inhibitor endostatin, a proteolytic fragment of collagen XVIII, or 27-amino acid endostatin peptides significantly inhibited the growth of endometriosis-like lesions.20,21 Similar results were seen with the TNP-470 derivate caplostatin.24 Because VEGF levels are increased in the peritoneal fluid of women with endometriosis compared with controls,12,13 the inhibition of VEGF has been suggested as a means of influencing angiogenesis in endometriosis.65 Soluble VEGF receptors and anti-VEGF antibodies showed efficacy in animal models.62,66
In the present study, we used 2-ME2 to treat mice with induced endometriosis. 2-ME2 is an angiogenesis inhibitor currently being tested in phase II trials for cancer. We showed that 5 weeks of systemic 2-ME2 treatment suppressed endometriotic growth in a dose-dependent manner. When 2-ME2 was given at a dose of 30 mg/kg, a 39% reduction in growth was seen. By increasing the dose to 100 mg/kg, the lesion area was significantly reduced by 64%. In addition, the inhibitory effect was apparent by the end of the 1st week after transplantation. Interestingly, we found that lesions from the control mice became smaller during the 1st week, which was probably due to apoptosis and tissue necrosis. However, the effect of 2-ME2 was significantly greater at that time point and increased to 58% during that experiment. The experiments were performed on nonestablished lesions, therefore making it a preventative therapy, ie, before lesion establishment. Other groups have shown that antiangiogenic therapy in different rodent models can inhibit growth of established lesions.62,66 We previously demonstrated that the use of endostatin does not significantly inhibit growth of established lesions in our mouse model of surgically induced endometriosis.20 In our model, we believe that angiogenesis inhibitors work best during early stages of vessel growth (migration and sprouting of endothelial cells), and vascular growth has been demonstrated during the 1st week after transplantation. However, because other models have demonstrated efficacy in established lesions, it is possible that angiogenesis inhibitors, such as 2-ME2, may work both in “prevention” and “treatment” settings. In a clinical scenario, patients may undergo surgical removal of lesions or conventional hormonal treatment, which is then supplemented by adjuvant antiangiogenic therapy.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining of endometriotic lesions removed at 1, 6, and 96 hours showed increase immunoreactivity in tissue from animals systemically treated with 2-ME2 (Supplemental Figure S1, see http://ajp.amjpathol.org). The effect appears to be strongest during the early time points. This finding could be explained by the fact that the 2-ME2 concentration in the transplanted tissue is likely to remain high immediately after transplantation. The longer no connection to the host vascular system exists, the less drug is available locally, which makes the difference of apoptotic cells between the treated and nontreated mice less apparent. In addition, our Western blot and RNA data suggest that HIF-1α and target gene expression levels fall after 2 to 3 days in endometriotic lesions. It is conceivable that, in addition to decreased 2-ME2 levels, less “substrate” for 2-ME2 may result in less obvious differences in apoptosis. This data and the inhibitory response on lesion growth are well in line with our experience with other angiogenesis inhibitors in this model. Endostatin and endostatin peptides inhibited the growth of endometriosis-like lesions by up to 47%, and the N-(2-hydroxypropyl)methacrylamide copolymer-linked TNP-470 compound caplostatin was 59% effective.20,21,24 However, 2-ME2 has the advantage of being orally bioavailable.
2-ME2 has been shown to suppress endothelial cell function by inhibiting microtubule dynamics.67 Recently, an additional pathway to explain some of the action of the drug has been described. Mabjeesh et al68 showed that 2-ME2 down-regulates HIF-1α at a posttranscriptional level in vitro. It has also been reported that 2-ME2 decreases nuclear binding activity of HIF-1α in head and neck squamous cell carcinoma.69 In the present study, we showed for the first time that 2-ME2 inhibits the in vivo expression of HIF-1α-inducible genes VEGF, Glut-1, and PGK as measured by real-time PCR during the first few hours after transplantation. These results are supported by our findings that HIF-1α was also reduced in endometriotic tissues at the protein level.
These data suggest that 2-ME2 may act through a dual mechanism in endometriosis. The indirect effect is through the inhibition of HIF-1α. The inhibition of HIF-1α leads to suppressed VEGF levels, as shown by the finding of reduced VEGF mRNA expression, which removes the stimulus for angiogenesis. The direct effect is via inhibition of endothelial cell function. The observation that 2-ME2 produces a marked reduction in vascular permeability in response to a fixed amount of injected VEGF in the Miles assay demonstrates the direct inhibitory effect. VEGF is known to have approximately 50,000 times higher permeabilizing activity than histamine.10 Therefore, the diminished extravasation of Evans Blue in 2-ME2-treated mice implies an inhibitory effect of the drug on VEGF stimulation of the endothelium. This is also illustrated by previous experiments that showed that 2-ME2 inhibits angiogenesis induced by a pellet of VEGF placed in the cornea.70 In addition, it is conceivable that the herein described suppressive effects on endometriotic growth are supported by the known inhibition of 2-ME2 on tubulin polymerization by interacting at the colchicine site as previously demonstrated in tumors.67,70 However, although VEGF is a major player in endometriosis, other cytokines have been demonstrated to induce angiogenesis in endometriosis both directly and indirectly through an up-regulation of VEGF expression (for review, see Ref. 71).
In summary, we have shown that hypoxia plays an important role in induced endometriosis-like lesions in this established mouse model. New blood vessel growth that is essential for the survival and development of this tissue is induced by the expression of angiogenic factors, such as VEGF, which is up-regulated by oxygen deprivation and subsequent production of HIF-1α. The antiangiogenic compound 2-methoxyestradiol inhibits HIF-1α expression in vivo and angiogenesis, which is likely to contribute to suppression of the growth of the lesions. It may therefore be a valuable novel therapeutic approach to the treatment of endometriosis.
Acknowledgments
We thank Kristin Johnson for excellent work with graphics and photography. We are also very thankful to David Sampson for technical assistance and Stephen Kennedy for useful comments about the manuscript.
Footnotes
Address reprint requests to Christian M. Becker, Nuffield Department of Obstetrics and Gynaecology, John Radcliffe Hospital, Womens Centre Headington, University of Oxford, Oxford, OX3 9DU, UK. E-mail: christian.becker@obs-gyn.ox.ac.uk.
Supported by a postdoctoral research exchange grant from the Max Kade Foundation (to C.M.B.), a grant from the Sidney Swensrud Foundation (to R.J.D.), a grant from the Deutsche Forschungsgemeinschaft (grant CR133/2-2, to T.C.), and a NIH/NCI Program Project Grant from the National Institutes of Health/National Cancer Institute (grant P01-CA45548, to T.C.).
Supplemental material for this article can be found on http:// ajp.amjpathol.org.
References
- ASRM: Endometriosis and infertility. Fertil Steril. 2004;81:1441–1446. doi: 10.1016/j.fertnstert.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endothelial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469. [Google Scholar]
- Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann NY Acad Sci. 2002;955:89–100. doi: 10.1111/j.1749-6632.2002.tb02769.x. discussion 118, 396–406. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- Senger DR, Perruzzi CA, Feder J, Dvorak HF. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. [PubMed] [Google Scholar]
- Dvorak HF. Rous-Whipple Award Lecture. How tumors make bad blood vessels and stroma. Am J Pathol. 2003;162:1747–1757. doi: 10.1016/s0002-9440(10)64309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. 1998;13:1686–1690. doi: 10.1093/humrep/13.6.1686. [DOI] [PubMed] [Google Scholar]
- McLaren J, Prentice A, Charnock-Jones DS, Smith SK. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Hum Reprod. 1996;11:220–223. doi: 10.1093/oxfordjournals.humrep.a019023. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993;82:3610–3615. [PubMed] [Google Scholar]
- Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CM, Sampson DA, Rupnick MA, Rohan RM, Efstathiou JA, Short SM, Taylor GA, Folkman J, D’Amato RJ. Endostatin inhibits the growth of endometriotic lesions but does not affect fertility. Fertil Steril. 2005;84(Suppl 2):1144–1155. doi: 10.1016/j.fertnstert.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Becker CM, Sampson DA, Short SM, Javaherian K, Folkman J, D’Amato RJ. Short synthetic endostatin peptides inhibit endothelial migration in vitro and endometriosis in a mouse model. Fertil Steril. 2006;85:71–77. doi: 10.1016/j.fertnstert.2005.07.1290. [DOI] [PubMed] [Google Scholar]
- Cummings AM, Metcalf JL. Induction of endometriosis in mice: a new model sensitive to estrogen. Reprod Toxicol. 1995;9:233–238. doi: 10.1016/0890-6238(95)00004-t. [DOI] [PubMed] [Google Scholar]
- Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44:684–694. [PubMed] [Google Scholar]
- Becker CM, Wright RD, Satchi-Fainaro R, Funakoshi T, Folkman J, Kung AL, D’Amato RJ. A novel noninvasive model of endometriosis for monitoring the efficacy of antiangiogenic therapy. Am J Pathol. 2006;168:2074–2084. doi: 10.2353/ajpath.2006.051133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa T, Puder M, Wood M, Schaefer BC, D’Amato RJ. Analysis of tumor-associated stromal cells using SCID GFP transgenic mice: contribution of local and bone marrow-derived host cells. FASEB J. 2006;20:95–102. doi: 10.1096/fj.04-3669com. [DOI] [PubMed] [Google Scholar]
- Claffey KP, Brown LF, del Aguila LF, Tognazzi K, Yeo KT, Manseau EJ, Dvorak HF. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res. 1996;56:172–181. [PubMed] [Google Scholar]
- Miles AA, Miles EM. Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of guinea-pigs. J Physiol. 1952;118:228–257. doi: 10.1113/jphysiol.1952.sp004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satchi-Fainaro R, Mamluk R, Wang L, Short SM, Nagy JA, Feng D, Dvorak AM, Dvorak HF, Puder M, Mukhopadhyay D, Folkman J. Inhibition of vessel permeability by TNP-470 and its polymer conjugate, caplostatin. Cancer Cell. 2005;7:251–261. doi: 10.1016/j.ccr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Streit M, Velasco P, Riccardi L, Spencer L, Brown LF, Janes L, Lange-Asschenfeldt B, Yano K, Hawighorst T, Iruela-Arispe L, Detmar M. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 2000;19:3272–3282. doi: 10.1093/emboj/19.13.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmowski WP, Steele RW, Baker GF. Deficient cellular immunity in endometriosis. Am J Obstet Gynecol. 1981;141:377–383. doi: 10.1016/0002-9378(81)90598-6. [DOI] [PubMed] [Google Scholar]
- van der Linden PJ, de Goeij AF, Dunselman GA, Arends JW, Evers JL. P-cadherin expression in human endometrium and endometriosis. Gynecol Obstet Invest. 1994;38:183–185. doi: 10.1159/000292475. [DOI] [PubMed] [Google Scholar]
- van der Linden PJ, de Goeij AF, Dunselman GA, van der Linden EP, Ramaekers FC, Evers JL. Expression of integrins and E-cadherin in cells from menstrual effluent, endometrium, peritoneal fluid, peritoneum, and endometriosis. Fertil Steril. 1994;61:85–90. doi: 10.1016/s0015-0282(16)56457-7. [DOI] [PubMed] [Google Scholar]
- Viganó P, Pardi R, Magri B, Busacca M, Di Blasio AM, Vignali M. Expression of intercellular adhesion molecule-1 (ICAM-1) on cultured human endometrial stromal cells and its role in the interaction with natural killers. Am J Reprod Immunol. 1994;32:139–145. doi: 10.1111/j.1600-0897.1994.tb01104.x. [DOI] [PubMed] [Google Scholar]
- Donnez J, Nisolle M, Smoes P, Gillet N, Beguin S, Casanas-Roux F. Peritoneal endometriosis and “endometriotic” nodules of the rectovaginal septum are two different entities. Fertil Steril. 1996;66:362–368. [PubMed] [Google Scholar]
- Donnez J, Van Langendonckt A, Casanas-Roux F, Van Gossum JP, Pirard C, Jadoul P, Squifflet J, Smets M. Current thinking on the pathogenesis of endometriosis. Gynecol Obstet Invest. 2002;54 Suppl 1:52–58. doi: 10.1159/000066295. discussion 59–62. [DOI] [PubMed] [Google Scholar]
- Heilier JF, Donnez O, Van Kerckhove V, Lison D, Donnez J. Expression of aromatase (P450 aromatase/CYP19) in peritoneal and ovarian endometriotic tissues and deep endometriotic (adenomyotic) nodules of the rectovaginal septum. Fertil Steril. 2006;85:1516–1518. doi: 10.1016/j.fertnstert.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Dunselman GA, Groothuis PG. Etiology of endometriosis: hypotheses and facts. Gynecol Obstet Invest. 2004;57:42–43. [PubMed] [Google Scholar]
- Maas JW, Groothuis PG, Dunselman GA, de Goeij AF, Struijker-Boudier HA, Evers JL. Development of endometriosis-like lesions after transplantation of human endometrial fragments onto the chick embryo chorioallantoic membrane. Hum Reprod. 2001;16:627–631. doi: 10.1093/humrep/16.4.627. [DOI] [PubMed] [Google Scholar]
- Witz CA, Cho S, Centonze VE, Montoya-Rodriguez IA, Schenken RS. Time series analysis of transmesothelial invasion by endometrial stromal and epithelial cells using three-dimensional confocal microscopy. Fertil Steril. 2003;79(Suppl 1):770–778. doi: 10.1016/s0015-0282(02)04834-3. [DOI] [PubMed] [Google Scholar]
- Evers JL, Dunselman GA, Groothuis P. Now you see them, now you don’t. Fertil Steril. 2005;84:31–32. doi: 10.1016/j.fertnstert.2005.01.122. discussion 38–39. [DOI] [PubMed] [Google Scholar]
- Lucidi RS, Witz CA, Chrisco M, Binkley PA, Shain SA, Schenken RS. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil Steril. 2005;84:16–21. doi: 10.1016/j.fertnstert.2004.10.058. [DOI] [PubMed] [Google Scholar]
- Mayr D, Amann G, Siefert C, Diebold J, Anderegg B. Does endometriosis really have premalignant potential? A clonal analysis of laser-microdissected tissue. FASEB J. 2003;17:693–695. doi: 10.1096/fj.02-0562fje. [DOI] [PubMed] [Google Scholar]
- Prowse AH, Manek S, Varma R, Liu J, Godwin AK, Maher ER, Tomlinson IP, Kennedy SH. Molecular genetic evidence that endometriosis is a precursor of ovarian cancer. Int J Cancer. 2006;119:556–562. doi: 10.1002/ijc.21845. [DOI] [PubMed] [Google Scholar]
- Wu Y, Basir Z, Kajdacsy-Balla A, Strawn E, Macias V, Montgomery K, Guo SW. Resolution of clonal origins for endometriotic lesions using laser capture microdissection and the human androgen receptor (HUMARA) assay. Fertil Steril. 2003;79(Suppl 1):710–717. doi: 10.1016/s0015-0282(02)04821-5. [DOI] [PubMed] [Google Scholar]
- Almog N, Henke V, Flores L, Hlatky L, Kung AL, Wright RD, Berger R, Hutchinson L, Naumov GN, Bender E, Akslen LA, Achilles EG, Folkman J. Prolonged dormancy of human liposarcoma is associated with impaired tumor angiogenesis. FASEB J. 2006;20:947–949. doi: 10.1096/fj.05-3946fje. [DOI] [PubMed] [Google Scholar]
- Naumov GN, Bender E, Zurakowski D, Kang SY, Sampson D, Flynn E, Watnick RS, Straume O, Akslen LA, Folkman J, Almog N. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst. 2006;98:316–325. doi: 10.1093/jnci/djj068. [DOI] [PubMed] [Google Scholar]
- Folkman J. Seminars in Medicine of the Beth Israel Hospital: Boston Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- Laderoute KR, Alarcon RM, Brody MD, Calaoagan JM, Chen EY, Knapp AM, Yun Z, Denko NC, Giaccia AJ. Opposing effects of hypoxia on expression of the angiogenic inhibitor thrombospondin 1 and the angiogenic inducer vascular endothelial growth factor. Clin Cancer Res. 2000;6:2941–2950. [PubMed] [Google Scholar]
- Tsuzuki Y, Fukumura D, Oosthuyse B, Koike C, Carmeliet P, Jain RK. Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1alpha→ hypoxia response element→VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res. 2000;60:6248–6252. [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleadle JM, Ratcliffe PJ. Induction of hypoxia-inducible factor-1, erythropoietin, vascular endothelial growth factor, and glucose transporter-1 by hypoxia: evidence against a regulatory role for Src kinase. Blood. 1997;89:503–509. [PubMed] [Google Scholar]
- Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells: identification of a 5′ enhancer. Circ Res. 1995;77:638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- Pugh CW, O’Rourke JF, Nagao M, Gleadle JM, Ratcliffe PJ. Activation of hypoxia-inducible factor-1: definition of regulatory domains within the alpha subunit. J Biol Chem. 1997;272:11205–11214. doi: 10.1074/jbc.272.17.11205. [DOI] [PubMed] [Google Scholar]
- Salceda S, Beck I, Caro J. Absolute requirement of aryl hydrocarbon receptor nuclear translocator protein for gene activation by hypoxia. Arch Biochem Biophys. 1996;334:389–394. doi: 10.1006/abbi.1996.0469. [DOI] [PubMed] [Google Scholar]
- Chapman JD, Franko AJ, Sharplin J. A marker for hypoxic cells in tumours with potential clinical applicability. Br J Cancer. 1981;43:546–550. doi: 10.1038/bjc.1981.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varia MA, Calkins-Adams DP, Rinker LH, Kennedy AS, Novotny DB, Fowler WC, Jr, Raleigh JA. Pimonidazole: a novel hypoxia marker for complementary study of tumor hypoxia and cell proliferation in cervical carcinoma. Gynecol Oncol. 1998;71:270–277. doi: 10.1006/gyno.1998.5163. [DOI] [PubMed] [Google Scholar]
- Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- Bruner-Tran KL, Webster-Clair D, Osteen KG. Experimental endometriosis: the nude mouse as a xenographic host. Ann NY Acad Sci. 2002;955:328–339. doi: 10.1111/j.1749-6632.2002.tb02793.x. discussion 340–322, 396–406. [DOI] [PubMed] [Google Scholar]
- Eggermont J, Donnez J, Casanas-Roux F, Scholtes H, Van Langendonckt A. Time course of pelvic endometriotic lesion revascularization in a nude mouse model. Fertil Steril. 2005;84:492–499. doi: 10.1016/j.fertnstert.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Grümmer R, Schwarzer F, Bainczyk K, Hess-Stumpp H, Regidor PA, Schindler AE, Winterhager E. Peritoneal endometriosis: validation of an in-vivo model. Hum Reprod. 2001;16:1736–1743. doi: 10.1093/humrep/16.8.1736. [DOI] [PubMed] [Google Scholar]
- Hull ML, Charnock-Jones DS, Chan CL, Bruner-Tran KL, Osteen KG, Tom BD, Fan TP, Smith SK. Antiangiogenic agents are effective inhibitors of endometriosis. J Clin Endocrinol Metab. 2003;88:2889–2899. doi: 10.1210/jc.2002-021912. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- Taylor RN, Mueller MD. Anti-angiogenic treatment of endometriosis: biochemical aspects. Gynecol Obstet Invest. 2004;57:54–56. [PubMed] [Google Scholar]
- Nap AW, Griffioen AW, Dunselman GA, Bouma-Ter Steege JC, Thijssen VL, Evers JL, Groothuis PG. Antiangiogenesis therapy for endometriosis. J Clin Endocrinol Metab. 2004;89:1089–1095. doi: 10.1210/jc.2003-031406. [DOI] [PubMed] [Google Scholar]
- D’Amato RJ, Lin CM, Flynn E, Folkman J, Hamel E. 2-Methoxyestradiol, an endogenous mammalian metabolite, inhibits tubulin polymerization by interacting at the colchicine site. Proc Natl Acad Sci USA. 1994;91:3964–3968. doi: 10.1073/pnas.91.9.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- Ricker JL, Chen Z, Yang XP, Pribluda VS, Swartz GM, Van Waes C. 2-Methoxyestradiol inhibits hypoxia-inducible factor 1alpha, tumor growth, and angiogenesis and augments paclitaxel efficacy in head and neck squamous cell carcinoma. Clin Cancer Res. 2004;10:8665–8673. doi: 10.1158/1078-0432.CCR-04-1393. [DOI] [PubMed] [Google Scholar]
- Klauber N, Parangi S, Flynn E, Hamel E, D’Amato RJ. Inhibition of angiogenesis and breast cancer in mice by the microtubule inhibitors 2-methoxyestradiol and taxol. Cancer Res. 1997;57:81–86. [PubMed] [Google Scholar]
- Becker CM, D’Amato RJ. Angiogenesis and antiangiogenic therapy in endometriosis. Microvasc Res. 2007;74:121–130. doi: 10.1016/j.mvr.2007.04.008. [DOI] [PubMed] [Google Scholar]