Abstract
Coagulation proteases have been suggested to play a role in the pathogenesis of tissue remodeling and fibrosis. We therefore assessed the proinflammatory and fibroproliferative effects of coagulation protease factor (F)Xa. We show that FXa elicits a signaling response in C2C12 and NIH3T3 fibroblasts. FXa-induced ERK1/2 phosphorylation was dependent on protease-activated receptor (PAR)-2 cleavage because desensitization with a PAR-2 agonist (trypsin) but not a PAR-1 agonist (thrombin) abolished FXa-induced signal transduction and PAR-2 siRNA abolished FXa-induced ERK1/2 phosphorylation. The PAR-2-dependent cellular effects of FXa led to fibroblast proliferation, migration, and differentiation into myofibroblasts, as demonstrated by the expression of α-smooth muscle actin and desmin, followed by the secretion of the cytokines monocyte chemotactic protein-1 and interleukin-6 as well as the expression of the fibrogenic proteins transforming growth factor-β and fibronectin. To assess the relevance of FXa-induced proliferation and cell migration, we examined the effect of FXa in a wound scratch assay. Indeed, FXa facilitated wound healing in a PAR-2- and ERK1/2-dependent manner. Taken together, these results support the notion that, beyond its role in coagulation, FXa-dependent PAR-2 cleavage might play a role in the progression of tissue fibrosis and remodeling.
Fibroblasts comprise the most abundant cell type in connective tissues and are central players in organ homeostasis. Their main function is to maintain the structural integrity of connective tissue by secreting collagen and fibronectin, which are principal components of the extracellular matrix (ECM). On injury, the fibrotic response orchestrated by fibroblasts is a critical component of tissue restoration and wound healing. Fibroblasts are frequently exposed to coagulation proteases after vascular damage. As fibroblasts constitutively express tissue factor, vascular injury exposes tissue factor to circulating factor (F)VII.1 After activation, FVIIa converts FX into its active form, FXa, which subsequently converts prothrombin into thrombin leading to the formation of a fibrin clot.
On the action of cytokines and growth factors secreted by monocytes, fibroblasts migrate into the wound and subsequently proliferate and concomitantly differentiate into myofibroblasts2,3 that develop ultrastructural and phenotypic characteristics of smooth muscle (SM) cells. The neoexpression of α-SM actin (α-SMA), typical of SM cells located in the vessel wall, is the hallmark of myofibroblastic cells.4 Myofibroblasts, which have enhanced metabolic activity as compared to undifferentiated fibroblasts, synthesize components of the new ECM, thereby restoring the structure and function of the injured tissue. In addition, myofibroblasts amplify the healing process by secreting inflammatory mediators.5
Fibroblast migration, proliferation, and differentiation in combination with enhanced ECM synthesis require tight regulation. The escape to self-limiting control results in a broad range of pathologies characterized by fibrotic disorders attributable to excessive fibroblast proliferation and deregulated ECM deposition.5,6 Eventually, the accumulation of connective tissue compromises organ function and often leads to premature death. Additionally, vascular remodeling, which is characterized by deregulated fibroproliferative responses represents a common theme of many cardiovascular abnormalities. Mechanistic studies suggest that transforming growth factor (TGF)-β is the most potent cytokine inducing ECM synthesis by myofibroblasts.7
Strikingly, many of the tissue fibrosis and remodeling disorders are associated with activation of the coagulation cascade. Persistent fibrin deposition has been observed in renal fibrotic disease.8 In lung fibrosis patients, thrombin levels but also zymogen FX levels are increased together with enhanced fibrin deposition.9 Abundant fibrin deposition is also observed in atherosclerosis,10 and patients with a thrombus after coronary angioplasty (which is known to contain thrombin and FXa11) at the site of vascular injury are at higher risk of restenosis.12
The identification of protease-activated receptors (PARs), a family of G-protein-coupled receptors, constitutes a potential link between activation of the coagulation cascade and the progression of tissue remodeling or fibrotic disease. Indeed, besides its properties in blood coagulation, FXa and thrombin induce intracellular signaling via proteolytic cleavage of PARs. PAR-1, -3, and -4 are cleaved by thrombin,13 whereas FXa can activate both PAR-1 and PAR-2.14
Although the role of thrombin-induced PAR-1 activation in tissue repair has been well documented,15,16 less is known about the contribution of FXa. In addition, the involvement of PAR-2 in wound healing and fibrotic disease has only recently begun to unfold. For instance, α-SMA and PAR-2 expression are correlated in a renal interstitial fibrosis model.17 Pulmonary PAR-2 is highly expressed in acute and chronic lung injury, which suggests that PAR-2 may participate in inflammation and fibroproliferation.18 PAR-2 furthermore maintains pancreatic fibrosis through increased proliferation and collagen production in pancreatic stellate cells.19 Finally, IgA nephropathy biopsies revealed the presence of PAR-2.20
Interestingly, FXa triggers signaling pathways involved in the regulation of cell growth and ECM deposition: it stimulates proliferation of fibroblasts and SM cells21,22,23,24 and induces the expression of interleukin (IL)-6, IL-8, monocyte chemotactic protein (MCP-1),25 and TGF-β.20 Moreover, FXa induces ERK1/2 phosphorylation in several cell lines, both murine and human, such as SM cells,26,27 cancer cells,14,21,28 endothelial cells,29 mesangial cells,30 and fibroblasts.22,31 The phosphorylation of ERK1/2 is widely used as a surrogate marker for PAR-1 and PAR-2 activation.32 Moreover, the activation of ERK1/2 has been closely associated with cellular proliferation,33,34 protein synthesis,35 and more recently wound healing.36 Taken together, these data support the hypothesis that FXa is involved in the pathogenesis of tissue fibrosis and remodeling. Therefore, we studied FXa-induced intracellular signaling and determined the potential consequence on proinflammatory and profibrotic responses.
Materials and Methods
Cell Culture
Growth-arrested fibroblasts NIH3T3 (CRL-1658) and myoblasts C2C12 (CRL-1772) were purchased from American Type Culture Collection (Manassas, VA), and human dermal fibroblasts were obtained from Promocell (Heidelberg, Germany). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS). Unless stated otherwise, cells were washed twice with phosphate-buffered saline (PBS), serum-starved for 4 hours, and subsequently stimulated as described.
Antibodies
Primary antibodies against α-actin, β-actin, desmin, TGF-β, fibronectin, PAR-1 (H-111), and PAR-2 (SAM11) were from Santa Cruz Biotechnology, Santa Cruz, CA. Phospho-FAK, phospho-p42/p44 MAP kinase, and phospho-Src antibodies were purchased from Cell Signaling Technology (Beverly, MA).
Reagents
Human FXa and thrombin were obtained from Kordia (Leiden, the Netherlands). Tick anticoagulant peptide (TAP) was kindly provided by Dr. Georges Vlasuk (Corvas International Inc., San Diego, CA). Hirudin was purchased from Calbiochem (San Diego, CA). All experiments involving 24-hour incubation with FXa included hirudin to block any thrombin signaling as described.14 Control experiments demonstrated that hirudin alone had no effect in our assays. ERK1/2 inhibitor U0126 was purchased from Cell Signaling Technology. Phalloidin-fluorescein isothiocyanate and phalloidin-tetramethyl-rhodamine isothiocyanate were purchased from Sigma (St. Louis, MO). Cell tracker green CMFDA (5-chloromethylfluorescein diacetate) was obtained from Molecular Probes (Eugene, OR). PP1 was from Biomol (Plymouth Meeting, PA). PAR-1 cleavage-blocking monoclonal antibody ATAP2 was obtained from Santa Cruz Biotechnology and used as previously reported.37 Predesigned PAR-2 (no. 158457) and control siRNA (no. 4611) was obtained from Ambion Inc. (Austin, TX). One U/ml FXa corresponds to 174 nmol/L.
Western Blot
Cells were lysed in Laemmli lysis buffer and incubated for 5 minutes at 95°C, and whole cell lysates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis, proteins were transferred to an immobilon-P polyvinylidene difluoride membrane (Millipore, Billerica, MA). Membranes were incubated overnight at 4°C with primary antibodies. All secondary antibodies were horseradish peroxidase-conjugated from DakoCytomation (Glostrup, Denmark). Blots were imaged using Lumilight Plus ECL substrate from Roche (Basel, Switzerland) on a GeneGnome imager (Syngene, Cambridge, UK). Densitometry was performed in Photoshop 7.0 (Adobe Systems, San Jose, CA) using the histogram function in a selected area of constant size for each band. Background was subtracted and values for the protein of interest were corrected for those of β-actin.
Immunofluorescence
Cells were grown on coverslips at 20 to 30% confluence. After pretreatment (when indicated), and subsequent stimulation with 0.75 U/ml FXa or PBS for the indicated time periods, cells were washed with PBS and fixed with 3.7% formaldehyde for 20 minutes at room temperature. Fixed cells were permeabilized and blocked in PBS/0.1% Triton X-100 (PBS-T) supplemented with 10% FCS for 1 hour. Cell nuclei were stained with 4,6-diamidino-2-phenylindole (200 ng/ml, Roche) in PBS-T for 30 minutes. The actin cytoskeleton was stained with 10 μg/ml of phalloidin-tetramethyl-rhodamine isothiocyanate in PBS-T supplemented with 1% bovine serum albumin. For imaging FAK or Src phosphorylation, cells were blocked as for phalloidin staining but subsequently incubated with the appropriate antibody and subsequent fluorescein isothiocyanate-conjugated secondary antibody (both in 3% bovine serum albumin/PBS-T). After staining, the cells were washed and mounted in Mowiol/DABCO aqueous mounting medium (Vector Laboratories, Burlingame, CA). Images were visualized using an epifluorescence microscope (Leica DMRA, Wetzlar, Germany) and captured on a cooled charge-coupled camera (KX Series; Apogee, Auburn, CA) operated by ImagePro Plus software (Media Cybernetics, Silver Spring, MD).
Proliferation Assay (MTT)
Cells seeded at a density of 104/cm2 in 96-well plates in DMEM supplemented with 1% FCS, were (if indicated) pretreated with TAP (200 nmol/L), hirudin (100 nmol/L), or the ERK1/2 inhibitor U0126 (10 μmol/L), after which they were stimulated with FXa (1 U/ml) or PBS as a control. Cell survival was determined at the indicated intervals using a MTT assay as described before.38
Transfections
Transfections of predesigned siRNAs were performed using HiPerfect kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. For transfections in six-well plates, 75 ng of siRNA was used at a 1:3 ratio of RNA/HiPerfect reagent. Cells were incubated with transfection complexes for 16 hours, after which fresh medium was added for 6 hours preceding further experimentation.
Cell Migration Assay
Cells were grown to 70% confluence in six-well plates and before experimentation labeled for 1 hour with 10 μmol/L CellTracker Green in serum-free medium. The dye was fixed by a 1-hour incubation in medium with 10% FCS. Subsequently, cells were washed and detached with 2 mmol/L ethylenediaminetetraacetic acid in PBS. Next, cells were resuspended in serum-free medium and transferred to 8-μm pore size HTS FluoroBlok cell culture inserts (BD Falcon, Franklin Lakes, NJ). FXa (1 U/ml) or PBS (control), were added to the bottom well and cell migration was assessed as described before.39 Briefly, fluorescence values representing the number of cells on the bottom side of the insert were read during 30 cycles (each cycle comprising four readings spanning 2 minutes) at 37°C on a Series 4000 CytoFluor multiwell plate reader (Perseptive Biosystems, Framingham, MA). The raw fluorescence data were corrected for background fluorescence and fading of the fluorophore, and the data were plotted with GraphPad Prism-4 (San Diego, CA).
Wound Scratch Assay
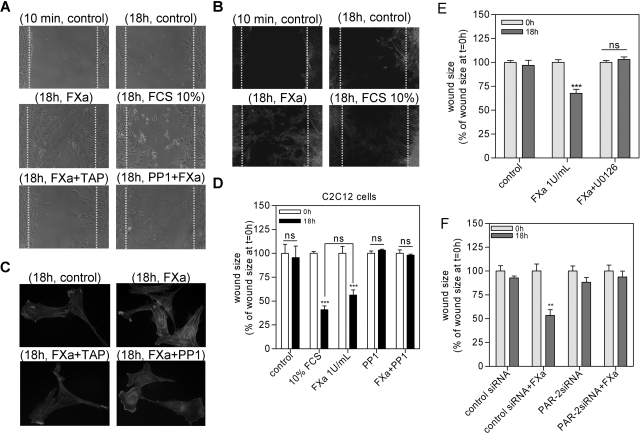
Cells were plated in six-well plates and maintained in DMEM supplemented with 10% FCS. After the cells reached 80 to 90% confluence, a wound was created in the center of the cell monolayer by a sterile plastic pipette tip. Immediately thereafter, the cells were washed with PBS to remove floating cellular debris and reincubated for an additional 18 hours with either serum-free medium (for use as a negative control), DMEM medium supplemented with 10% FCS (as positive control), or serum-free medium containing FXa. When indicated, cells were preincubated with PP1 (10 μmol/L), U0126 (10 μmol/L), or TAP (200 nmol/L) 30 minutes before wounding the cells. The ability of cells to proliferate and migrate into the wound area was assessed after 18 hours by comparing the 0- and 18-hour phase-contrast micrographs of six marked points along the wounded area at each plate. The percentage of nonrecovered wound area was calculated by dividing the nonrecovered area after 18 hours by the initial wound area at 0 time.
Cytokine/Chemokine Assay
IL-6, MCP-1, interferon-γ, tumor necrosis factor (TNF)-α, and IL-10 were measured using the BD Cytometric bead array mouse inflammation kit (Becton Dickinson, Franklin Lakes, NJ) as described before.40 Detection limits were 10 pg/ml.
Statistics
Statistical analyses were conducted using GraphPad Prism version 4.00 software. Data are expressed as means ± SEM. Comparisons between two conditions were analyzed using Mann-Whitney t-tests.
Results
Expression of PAR-1 and PAR-2 by NIH3T3 and C2C12 Cells
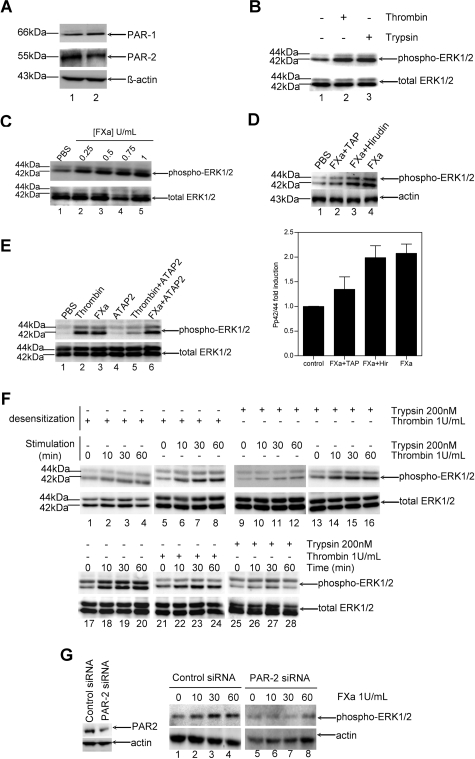
For efficient signal transduction of FXa, it is essential that its cellular receptors are expressed. Therefore, we assessed the presence of PAR-1 and PAR-2 on the NIH3T3 and C2C12 fibroblast cells used in this study. As shown in Figure 1A, both NIH3T3 (lane 1) and C2C12 (lane 2) cells constitutively expressed PAR-1 and PAR-2.
Figure 1.
FXa elicits specific phosphorylation of ERK1/2 in fibroblasts via PAR-2 activation. A: Western blot analysis on cell lysates of NIH3T3 (lane 1) and C2C12 (lane 2) cells showing that both cell lines constitutively express PAR-1 and PAR-2. Actin was used as a loading control. B: Serum-starved C2C12 cells (4 hours) treated with PBS (lane 1), thrombin (lane 2), or trypsin (lane 3) for 30 minutes. Western blot analysis of phospho-ERK1/2 indicates the functionality of both PARs. C: Effect of different concentrations of FXa on the phosphorylation of ERK1/2 in C2C12 cells. Cells stimulated for 30 minutes with PBS (lane 1) and FXa (lanes 2 to 5). D: Top: Western blot of C2C12 cells pretreated for 30 minutes with 200 nmol/L TAP (lane 2) or 100 nmol/L hirudin (lane 3) and stimulated for 30 minutes with PBS (lane 1) or 1 U/ml FXa (lanes 2 to 4) indicate that FXa-induced ERK1/2 phosphorylation is specific. Bottom: Densitometric analysis of three independent Western blots (mean ± SEM). E: FXa-induced ERK1/2 phosphorylation is not mediated by PAR-1. Western blots of C2C12 cells incubated for 30 minutes with PBS (lanes 1 to 3) or the anti-PAR-1 antibody ATAP2 (lanes 4 to 6), which were subsequently stimulated for 30 minutes with PBS (lane 1), 1 U/ml thrombin (lanes 2 and 5), or 1 U/ml FXa (lanes 3 and 6). F: FXa-induced ERK1/2 phosphorylation is mediated by PAR-2. Lanes 1 to 16: Western blots of fibroblasts exposed for 150 minutes (desensitization) to thrombin (lanes 1 to 8) or trypsin (lanes 9 to 16), subsequently stimulated with thrombin (lanes 1 to 4 and 13 to 16) or trypsin (lanes 5 to 12) for the indicated time points. Western blots of fibroblasts exposed for 150 minutes to serum-free control medium (lanes 17 to 20), thrombin (lanes 21 to 24), or trypsin (lanes 25 to 28) subsequently stimulated with FXa (1 U/ml) for the indicated time points. B–F: Total ERK1/2 is used as a loading control. G: PAR-2-specific siRNA causes loss of receptor-stimulated ERK1/2 phosphorylation. Left: Western blot of C2C12 cells transfected with control siRNA or PAR-2 siRNA. Right: Western blot of C2C12 cells transfected with control siRNA (lanes 1 to 4) or PAR-2 siRNA (lanes 5 to 8), stimulated with FXa for the indicated time points (minutes). Actin served as a loading control. Results presented are representative of three independent experiments.
PAR-1 and PAR-2 Expressed by NIH3T3 and C2C12 Are Functional
We next determined whether the expression of PAR-1 and PAR-2 was associated with functional responses in both cell lines. As read-out we examined the phosphorylation of ERK1/2, which is widely used as a surrogate marker for PAR-1 and PAR-2 activation.32 Cells were serum-starved for 4 hours, stimulated with thrombin (1 U/ml corresponding to 10 nmol/L), a specific PAR-1 agonist; trypsin (200 nmol/L), a specific PAR-2 agonist; or PBS as negative control. As shown in Figure 1B for C2C12 cells, stimulation with thrombin (lane 2) or trypsin (lane 3) induced strong phosphorylation of ERK1/2 as compared to the negative control (lane1). Similar results were obtained with NIH3T3 cells (data not shown). Hence, both PAR-1 and PAR-2 are functionally active on the fibroblasts.
FXa Elicits Signal Transduction in NIH3T3 and C2C12 Cells
To assess the capacity of FXa to induce intracellular signaling in fibroblasts, we examined FXa-induced phosphorylation of ERK1/2. Figure 1C shows that signaling induced by FXa was already detectable at 0.25 U/ml (lane 2). ERK1/2 phosphorylation strongly increased using 0.5 U/ml FXa (lane 3) and was maximal at 1 U/ml (lane 5). The same results were obtained in NIH3T3 cells (data not shown). To verify the specificity of ERK1/2 phosphorylation induced by FXa, cells were preincubated with TAP, an FXa inhibitor, or hirudin, a thrombin inhibitor. As shown in Figure 1D for C2C12 cells, pretreatment with TAP (lane 2), but not with hirudin (lane 3), almost completely inhibited FXa-induced ERK1/2 phosphorylation. Similar results were obtained with NIH3T3 cells (data not shown). Hence, FXa specifically induces ERK1/2 phosphorylation, which is independent of thrombin formation.
FXa Signals via PAR-2 Activation
To identify the receptor by which FXa induces signaling, we tested whether the activation of PAR-1 is required for FXa signaling. Cells were treated with FXa in the absence or presence of the PAR-1-blocking antibody ATAP2. As shown in Figure 1E, ERK1/2 phosphorylation was strongly induced by FXa (lane 3) independently of the blocking antibody (compare lanes 3 and 6). In contrast, nearly 70% of ERK1/2 phosphorylation induced by thrombin (lane 2) was inhibited by antibody treatment (lane 5). These results suggest that PAR-1 activation is not required for FXa-mediated signaling (in contrast to thrombin-mediated signaling) and that PAR-2 is likely to be involved. To confirm the involvement of PAR-2, we studied FXa-induced signaling in thrombin- or trypsin-desensitized fibroblasts. To validate the experimental set-up, we first verified the specificity of our desensitization experiments. As shown in Figure 1F, thrombin desensitization abolished subsequent stimulation by thrombin (lanes 1 to 4) but not by trypsin (lanes 5 to 8). Reciprocally, trypsin pretreatment prevented subsequent ERK1/2 phosphorylation when the fibroblasts were treated with trypsin (lanes 9 to 12) but not when treated with thrombin (lanes 13 to 16). Because thrombin and trypsin indeed specifically desensitized cells, we next assessed FXa-induced signaling in desensitized cells. As shown in Figure 1F, FXa still induced ERK1/2 phosphorylation in thrombin-desensitized cells (lanes 21 to 24). In contrast, fibroblasts pretreated with trypsin failed to respond to subsequent FXa stimulation (lanes 25 to 28 as compared to lanes 17 to 20), indicating both agonists activate the same receptor (ie, PAR-2). Similar results were obtained with NIH3T3 cells. To confirm that PAR-2 is the receptor mediating FXa signaling, cells were transfected with PAR-2 siRNA. As shown in Figure 1G, PAR-2 siRNA, but not control siRNA, impaired FXa-induced phosphorylation of ERK1/2. Taken together, these results strongly suggest that PAR-2 mediates FXa signaling in fibroblasts.
FXa Increases α-SMA and Desmin Expression in Fibroblasts
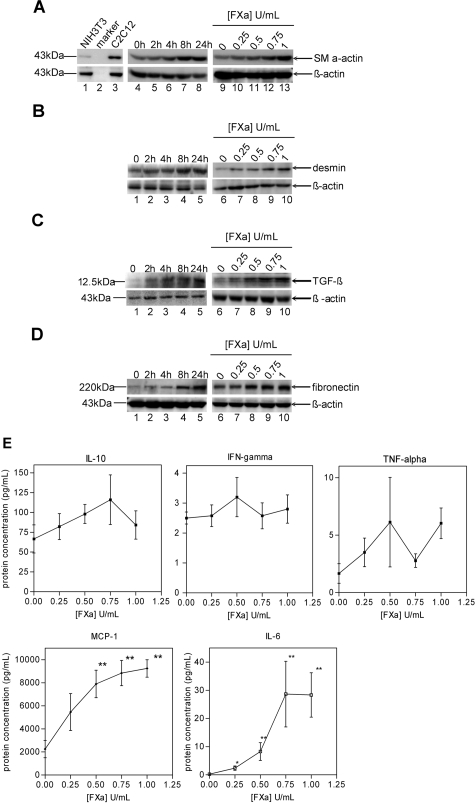
We assessed the consequences of PAR-2-mediated FXa-induced signaling for processes involved in wound healing and fibrosis, which are both characterized by the formation of a myofibroblast-enriched provisional ECM. Therefore, we examined whether FXa induced the differentiation of fibroblasts into myofibroblasts. Because α-SMA expression is a hallmark characteristic for the conversion of fibroblasts into myofibroblasts, we assessed α-SMA levels in the fibroblast cells. As shown in Figure 2A, growth-arrested NIH3T3 fibroblasts (lane 1) expressed low constitutive levels of α-SMA, whereas the differentiated C2C12 cells expressed higher basal levels (lane 3). Stimulation of NIH3T3 cells with FXa strongly increased α-SMA expression in a time-dependent (lanes 4 to 8) and dose-dependent (lanes 9 to 13) manner, indeed suggesting that FXa induces the differentiation of fibroblasts into myofibroblasts.
Figure 2.
FXa induces a profibrotic and proinflammatory phenotype in fibroblasts. A: FXa increases α-SMA expression in NIH3T3 fibroblasts. Lanes 1 and 3: Constitutive expression of α-SMA. Lanes 4 to 8: Cells treated with 1 U/ml FXa. Lanes 9 to 13: Cells treated for 24 hours with various concentrations of FXa. B: FXa increases desmin expression in NIH3T3 fibroblasts. Lanes 1 to 5: Cells treated with 1 U/ml FXa. Lanes 6 to 10: Cells treated for 24 hours with various concentrations of FXa. C: FXa stimulates TGF-β production in C2C12 cells. Lanes 1 to 5: Cells treated with 1 U/ml FXa. Lanes 6 to 10: Cells treated for 24 hours with various concentrations of FXa. D: FXa induces fibronectin production. Lanes 1 to 5: Cells treated with 1 U/ml FXa. Lanes 6 to 10: Cells treated for 24 hours with various concentrations of FXa. A–D: β-Actin served as loading control and results are representative of three independent experiments. E: FXa induces the secretion of MCP-1 and IL-6. IL-10, interferon-γ, TNF-α, IL-6, and MCP-1 expression in supernatants of C2C12 cells stimulated with FXa for 24 hours. Shown is the mean ± SEM (n = 4).
In addition to α-SMA expression, the intermediate filament protein desmin might be induced during fibroblast differentiation into myofibroblast.41 Therefore, we also determined desmin expression in NIH3T3 fibroblasts. As shown in Figure 2B, FXa also induced a time-dependent (lanes 1 to 5) and concentration-dependent (lanes 6 to 10) expression of desmin, further supporting the hypothesis that FXa induces fibroblast differentiation.
FXa Enhances Profibrotic Protein Production by C2C12 Myoblasts
We next examined FXa-induced synthesis of the profibrotic cytokine TGF-β. As shown in Figure 2C, C2C12 cells constitutively expressed very low levels of TGF-β. Stimulation with FXa showed a time-dependent increase in TGF-β levels (lanes 1 to 5), with maximal levels reached at 24 hours (lane 5). TGF-β was already induced by 0.5 U/ml FXa, but maximal expression was observed using 1 U/ml indicating a dose-response effect (lanes 6 to 10). Similar results were observed in the NIH3T3 cells, although protein synthesis was slightly less efficient in these cells (data not shown).
Fibronectin is one of the first matrix proteins produced in response to cell injury and inflammation. Therefore, we investigated whether FXa induced fibronectin levels in C2C12 cells. As shown in Figure 2D, FXa increased fibronectin levels in a time-dependent (lanes 1 to 5) and dose-dependent (lanes 6 to 10) manner. FXa induced fibronectin synthesis in NIH3T3 cells also, although to a lesser extent (data not shown). As shown in Supplementary Figure S1 at http://ajp.amjpathol.org, PAR-2, but not control, siRNA transfection inhibited fibronectin synthesis in C2C12 cells stimulated with FXa.
FXa Enhances the Expression of Proinflammatory Mediators
To determine the potential proinflammatory effect of FXa, the levels of TNF-α, interferon-γ, IL-6, IL-10, and MCP-1 in the supernatant of C2C12 fibroblasts stimulated with FXa were determined. As shown in Figure 2E, FXa did not modify the expression of IL-10, TNF-α, and interferon-γ. In contrast, FXa induced IL-6 expression in a dose-dependent manner. IL-6 levels were detectable after stimulation with 0.25 U/ml FXa, whereas maximal levels were obtained at a concentration of 0.75 U/ml. Finally, FXa strongly enhanced the expression of MCP-1, which peaked at a FXa concentration of 1 U/ml.
FXa Stimulates Fibroblast Proliferation
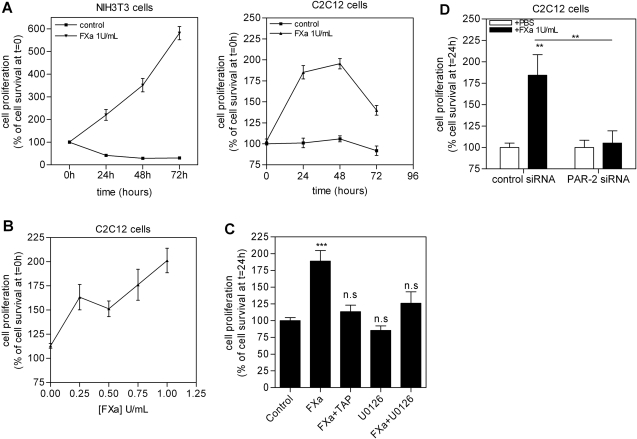
Because fibroblast proliferation is an important process in wound healing, we assessed the functional consequences of FXa-induced signaling on fibroblast proliferation. As shown in Figure 3A (left), FXa enhanced the proliferation of NIH3T3 cells by 334 ± 10% after 24 hours. This induction was sustained after a 48-hour incubation (319 ± 17%) and further increased after 72 hours (600 ± 35%). As shown in Figure 3A (right), FXa also stimulated proliferation of C2C12 fibroblasts (195 ± 6% after 24 hours, 202 ± 4% after 48 hours, and 140 ± 6% after 72 hours), but to a lesser extent (approximately three times less proliferation than in NIH3T3 fibroblasts). Furthermore, we demonstrated that the effect of FXa on cell proliferation was concentration-dependent. As shown in Figure 3B, a 24-hour incubation with FXa enhanced C2C12 proliferation by 168 ± 6% at a concentration as low as 0.25 U/ml, whereas maximal induction of proliferation was observed using 1 U/ml (191 ± 5%).
Figure 3.
FXa induces NIH3T3 and C2C12 proliferation. A: Cell survival of NIH3T3 and C2C12 cells seeded in medium containing 1% FCS treated with FXa or PBS. B: Proliferation of C2C12 cells stimulated for 24 hours with different FXa concentrations. C: Proliferation of C2C12 cells pretreated with 200 nmol/L TAP or 10 μmol/L U0126 for 2 hours, then stimulated for 24 hours with 1 U/ml FXa. D: Proliferation of C2C12 cells transfected with control or PAR-2 siRNA and incubated for 24 hours with 1 U/ml FXa. Results are shown as mean ± SEM of two independent experiments performed in octuplicate. **P < 0.01; ***P < 0.001.
To verify that FXa-induced cell proliferation is specific, cells were preincubated with TAP for 2 hours and subsequently incubated with FXa for 24 hours. As shown in Figure 3C, TAP pretreatment almost completely inhibited the FXa effect on cell proliferation. Furthermore, we verified the involvement of ERK1/2 in FXa-induced cell proliferation by pretreatment with the ERK1/2 inhibitor U0126. As shown in Figure 3C, ERK1/2 inhibition abolished FXa-induced proliferation. To assess the role of PAR-2 in FXa-induced cell proliferation, cells were transfected with PAR-2 and/or control siRNA. As shown in Figure 3D, PAR-2 siRNA blocked FXa-induced proliferation, whereas control siRNA did not modify the FXa effect on proliferation.
FXa Enhances Fibroblast Migration
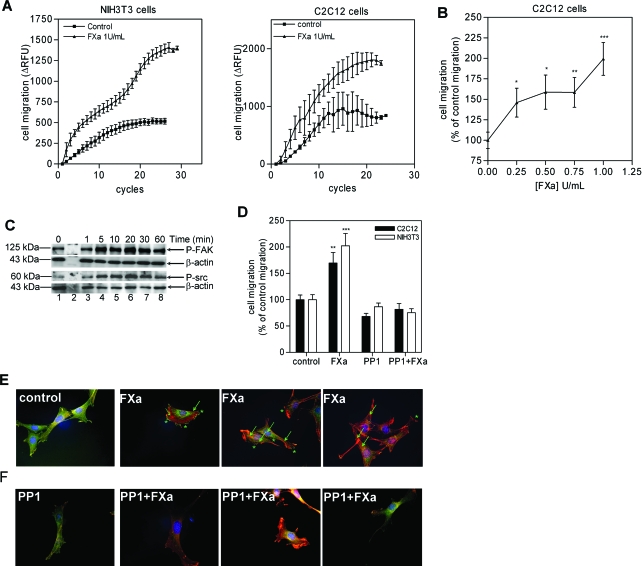
Next to proliferation, another important process in wound healing and fibrotic disease is the recruitment of fibroblasts toward the site of injury/inflammation. Therefore, we assessed transmigration of NIH3T3 cells toward a gradient of FXa. As shown in Figure 4A (left), FXa enhanced NIH3T3 migration by 249 ± 23%, as compared to PBS-negative controls. FXa also enhanced C2C12 cell migration as compared to the PBS control (195 ± 19%; Figure 4A, right), but again to a lesser extent as compared to the NIH3T3 fibroblasts. Again, FXa exerted its effect in a concentration-dependent manner: As shown in Figure 4B, 0.25 U/ml FXa was already a powerful chemoattractant inducing migration by 146 ± 18%, but maximal induction was observed at 1 U/ml FXa (199 ± 20%).
Figure 4.
Effect of FXa signaling on migration and cytoskeletal reorganization of NIH3T3 and C2C12 cells. A: Migration of NIH3T3 and C2C12 cells toward DMEM (control) or DMEM supplemented with FXa. Results represent the mean ± SEM (n = 3). B: Migration of C2C12 cells in the presence of FXa. Results are shown as mean ± SEM and are representative of two independent experiments performed in duplicate. C: FXa (1 U/ml) induced phosphorylation of FAK and Src. β-Actin serves as loading control. The blots are representative of two independent experiments. D: Inhibition of Src abolishes FXa-induced stimulation of cell migration, as demonstrated by pretreatment of cells with PP1. Results represent the number of cells migrated as percentage of the control and are shown as mean ± SEM (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001. E: Fluorescent microscopy of FXa stimulated C2C12 cells (for 15 minutes) showing that FXa induces phosphorylation of tyrosine residues. Stress fibers are indicated by arrows and the spikes by asterisks. F: Effect of FXa (15-minute incubation) on stress fiber assembly and tyrosine-phosphorylation in the absence or presence of PP1. Red, actin; blue, nucleus; green, phosphotyrosine. Experiments were repeated twice.
A crucial mediator involved in cell migration is the nonreceptor protein tyrosine kinase FAK (focal adhesion kinase). When activated, FAK phosphorylates and/or associates with other cytoskeletal components, including Src, and this complex is involved in actin stress fiber formation and cytoskeletal reorganization. Therefore, we studied the effect of FXa on the FAK/Src pathway. As shown in Figure 4C for C2C12 cells, FXa induced phosphorylation of FAK with maximal phosphorylation at 20 minutes (lane 6). In addition, FXa induced a robust phosphorylation of Src, which also peaked at 20 minutes. To assess further the importance of the FAK/Src pathway in FXa-induced migration, cells were pretreated with PP1, a Src-inhibitor, before performing the migration assay. As shown in Figure 4D, PP1 pretreatment abolished the FXa effect. Taken together, these data suggest that FXa induces fibroblast migration via the FAK/Src pathway. Similar results were obtained with the NIH3T3 cells.
FXa Mediates Cytoskeletal Reorganization and Stress Fiber Formation
To confirm the activation of the FAK/Src pathway by FXa, we used fluorescent microscopy on C2C12 cells stimulated with FXa. As shown in Figure 4E, after 15 minutes of stimulation, spikes (indicated by asterisks) representing phosphorylated tyrosine residues could be observed at the cell membrane. Concomitant with the activation of these kinases, FXa induced actin cytoskeletal reorganization with an increase in stress fiber formation (indicated by arrows) as compared to the control. To assess the involvement of FXa-induced FAK/Src activation in stress fiber formation, cells were pretreated with PP1 and subsequently stimulated with FXa. As shown in Figure 4F, pretreatment with PP1 reduced the amount of cells with stress fibers and with phosphorylated tyrosines at the membrane as compared to the previous conditions (Figure 4E). Overall these data confirm the involvement of the FAK/Src pathway in FXa-mediated cytoskeletal reorganization leading to cell migration.
FXa Facilitates Wound Healing
To assess further the relevance of FXa-induced proliferation and cell migration, we examined the effect of FXa in wound scratch assays. In such assays, C2C12 cells are grown to confluence in monolayers, and their ability to migrate into and across a denuded area of the monolayer is followed throughout time. Control-treated cells hardly migrated into the wounded area within 18 hours (Figure 5A, control), whereas FXa-treated cells did migrate into the wounded area within this time period (Figure 5A, FXa). Both FXa-treated cells as well as 10% FCS-treated cells (positive control) reduced the wound size by ∼50% within 18 hours as compared to no significant reduction in wound size for control-treated cells (Figure 5, A and D). To assess the role of ERK1/2 phosphorylation in FXa-induced wound healing, cells were pretreated with U0126 before FXa treatment. As shown in Figure 5E, ERK1/2 inhibition abolishes the effect of FXa on wound healing. Furthermore, cells were transfected with PAR-2 and/or control siRNA to assess the role of PAR-2 in FXa-induced wound healing. As shown in Figure 5F, PAR-2 siRNA strongly reduced FXa-induced wound healing, whereas control siRNA did not modify the FXa effect.
Figure 5.
FXa facilitates wound healing in a wound scratch assay. A: Wound size of C2C12 fibroblast monolayers after treatment with either PBS (control), FXa (1 U/ml), FCS (10%), FXa/TAP (200 nmol/L), or FXa/PP1 (1 μmol/L) for 18 hours. Shown are photographs of representative microscopic fields. B: Actin staining of fibroblasts treated with PBS (control), FXa (1 U/ml), or FCS (10%) after wound induction. C: Detail of the effect of FXa, in the presence or in the absence of TAP or PP1, on stress fiber formation. D: Quantification of the results depicted in A as described in the Materials and Methods section. E: Involvement of ERK1/2 in FXa-mediated wound healing. Quantification of wound size of fibroblast monolayers after treatment with either PBS (control), FXa (1 U/ml), or FXa/U0126 for 18 hours. F: Wound size of C2C12 cells transfected with control or PAR-2 siRNA, after treatment with either PBS (control) or FXa (1 U/ml) for 18 hours. Results are shown as mean ± SEM of two independent experiments performed in octuplicate. **P < 0.01; ***P < 0.001.
As indicated before, FXa induces stress fiber formation via the FAK/Src pathway and inhibition of Src abolished FXa-induced fibroblast migration. Indeed, fluorescent staining of cells in the wound scratch assay confirmed the induction of stress fibers of cells treated with FXa or 10% FCS, as compared to control-treated cells (Figure 5, B and C). Importantly, PP1 treatment abolished the effect of FXa on wound healing (Figure 5, A and D), which suggests that during the first hours after wound induction, fibroblasts migrate, whereas proliferation becomes prominent only later. To confirm that FXa-induced stress fiber formation is specific and mediated by Src, we pretreated the cells with PP1 or TAP before incubating them with FXa for 18 hours. As shown in Figure 5C, both inhibitors strongly diminished FXa-induced stress fiber formation.
Discussion
In this study, we demonstrated that FXa initiated both proinflammatory and fibroproliferative responses in fibroblasts via PAR-2 activation. The signaling effects of FXa were specific and required its proteolytic activity. We also demonstrated that FXa acted as a chemoattractant. The recruitment of fibroblasts, as well as their subsequent proliferation and synthesis of the ECM are essential in wound healing but are also central features in pathological mechanisms leading to tissue fibrosis and remodeling. The consequences of FXa on fibroblast activation were shown by using a wound scratch model in which FXa induced wound healing. The Src inhibitor PP1, which inhibited migration, also prevented FXa-induced wound healing suggesting that cell migration occurs before proliferation during the wound healing process.
ERK1/2 phosphorylation is widely used as a surrogate marker for PAR-1 and PAR-2 activation.32 Indeed, FXa-induced PAR-2 cleavage results in phosphorylation of ERK1/2 also in our cell lines. As the activation of ERK1/2 has been closely associated with cellular proliferation,33,34 protein synthesis, and more recently wound healing,36 it is tempting to speculate that the ERK1/2 pathway is critically involved in mediating the profibrotic effects of FXa. The fact that the ERK1/2 inhibitor U0126 completely blocked FXa-induced proliferation (Figure 3C) and wound healing (Figure 5E) strongly suggests that the profibrotic response induced by FXa is ERK1/2-dependent.
We established a potential role for coagulation FXa signaling in the progression of tissue fibrosis/wound healing. Fibroblasts can become exposed to coagulation proteases after vascular damage, and activation of the coagulation cascade occurs in many pathological conditions involving wound healing and fibrosis. Moreover, there are a number of potential mechanisms that may lead to increased expression of FXa in inflammatory contexts like atherosclerotic plaque formation or bleomycin-induced lung injury. For instance, FXa may leak into the connective tissue from the vascular compartment as a result of chronic activation of the coagulation cascade, after extensive and continued endothelial injury.
Because activation of the coagulation cascade is relevant to organs where the interstitial compartment is in close contact with the microvasculature bed, one could hypothesize from our data that locally generated FXa first acts as a chemoattractant and contributes to the initiation of fibroblast migration toward the wound or the site of inflammation. Further exposure of the fibroblasts to FXa leads to fibroblast activation, which includes enhancement of the expression of α-actin, TGF-β, fibronectin, and secretion of IL-6 and MCP-1, all of which are implicated in tissue fibrosis and wound healing (Figure 6). Of these, IL-6 and MCP-125 expression has already been reported in fibroblasts and SM cells after stimulation with FXa. IL-6 and MCP-1 expression are suggested to contribute to fibrosis and wound healing as the expression and secretion of IL-6 correlates with fibrosis in the liver,42 skin,43 and lung.44 The development of subepithelial fibrosis in transgenic mice with targeted overexpression of IL-6 in the lung,45 as well as the significant reduction of fibrosis in IL-6-deficient mice in a model of hepatic fibrosis,46 also argues for an important role of IL-6 in fibrosis. MCP-1 stimulates and induces the recruitment of monocytes that in turn secrete cytokines and chemokines thereby promoting inflammation and fibrosis. In addition, MCP-1-deficient mice show less skin fibrosis,47 and anti-MCP-1 therapy attenuates intimal hyperplasia and vein graft thickening by inhibiting vascular SM cell proliferation.48 Our finding that FXa enhanced MCP-1 secretion by fibroblasts supports the view that FXa enhances MCP-1-induced infiltration of inflammatory cells during tissue remodeling.
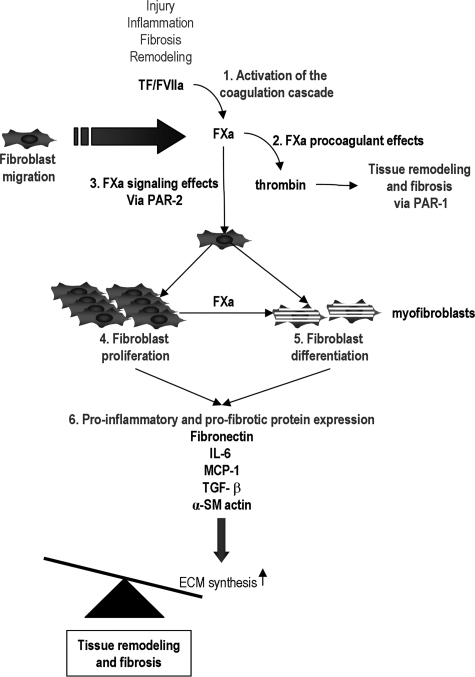
Figure 6.
Proposed role of FXa in wound healing and fibrosis. After activation of the coagulation cascade (1), FXa induces fibrosis via thrombin generation (2) or via PAR-2-dependent signaling (3). Signaling leads to fibroblast proliferation (4) and differentiation into myofibroblasts (5) followed by the secretion of MCP-1, IL-6, TGF-β, and fibronectin (6). Ultimately this leads to the accumulation of ECM thereby disrupting the tight balance between production and degradation of ECM, leading to wound healing and fibrosis.
We observed that FXa induced a time- and concentration-dependent increase in α-SMA expression, which is the hallmark of differentiated myofibroblasts.2 The presence of myofibroblasts, associated with excessive ECM protein biosynthesis, hypercontractility, and organ-destructive remodeling, has been extensively documented in active fibrotic lesions and vascular remodeling.49,50 Differentiation of fibroblasts into myofibroblasts contributed to the progression of, among others, restenosis,6,51,52 as well as intimal hyperplasia and vein graft remodeling53 or atherogenesis.2,54 Therefore, the finding that FXa led to activation of fibroblasts and to the appearance of a myofibroblast phenotype provides additional support for the notion that FXa is an important mediator in tissue remodeling.
We also observed a significant increase in TGF-β expression in fibroblasts exposed to FXa. Because TGF-β is a well established mediator of interstitial fibrosis,7 our observation supports the hypothesis that FXa plays a pathogenic role in the progression of fibrotic diseases. TGF-β is known to induce fibroblast proliferation, differentiation into myofibroblasts,55 and to enhance fibronectin expression. However, it is likely that the expression of these proteins by FXa via PAR-2 activation does not depend on the autocrine effect of TGF-β. Indeed, the time courses for fibronectin, α-SMA, and TGF-β expression did not differ significantly, suggesting that newly synthesized TGF-β did not play a significant role in FXa-induced fibronectin and α-SMA expression. However, it is likely that TGF-β plays a contributing role to these processes at later time points.
Fibronectin is a major component of the ECM, expression of which markedly increases after tissue injury.56 In keeping with its properties in connective tissue remodeling that takes place during wound healing and fibrosis, increased fibronectin expression occurs in pulmonary fibroproliferative disease.57 Furthermore, there is a correlation between atherosclerotic plaque development and fibronectin expression,58 and fibronectin deposition increases after angioplasty during vascular remodeling.59 Because aberrant accumulation of ECM is primarily responsible for the destruction of architecture and loss of function during organ remodeling, FXa-induced fibronectin expression likely contributes to profibrotic responses.
We showed that FXa mediates its effects via PAR-2 by using several approaches. First, we showed that a PAR-1-inhibiting antibody did not block the FXa response, suggesting that PAR-1 was not involved in mediating FXa effects. Second, we showed that trypsin but not thrombin desensitized FXa-mediated cellular effects, thereby suggesting that PAR-2 activation mediated these effects. Finally, to assess definitely the role of PAR-2, we transfected cells with PAR-2 siRNA and showed that FXa effects on cell proliferation, migration (determined in a wound scratch assay), protein synthesis, and ERK1/2 phosphorylation were diminished. FXa-induced signaling has been documented in many cell types originating from different species; however, the receptor involved remained controversial. In human coronary artery SM cells, FXa signaling was mediated via PAR-2 exclusively23 or via the activation of PAR-1 and PAR-2.60 Other studies suggest that in human and murine fibroblasts FXa signals exclusively via PAR-1.22 Finally, studies using rat, human, and rabbit vascular SM cells showed that FXa signaled independent of PARs.24,27 Importantly, the profibrotic and proinflammatory effects induced by FXa-dependent PAR-2 signaling are not redundant of the profibrotic effects of thrombin, including fibronectin, IL-6, MCP-1, and TGF-β expression, which are mediated by PAR-1.15,16
The studies described in this paper were performed using murine fibroblasts, and one could therefore argue that our data are less informative for fibrosis in humans. However, FXa also induces phosphorylation of ERK1/2 and synthesis of profibrotic and proinflammatory proteins in human dermal fibroblasts (Supplementary Figure S2 at http://ajp.amjpathol.org). Furthermore, these human dermal fibroblasts show increased proliferation and enhanced wound healing after FXa stimulation. It therefore seems that the profibrotic effects of FXa are not species-specific and hold true for human cells as well. Obviously, future experiments should elucidate the in vivo relevance of FXa in fibrotic disease.
In conclusion, we have demonstrated that coagulation of FXa stimulates proinflammatory and fibroproliferative responses in fibroblasts and their differentiation into myofibroblasts. Further studies are needed to assess the in vivo relevance of these results, but it can be hypothesized that these responses, which are mediated by PAR-2, may help explain the extent of fibrosis and remodeling observed in association with activation of the coagulation cascade. Although therapies that specifically block PAR-1 activation partly limit the progression of fibrosis, combined strategies targeting both PAR-1 (induced by thrombin) and PAR-2 (induced by FXa) activation may be more effective.
Footnotes
Address reprint requests to Keren S. Borensztajn, Center for Experimental and Molecular Medicine, Academic Medical Center, Meibergdreef 9, NL-1105 AZ, Amsterdam, The Netherlands. E-mail: k.s.borensztajn@amc.uva.nl.
Supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (to K.S.B.).
Supplemental for this article can be found on http://ajp.amjpathol.org.
References
- Furie B, Furie BC. The molecular basis of blood coagulation. Cell. 1988;53:505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164:1007–1019. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63:21–29. [PubMed] [Google Scholar]
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- Christen T, Verin V, Bochaton-Piallat M, Popowski Y, Ramaekers F, Debruyne P, Camenzind E, van Eys G, Gabbiani G. Mechanisms of neointima formation and remodeling in the porcine coronary artery. Circulation. 2001;103:882–888. doi: 10.1161/01.cir.103.6.882. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Eneström S, Druid H, Rammer L. Fibrin deposition in the kidney in post-ischaemic renal damage. Br J Exp Pathol. 1988;69:387–394. [PMC free article] [PubMed] [Google Scholar]
- Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003;31:S213–S220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- Spronk HM, van der Voort D, Ten Cate H. Blood coagulation and the risk of atherothrombosis: a complex relationship. Thromb J. 2004;2:12. doi: 10.1186/1477-9560-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager NA, Abendschein DR, McKenzie CR, Eisenberg PR. Role of thrombin compared with factor Xa in the procoagulant activity of whole blood clots. Circulation. 1995;92:962–967. doi: 10.1161/01.cir.92.4.962. [DOI] [PubMed] [Google Scholar]
- Bauters C, Lablanche JM, McFadden EP, Hamon M, Bertrand ME. Relation of coronary angioscopic findings at coronary angioplasty to angiographic restenosis. Circulation. 1995;92:2473–2479. doi: 10.1161/01.cir.92.9.2473. [DOI] [PubMed] [Google Scholar]
- Coughlin SR. Protease-activated receptors in vascular biology. Thromb Haemost. 2001;86:298–307. [PubMed] [Google Scholar]
- Riewald M, Kravchenko VV, Petrovan RJ, O’Brien PJ, Brass LF, Ulevitch RJ, Ruf W. Gene induction by coagulation factor Xa is mediated by activation of protease-activated receptor 1. Blood. 2001;97:3109–3116. doi: 10.1182/blood.v97.10.3109. [DOI] [PubMed] [Google Scholar]
- Bogatkevich GS, Tourkina E, Silver RM, Ludwicka-Bradley A. Thrombin differentiates normal lung fibroblasts to a myofibroblast phenotype via the proteolytically activated receptor-1 and a protein kinase C-dependent pathway. J Biol Chem. 2001;276:45184–45192. doi: 10.1074/jbc.M106441200. [DOI] [PubMed] [Google Scholar]
- Zhang A, Liu X, Cogan JG, Fuerst MD, Polikandriotis JA, Kelm RJ, Jr, Strauch AR. YB-1 coordinates vascular smooth muscle alpha-actin gene activation by transforming growth factor beta1 and thrombin during differentiation of human pulmonary myofibroblasts. Mol Biol Cell. 2005;16:4931–4940. doi: 10.1091/mbc.E05-03-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Zhu Z, Liu J, Wang Y, Li Z. Role of protease activated receptor-2 expression in renal interstitial fibrosis model in mice. J Huazhong Univ Sci Technolog Med Sci. 2005;25:523–526. doi: 10.1007/BF02896006. [DOI] [PubMed] [Google Scholar]
- Cederqvist K, Haglund C, Heikkila P, Hollenberg MD, Karikoski R, Andersson S. High expression of pulmonary proteinase-activated receptor 2 in acute and chronic lung injury in preterm infants. Pediatr Res. 2005;57:831–836. doi: 10.1203/01.PDR.0000161416.63314.70. [DOI] [PubMed] [Google Scholar]
- Masamune A, Kikuta K, Satoh M, Suzuki N, Shimosegawa T. Protease-activated receptor-2-mediated proliferation and collagen production of rat pancreatic stellate cells. J Pharmacol Exp Ther. 2005;312:651–658. doi: 10.1124/jpet.104.076232. [DOI] [PubMed] [Google Scholar]
- Grandaliano G, Pontrelli P, Cerullo G, Monno R, Ranieri E, Ursi M, Loverre A, Gesualdo L, Schena FP. Protease-activated receptor-2 expression in IgA nephropathy: a potential role in the pathogenesis of interstitial fibrosis. J Am Soc Nephrol. 2003;14:2072–2083. doi: 10.1097/01.asn.0000080315.37254.a1. [DOI] [PubMed] [Google Scholar]
- Borensztajn KS, Bijlsma MF, Groot AP, Bruggemann LW, Versteeg HH, Reitsma PH, Peppelenbosch MP, Spek CA. Coagulation factor Xa drives tumour cells into apoptosis through BH3-only protein Bim up-regulation. Exp Cell Res. 2007;313:2622–2633. doi: 10.1016/j.yexcr.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Blanc-Brude OP, Archer F, Leoni P, Derian C, Bolsover S, Laurent GJ, Chambers RC. Factor Xa stimulates fibroblast procollagen production, proliferation, and calcium signaling via PAR1 activation. Exp Cell Res. 2005;304:16–27. doi: 10.1016/j.yexcr.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Koo BH, Chung KH, Hwang KC, Kim DS. Factor Xa induces mitogenesis of coronary artery smooth muscle cell via activation of PAR-2. FEBS Lett. 2002;523:85–89. doi: 10.1016/s0014-5793(02)02948-4. [DOI] [PubMed] [Google Scholar]
- Rauch BH, Millette E, Kenagy RD, Daum G, Clowes AW. Thrombin- and factor Xa-induced DNA synthesis is mediated by transactivation of fibroblast growth factor receptor-1 in human vascular smooth muscle cells. Circ Res. 2004;94:340–345. doi: 10.1161/01.RES.0000111805.09592.D8. [DOI] [PubMed] [Google Scholar]
- Bachli EB, Pech CM, Johnson KM, Johnson DJ, Tuddenham EG, McVey JH. Factor Xa and thrombin, but not factor VIIa, elicit specific cellular responses in dermal fibroblasts. J Thromb Haemost. 2003;1:1935–1944. doi: 10.1046/j.1538-7836.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- Millette E, Rauch BH, Defawe O, Kenagy RD, Daum G, Clowes AW. Platelet-derived growth factor-BB-induced human smooth muscle cell proliferation depends on basic FGF release and FGFR-1 activation. Circ Res. 2005;96:172–179. doi: 10.1161/01.RES.0000154595.87608.db. [DOI] [PubMed] [Google Scholar]
- Koo BH, Kim DS. Factor Xa induces mitogenesis of vascular smooth muscle cells via autocrine production of epiregulin. J Biol Chem. 2003;278:52578–52586. doi: 10.1074/jbc.M310007200. [DOI] [PubMed] [Google Scholar]
- Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J. Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res. 2006;66:307–314. doi: 10.1158/0008-5472.CAN-05-1735. [DOI] [PubMed] [Google Scholar]
- Camerer E, Kataoka H, Kahn M, Lease K, Coughlin SR. Genetic evidence that protease-activated receptors mediate factor Xa signaling in endothelial cells. J Biol Chem. 2002;277:16081–16087. doi: 10.1074/jbc.M108555200. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Arai H, Liu N, Nogaki F, Nomura K, Kasuno K, Oida E, Kita T, Ono T. Role of coagulation factor Xa and protease-activated receptor 2 in human mesangial cell proliferation. Kidney Int. 2005;67:2123–2133. doi: 10.1111/j.1523-1755.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- Versteeg HH, Hoedemaeker I, Diks SH, Stam JC, Spaargaren M, van Bergen En Henegouwen PM, van Deventer SJ, Peppelenbosch MP. Factor VIIa/tissue factor-induced signaling via activation of Src-like kinases, phosphatidylinositol 3-kinase, and Rac. J Biol Chem. 2000;275:28750–28756. doi: 10.1074/jbc.M907635199. [DOI] [PubMed] [Google Scholar]
- Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- Dunn C, Wiltshire C, MacLaren A, Gillespie DA. Molecular mechanism and biological functions of c-Jun N-terminal kinase signalling via the c-Jun transcription factor. Cell Signal. 2002;14:585–593. doi: 10.1016/s0898-6568(01)00275-3. [DOI] [PubMed] [Google Scholar]
- Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- Padmasekar M, Nandigama R, Wartenberg M, Schluter KD, Sauer H. The acute phase protein alpha2-macroglobulin induces rat ventricular cardiomyocyte hypertrophy via ERK1,2 and PI3-kinase/Akt pathways. Cardiovasc Res. 2007;75:118–128. doi: 10.1016/j.cardiores.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Fitsialos G, Chassot AA, Turchi L, Dayem MA, LeBrigand K, Moreilhon C, Meneguzzi G, Busca R, Mari B, Barbry P, Ponzio G. Transcriptional signature of epidermal keratinocytes subjected to in vitro scratch wounding reveals selective roles for ERK1/2, p38, and phosphatidylinositol 3-kinase signaling pathways. J Biol Chem. 2007;282:15090–15102. doi: 10.1074/jbc.M606094200. [DOI] [PubMed] [Google Scholar]
- Feistritzer C, Lenta R, Riewald M. Protease-activated receptors-1 and -2 can mediate endothelial barrier protection: role in factor Xa signaling. J Thromb Haemost. 2005;3:2798–2805. doi: 10.1111/j.1538-7836.2005.01610.x. [DOI] [PubMed] [Google Scholar]
- Versteeg HH, Spek CA, Richel DJ, Peppelenbosch MP. Coagulation factors VIIa and Xa inhibit apoptosis and anoikis. Oncogene. 2004;23:410–417. doi: 10.1038/sj.onc.1207066. [DOI] [PubMed] [Google Scholar]
- Bijlsma MF, Borensztajn KS, Roelink H, Peppelenbosch MP, Spek CA. Sonic hedgehog induces transcription-independent cytoskeletal rearrangement and migration regulated by arachidonate metabolites. Cell Signaling. 2007;19:2596–2604. doi: 10.1016/j.cellsig.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Slofstra SH, Cate HT, Spek CA. Low dose endotoxin priming is accountable for coagulation abnormalities and organ damage observed in the Shwartzman reaction. A comparison between a single-dose endotoxemia model and a double-hit endotoxin-induced Shwartzman reaction. Thromb J. 2006;4:13. doi: 10.1186/1477-9560-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyden B. The myofibroblast: a study of normal, reactive and neoplastic tissues, with an emphasis on ultrastructure. Part 2—tumours and tumour-like lesions. J Submicrosc Cytol Pathol. 2005;37:231–296. [PubMed] [Google Scholar]
- Kayano K, Okita K. Does IL-6 regulate liver fibrosis/cirrhosis directly and indirectly? J Gastroenterol. 2000;35:250–251. doi: 10.1007/s005350050339. [DOI] [PubMed] [Google Scholar]
- Sato S, Hasegawa M, Takehara K. Serum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosis. J Dermatol Sci. 2001;27:140–146. doi: 10.1016/s0923-1811(01)00128-1. [DOI] [PubMed] [Google Scholar]
- Fries KM, Felch ME, Phipps RP. Interleukin-6 is an autocrine growth factor for murine lung fibroblast subsets. Am J Respir Cell Mol Biol. 1994;11:552–560. doi: 10.1165/ajrcmb.11.5.7946384. [DOI] [PubMed] [Google Scholar]
- DiCosmo B, Geba G, Picarella D, Elias JA, Rankin JA, Stripp B, Whitsett JA, Flavell RA. Expression of interleukin-6 by airway epithelial cells. Effects on airway inflammation and hyperreactivity in transgenic mice. Chest. 1995;107:131S. doi: 10.1378/chest.107.3_supplement.131s. [DOI] [PubMed] [Google Scholar]
- Natsume M, Tsuji H, Harada A, Akiyama M, Yano T, Ishikura H, Nakanishi I, Matsushima K, Kaneko S, Mukaida N. Attenuated liver fibrosis and depressed serum albumin levels in carbon tetrachloride-treated IL-6-deficient mice. J Leukoc Biol. 1999;66:601–608. [PubMed] [Google Scholar]
- Ferreira AM, Takagawa S, Fresco R, Zhu X, Varga J, DiPietro LA. Diminished induction of skin fibrosis in mice with MCP-1 deficiency. J Invest Dermatol. 2006;126:1900–1908. doi: 10.1038/sj.jid.5700302. [DOI] [PubMed] [Google Scholar]
- Schepers A, Eefting D, Bonta PI, Grimbergen JM, de Vries MR, van Weel V, de Vries CJ, Egashira K, van Bockel JH, Quax PH. Anti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2006;26:2063–2069. doi: 10.1161/01.ATV.0000235694.69719.e2. [DOI] [PubMed] [Google Scholar]
- Heeneman S, Cleutjens JP, Faber BC, Creemers EE, van Suylen RJ, Lutgens E, Cleutjens KB, Daemen MJ. The dynamic extracellular matrix: intervention strategies during heart failure and atherosclerosis. J Pathol. 2003;200:516–525. doi: 10.1002/path.1395. [DOI] [PubMed] [Google Scholar]
- Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- Shi Y, O’Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- Aikawa M, Sakomura Y, Ueda M, Kimura K, Manabe I, Ishiwata S, Komiyama N, Yamaguchi H, Yazaki Y, Nagai R. Redifferentiation of smooth muscle cells after coronary angioplasty determined via myosin heavy chain expression. Circulation. 1997;96:82–90. doi: 10.1161/01.cir.96.1.82. [DOI] [PubMed] [Google Scholar]
- Hao H, Gabbiani G, Camenzind E, Bacchetta M, Virmani R, Bochaton-Piallat ML. Phenotypic modulation of intima and media smooth muscle cells in fatal cases of coronary artery lesion. Arterioscler Thromb Vasc Biol. 2006;26:326–332. doi: 10.1161/01.ATV.0000199393.74656.4c. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M, Takayanagi T, Egashira K, Takeshita A. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. J Clin Invest. 1996;97:769–776. doi: 10.1172/JCI118476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1). Am J Respir Cell Mol Biol. 1999;21:658–665. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- Dang Y, Cole AA, Homandberg GA. Comparison of the catabolic effects of fibronectin fragments in human knee and ankle cartilages. Osteoarthritis Cartilage. 2003;11:538–547. doi: 10.1016/s1063-4584(03)00085-2. [DOI] [PubMed] [Google Scholar]
- Limper AH, Roman J. Fibronectin. A versatile matrix protein with roles in thoracic development, repair and infection. Chest. 1992;101:1663–1673. doi: 10.1378/chest.101.6.1663. [DOI] [PubMed] [Google Scholar]
- Matter CM, Schuler PK, Alessi P, Meier P, Ricci R, Zhang D, Halin C, Castellani P, Zardi L, Hofer CK, Montani M, Neri D, Luscher TF. Molecular imaging of atherosclerotic plaques using a human antibody against the extra-domain B of fibronectin. Circ Res. 2004;95:1225–1233. doi: 10.1161/01.RES.0000150373.15149.ff. [DOI] [PubMed] [Google Scholar]
- Buerke M, Guckenbiehl M, Schwertz H, Buerke U, Hilker M, Platsch H, Richert J, Bomm S, Zimmerman GA, Lindemann S, Mueller-Werdan U, Werdan K, Darius H, Weyrich AS. Intramural delivery of Sirolimus prevents vascular remodeling following balloon injury. Biochim Biophys Acta. 2007;1774:5–15. doi: 10.1016/j.bbapap.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean K, Schirm S, Johns A, Morser J, Light DR. FXa-induced responses in vascular wall cells are PAR-mediated and inhibited by ZK-807834. Thromb Res. 2001;103:281–297. doi: 10.1016/s0049-3848(01)00330-9. [DOI] [PubMed] [Google Scholar]