Abstract
Human and mouse Cripto-1 (CR-1/Cr-1) proteins play an important role in mammary gland development and tumorigenesis. In this study, we examined the relationship between Cripto-1 and caveolin-1 (Cav-1), a membrane protein that acts as a tumor suppressor in the mammary gland. Cripto-1 was found to interact with Cav-1 in COS7 cells and mammary epithelial cells. Using EpH4 mouse mammary epithelial cells expressing Cr-1 (EpH4 Cr-1) or Cr-1 and Cav-1 (EpH4 Cr-1/Cav-1), we demonstrate that Cav-1 expression markedly reduced the ability of Cr-1 to enhance migration, invasion, and formation of branching structures in EpH4 Cr-1/Cav-1 cells as compared to EpH4 Cr-1 cells. Furthermore, coexpression of Cav-1 together with Cr-1 in EpH4 Cr-1/Cav-1 cells inhibited Cr-1-mediated activation of c-src and mitogen-activated protein kinase signaling pathways. Conversely, primary mammary epithelial cells isolated from Cav-1 null−/−/mouse mammary tumor virus-CR-1 transgenic animals showed enhanced motility and activation of mitogen-activated protein kinase and c-src as compared to Cav-1+/−/CR-1 mammary cells. Finally, mammary tumors derived from mouse mammary tumor virus-CR-1 mice showed a dramatic reduction of Cav-1 expression as compared to mammary tissue from normal FVB/N mice, suggesting that in vivo Cav-1 is down-regulated during the process of CR-1-mediated mammary tumorigenesis.
Human and mouse Cripto-1 (CR-1/Cr-1) are members of the epidermal growth factor (EGF)-CFC (Cripto in humans, FRL1 in Xenopus, and Cryptic in mice) protein family.1,2,3 EGF-CFC family members contain a modified EGF-like domain, a conserved cysteine-rich domain (CFC domain) and a short hydrophobic carboxyl terminus that contains a consensus sequence for glycosylphosphatidylinositol (GPI) cleavage and attachment.4 Cripto-1 is mostly found to be cell membrane associated. However, Cripto-1 can also be detected in the conditioned medium of several cell lines and in the plasma of patients with colon and breast cancer, due to cleavage of the GPI linkage by GPI-phospholipase D.5,6,7,8,9,10 Cripto-1 plays a crucial role in early embryonic development and during cellular transformation.1,2,3 During embryogenesis, Cripto-1 functions as an essential co-receptor for the transforming growth factor β-related proteins Nodal and Vg1/growth and differentiation factor-1 and -3 and regulates the formation of the primitive streak during gastrulation, patterning of the anterior/posterior axis, specification of mesoderm and endoderm and establishment of left/right asymmetry of developing organs.11,12,13 EGF-CFC proteins bind directly to Nodal through the EGF-like domain and to the activin type I receptor Alk4 through the CFC domain, recruiting the activin type II receptor and inducing activation of Smad2/Smad3/Smad4 intracellular signaling pathway.14,15 Cripto-1 has also been shown to regulate mouse and human embryonic stem cell self-renewal and pluripotentiality, and it is therefore considered a stem cell marker.16,17,18 In the adult, CR-1 is overexpressed in different types of primary human carcinomas, including breast, colon, stomach, pancreas, ovary, cervix, endometrium, and testis.19 Overexpression of mouse Cr-1 can lead to the in vitro transformation of EpH4 mouse mammary epithelial cells and can increase migration, invasion, branching morphogenesis, and epithelial-to-mesenchymal transition of several human and mouse mammary epithelial cells.20,21,22,23 Cripto-1 can also enhance endothelial cell migration and invasion in vitro and stimulate angiogenesis in vivo.24 In mouse and human mammary epithelial cells and in cervical and breast carcinoma cells, Cripto-1 has been shown to activate mitogen-activated protein kinase (MAPK), phosphatidylinositol 3′ kinase (PI3K)/AKT and the cytoplasmic tyrosine kinase c-src, through binding of CR-1 to the GPI cell surface-linked heparan sulfate proteoglycan glypican-1.25,26,27 Furthermore, transgenic mouse models have demonstrated that overexpression of a human CR-1 transgene in the mammary gland under the control of the mouse mammary tumor virus (MMTV) or the whey acidic protein promoter results in multifocal mammary hyperplasias and adenocarcinomas in approximately 30 to 50% of multiparous mice.28,29 MMTV-CR-1 mice have also been recently shown to develop uterine leiomyosarcomas.30
The presence of a GPI anchor in the mouse and human Cripto-1 protein suggests that this molecule might localize to lipid rafts, which are cell membrane regions that function as microdomains for a number of signaling molecules that are GPI-linked.31 A subclass of lipid rafts is represented by caveolae.32 Caveolae are vesicular invaginations of the plasma membrane characterized by the presence of membrane proteins of the caveolin family.33,34 Caveolae have been involved in several cellular events including endocytosis, regulation and transport of cholesterol, and signal transduction.35 In particular, caveolin-1 (Cav-1) has been shown to function as a negative effector of the basal activity of several signaling pathways, by directly interacting with signaling molecules, such as the EGF receptor, c-src, Ha-Ras, transforming growth factor β receptors, and MAPK through a 20-amino- acid region, the caveolin scaffolding domain (CSD, amino acids 81-101).34,36 Synthetic peptides that correspond to the amino acid sequence of the CSD can function as inhibitors of several different signaling molecules that localize to caveolae.34,37 Several studies have shown that Cav-1 has a tumor suppressor function in the mammary gland. In this regard, Cav-1 mRNA and protein are down-regulated in human primary breast carcinomas and cell lines and reintroduction of Cav-1 in vitro is sufficient to inhibit the tumorigenic properties of breast cancer cells, including anchorage-independent growth and invasiveness.38,39,40,41,42 More recently, Cav-1 null mice have provided a more complete delineation of the role of Cav-1 in the pathogenesis of mammary epithelial cell tumorigenesis in an in vivo setting. Under physiological conditions, Cav-1 null mice develop hyperplastic lesions in the virgin mammary gland and exhibit precocious lobuloalveolar development and lactation during pregnancy.43,44 Although Cav-1 null mice do not develop mammary tumors, they are more susceptible to tumor formation induced by different oncogenes. For example, homozygous loss of Cav-1 in MMTV-PyMT mice results in an accelerated rate of mammary tumor development and an increase in the number of tumors as compared to MMTV-PyMT mice that posses a single wild-type (WT) Cav-1 allele.45,46
In the present study we have investigated the functional interaction between Cav-1 and Cripto-1, with respect to the ability of Cripto-1 to stimulate mammary epithelial cell migration and invasion and activate intracellular signaling molecules, such as c-src and MAPK, in the presence or absence of Cav-1 expression. Finally, we have evaluated Cav-1 expression in mammary adenocarcinomas that arise in MMTV-CR-1 transgenic mice.
Materials and Methods
Cell Cultures and Transfections
EpH4 mouse mammary epithelial, monkey kidney COS7 and human 293 embryonal kidney cells (HEK 293) were grown in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. MCF-10A human mammary epithelial cells were grown as previously described.47 EpH4 WT and EpH4-overexpressing Cr-1 (EpH4 Cr-1) cells21 were transfected with a mouse Cav-1 cDNA expression vector (UB6-Cav-1-V5/His) using Fugene 6 (Roche, Indianapolis, IN), as recommended by the supplier, and the polyclonal cell lines were selected with blasticidin (5 μg/ml) (Invitrogen, Carlsbad, CA) for two weeks. Expression of Cav-1 in the transfected cells (EpH4 Cav-1 and EpH4 Cr-1/Cav-1 cells) was confirmed by Western blot analysis using a mouse monoclonal anti-V5 antibody (1:5000, Invitrogen) to detect Cav-1-V5/His fusion protein. We also assessed Cr-1 expression in these cells by Western blot analysis as previously described.21 An anti-β-actin mouse monoclonal antibody (Sigma, St. Louis, MO) was used to ensure equal loading. HEK 293 cells and COS7 cells (6 × 106 cells in 100-mm-diameter plates) were transiently transfected with 8 μg of an expression plasmid encoding human CR-125 and Alk4-Flag14 or human CR-1 alone, respectively, using Fugene 6 (Roche). Forty-eight hours after transfection, cells were lysed and membrane fractions isolated (see below).
Expression Vectors
The expression construct containing mouse Cav-1 cDNA in pcDNA3-myc/His expression vector was kindly provided by Dr. Richard G.W. Anderson (Department of Cell Biology, University of Texas Southwestern Medical Center, Dallas, TX). Mouse Cav-1 cDNA was also subcloned into the EcoRI/XhoI sites of pUB6-V5/His plasmid (Invitrogen) to generate EpH4 Cav-1 and EpH4 Cr-1/Cav-1 cells. Mouse and human Cripto-1, Alk4-Flag, c-src, and glypican-1-Fc expression vectors have been previously described.14,25
Isolation of Low-Density Triton X-100-Insoluble Complexes by Sucrose Density Centrifugation
CR-1 and Alk4-Flag transiently transfected 293 cells or CR-1 transiently transfected COS7 cells were grown in 100-mm-diameter plates to prepare membrane fractions. An established method using the nonionic detergent Triton X-100 was used to prepare low-density detergent insoluble membrane fractions.48,49 After two washes with phosphate-buffered saline, plates were scraped in 2 ml of 2-N-morpholinoethanesulfonic acid-buffered saline containing 25 mmol/L 2-N-morpholinoethanesulfonic acid, pH 6.5, 0.15 mol/L NaCl, and 1% Triton X-100 and solubilized for 20 minutes at 4°C. The cells were homogenized by a tight-fitting Dounce homogenizer, and the homogenates were adjusted to 40% sucrose by adding 2 ml of 80% sucrose in 2-N-morpholinoethanesulfonic acid-buffered saline. A 5 to 40% discontinuous gradient was formed and centrifuged at 40,000 rpm for 20 hours in a SW40Ti rotor (Beckmann Instruments, Palo Alto, CA). Twelve 1-ml fractions were removed from the top of the tubes and analyzed by Western blotting using anti-CR-1 mouse monoclonal antibody (B3F6, Biogen-Idec, Cambridge, MA), anti-Flag monoclonal antibody (Sigma), anti-Cav-1 monoclonal antibody (1:1000, BD Bioscience PharMingen, San Jose, CA), and anti-β-actin monoclonal antibody (1:1000, Sigma), as previously described.14 Dot blot for the lipid raft marker GM1 was performed on 96-well Bio-blot microfilter apparatus (BioRad, Hercules, CA). Fifty microliters of each sample was blotted on nitrocellulose membrane by passive filtration and the filter incubated with horseradish peroxidase-conjugated cholera toxin subunit B (Sigma) and visualized with enhanced chemiluminescence detection reagent (Amersham, Piscataway, NJ).
Coimmunoprecipitation in COS7, MCF-10A, and FVB/N Mammary Epithelial Cells
COS7 cells (8 × 105 cells in 60-mm-diameter plates) were transiently transfected with CR-1, Cav-1-myc/His, c-src, and glypican-1-Fc expression vectors either alone or in various indicated combinations using Fugene 6 (Roche). Forty-eight hours after transfection, the cells were lysed as previously described,14 and 800 μg of proteins were immunoprecipitated for 2 hours at 4°C with anti-c-myc monoclonal antibody agarose beads (Sigma) to immunoprecipitate Cav-1-myc/His fusion protein or with protein G-agarose beads to immunoprecipitate glypican-1-Fc fusion protein. The bound proteins were eluted with sample buffer and analyzed by Western blot analysis using a rabbit polyclonal anti-CR-1 antibody (Biocon, Frederick, MD), a mouse monoclonal anti-c-src antibody (GD11, Upstate Biotechnology, Chicago, IL), an anti-human IgG horseradish peroxidase-linked whole antibody (Amersham), an anti-myc monoclonal antibody (1:1000, 9E10, Covance, Princeton, NJ), or an anti-Flag mouse monoclonal antibody (Sigma).14,25 Before immunoprecipitation COS7 cell lysates were also analyzed by Western blot to check for expression of the transfected plasmids in the cells using the above-mentioned antibodies. In MCF-10A and FVB/N mammary epithelial cells, endogenous CR-1 protein was immunoprecipitated with anti-CR-1 rabbit polyclonal antibody (Biocon) and analyzed by Western blot using an anti-Cav-1 rabbit polyclonal antibody (1:1000, N-20, Santa Cruz Biotechnology, Santa Cruz, CA), as described above.
Generation of Cav-1 Null−/−/MMTV-CR-1 Double Transgenic Mice
Cav-1 null and MMTV-CR-1 mice, both in the FVB/N background, have been previously described.28,50 To generate Cav-1 null−/−/MMTV-CR-1 double transgenic animals, Cav-1 null female mice were crossed with MMTV/CR-1 homozygous male mice. Approximately 20% of the offspring had the correct genotype as assessed by PCR reaction using previously published primers sets.28,50 Cav-1 heterozygous+/−/MMTV-CR-1 mice were also generated and used in our study.
Isolation of Primary Mammary Epithelial Cells
Primary mammary epithelial cell clusters (organoids) from the fourth and fifth mammary glands of 2- to 3-month-old virgin mice were isolated as previously described.42 Briefly, after aseptically removing the mammary glands of each genotype (FVB/N control, Cav-1+/−/CR-1 and Cav-1−/−/CR-1), the glands were minced with surgical blades and incubated for 3 hours at 37°C in a shaker in 35 ml of growth medium51 containing 2 mg/ml of collagenase type I and 50 μg/ml gentamicin. Cells were then centrifuged to eliminate floating fat cells, and pellets were resuspended in 10 ml of assay medium.51 To eliminate single cells, such as fibroblasts, endothelial, and smooth muscle cells, pellets underwent differential centrifugations (1000 rpm for 45 seconds repeated 10 times). After the last wash, organoids were resuspened in growth medium and repeatedly pipetted with a 1-ml blue tip to disrupt cell clusters. Cells were then plated in two-well chamber slides for immunofluorescence studies and in 100- or 60-mm plates to allow them to grow as a monolayer. Animal care was in accordance with institutional guidelines, and all experiments were performed under an approved protocol by the National Institutes of Health.
Western Blot Analysis
EpH4 WT, EpH4 Cr-1, EpH4 Cav-1 and EpH4 Cr-1/Cav-1 were seeded in 60-mm-diameter plates (8 × 105 cells/plate) and the following day were serum-starved for 6 hours in the presence or absence of a cell-permeable Cav-1 scaffolding domain peptide (cavtratin, aa 82-101, Calbiochem, San Diego, CA), 10 μmol/L, or a scrambled Cav-1 scaffolding domain used as negative control (control peptide, Calbiochem), 10 μmol/L. We also analyzed primary mammary epithelial cells isolated from FVB/N control mice, Cav-1+/−/ CR-1 or Cav-1−/−/CR-1 transgenic mice growing in 60-mm plates (passage 1) with growth medium containing EGF (20 ng/ml) (Biosource, Camarillo, CA). EpH4 cells and primary mammary epithelial cells were lysed and Western blot analysis was performed for phosphorylated and total MAPK or c-src, as previously described.25 Primary mammary FVB/N, Cav-1+/−/CR-1 or Cav-1−/−/CR-1 cell lysates were also analyzed by Western blot for expression of Cav-1 and CR-1 using anti-Cav-1 rabbit polyclonal antibody (1:1000, N-20, Santa Cruz Biotechnology) and anti-CR-1 B3.F6 mouse monoclonal antibody (Biogen-Idec). Frozen mammary tissues from normal FVB/N or MMTV-CR-1 virgin mice and from four mammary tumors (T1, T2, T3, and T4) found in MMTV-CR-1 multiparous mice were homogenized, as previously described.20 Western blot analysis was performed using an anti-Cav-1 mouse monoclonal antibody (1:1000, N20, Santa Cruz Biotechnology). For control for equal loading, the blot was reprobed with β-actin antibody (1:5000, Sigma). Densitometric analysis of the bands on the Western blots was performed with the NIH Image program (http://rbs.info.nih.gov/nih-image/, last accessed June 4, 2007). Density of the bands was normalized to total protein expression of the nonphosphorylated forms of each protein.
Migration and Invasion Assays
Migration and invasion assays, respectively, were performed in fibronectin-coated or Matrigel-coated Boyden chambers (QCM-FN Quantitative Cell Migration Assay and Cell Invasion Assay Kit, Chemicon, Temecula, CA), as previously described.24,25 Briefly, EpH4 WT, EpH4 Cr-1, EpH4 Cav-1, and EpH4 Cr-1/Cav-1 cells or FVB/N, Cav-1+/−/CR-1, and Cav-1−/−/CR-1 primary cells at passage 2 were seeded in 12-well plates at 2 × 105 per well. The Boyden chambers were incubated overnight at 37°C, and the cells that had migrated or invaded the Matrigel through the filter and attached to the bottom of the membrane were stained with a crystal violet solution. The stain solution was eluted, and the absorbance was read at 595 nm. For the migration and invasion assays with cavtratin or control peptide, EpH4 Cr-1 cells or Cav-1−/−/CR-1 primary mammary epithelial cells were seeded in 12-well plates (2 × 105 per well) and incubated in the upper Boyden chambers with cavtratin (5 or 10 μmol/L) or control peptide (5 or 10 μmol/L) (Calbiochem). In all of the experiments, 5% fetal bovine serum was used as chemoattractant in the lower chamber. These experiments were repeated three times with duplicate samples.
In Vitro Matrigel Branching Assay
EpH4 WT, EpH4 Cr-1, EpH4 Cav-1, and EpH4 Cr-1/Cav-1 cells were seeded in Matrigel matrix growth factor reduced (Becton Dickinson, San Jose, CA) coated 24-well plates at 5 × 104 cells per well. After 1 week, colonies with branching structures were counted and photographed. The experiment was repeated two times with triplicate samples.
Immunofluorescence and Confocal Microscopy
Approximately 2 × 104 EpH4 Cr-1 or EpH4 Cr-1/Cav-1 cells were cultured overnight in Lab-Tek dual chamber slides (Nalge Nunc International, Naperville, IL). COS7 cells were transiently transfected with mouse Cr-1 and Cav-1 expression vectors using Fugene 6, and 48 hours after transfection they were seeded in dual chamber slides. Primary FVB/N mammary epithelial cells were directly seeded in dual chamber slides after isolation and purification from the mammary gland, and they were analyzed after they reached approximately 50% confluence. Culture medium was removed, and cells were incubated with growth medium containing 1 μg/ml cholera toxin subunit B (CT-B) labeled with Alexa Fluor 594 dye (Invitrogen) for 10 minutes at 4°C. Anti-CT-B rabbit serum (Invitrogen) was then added to the cells for 15 minutes at 4°C to cross-link the CT-B to the membrane ganglioside GM1. Cells were washed three times with phosphate-buffered saline and fixed with 4% formaldehyde for 15 minutes at 4°C. Slides were washed again three times with phosphate-buffered saline, blocked for 30 minutes with 5% bovine serum albumin, and incubated overnight at 4°C with primary goat polyclonal antibody against mouse Cr-1 (F20, 1:100, Santa Cruz Biotechnology), with rabbit polyclonal anti-Cav-1 antibody fluorescein isothiocyanate conjugated (1:100, Santa Cruz) for colocalization in EpH4 Cr-1/Cav-1 cells of Cav-1 and GM1 or with rabbit polyclonal anti-Cav-1 antibody (1:100, N-20, Santa Cruz Biotechnology) for all of the other experiments. Cells were then washed three times with phosphate-buffered saline and incubated for 30 minutes with donkey anti-goat Alexa Fluor-conjugated secondary antibody (1:800, Molecular Probes, Eugene, OR) and with rabbit anti-mouse rhodamine-conjugated secondary antibody (1:800, Molecular Probes). After final washes with phosphate-buffered saline, slides were mounted with ProLong antifade mounting solution (Molecular Probes). Negative controls were obtained by replacing the primary antibodies with isotype nonspecific Ig. The slides were observed with a Zeiss Axioplan 2 microscope, and images were captured using a Perkin/Elmer Ultra View CSU 10 confocal head and IP Lab 3.9 software.
Immunohistochemistry
Immunohistochemistry for CR-1 in mammary tissue serial sections from FVB/N control and MMTV-CR-1 mice was performed as previously described.20,28 For Cav-1 immunodetection, the sections were incubated with an anti-Cav-1 rabbit polyclonal antibody (N-20, Santa Cruz Biotechnology) diluted at 1:100. Negative controls were obtained by replacing the primary antibody with irrelevant control isotype IgG.
Statistical Analysis
The nonparametric Wilcoxon rank sum test was used to assess the statistical significances of the differences between groups. Statistical calculations were done with the use of Statistical Package for Social Sciences software package, version 11.0 (SPSS, Chicago, IL). The statistical tests were two-sided, and data were considered statistically significant at P < 0.05.
Results
CR-1 Localizes in Low-Density Triton X-100-Insoluble Complexes Together with Cav-1 and GM1
We assessed whether the presence of a GPI anchor at the carboxyl terminus of human CR-1 protein could localize CR-1 in caveolae, which form low-density Triton X-100-insoluble complexes enriched with Cav-1 protein. Insoluble, low-density complexes from 293 cells transiently transfected with CR-1 and Alk4-Flag expression vectors or CR-1-transfected COS7 cells were prepared using a 1% Triton X-100 sucrose density gradient fractionation method.48,49 The gradients were divided into 12 fractions ranging from 5% to 40% sucrose, and Western blot analysis using an anti-CR-1 mouse monoclonal antibody was performed to visualize the distribution of CR-1. Approximately 20% of the total CR-1 signal was detected in fractions 5 and 6, corresponding to the interface between 5% and 30% density range in both 293 and COS7 cells (Figure 1, A and B). In contrast, the transmembrane receptor Alk4 (Figure 1A) and the cytoskeleton protein β-actin (Figure 1, A and B), used as controls for non-lipid raft proteins, were detected only in the high-density detergent-soluble fractions (8–12). Western blot of the endogenously produced Cav-1 protein with a mouse monoclonal anti-Cav-1 antibody showed a distribution similar to CR-1 with approximately 10% of the total Cav-1 signal in fractions 4 to 6 in both cell lines (Figure 1, A and B). The lipid raft marker GM1 was also detected by dot blot in the low-density fractions (Figure 1, A and B). CR-1 and Cav-1 were also present in higher density detergent-soluble fractions, as previously reported for Cav-1.48,52 In conclusion, a portion of CR-1 protein localizes in low-density Triton X-100-resistant membrane fractions together with Cav-1 and GM1, whereas Alk4 is only present in high-density sucrose fractions.
Figure 1.

Colocalization of human CR-1 and Cav-1 in low-density sucrose fractions in 293 and COS7 cells. Western blot analysis on 5% to 40% sucrose gradient fractions isolated from 293 cells transiently transfected with expression plasmids encoding human CR-1 and Alk4-Flag (A) or from COS7 cells transiently transfected with a human CR-1 expression vector (B). Western blot analysis was performed using anti-Flag monoclonal antibody to detect Alk4-Flag fusion protein, anti-CR-1 mouse monoclonal antibody, anti-Cav-1 mouse monoclonal antibody, and anti-β-actin mouse monoclonal antibody. The lipid raft marker GM1 was detected with horseradish peroxidase-conjugated CT-B by dot blot. Low-density sucrose fractions: 4, 5, and 6.
CR-1 Coimmunoprecipitates with Cav-1 in COS7 Monkey Kidney Cells and in Mouse and Human Mammary Epithelial Cells
To determine whether CR-1 could associate with Cav-1 within caveolae, COS7 cells were transiently transfected with CR-1 and Cav-1 expression vectors. Because c-src has been shown to be activated by CR-1 and also to be localized in caveolae,25,53 we coexpressed c-src together with CR-1 and Cav-1 in COS7 cells. CR-1 and c-src could be immunoprecipitated by Cav-1 from transiently transfected COS7 cells as detected by Western blot analysis using an anti-CR-1 rabbit polyclonal antibody and c-src mouse monoclonal antibody (Figure 2A). Furthermore, we assessed whether glypican-1, a GPI-linked membrane heparan sulfate proteoglycan, can also interact with Cav-1. COS7 cells were transiently transfected with glypican-1 containing an Fc-tag, Cav-1, and CR-1 expression vectors. Glypican-1-Fc was then immunoprecipitated using protein G-agarose gel that binds to the Fc portion of the glypican-1-Fc fusion protein, and immunoprecipitated proteins were analyzed for the presence of CR-1 and Cav-1. Cav-1 was found to interact with glypican-1 in the presence or absence of CR-1 (Figure 2B). CR-1 was also found to coimmunoprecipitate with glypican-1, as we have previously demonstrated (Figure 2B).25 Thus, CR-1 and its signaling molecules, glypican-1 and c-src, can coimmunoprecipitate with Cav-1 when they are expressed together in COS7 cells. CR-1, Cav-1, c-src, and glypican-1 were all expressed in COS7 cells as detected by Western blot analysis on cell lysates of COS7-transfected cells (Figure 2, C and D). In contrast, no interaction between Cav-1 and Alk4-Flag was detected in a coimmunoprecipitation assay in COS7 cells coexpressing Cav-1, Alk4-Flag, and CR-1 (data not shown). To confirm that CR-1 and Cav-1 endogenous proteins can also interact with each other, a coimmunoprecipitation assay was performed in primary FVB/N mouse mammary epithelial cells and MCF-10A human mammary epithelial cells, which have been previously shown to express endogenous Cav-1 and Cripto-1 proteins.14,38,54 Following immunoprecipitation with an anti-CR-1 rabbit polyclonal antibody, Cav-1 was found to be associated with CR-1 in both mouse and human mammary epithelial cells, as assessed by Western blot analysis using an anti-Cav-1 antibody (Figure 2E). No specific bands in the range of Cav-1 or CR-1 molecular weight were detected when a control immunoglobulin was used in the immunoprecipitation assay in FVB/N or MCF-10A cells (data not shown).
Figure 2.
Binding of Cripto-1 to Cav-1 in coimmunoprecipitation experiments in COS7 cells and in FVB/N and MCF-10A mammary epithelial cells. A: COS7 cells were transiently transfected with Cav-1-myc/His, CR-1 and c-src expression vectors either alone or in various combinations, and 48 hours after transfection the cells were lysed and proteins were immunoprecipitated with anti-myc monoclonal antibody agarose beads. The myc-tagged immunoprecipitated proteins were then analyzed by Western blot analysis using anti-CR-1 rabbit polyclonal antibody, anti-c-src mouse monoclonal antibody or anti-His mouse monoclonal antibody to detect Cav-1-myc/His fusion protein. B: COS7 cells were transiently transfected with Cav-1-myc/His, CR-1 and glypican-1-Fc expression vectors either alone or in various combinations and 48 hours after transfection the cells were lysed and protein G-agarose beads were used to immunoprecipitate glypican-1-Fc fusion protein. The bound proteins were analyzed by Western blotting with anti-CR-1 rabbit polyclonal antibody, anti-His mouse monoclonal antibody, or anti-human IgG horseradish peroxidase-linked whole antibody to detect glypican-1-Fc fusion protein. C and D: Western blot analysis for CR-1, Cav-1, c-src, and glypican-1 on cell lysates of COS7 cells transiently transfected with various expression vectors as described above. E: FVB/N primary mouse mammary epithelial cells and MCF-10A human mammary epithelial cells were lysed and immunoprecipitated with an anti-CR-1 rabbit polyclonal antibody. The immunoprecipitated proteins were then analyzed by Western blotting using an anti-Cav-1 rabbit polyclonal antibody and anti-CR-1 rabbit polyclonal antibody. IP, immunoprecipitation; WB, Western blot.
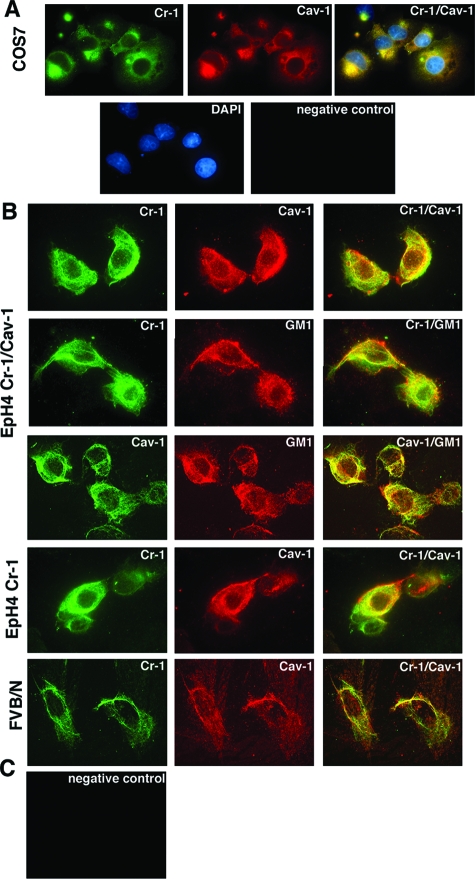
Cripto-1 Colocalizes with Cav-1 in EpH4 and FVB/N Mouse Mammary Epithelial Cells
We have previously demonstrated that overexpression of mouse Cr-1 in mammary epithelial cells induces an increase in cell proliferation, anchorage-independent growth in soft agar, migration, and invasion.21 We next investigated the effect of Cav-1 expression in EpH4 mouse mammary epithelial cells transformed by Cr-1. EpH4 WT, and EpH4-overexpressing mouse Cr-1 (EpH4 Cr-1) were transfected with an expression vector encoding for mouse Cav-1 (pUB6-Cav-1-V5/His). Following 2 weeks of selection with blasticidin, Western blot analysis was performed to confirm Cav-1 expression. As shown in Figure 3, Cav-1 could be detected in EpH4 Cav-1 and EpH4 Cr-1/Cav-1 cells using an anti-V5 monoclonal antibody. We also confirmed Cr-1 expression in EpH4 Cr-1 cells and double transfected EpH4 Cr-1/Cav-1 cells using an anti-CR-1 mouse monoclonal antibody (Figure 3). Western blot analysis for β-actin was performed to ensure that equal amounts of proteins were loaded on the gel (Figure 3). To further support our coimmunoprecipitation studies showing binding of human and mouse Cripto-1 to Cav-1, we used immunofluorescence double labeling to demonstrate colocalization of Cr-1 with Cav-1 in COS7 cells transiently transfected with Cr-1 and Cav-1 expression vectors and in EpH4 Cr-1/Cav-1 cells. Laser scanning confocal microscopy showed a punctate staining pattern for Cr-1 (green) and Cav-1 (red) in Cr-1/Cav-1 transfected COS7 cells and in EpH4 Cr-1/Cav-1 cells (Figure 4, A and B). When Cr-1/Cav-1 expressing COS7 cells and EpH4 Cr-1/Cav-1 cells were costained with anti-Cr-1 and anti-Cav-1 antibodies, a significant amount of Cr-1 colocalized (yellow) with Cav-1 (Figure 4, A and B). Cr-1 (green) and Cav-1 (green) also colocalized with the lipid raft marker GM1, in EpH4 Cr-1/Cav-1 cells, as previously reported for Cav-155 (Figure 4B). Colocalization of Cr-1 (green) and endogenous Cav-1 (red) protein was also detected in EpH4 Cr-1 cells, without any evident difference in the distribution pattern of the colocalized protein complex (Figure 4B). Finally, to ensure that interaction occurs also between endogenous proteins, FVB/N primary mouse mammary cells were stained with anti-Cr-1 and anti-Cav-1 antibodies. Staining for Cr-1 and Cav-1 was detected in FVB/N primary mammary epithelial cells and Cr-1 fluorescence (green) extensively overlapped with Cav-1 fluorescence (red) (Figure 4B).
Figure 3.

Stable Cav-1 expression in EpH4 mouse mammary epithelial cells. Cell lysates from EpH4 WT, EpH4 Cav-1, EpH4 Cr-1, and EpH4 Cr-1/Cav-1 cells were analyzed by Western blotting to confirm gene expression using anti-CR-1 mouse monoclonal antibody, anti-V5 mouse monoclonal antibody to detect Cav-1-V5/His fusion protein, or anti-β-actin monoclonal antibody to ensure equal loading of the protein samples. WB, Western blot.
Figure 4.
Colocalization of Cripto-1 and Cav-1 in COS7 cells expressing Cr-1 and Cav-1, in EpH4 Cr-1/Cav-1, EpH4 Cr-1, and FVB/N primary mammary epithelial cells. A: Immunofluorescence staining for Cr-1 and Cav-1 in COS7 cells transiently transfected with Cr-1 and Cav-1 expression vectors. B: Immunofluorescence staining for Cr-1, Cav-1, and GM-1 in EpH4 Cr-1/Cav-1 and EpH4 Cr-1 cells and immunofluorescence staining for Cr-1 and Cav-1 in primary FVB/N mouse mammary epithelial cells. C: Negative controls were obtained by replacing the primary antibody with irrelevant control isotype Ig. Fluorescence images were acquired by laser scanner confocal microscopy.
Cav-1 Blocks Cr-1-Induced Migration, Invasion, and Branching in Matrigel in EpH4 Mouse Mammary Epithelial Cells
We have previously demonstrated that Cr-1 is able to stimulate cell motility in several mammary epithelial cell lines and to enhance branching morphogenesis when mammary epithelial cells are grown in a three-dimensional matrix in vitro.21 To determine whether Cav-1 expression affects EpH4 cell migration and invasion stimulated by Cr-1, EpH4 WT, EpH4 Cr-1, EpH4 Cav-1, and EpH4 Cr-1/Cav-1 cells were seeded in Boyden chambers coated with the extracellular matrix protein fibronectin for the migration assay (Figure 5A) and with Matrigel for the invasion assay (Figure 5B). In agreement with previous results, EpH4 Cr-1 exhibited a threefold increase in their migratory and invasive behavior when compared to EpH4 WT cells (P < 0.05) (Figure 5, A and B). In contrast, expression of Cav-1 in EpH4 Cr-1/Cav-1 cells significantly inhibited migration and invasion of these cells (P < 0.05) when compared to EpH4 Cr-1 cells (Figure 5, A and B). EpH4 WT and EpH4 Cav-1 cells had comparable levels of migration and invasion (Figure 5, A and B). In addition, when EpH4 Cr-1 cells were grown in a Matrigel matrix in vitro, they started to form branching tubules with elongated cells with a fibroblastic morphology (Figure 5, C and D). Interestingly, this morphology was lost in the EpH4 Cr-1/Cav-1 cells (P < 0.0001, compared with EpH4 Cr-1 cells), which formed only small cyst-like structures similarly to what was observed in EpH4 WT cells (Figure 5, C and E). Similarly to EpH4 WT control cells, EpH4 Cav-1 cells did not show any branching structures (Figure 5, C and E). We next tested a cell-permeable peptide corresponding to the Cav-1 scaffolding domain that mimics Cav-1 inhibitory function. This peptide, also known as cavtratin, has been shown to block nitric oxide production in vitro and inhibit angiogenesis in vivo.37 As shown in Figure 6, A and B, Cr-1-enhanced migration and invasion of EpH4 Cr-1 cells were significantly inhibited in a dose-dependent manner by adding cavtratin (P < 0.05), when compared to EpH4 Cr-1 incubated with a scrambled Cav-1 scaffolding domain (control peptide). Thus, Cav-1 expression in EpH4 Cr-1/Cav-1 cells or treatment of EpH4 Cr-1 cells with cavtratin can interfere with Cr-1 function in EpH4 cells by inhibiting Cr-1-induced mammary epithelial cell motility and branching in Matrigel.
Figure 5.
Cav-1 inhibits Cr-1 enhanced migration, invasion, and branching of EpH4 mouse mammary epithelial cells. A: Migration or invasion assay (B) of EpH4 WT, EpH4 Cav-1, EpH4 Cr-1, and EpH4 Cr-1/Cav-1 cells. OD, optical density. *P < 0.05 compared to EpH4 WT cells; **P < 0.05 compared to EpH4 Cr-1 cells. C–E: Growth in Matrigel of EpH4 WT, EpH4 Cav-1, EpH4 Cr-1, and EpH4 Cr-1/Cav-1 cells. After 1 week, colonies with branching structures were photographed (C) and counted (E). D: Magnification of EpH4 Cr-1 branching structures. *P < 0.0001 compared to EpH4 WT cells; **P < 0.0001 compared to EpH4 Cr-1 cells.
Figure 6.
Cavtratin inhibits migration and invasion of EpH4 Cr-1 cells. Migration (A) and invasion (B) assay of EpH4 Cr-1 cells incubated in the presence of cavtratin. OD, optical density. *P < 0.05, compared to control, 5 μmol/L; **P < 0.05, compared to control, 10 μmol/L. Cav-1 interferes with the ability of Cr-1 to activate MAPK and c-src in EpH4 mouse mammary epithelial cells. Serum-starved EpH4 WT, EpH4 Cav-1, EpH4 Cr-1, and EpH4 Cr-1/Cav-1 cells were lysed and analyzed by Western blot analysis using phospho- and nonphospho-specific anti-MAPK (C) and anti-c-src antibodies (D). Serum-starved EpH4 WT and EpH4 Cr-1 cells were treated with cavtratin (10 μmol/L) or a control peptide (10 μmol/L) and analyzed by Western blotting using phospho- and nonphospho-specific anti-MAPK (E) and anti-c-src (F) antibodies. WB, Western blot.
Cav-1 Strongly Inhibits MAPK and c-src Activation by Cr-1 in EpH4 Mouse Mammary Epithelial Cells
We next assessed whether the inhibitory effect of Cav-1 on Cr-1-stimulated migration, invasion and branching in Matrigel in mouse mammary epithelial cells correlates with changes in the activation of intracellular signaling molecules activated by Cr-1, such as MAPK and c-src. When cultured in serum-free conditions, EpH4 Cr-1 cells showed an approximately 2.5-fold increase in the phosphorylation of MAPK (Figure 6C) and c-src (Figure 6D) as compared to EpH4 WT cells. Interestingly, EpH4 Cr-1/Cav-1 cells showed an approximately 60% reduction in the phosphorylation of MAPK and c-src as compared to EpH4 Cr-1 cells (Figure 6, C and D). Furthermore, EpH4 Cav-1 cells showed only a slight increase in phospho-MAPK and phospho-c-src as compared to EpH4 WT cells (Figure 6, C and D). Thus, Cav-1 expression strongly inhibits intracellular signaling pathways activated by Cr-1 in EpH4 Cr-1/Cav-1 cells. Similarly, addition of cavtratin (10 μmol/L) to EpH4 Cr-1 cells decreased MAPK (approximately 50%) and c-src phosphorylation (approximately 60%) induced by Cr-1, whereas cavtratin had no effect on the levels of MAPK and c-src phosphorylation in EpH4 WT cells (Figure 6, E and F). In contrast, addition of a control peptide (10 μmol/L) to EpH4 Cr-1 cells did not interfere with the ability of Cr-1 to enhance MAPK and c-src phosphorylation as compared to control peptide-treated EpH4 WT cells (Figure 6, E and F). We also investigated whether Cav-1 can inhibit Alk4/Nodal-dependent Cripto-1 signaling using a Nodal (2n)7-lucifersase reporter gene containing FoxHI-binding sequences, which has been shown to be induced by Cripto-1 and Nodal in Mv-1Lu mink cells responsive to transforming growth factor β.56 Alk4, CR-1, Nodal, and FoxHI when transfected together in 293 cells induced a strong increase in the (2n)7-luciferase activity, and addition of increasing concentrations of a Cav-1 expression vector ranging from 5 to 100 ng/well did not interfere with activation of (2n)7-luciferase induced by Alk4, CR-1, Nodal, and FoxHI (data not shown).
Loss of Cav-1 Enhances CR-1-Mediated Migration, Invasion, and Activation of c-src and MAPK in Primary Mammary Epithelial Cells
The identification of an antagonistic effect of Cav-1 on Cr-1 in mammary epithelial cells prompted us to investigate the effect of a partial or complete loss of Cav-1 expression on Cripto-1 biological function. Previous reports have shown that a complete loss of Cav-1 is required to accelerate tumorigenesis in vivo, whereas mice with a partial loss of Cav-1 (+/−) behave as Cav-1 (+/+) control mice.46 Thus we interbred MMTV-CR-1 mice with Cav-1 null mice to generate female Cav-1 null−/−/CR-1 and Cav-1 heterozygous+/−/CR-1 double transgenic mice. From 2- to 3-month-old FVB/N control mice and Cav-1−/−/CR-1 and Cav-1+/−/CR-1 transgenic mice we isolated primary mammary epithelial cells and evaluated their behavior in migration and invasion assays in vitro. Western blot analysis confirmed loss of Cav-1 protein expression in the Cav-1 null cells, whereas CR-1 transgene was expressed in both Cav-1−/−/CR-1 and Cav-1+/−/CR-1 cells (Figure 7A). The anti-CR-1 mouse monoclonal antibody could not detect endogenous mouse Cr-1 protein in FVB/N mammary cells, since it specifically interacts with human CR-1 protein only.57 As shown in Figure 7, B and C, Cav-1−/−/CR-1 and Cav-1+/−/CR-1 mammary epithelial cells showed enhanced migration and invasion as compared to control FVB/N mammary epithelial cells. Interestingly, complete loss of Cav-1 in Cav-1−/−/CR-1 mammary epithelial cells further enhanced the levels of CR-1-induced migration and invasion as compared to Cav-1+/−/CR-1 mammary epithelial cells (P < 0.05) (Figure 7, B and C). Addition of cavtratin to Cav-1−/−/CR-1 mammary epithelial cells significantly inhibited migration and invasion enhanced by CR-1 in the absence of Cav-1 expression, suggesting that cavtratin was able to restore Cav-1 inhibitory effect on CR-1 in Cav-1−/−/CR-1 mammary epithelial cells (P < 0.05) (Figure 7, B and C). Treatment of Cav-1−/−/CR-1 mammary epithelial cells with a control peptide had no effect on the migratory or invasive behavior of these cells (Figure 7, B and C). To determine the molecular basis for the increased migration and invasion of Cav-1−/−/CR-1 mammary cells compared to FVB/N control and Cav-1+/−/CR-1 mammary cells, we performed immunoblotting on cell lysates derived from these primary cultures for the activated phosphorylated forms of MAPK and c-src signaling molecules. As shown in Figure 7D, an approximately twofold increase in the phosphorylation of both MAPK and c-src was observed in Cav-1+/−/CR-1 mammary cells as compared to control FVB/N mammary epithelial cells. Complete loss of Cav-1 expression in Cav-1−/−/CR-1 mammary epithelial cells increased even more dramatically the phosphorylation status of MAPK and c-src as compared to control FVB/N and Cav-1+/−/CR-1 mammary epithelial cells (Figure 7D).
Figure 7.
Loss of Cav-1 enhances migration and invasion and induces hyperactivation of MAPK and c-src signaling molecules in primary mammary epithelial cells derived from Cav-1 null/MMTV-CR-1 double transgenic mice. A: Western blot analysis for Cav-1, CR-1, and β-actin in cell lysates derived from primary mammary epithelial cells isolated from FVB/N, Cav-1+/−/CR-1, and Cav-1−/−/CR-1 mice. Migration (B) and invasion (C) assay of FVB/N, Cav-1+/−/CR-1, and Cav-1−/−/CR-1 primary mammary epithelial cells. Cav-1−/−/CR-1 primary mammary epithelial cells were also treated with cavtratin (10 μmol/L) or with a control peptide (10 μmol/L). *P < 0.05 compared to FVB/N cells; **P < 0.05 compared to Cav-1+/−/CR-1 cells; ***P < 0.05 compared to control 10 μmol/L treated Cav-1−/−/CR-1 cells. D: Protein lysates from FVB/N, Cav-1+/−/CR-1, and Cav-1−/−/CR-1 primary mammary epithelial cells were subjected to immunoblotting with antibodies directed against phospho- and total-MAPK and phospho- and total-c-src.
Expression of Cav-1 in MMTV-CR-1 Transgenic Mice
We next evaluated the expression of Cav-1 in the mammary gland and tumors of transgenic mice that overexpress the human CR-1 transgene under the control of the MMTV promoter.28 Compared to mammary tissues obtained from wild-type multiparous FVB/N and MMTV-CR-1 virgin animals, a strong reduction of Cav-1 expression was observed by Western blot analysis in tissue extracts from mammary tumors of MMTV-CR-1 mice (Figure 8A). Immunohistochemical analysis of serial mammary tissue sections of FVB/N virgin and multiparous animals showed a strong positivity for Cav-1 in epithelial cells, adipocytes, and stroma, whereas CR-1 was expressed at moderate levels in the virgin mammary gland, and its expression increased in the multiparous gland (Figure 8B). Interestingly, Cav-1 staining was lost in the epithelial compartment of MMTV-CR-1 mammary papillary adenocarcinomas, whereas moderate staining for Cav-1 could still be detected in adipocytes and stroma (Figure 8B). In contrast, CR-1 was highly expressed in the mammary tumor, as expected (Figure 8B). Thus, mammary tumors that are induced by CR-1 overexpression have a reduction in the expression of Cav-1 in the epithelial compartment of the tumor, suggesting that during the process of transformation CR-1 escapes the inhibitory function of Cav-1, possibly by down-regulating Cav-1 expression.
Figure 8.
Cav-1 expression is reduced in mammary tumors of MMTV-CR-1 transgenic mice. A: Western blot analysis for Cav-1 in tissues from one normal FVB/N or two MMTV-CR-1 virgin mice and from mammary tumors found in four MMTV-CR-1 multiparous mice (T1, T2, T3 and T4). For equal loading, the blot was reprobed with anti-β-actin antibody. B: Immunohistochemistry for Cav-1 and CR-1 in mammary tissue serial sections derived from FVB/N virgin or multiparous mice and in mammary tumor of MMTV-CR-1 mice. These sections were incubated with an anti-Cav-1 or with anti-CR-1 antibodies as described in Materials and Methods. Magnification, ×400. Negative controls are shown in the insets. WB, Western blot.
Discussion
In the present study we have investigated the physical and functional interaction between Cripto-1 and Cav-1, two critical regulators of mouse mammary gland development and tumorigenesis. Regarding the importance of Cripto-1 in the mammary gland, we have previously reported that mouse Cr-1 is expressed primarily in the body cells of the growing terminal end buds of the pubescent mouse mammary gland, and its expression is elevated in ductal epithelial cells from pregnant and early lactating mice.58,59 Biologically active human CR-1 protein is present in human milk samples, suggesting that CR-1 in milk may be important in regulating mammary gland development during lactation.60 We have also found that Cripto-1 is overexpressed in a majority of mouse mammary carcinoma cell lines in several different spontaneous and carcinogen-induced mouse mammary tumors and in human breast carcinomas.61,62,63 On the contrary, Cav-1 is normally expressed at high levels in adipocytes and stromal cells and at moderate levels in mammary epithelial cells in the virgin mouse mammary gland, and Cav-1 expression levels are down-regulated by the hormone prolactin during pregnancy.38,39,54 Unlike Cripto-1, Cav-1 is a negative regulator of mammary epithelial cell proliferation, as clearly demonstrated by the enhanced mammary gland hyperplasia of Cav-1 knockout mice and by experiments with primary cultures of Cav-1 null mouse mammary epithelial cells.42,43 When embedded in Matrigel, Cav-1-deficient mammary epithelial cells exhibit premalignant changes such as hyperproliferation, EGF-independent growth, defects in cell substrate attachment, and increased cell invasiveness.42
The presence of a GPI anchor within the carboxyl terminus of mouse and human Cripto-1 have lead us to investigate the subcellular distribution of CR-1 within membrane microdomains such as caveolae. GPI-linked proteins have been described to cluster in caveolae that provide an environment for signaling molecules.64 With the use of a detergent-based isolation procedure, we demonstrate that GPI-anchored human CR-1 protein cofractionates with Cav-1-rich membranes. GM1 was also found in the same detergent-insoluble fractions, indicating that CR-1 and Cav-1 are localized in lipid rafts in 293 and COS7 cells. However, a substantial amount of Cav-1 and CR-1 were also associated with higher density detergent-soluble fractions. These data are consistent with previous reports showing that only a proportion of Cav-1 is Triton X-100-insoluble using this isolation procedure, suggesting that a dynamic equilibrium might regulate the presence of Cav-1 and GPI-linked proteins within caveolae.48,52 In addition, when visualized by immunofluorescence, an anti-Cripto-1 antibody stained EpH4 Cr-1/Cav-1 and EpH4 Cr-1 cells in a punctate pattern, which partially colocalized with the signal from an anti-Cav-1 antibody and fluorescence-labeled CT-B. We further demonstrate that CR-1 together with glypican-1 and c-src can also coimmunoprecipitate with Cav-1 in transiently transfected COS7 cells, whereas we were unable to detect any binding of Cav-1 to Alk4. Furthermore, Cripto-1 and Cav-1 endogenous proteins can also interact with each other in FVB/N and MCF-10A mammary epithelial cells as demonstrated by coimmunoprecipitation and immunofluorescence experiments (Figures 2E and 4B). Interaction of Cav-1 with a number of intracellular signaling proteins occurs through the CSD, which recognizes a Cav-1-binding sequence within the catalytic binding domains of several tyrosine protein kinases, including c-src.34 However, the mechanism(s) by which GPI-linked proteins that are localized in the outer leaflet of the cellular membrane can directly or indirectly interact with the CSD of Cav-1 or with other domains within Cav-1 is unclear.64,65 In this respect, a recent article has demonstrated that Cav-1 can function as an adapter membrane protein that allows GPI-linked proteins to signal within the cell.66 Regardless of the mechanism by which binding of Cripto-1 to Cav-1 occurs, we demonstrate that interaction between these proteins is functionally significant, since overexpression of Cav-1 in EpH4 Cr-1/Cav-1 cells can reduce the in vitro migration and invasion of these cells and can inhibit branching morphogenesis in Matrigel as compared to EpH4 Cr-1 cells. These effects of Cav-1 overexpression can also be recapitulated in EpH4 Cr-1 cells by treatment with cavtratin, suggesting that the Cav-1 CSD mediates the inhibitory effects of Cav-1. In contrast, primary mouse mammary epithelial cells derived from Cav-1 null/MMTV-CR-1 double transgenic animals show an increased migration and invasion behavior as compared to Cav-1 heterozygous+/−/CR-1 mammary epithelial cells, indicating that Cav-1 deficiency exacerbates cell migration and invasiveness driven by CR-1 in primary mammary epithelial cells. We further demonstrate that Cav-1 expression or cavtratin treatment impairs Cr-1 signaling through MAPK and c-src. Consistent with the inhibition of MAPK and c-src signaling in EpH4 cells, we observed increased MAPK and c-src signaling in Cav-1 null−/−/CR-1 primary mouse mammary epithelial cells compared to Cav-1 heterozygous+/−/CR-1 mammary epithelial cells. Since c-src and MAPK pathways have been shown to be critical for Cr-1-mediated cell migration and invasion, Cav-1 might normally antagonize Cripto-1 function by impairing its ability to transmit downstream signaling through these intracellular signaling molecules.24,25 These observations are consistent with previous in vitro and in vivo studies demonstrating that Cav-1 negatively regulates the p42/p44 MAPK and c-src activity by directly binding to the catalytic domains of these kinases through the CSD.34 Furthermore, mammary gland tissues of Cav-1 null mice show hyperactivation of MAPK and up-regulation of cyclin D1, providing a molecular basis for the increased cell proliferation in Cav-1 null mammary epithelial cells.42,45 Interestingly, Cav-1 could not inhibit CR-1/Nodal/Alk4 signaling using a Nodal luciferase-responsive reporter assay in 293 cells. Since Alk4 does not localize in Cav-1-containing detergent-insoluble membrane fractions, as we show in Figure 1A, and does not bind to Cav-1 in a coimmunoprecipitation assay in COS7 cells, it is possible that Cav-1 cannot interact with Alk4 and therefore cannot exert an inhibitory effect on CR-1/Nodal signaling.
Finally, targeted expression of human CR-1 in mouse mammary epithelial cells in vivo is sufficient to down-regulate Cav-1 expression in mammary tumors as assessed by Western blot analysis and immunohistochemistry, suggesting that endogenous Cav-1 protein might be sensitive to down-regulation during CR-1-mediated cell transformation in vivo. Interestingly, while we observed weak or no staining for Cav-1 in mammary epithelial tumor cells in MMTV-CR-1 tumor sections, a strong staining for Cav-1 could still be detected in the adjacent stroma, containing adipocytes and vessels, suggesting that MMTV-driven expression of CR-1 in mammary epithelial cells specifically down-regulates Cav-1 expression only in the epithelial compartment. Down-regulation of Cav-1 expression is a common event in cancer, and different mechanisms have been described. The gene encoding Cav-1 is localized to the D7S522 region of human chromosome 7q31.1, and this locus is commonly deleted in a variety of human cancers.67,68,69,70 Methylation of CpG islands in the Cav-1 promoter region or a sporadic point mutation (P132L) in the Cav-1 protein have also been reported in breast cancer cell lines and in 16% of patients with primary breast cancer, respectively.71,72 Finally, several cellular oncogenes, such as c-Myc, Ha-Ras, c-src, and c-Neu/erbB2, have been shown to down-regulate Cav-1 expression through transcriptional regulation.73 In agreement with our results, Engelman and colleagues have shown that while overexpression of Cav-1 blocks c-Neu tyrosine kinase activity in vitro, a dramatic reduction of Cav-1 expression occurs in vivo in mammary tumors derived from c-Neu-expressing transgenic mice and other transgenic animals expressing downstream effectors of c-Neu-mediated signal transduction, such as c-src and Ha-Ras.39 These results together with our findings suggest that Cav-1 inactivation is a common event during oncogene-driven mouse mammary transformation.39
In conclusion, we have demonstrated for the first time a functional interaction between Cripto-1 and Cav-1. While forced Cav-1 expression strongly interferes with Cr-1 oncogenic activity in EpH4 cells, in contrast loss of Cav-1 potentiates CR-1-induced migration and invasion of primary mammary epithelial cells derived from Cav-1 null/MMTV-CR-1 double transgenic mice, suggesting that mammary epithelial cells that lack Cav-1 expression might be more sensitive to the biological effects of Cripto-1. We are currently characterizing the mammary gland phenotype of Cav-1 null/MMTV-CR-1 double transgenic mice to evaluate if there are any significant differences in the frequency and number of premalignant mammary lesions (hyperplasias) and tumors in these bitransgenic mice.
Footnotes
Address reprint requests to David S. Salomon, Ph.D., National Cancer Institute, 37 Convent Drive, Building 37, Room 1118, Bethesda, MD 20892. E-mail: salomond@mail.nih.gov.
Supported by Intramural Research program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and by a grant from the Associazione Italiana per la Ricerca sul Cancro to Nicola Normanno.
References
- Bianco C, Strizzi L, Normanno N, Khan N, Salomon DS. Cripto-1: an oncofetal gene with many faces. Curr Top Dev Biol. 2005;67:85–133. doi: 10.1016/S0070-2153(05)67003-2. [DOI] [PubMed] [Google Scholar]
- Strizzi L, Bianco C, Normanno N, Salomon D. Cripto-1: a multifunctional modulator during embryogenesis and oncogenesis. Oncogene. 2005;24:5731–5741. doi: 10.1038/sj.onc.1208918. [DOI] [PubMed] [Google Scholar]
- Bianco C, Normanno N, Salomon DS, Ciardiello F. Role of the cripto (EGF-CFC) family in embryogenesis and cancer. Growth Factors. 2004;22:133–139. doi: 10.1080/08977190410001723290. [DOI] [PubMed] [Google Scholar]
- Salomon DS, Bianco C, Ebert AD, Khan NI, De Santis M, Normanno N, Wechselberger C, Seno M, Williams K, Sanicola M, Foley S, Gullick WJ, Persico G. The EGF-CFC family: novel epidermal growth factor-related proteins in development and cancer. Endocr Relat Cancer. 2000;7:199–226. doi: 10.1677/erc.0.0070199. [DOI] [PubMed] [Google Scholar]
- Minchiotti G, Parisi S, Liguori G, Signore M, Lania G, Adamson ED, Lago CT, Persico MG. Membrane-anchorage of Cripto protein by glycosylphosphatidylinositol and its distribution during early mouse development. Mech Dev. 2000;90:133–142. doi: 10.1016/s0925-4773(99)00235-x. [DOI] [PubMed] [Google Scholar]
- Normanno N, De Luca A, Bianco C, Maiello MR, Carriero MV, Rehman A, Wechselberger C, Arra C, Strizzi L, Sanicola M, Salomon DS. Cripto-1 overexpression leads to enhanced invasiveness and resistance to anoikis in human MCF-7 breast cancer cells. J Cell Physiol. 2004;198:31–39. doi: 10.1002/jcp.10375. [DOI] [PubMed] [Google Scholar]
- Brandt R, Normanno N, Gullick WJ, Lin JH, Harkins R, Schneider D, Jones BW, Ciardiello F, Persico MG, Armenante F, Kim N, Salomon DS. Identification and biological characterization of an epidermal growth factor-related protein: cripto-1. J Biol Chem. 1994;269:17320–17328. [PubMed] [Google Scholar]
- Bianco C, Strizzi L, Mancino M, Rehman A, Hamada S, Watanabe K, De Luca A, Jones B, Balogh G, Russo J, Mailo D, Palaia R, D’Aiuto G, Botti G, Perrone F, Salomon DS, Normanno N. Identification of cripto-1 as a novel serologic marker for breast and colon cancer. Clin Cancer Res. 2006;12:5158–5164. doi: 10.1158/1078-0432.CCR-06-0274. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Hamada S, Bianco C, Mancino M, Nagaoka T, Gonzales M, Bailly V, Strizzi L, Salomon DS. Requirement of glycosylphosphatidylinositol anchor of cripto-1 for “trans” activity as a nodal co-receptor. J Biol Chem. 2007;282:35772–35786. doi: 10.1074/jbc.M707351200. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Bianco C, Strizzi L, Hamada S, Mancino M, Bailly V, Mo W, Wen D, Miatkowski K, Gonzales M, Sanicola M, Seno M, Salomon DS. Growth factor induction of cripto-1 shedding by GPI-phospholipase D and enhancement of endothelial cell migration. J Biol Chem. 2007;282:31643–31655. doi: 10.1074/jbc.M702713200. [DOI] [PubMed] [Google Scholar]
- Chen C, Ware SM, Sato A, Houston-Hawkins DE, Habas R, Matzuk MM, Shen MM, Brown CW. The Vg1-related protein Gdf3 acts in a Nodal signaling pathway in the pre-gastrulation mouse embryo. Development. 2006;133:319–329. doi: 10.1242/dev.02210. [DOI] [PubMed] [Google Scholar]
- Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- Cheng SK, Olale F, Bennett JT, Brivanlou AH, Schier AF. EGF-CFC proteins are essential coreceptors for the TGF-beta signals Vg1 and GDF1. Genes Dev. 2003;17:31–36. doi: 10.1101/gad.1041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C, Adkins HB, Wechselberger C, Seno M, Normanno N, De Luca A, Sun Y, Khan N, Kenney N, Ebert A, Williams KP, Sanicola M, Salomon DS. Cripto-1 activates nodal- and ALK4-dependent and - independent signaling pathways in mammary epithelial cells. Mol Cell Biol. 2002;22:2586–2597. doi: 10.1128/MCB.22.8.2586-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo C, Whitman M. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol Cell. 2001;7:949–957. doi: 10.1016/s1097-2765(01)00249-0. [DOI] [PubMed] [Google Scholar]
- Minchiotti G, Parisi S, Persico MG. Cripto signaling in differentiating embryonic stem cells. Methods Mol Biol. 2006;329:151–169. doi: 10.1385/1-59745-037-5:151. [DOI] [PubMed] [Google Scholar]
- Minchiotti G. Nodal-dependent Cripto signaling in ES cells: from stem cells to tumor biology. Oncogene. 2005;24:5668–5675. doi: 10.1038/sj.onc.1208917. [DOI] [PubMed] [Google Scholar]
- Assou S, Lecarrour T, Tondeur S, Strom S, Gabelle A, Marty S, Nadal L, Pantesco V, Reme T, Hugnot JP, Gasca S, Hovatta O, Hamamah S, Klein B, De Vos J. A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells. 2007;25:961–973. doi: 10.1634/stemcells.2006-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanno N, Bianco C, De Luca A, Salomon DS. The role of EGF-related peptides in tumor growth. Front Biosci. 2001;6:D685–D707. doi: 10.2741/normano. [DOI] [PubMed] [Google Scholar]
- Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y, Sanicola M, Salomon DS. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Cripto-1 transgenic mice. J Cell Physiol. 2004;201:266–276. doi: 10.1002/jcp.20062. [DOI] [PubMed] [Google Scholar]
- Wechselberger C, Ebert AD, Bianco C, Khan NI, Sun Y, Wallace-Jones B, Montesano R, Salomon DS. Cripto-1 enhances migration and branching morphogenesis of mouse mammary epithelial cells. Exp Cell Res. 2001;266:95–105. doi: 10.1006/excr.2001.5195. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Wechselberger C, Nees M, Clair T, Schaller G, Martinez-Lacaci I, Wallace-Jones B, Bianco C, Weitzel HK, Salomon DS. Cripto-1-induced increase in vimentin expression is associated with enhanced migration of human Caski cervical carcinoma cells. Exp Cell Res. 2000;257:223–229. doi: 10.1006/excr.2000.4881. [DOI] [PubMed] [Google Scholar]
- Ciardiello F, Dono R, Kim N, Persico MG, Salomon DS. Expression of cripto, a novel gene of the epidermal growth factor gene family, leads to in vitro transformation of a normal mouse mammary epithelial cell line. Cancer Res. 1991;51:1051–1054. [PubMed] [Google Scholar]
- Bianco C, Strizzi L, Ebert A, Chang C, Rehman A, Normanno N, Guedez L, Salloum R, Ginsburg E, Sun Y, Khan N, Hirota M, Wallace-Jones B, Wechselberger C, Vonderhaar BK, Tosato G, Stetler-Stevenson WG, Sanicola M, Salomon DS. Role of human cripto-1 in tumor angiogenesis. J Natl Cancer Inst. 2005;97:132–141. doi: 10.1093/jnci/dji011. [DOI] [PubMed] [Google Scholar]
- Bianco C, Strizzi L, Rehman A, Normanno N, Wechselberger C, Sun Y, Khan N, Hirota M, Adkins H, Williams K, Margolis RU, Sanicola M, Salomon DS. A Nodal- and ALK4-independent signaling pathway activated by Cripto-1 through glypican-1 and c-Src. Cancer Res. 2003;63:1192–1197. [PubMed] [Google Scholar]
- Kannan S, De Santis M, Lohmeyer M, Riese DJ, 2nd, Smith GH, Hynes N, Seno M, Brandt R, Bianco C, Persico G, Kenney N, Normanno N, Martinez-Lacaci I, Ciardiello F, Stern DF, Gullick WJ, Salomon DS. Cripto enhances the tyrosine phosphorylation of Shc and activates mitogen-activated protein kinase (MAPK) in mammary epithelial cells. J Biol Chem. 1997;272:3330–3335. doi: 10.1074/jbc.272.6.3330. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Wechselberger C, Frank S, Wallace-Jones B, Seno M, Martinez-Lacaci I, Bianco C, De Santis M, Weitzel HK, Salomon DS. Cripto-1 induces phosphatidylinositol 3′-kinase-dependent phosphorylation of AKT and glycogen synthase kinase 3beta in human cervical carcinoma cells. Cancer Res. 1999;59:4502–4505. [PubMed] [Google Scholar]
- Wechselberger C, Strizzi L, Kenney N, Hirota M, Sun Y, Ebert A, Orozco O, Bianco C, Khan NI, Wallace-Jones B, Normanno N, Adkins H, Sanicola M, Salomon DS. Human Cripto-1 overexpression in the mouse mammary gland results in the development of hyperplasia and adenocarcinoma. Oncogene. 2005;24:4094–4105. doi: 10.1038/sj.onc.1208417. [DOI] [PubMed] [Google Scholar]
- Sun Y, Strizzi L, Raafat A, Hirota M, Bianco C, Feigenbaum L, Kenney N, Wechselberger C, Callahan R, Salomon DS. Overexpression of human Cripto-1 in transgenic mice delays mammary gland development and differentiation and induces mammary tumorigenesis. Am J Pathol. 2005;167:585–597. doi: 10.1016/S0002-9440(10)63000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strizzi L, Bianco C, Hirota M, Watanabe K, Mancino M, Hamada S, Raafat A, Lawson S, Ebert A, D’Antonio A, Losito S, Normanno N, Salomon D. Development of leiomyosarcoma of the uterus in MMTV-CR-1 transgenic mice. J Pathol. 2007;211:36–44. doi: 10.1002/path.2083. [DOI] [PubMed] [Google Scholar]
- Quest AF, Leyton L, Parraga M. Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease. Biochem Cell Biol. 2004;82:129–144. doi: 10.1139/o03-071. [DOI] [PubMed] [Google Scholar]
- Hnasko R, Lisanti MP. The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol Interv. 2003;3:445–464. doi: 10.1124/mi.3.8.445. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Scherer PE, Tang Z, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994;4:231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- Schwartz EA, Reaven E, Topper JN, Tsao PS. Transforming growth factor-beta receptors localize to caveolae and regulate endothelial nitric oxide synthase in normal human endothelial cells. Biochem J. 2005;390:199–206. doi: 10.1042/BJ20041182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton JP, Lin MI, Yu J, Weiss ED, Jiang ZL, Fairchild TA, Iwakiri Y, Groszmann R, Claffey KP, Cheng YC, Sessa WC. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell. 2003;4:31–39. doi: 10.1016/s1535-6108(03)00168-5. [DOI] [PubMed] [Google Scholar]
- Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16:1391–1397. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Lee RJ, Karnezis A, Bearss DJ, Webster M, Siegel P, Muller WJ, Windle JJ, Pestell RG, Lisanti MP. Reciprocal regulation of neu tyrosine kinase activity and caveolin-1 protein expression in vitro and in vivo: implications for human breast cancer. J Biol Chem. 1998;273:20448–20455. doi: 10.1074/jbc.273.32.20448. [DOI] [PubMed] [Google Scholar]
- Fiucci G, Ravid D, Reich R, Liscovitch M. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene. 2002;21:2365–2375. doi: 10.1038/sj.onc.1205300. [DOI] [PubMed] [Google Scholar]
- Sloan EK, Stanley KL, Anderson RL. Caveolin-1 inhibits breast cancer growth and metastasis. Oncogene. 2004;23:7893–7897. doi: 10.1038/sj.onc.1208062. [DOI] [PubMed] [Google Scholar]
- Sotgia F, Williams TM, Schubert W, Medina F, Minetti C, Pestell RG, Lisanti MP. Caveolin-1 deficiency (−/−) conveys premalignant alterations in mammary epithelia, with abnormal lumen formation, growth factor independence, and cell invasiveness. Am J Pathol. 2006;168:292–309. doi: 10.2353/ajpath.2006.050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Park DS, Razani B, Russell RG, Pestell RG, Lisanti MP. Caveolin-1 mutations (P132L and null) and the pathogenesis of breast cancer: caveolin-1 (P132L) behaves in a dominant-negative manner and caveolin-1 (−/−) null mice show mammary epithelial cell hyperplasia. Am J Pathol. 2002;161:1357–1369. doi: 10.1016/S0002-9440(10)64412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DS, Lee H, Frank PG, Razani B, Nguyen AV, Parlow AF, Russell RG, Hulit J, Pestell RG, Lisanti MP. Caveolin-1-deficient mice show accelerated mammary gland development during pregnancy, premature lactation, and hyperactivation of the Jak-2/STAT5a signaling cascade. Mol Biol Cell. 2002;13:3416–3430. doi: 10.1091/mbc.02-05-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Cheung MW, Park DS, Razani B, Cohen AW, Muller WJ, Di Vizio D, Chopra NG, Pestell RG, Lisanti MP. Loss of caveolin-1 gene expression accelerates the development of dysplastic mammary lesions in tumor-prone transgenic mice. Mol Biol Cell. 2003;14:1027–1042. doi: 10.1091/mbc.E02-08-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, Chopra NG, Scherer PE, Pestell RG, Lisanti MP. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo: role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279:51630–51646. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- Martinez-Lacaci I, De Santis M, Kannan S, Bianco C, Kim N, Wallace-Jones B, Wechselberger C, Ebert AD, Salomon DS. Regulation of heparin-binding EGF-like growth factor expression in Ha-ras transformed human mammary epithelial cells. J Cell Physiol. 2001;186:233–242. doi: 10.1002/1097-4652(200002)186:2<233::AID-JCP1017>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- McCabe JB, Berthiaume LG. N-terminal protein acylation confers localization to cholesterol, sphingolipid-enriched membranes but not to lipid rafts/caveolae. Mol Biol Cell. 2001;12:3601–3617. doi: 10.1091/mbc.12.11.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Sargiacomo M, Galbiati F, Parenti M, Lisanti MP. Targeting of a G alpha subunit (Gi1 alpha) and c-Src tyrosine kinase to caveolae membranes: clarifying the role of N-myristoylation. Cell Mol Biol (Noisy-le-grand) 1997;43:293–303. [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Joliot A, Trembleau A, Raposo G, Calvet S, Volovitch M, Prochiantz A. Association of Engrailed homeoproteins with vesicles presenting caveolae-like properties. Development. 1997;124:1865–1875. doi: 10.1242/dev.124.10.1865. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Park DS, Lee H, Riedel C, Hulit J, Scherer PE, Pestell RG, Lisanti MP. Prolactin negatively regulates caveolin-1 gene expression in the mammary gland during lactation, via a Ras-dependent mechanism. J Biol Chem. 2001;276:48389–48397. doi: 10.1074/jbc.M108210200. [DOI] [PubMed] [Google Scholar]
- Pang H, Le PU, Nabi IR. Ganglioside GM1 levels are a determinant of the extent of caveolae/raft-dependent endocytosis of cholera toxin to the Golgi apparatus. J Cell Sci. 2004;117:1421–1430. doi: 10.1242/jcs.01009. [DOI] [PubMed] [Google Scholar]
- Saijoh Y, Adachi H, Sakuma R, Yeo CY, Yashiro K, Watanabe M, Hashiguchi H, Mochida K, Ohishi S, Kawabata M, Miyazono K, Whitman M, Hamada H. Left-right asymmetric expression of lefty2 and nodal is induced by a signaling pathway that includes the transcription factor FAST2. Mol Cell. 2000;5:35–47. doi: 10.1016/s1097-2765(00)80401-3. [DOI] [PubMed] [Google Scholar]
- Adkins HB, Bianco C, Schiffer SG, Rayhorn P, Zafari M, Cheung AE, Orozco O, Olson D, De Luca A, Chen LL, Miatkowski K, Benjamin C, Normanno N, Williams KP, Jarpe M, LePage D, Salomon D, Sanicola M. Antibody blockade of the Cripto CFC domain suppresses tumor cell growth in vivo. J Clin Invest. 2003;112:575–587. doi: 10.1172/JCI17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington EE, Ram TG, Salomon DS, Johnson GR, Gullick WJ, Kenney N, Hosick HL. Expression of epidermal growth factor-related proteins in the aged adult mouse mammary gland and their relationship to tumorigenesis. J Cell Physiol. 1997;170:47–56. doi: 10.1002/(SICI)1097-4652(199701)170:1<47::AID-JCP6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Kenney NJ, Huang RP, Johnson GR, Wu JX, Okamura D, Matheny W, Kordon E, Gullick WJ, Plowman G, Smith GH, Salomon DS, Adamson ED. Detection and location of amphiregulin and Cripto-1 expression in the developing postnatal mouse mammary gland. Mol Reprod Dev. 1995;41:277–286. doi: 10.1002/mrd.1080410302. [DOI] [PubMed] [Google Scholar]
- Bianco C, Wechselberger C, Ebert A, Khan NI, Sun Y, Salomon DS. Identification of Cripto-1 in human milk. Breast Cancer Res Treat. 2001;66:1–7. doi: 10.1023/a:1010648923432. [DOI] [PubMed] [Google Scholar]
- Qi CF, Liscia DS, Normanno N, Merlo G, Johnson GR, Gullick WJ, Ciardiello F, Saeki T, Brandt R, Kim N, Kenney N, Salomon DS. Expression of transforming growth factor alpha, amphiregulin and cripto-1 in human breast carcinomas. Br J Cancer. 1994;69:903–910. doi: 10.1038/bjc.1994.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney NJ, Smith GH, Maroulakou IG, Green JH, Muller WJ, Callahan R, Salomon DS, Dickson RB. Detection of amphiregulin and Cripto-1 in mammary tumors from transgenic mice. Mol Carcinog. 1996;15:44–56. doi: 10.1002/(SICI)1098-2744(199601)15:1<44::AID-MC7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Niemeyer CC, Spencer-Dene B, Wu JX, Adamson ED. Preneoplastic mammary tumor markers: cripto and Amphiregulin are overexpressed in hyperplastic stages of tumor progression in transgenic mice. Int J Cancer. 1999;81:588–591. doi: 10.1002/(sici)1097-0215(19990517)81:4<588::aid-ijc14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Stahl A, Mueller BM. The urokinase-type plasminogen activator receptor, a GPI-linked protein, is localized in caveolae. J Cell Biol. 1995;129:335–344. doi: 10.1083/jcb.129.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker EM, Zaman MM, Freedman SD. GP2, a GPI-anchored protein in the apical plasma membrane of the pancreatic acinar cell, co-immunoprecipitates with src kinases and caveolin. Pancreas. 2000;21:219–225. doi: 10.1097/00006676-200010000-00001. [DOI] [PubMed] [Google Scholar]
- Chen Y, Thelin WR, Yang B, Milgram SL, Jacobson K. Transient anchorage of cross-linked glycosyl-phosphatidylinositol-anchored proteins depends on cholesterol: Src family kinases, caveolin, and phosphoinositides. J Cell Biol. 2006;175:169–178. doi: 10.1083/jcb.200512116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenklusen JC, Thompson JC, Troncoso P, Kagan J, Conti CJ. Loss of heterozygosity in human primary prostate carcinomas: a possible tumor suppressor gene at 7q31.1. Cancer Res. 1994;54:6370–6373. [PubMed] [Google Scholar]
- Koike M, Takeuchi S, Park S, Hatta Y, Yokota J, Tsuruoka N, Koeffler HP. Ovarian cancer: loss of heterozygosity frequently occurs in the ATM gene, but structural alterations do not occur in this gene. Oncology. 1999;56:160–163. doi: 10.1159/000011958. [DOI] [PubMed] [Google Scholar]
- Nishizuka S, Tamura G, Terashima M, Satodate R. Commonly deleted region on the long arm of chromosome 7 in differentiated adenocarcinoma of the stomach. Br J Cancer. 1997;76:1567–1571. doi: 10.1038/bjc.1997.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shridhar V, Sun QC, Miller OJ, Kalemkerian GP, Petros J, Smith DI. Loss of heterozygosity on the long arm of human chromosome 7 in sporadic renal cell carcinomas. Oncogene. 1997;15:2727–2733. doi: 10.1038/sj.onc.1201448. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zhang XL, Lisanti MP. Sequence and detailed organization of the human caveolin-1 and -2 genes located near the D7S522 locus (7q31.1): methylation of a CpG island in the 5′ promoter region of the caveolin-1 gene in human breast cancer cell lines. FEBS Lett. 1999;448:221–230. doi: 10.1016/s0014-5793(99)00365-8. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Matsuda S, Machida K, Yamamoto T, Fukuda Y, Nimura Y, Hayakawa T, Hamaguchi M. Invasion activating caveolin-1 mutation in human scirrhous breast cancers. Cancer Res. 2001;61:2361–2364. [PubMed] [Google Scholar]
- Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–C506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]