Abstract
CC chemokine receptor 1 (CCR1) is found on a variety of cells in the immune system and has been shown to play an important role in the host response to pathogens. These studies used a murine model of virus-induced exacerbation of allergic airway disease to examine the role of CCR1 on T cells associated with immune responses taking place in the lung. Lungs of virally exacerbated allergic animals contained elevated levels of interferon-γ and interleukin-13 and increased levels of CCR1 ligands CCL3 and CCL5. CCR1 expression on T cells was increased in virally exacerbated allergic animals over the level observed in mice sensitized to allergen or exposed to viral infection alone. Using mice deficient for CCR1, we observed decreased airway hyperreactivity and Th2 cytokine production from CD4+ T cells when this receptor was absent. Transfer studies demonstrated that neither CD4+ nor CD8+ T cells from CCR1−/− mice migrated to the lymph node as efficiently as wild-type T cells. Intracellular cytokine staining in wild-type mice revealed that CCR1+ CD4+ and CD8+ T cells are associated with interleukin-13 production. Thus, these studies identify CCR1 as a potential target for alleviating T-cell accumulation during exacerbation of asthmatic disease.
Previous research has demonstrated a clear link between CC chemokine receptor 1 (CCR1) and pulmonary inflammation. Some of the first studies examining the function of this receptor in inflammation demonstrated that its absence during an acute model of lung injury markedly reduced inflammation, likely through reduced neutrophil recruitment.1 Other studies have determined that CCR1−/− mice have reduced pulmonary immune responses to a variety of pathogens, including Aspergillis fumigatus2 and respiratory syncytial virus (RSV).3 Additionally, in a murine model in which mice were first infected with RSV and then sensitized and challenged with allergen, CCR1−/− mice had reduced inflammation and reduced T-cell recruitment to the lungs.4 This latter study demonstrated that there was no difference between CCR1−/− and wild-type mice in their response to allergen challenge alone. Additionally, interleukin (IL)-13 transgenic mice crossed with mice deficient in CCR1 displayed a diminished fibrotic response in the lung, thus providing another link between this receptor, chronic diseases, and Th2 cytokines.5
Because of the diversity of cell types expressing this receptor, it has been difficult to identify the subset(s) of CCR1-expressing cells that contribute to the pathogenesis seen in CCR1-dependent models of pulmonary inflammation. Some evidence suggests that CCR1 may be important in the trafficking of subsets of T cells during immune responses, although this has also been difficult to determine because the CCR1 ligands CCL3 and CCL5 can also bind to CCR5. Thus the expression pattern of CCR1 on T cells remains unclear. One study suggests that expression of this receptor is restricted to memory CD4+ T cells,6 but another found the receptor to be expressed equally by both naïve and memory populations of CD4+ T cells.7 Additionally, it has been shown that CCL3 and CCL5 caused the migration and adhesion of activated CD4+, but not CD8+, T cells in vitro.8 Furthermore, whereas CCR1 can mediate cellular adhesion events, CCR5 was shown to be responsible for diapedesis and changes in T-cell morphology.9
In the present studies a murine model of exacerbation of allergic asthma was used to study the function of CCR1 on T cells. In this model, mice were sensitized to allergen and subsequently infected with RSV and challenged with allergen during viral infection. Exacerbation of allergic asthma is a clinically relevant medical condition that can occur when a patient is exposed to both allergen and a viral infection at the same time.10 CCR1 ligands CCL3 and CCL5 have been found to be up-regulated during both allergic responses and respiratory viral infections in humans11,12; thus, we hypothesized that CCR1 may be important in mediating the adaptive pulmonary immune response that occurs during exacerbation of allergic asthma. We found that CCR1 was expressed on both CD4+ and CD8+ T cells during the exacerbated response in the draining lymph nodes and appeared to be associated with migration of these cells to sites of inflammation. The data demonstrate that whereas CCR1 appeared to be associated with decreased cytokine production in CD4+ T cells, CD8+ T cells lacking CCR1 did not proliferate in response to allergen. Importantly, IL-4 and IL-13 and interferon (IFN)-γ-expressing T cells from the lymph nodes of wild-type mice expressed CCR1, thus suggesting that CCR1 is an important receptor for inflammatory cells. These data demonstrate that CCR1 may be important in mediating migration of T-cell subsets to sites of inflammation and may also define distinct functional subsets of CD4+ and CD8+ T cells.
Materials and Methods
Mice
All mice used were supplied by Jackson Laboratories (Bar Harbor, ME) except for BALB/c CCR1−/− mice, which were the generous gift of Dr. Craig Gerard (Harvard Medical School, Boston, MA). Mice were sensitized at 6 to 8 weeks and were age-, strain-, and sex-matched in all experiments.
Cockroach Antigen (CRA) Sensitization
CRA sensitization was performed as previously described.13 Briefly, female mice, 6 to 8 weeks of age, were sensitized with a 1:1 mixture of CRA (Bayer Corp., Elkhart, IN) and incomplete Freund’s adjuvant (Sigma Chemical, St. Louis, MO), both subcutaneously and intraperitoneally on day 0. On day 14, mice received an intranasal challenge of CRA. All mice then received 40 μl of CRA intratracheally on day 21 of sensitization.
Box Plethysmography
AHR was assessed by box plethysmography, as previously described,14 using a Buxco system with Biosystem XA software (Buxco, Wilmington, NC). Briefly, mice were anesthetized with sodium pentobarbital and the trachea intubated with an 18-gauge metal tube. Mice were ventilated using a Harvard MiniVent (March, Germany) at 150 breaths/minute with a 125 μl stroke volume. Methacholine (0.1 mg/kg) was injected intravenously after taking a baseline measurement of airway resistance, which divides the area under the curve measurement of tracheal pressure by the area under the curve measurement of box pressure. Airway resistance was determined by subtracting the baseline measurement from the measurement taken after methacholine injection.
RSV
The line 19 strain of RSV was originally isolated from an infant at the University of Michigan hospital15 and grown on HEp2 cells as previously described.16 Briefly, cells were infected with 2.5 × 105 PFU and cultured for 3 days. Cells were then lysed, supernatants clarified by centrifugation, and virus plaqued on Vero cells using 1.8% methylcellulose Eagle’s modified essential medium media. Titer was determined by staining for RSV antigen as previously described.16 Mice were intratracheally infected with 40 μl of virus that contained between 3.0 and 4.0 × 106 PFU.
Exacerbation of Allergen-Sensitized Mice
On day 0, mice were sensitized subcutaneously and intraperitoneally with CRA. Mice were challenged intranasally with CRA on day 14, followed by RSV infection on day 16. On day 21 mice were challenged intratracheally with CRA, and data were collected on day 22.
Enzyme-Linked Immunosorbent Assay Analysis
Standardized sandwich enzyme-linked immunosorbent assays were run as previously described.17 Plates were coated with 15 μg/ml of polyclonal capture antibody (R&D Systems, Minneapolis, MN) overnight at 4°C, washed, and blocked with 2% bovine serum albumin [in phosphate-buffered saline (PBS)] for 1 hour at 37°C. Cell-free lung homogenate was then added to each well, incubated for 1 hour at 37°C, and washed again. A biotinylated polyclonal detection antibody was added at a concentration of 3.5 mg/ml and incubated for 45 minutes at 37°C. To obtain pg/mg protein, a Bradford assay was done (Bio-Rad, Hercules, CA) on lung homogenates to determine total protein concentration.
RNA Isolation and Quantitative Polymerase Chain Reaction (qPCR)
RNA was isolated from the upper right lobes of lung using Trizol (Invitrogen, Carlsbad, CA). Levels of mRNA were assessed using qPCR analysis (TaqMan) with predeveloped primers and probe sets from Applied Biosystems (Foster City, CA) (IFN-γ Mm00801778_m1, IL-13 Mm00434204_m1). Quantification of the genes of interests were normalized to GAPDH and expressed as fold increases over the naive control for each treatment.
Bioplex Assay
T cells were isolated by magnetic activated cell sorting (Millenyi Biotec, Auburn, CA) from the lymph nodes of exacerbated mice 24 hours after intratracheal challenge with CRA. Isolated subsets of T cells were then co-cultured with bone marrow-derived dendritic cells (BMDCs) grown for 5 days in GMCSF and selected by CD11c expression using magnetic activated cell sorting. DCs were then pulsed with CRA (30 μl/ml) or RSV (multiplicity of infection, 1) for 2 hours in a 96-well plate, centrifuged, and washed to remove antigen. T cells (2.0 × 105) were then added to each well so that the final volume per well was 200 μl. Supernatants were collected after 48 hours of co-culture.
Proliferation
Co-cultures were set up as described for the bioplex assay. Cultures were incubated for 4 days, after which 1 μCi of 3H thymidine was added per well, and cultures were then incubated for an additional 24 hours. Plates were harvested using a PHD harvester.
Cell Transfers
Purified T cells were collected from the lymph nodes of exacerbated mice using positive selection (Miltenyi Biotech, Auburn, CA). Of the positively selected cells 3.0 × 106 were used for transfer in all cases.
Flow Cytometry
Whole lungs from mice were dispersed in type IV collagenase, red blood cells were lysed, and total number of cells per lung was counted. To obtain a total lung cell count, a single cell suspension from the entire lung was resuspended in 3 ml of buffer (PBS, 1% fetal calf serum, and 0.1% sodium azide). Ten μl of this was diluted 1:100 in Turk’s stain (1 L PBS, 3 ml glacial acetic acid, 0.05 g crystal violet), and 10 μl of this dilution was loaded onto a hemocytometer. Three million cells were stained with CD4 and CD8 antibody (Pharmingen, San Diego, CA). Events, 8.0 × 105, were counted on a Cytomics FC 500 flow cytometer (Beckman-Coulter, Fullerton, CA) and the data analyzed with Flowjo software (Treestar, Ashland, OR). Single-cell suspensions were made from lymph nodes by pressing the lymph nodes through a 100-μm filter and then lysing red blood cells.
CCR1 staining was done after FC block by first incubating cells with rabbit anti-human CCR1 antibody (cross-reactive with murine CCR1) from Capralogics (catalog number CI101; Hardwick, MA) for 20 minutes at a dilution of 1:200. One μl of normal goat serum was then added to each sample and incubated for an additional 10 minutes. A fluorescein isothiocyanate-labeled goat anti-rabbit secondary (Jackson ImmunoResearch, West Grove, PA) was then added at a dilution of 1:100 and incubated for 20 minutes.
Carboxy Fluoroscein Succinimidyl Ester (CFSE) Labeling
CFSE was obtained from Invitrogen and brought to 10 mmol/L concentration in dimethyl sulfoxide. A 2 μmol/L stock solution was used to label cells at 5.0 × 106/ml at room temperature in the dark in PBS for 20 minutes. Cells were washed three times to remove excess.
Statistics
All statistics were done using Graphpad Prism 4 (San Diego, CA). Significance was determined using one-way or two-way analysis of variance with 95% confidence intervals where applicable.
Results
RSV Exacerbates the Allergic Response and Increases CCR1 Ligand Production
To determine the effects of RSV infection occurring after allergen sensitization, wild-type mice were sensitized with CRA on day 0 and then infected with RSV intratracheally on day 16. On day 21 mice received an intratracheal challenge of CRA allergen, and 1 day later (day 22 after sensitization) mice were sacrificed for whole lung analysis. The day 22 time point, at 24 hours after CRA challenge and 6 days after RSV infection, was chosen because at this time only modest increases in airway hyperreactivity (AHR) were observed with RSV infection alone.18 We observed both a decrease in the ratio of CD4:CD8 cells in the airways of RSV-exacerbated (CRA/RSV) mice and an increase in the percentage of both CD4+ and CD8+ T cells in the BAL of mice infected with RSV, regardless of whether these mice were exacerbated (data not shown). Additionally, there was a significant increase in AHR in exacerbated mice compared to mice that were either challenged with allergen (CRA) or infected with virus alone.
Chemokine and cytokine analysis by enzyme-linked immunosorbent assay of whole lung homogenates showed an overall increase in cytokine and chemokine production in CRA/RSV mice compared to CRA or RSV groups. IFN-γ and IL-13 levels in the lungs of CRA/RSV mice were significantly increased compared to mice receiving CRA sensitization or RSV infection alone (Figure 1, B and C). Moreover, CCR1 ligands (CCL3 and CCL5) were significantly elevated in the lungs of CRA/RSV mice compared to mice receiving CRA sensitization alone (Figure 1, D and E). However, there were no increases in the levels of IL-4 or IL-5 in whole lung homogenates. To assess further the differences between the three groups of animals, we examined histological sections of paraffin-embedded lungs taken 24 hours after the final challenge with CRA (Figure 1F). Although both CRA and CRA/RSV animals exhibited eosinophilic infiltrate, no difference was found between the two groups when the number of eosinophils associated with major airways was enumerated (data not shown). The cellular infiltrate observed in RSV-infected mice was primarily mononuclear in nature. Whereas alteration of airway epithelial cells was prominent in all three treatment groups, the damage to airway epithelium appeared most severe in the CRA/RSV animals, in which separation of cells from the basement membrane was noted. Thus, the combination of allergen and RSV appeared to increase the airway damage and alter the composition of the cellular infiltrate, which included both eosinophil and lymphocyte populations.
Figure 1.
Viral infection of allergen-sensitized mice increases AHR and inflammatory cytokines in the lung. A: AHR of mice sensitized to CRA, infected with RSV, or in a combined model. *P = 0.0189 when compared to CRA and 0.0043 when compared to RSV. The dashed line represents AHR of naïve mice. We also measured amounts of IFN-γ (B) *P = 0.0139 when compared to CRA, IL-13 (C) *P = 0.0001 when compared to CRA and 0.0025 when compared to RSV, CCL3 (D) *P = 0.0394 when compared to CRA. E: CCL5 *P = 0.0260 when compared to CRA. F: H&E staining of lung sections showing representative airways of CRA, RSV, and CRA/RSV mice. n = five mice/group/experiment, data are pooled from three experiments.
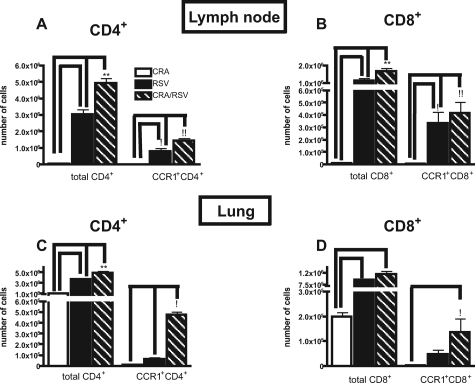
Because we observed increases in both IFN-γ and IL-13, two cytokines produced by T cells, in the lungs of CRA/RSV mice 24 hours after allergen challenge, we next assessed the contribution of T cells to the exacerbation of the asthmatic response. To better understand the T-cell response during exacerbation, we analyzed the number of CD4+ and CD8+ T cells in the lymph node and lung in CRA, RSV, and CRA/RSV mice at 0, 8, and 24 hours after CRA challenge (Figure 2, A–D). At all time points examined, the total number of T cells in the lungs and lymph nodes of CRA/RSV mice was significantly higher than the number found in CRA mice. There was also a significant difference in the number of CD4+ T cells in the lungs of CRA/RSV mice at 8 hours after allergen challenge when compared to mice infected with RSV alone (Figure 2C). However, the most striking differences between CRA/RSV mice and mice infected only with RSV occurred in the lymph node. At 8 hours after CRA challenge, there were significantly more CD4+ and CD8+ cells in the lymph nodes of CRA/RSV mice.
Figure 2.
Increased total T and CCR1+ T cells in the lung and lymph node during exacerbation. A and B: The number of total and CCR1+, CD4+, and CD8+ T cells was assessed in the lymph node of mice infected with RSV, sensitized to CRA, or receiving both treatments 8 hours after CRA challenge. **P < 0.001 compared to RSV. At this point both RSV and CRA/RSV treatments had significantly more total T cells in the lymph node than CRA treatment alone. !!P < 0.001 compared to CRA and RSV. !P < 0.01 compared to CRA. C and D: Total number of T cells in the lung under the same conditions as A and B. **P < 0.001. At all time points, both RSV and CRA/RSV treatments had significantly more total T cells in the lung than CRA treatment alone. C: !P < 0.01 compared to CRA and RSV. D: !P < 0.05 compared to CRA. n = three mice/group/experiment, data are pooled from three experiments.
When we examined the expression of CCR1 on T cells, we found that peak expression occurred 8 hours after antigen challenge. There was greater number of CCR1+ T cells in the lymph nodes of RSV mice than in the lymph nodes of CRA mice 8 hours after CRA challenge. Furthermore, an additional increase of CCR1+ CD4+ T cells was observed in the lymph nodes of CRA/RSV mice (Figure 2, A and B). We also found a significant difference in the number of CCR1+ CD4+ and CD8+ T cells in the lungs of CRA/RSV mice at 8 hours after challenge when compared to CRA mice or RSV mice (Figure 2, C and D). Collectively, these data suggest that CCR1 may be important in contributing to the exacerbated phenotype by mediating migration of T cells to both the lung and lymph node.
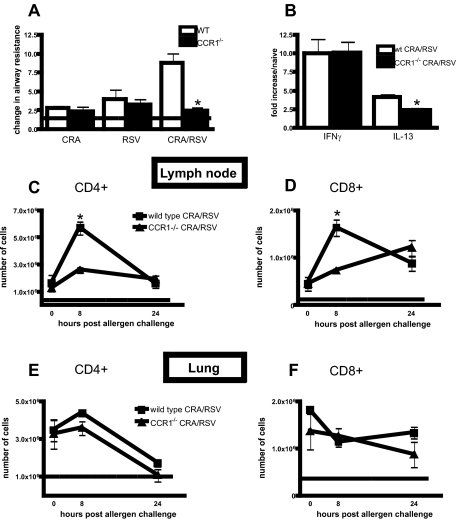
CCR1−/− CRA-Sensitized Mice Do Not Have an Exacerbated Phenotype on RSV Infection
It has previously been reported that the airway hyperresponsiveness of CCR1-deficient mice is not different from wild-type mice on allergen challenge alone.4 Because we observed an increase in CCR1+ T cells in the lungs of CRA/RSV mice, we next determined if the absence of CCR1 altered AHR in the CRA/RSV model. Figure 3A demonstrates that there was no difference in AHR measurements between CCR1−/− and wild-type CRA mice or RSV mice at day 6 after infection. However, although wild-type CRA/RSV mice had increased AHR, we observed no increase in AHR in CCR1−/− CRA/RSV mice. Analysis of whole lung mRNA revealed less IL-13 transcript in CCR1−/− lungs than in wild-type lungs but no difference in the amount of IFN-γ detected between the two groups (Figure 3B). The number of CD4+ and CD8+ T cells in the lungs and lymph nodes of CCR1−/− or wild-type CRA/RSV mice was assessed by flow cytometry (Figure 3, C–F). Whereas no difference in the numbers of CD4+ or CD8+ T cells in the lungs of CRA/RSV CCR1−/− mice was found when compared to wild type (Figure 3, E and F), there was a significant decrease in the number of T cells in the draining lymph nodes of CCR1−/− CRA/RSV mice at 8 hours after allergen challenge (Figure 3, C and D). Analysis of other cell populations by flow cytometry, including CD11b+/CD11c+, CD45R+/CD11c+, and F480+ cells revealed no difference between wild-type and CCR1−/− mice after CRA/RSV treatment. Additionally the number of eosinophils associated with major airways in CCR1−/− and wild-type CRA/RSV mice 24 hours after CRA was the same, as quantified by examination of histological sections.
Figure 3.
CCR1−/− mice have reduced AHR and T-cell recruitment during viral exacerbation of allergic airway disease. A: AHR of CCR1−/− and wild-type mice was assessed 24 hours after antigen challenge or 6 days after RSV infection. The dashed line represents AHR of naïve mice. *P = 0.0022. B: The amount of transcript for IL-13 and IFN-γ was assessed using whole lung mRNA by real-time PCR. *P = 0.024. C–F: The number of CD4+ and CD8+ T cells in the lungs and lymph nodes of exacerbated wild-type and CCR1−/− mice at 0, 8, and 24 hours after antigen challenge. The dashed line represents the number of T cells found in the lungs or lymph nodes of naïve mice. *P < 0.01. n = five mice/group/experiment, data are pooled from two experiments.
CCR1−/− T Cells Are Deficient in Lung and Lymph Node Migration
Because we observed a decrease in CD4+ T cells in the lymph nodes of CCR1−/− CRA/RSV mice, we next asked whether these cells were appropriately trafficking to the lungs and lymph nodes. In these experiments, we used the pan-T cell marker CD90 to purify T cells from the draining lymph nodes of wild-type and CCR1−/− CRA/RSV mice 24 hours after allergen challenge. T cells were then labeled with CFSE and transferred into exacerbated wild-type mice 1 day before final allergen challenge. Cells from both strains of mice were ≥95% viable by trypan blue staining before transfer. At 24 hours after allergen challenge, both lymph nodes and lungs were harvested from recipient CRA/RSV mice, and the number of CFSE+ CD4+ and CD8+ T cells were quantified. The number of CCR1−/− CD4+ and CD8+ T cells recruited to the lymph node was significantly lower compared to the number of wild-type T cells (Figure 4A). Additionally, the number of donor CD4+ from CCR1−/− mice recruited to the lung was less compared to the number of donor T cells recruited from wild-type mice (Figure 4B). Thus, CCR1 is an important receptor for recruiting T cells to both the lung and the lymph node.
Figure 4.
CCR1−/− T cells are deficient in migration to sites of inflammation. A and B: CD90+ T cells were isolated from the lymph nodes of CCR1−/− or wild-type mice 8 hours after antigen challenge and labeled with CFSE. Cells were transferred into wild-type mice 1 day before allergen challenge, and the number of CFSE+ CD4+ and CD8+ T cells per million events assessed 24 hours after antigen challenge. A: *P = 0.0081 for CD4+ cells and **P = 0.0085 for CD8+ cells. B: *P = 0.0003, n = four mice/group/experiment, data are pooled from two experiments.
CCR1−/− T Cells Produce Less Inflammatory Cytokines in Response to Antigen
Although several studies have examined mechanisms contributing to the exacerbation of allergic responses by viral infection, the cytokine profile of responding T cells has not previously been studied.19,20,21 We sought to determine whether T cells from exacerbated mice could produce cytokines in response to both allergen and viral antigens. Furthermore, we wanted to determine whether this cytokine profile was altered when using T cells from CCR1−/− mice. To do this we isolated T cells from the draining lymph nodes of CCR1−/− or wild-type CRA/RSV mice and co-cultured them with antigen-pulsed BMDCs. Cells were co-cultured for 48 hours and cytokines measured in cell-free supernatants by luminex assay (Figure 5, A–D). CD4+ and CD8+ T cells from exacerbated wild-type mice were able to produce cytokines when cultured with either allergen-pulsed or virally infected BMDCs. Interestingly, we found that CD4+ T cells from CCR1−/− CRA/RSV mice produced less Th1 and Th2 cytokines on exposure to RSV-pulsed BMDCs and less Th2 cytokines in response to CRA-pulsed DCs (Figure 5, A and C). There were no significant differences in the ability of CD8+ T cells from either strain to produce IFN-γ when stimulated by RSV-pulsed BMDCs (Figure 5D).
Figure 5.
CCR1−/− T cells have reduced cytokine production and proliferation in response to restimulation with allergen or virus. A–D: T cells isolated from the draining lymph node of exacerbated animals were restimulated with either RSV- or CRA-pulsed BMDCs for 48 hours. Shown is one representative of three experiments. #P = 0.0022, ##P < 0.0001, *P < 0.0001, **P = 0.0038, ***P = 0.0022. E and F: Proliferation of CD4+ and CD8+ T cells isolated from the lymph nodes of CCR1−/− or wild-type mice. T cells (2.0 × 105) were co-cultured with 4.0 × 104 pulsed BMDCs for 5 days and pulsed with 3H thymidine 24 hours before analysis. *P < 0.001 by one-way analysis of variance when compared to WT CD8+ T cells cultured with CRA-pulsed BMDCs. n = three mice/group/experiment, data are pooled from four experiments. G: Transfer of wild-type CD4+ or CD8+ T cells into CCR1−/− mice restores AHR. #P < 0.05 compared to CCR1−/− *P < 0.01 compared to CCR1−/−. n = four mice/group/experiment, data are pooled from three experiments.
To characterize further the differences between the wild-type and CCR1−/− strains, CD4+ and CD8+ T cells were again isolated from the lymph nodes of CCR1−/− and wild-type CRA/RSV mice and used in a proliferation assay. Proliferation was measured in vitro by 3H thymidine uptake after 4 days of co-culture of isolated T cells with BMDCs and allergen. CD4+ T cells from wild-type and CCR1−/− CRA/RSV mice proliferated equally well in response to allergen (Figure 5E). Interestingly, we found that whereas wild-type CD8+ T cells were able to proliferate in response to CRA, CCR1−/− CD8+ T cells did not proliferate when co-cultured with allergen-pulsed BMDCs (Figure 5F). Additionally, we found no difference in the ability of CD4+ or CD8+ T cells from either strain to proliferate in response to RSV (data not shown).
To determine whether the lower airway hyperresponsiveness observed in CCR1−/− CRA/RSV mice was attributable specifically to a defect in T cells, we transferred CD4+ or CD8+ T cells purified from the draining lymph nodes of wild-type CRA/RSV mice into CCR1−/− CRA/RSV mice. T cells were isolated from wild-type mice 24 hours after the final CRA challenge and transferred on the same day into CCR1−/− mice on day 20 of the exacerbation model. Results demonstrate that both CD4+ and CD8+ T cells could reconstitute the reduced AHR observed in CCR1−/− mice (Figure 5G).
CCR1 Is Expressed on Cytokine-Producing Cells
Because there was a deficiency in the ability of CCR1−/− CD4+ T cells to produce both Th1 and Th2 cytokines in response to antigen, the link between the expression of CCR1 and cytokine production was further investigated. To determine whether CCR1+ T cells were responsible for the production of Th1 or Th2 cytokines, lymph nodes from wild-type exacerbated mice were taken 8 hours after antigen challenge. Whole lymph node cells were stimulated with PMA and ionomycin for 6 hours and then stained for both CD4 and CD8 markers, as well as for the CCR1 receptor. Additionally, we stained intracellularly for cytokines IFN-γ, IL-4, or IL-13. We found that the CCR1+ CD4+ T-cell population had a higher frequency of cells producing cytokines than the bulk population of CD4+ T cells (Figure 6). Whereas IL-4- and IFN-γ-producing CD4+ cells were significantly enriched by gating on the CCR1+ population, IL-13+ CD4+ cells increased by nearly 10-fold when gating on this population (Figure 6G). To characterize further the role of CCR1 on CD8+ T cells, we examined the cytokine expression of this population after stimulation with PMA and ionomycin. Although we were unable to detect any IL-4 production from CD8+ T cells after stimulation, we did find a slight increase in the percentage of IL-13+ cells when gating on the CCR1+ population. Additionally, we observed a negative association between CCR1 expression and the production of IFN-γ. These data clearly demonstrate that CCR1 is associated with subsets of CD4+ T cells that produce cytokines in response to allergen and viral antigen and of CD8+ T cells that produce IL-13 and less IFN-γ than the bulk population.
Figure 6.
CCR1 is associated with cytokine production from CD4+ T cells. Lymph node cells (2.0 × 106) were taken from exacerbated wild-type mice 8 hours after allergen challenge and stimulated with PMA and ionomycin for 6 hours in the presence of brefeldin A. Intracellular staining for IL-4, IL-13, and IFN-γ was performed to assess cytokine production. A, C, E: Percentage of total CD4+ T cells producing IL-4, IL-13, or IFN-γ. B, D, F: Percentage of CCR1+ CD4+ T cells producing these same cytokines. G: Quantification of the percentage of total and CCR1+ CD4+ T cells producing cytokines. *P = 0.0015 **P = 0.0025. F: Percentage of total or CCR1+ CD8+ T cells producing cytokines. *P = 0.0001. ND = not detected n = three mice/group/experiment, data are pooled from two experiments.
Discussion
The studies presented here indicate that CCR1 expression on T cells is linked to the exacerbation of allergic airway disease induced by viral infection through increased recruitment of T cells to the lung and lymph node. Although several studies have linked the absence of CCR1 to a decreased Th2 response2,18 and emphasized a link between IL-13-driven fibrotic responses and CCR1,5 no previous research has specifically shown that T cells are responsible for this phenotype. The above studies demonstrate that allergic CCR1−/− mice are not exacerbated when infected with RSV, and this may be attributable to a lack of activated T cells being recruited to both the lung and the lymph node. No previous study has linked CCR1 expression on T cells to a change in phenotype of a respiratory inflammation model. These data imply that CCR1 may be an important T-cell chemokine receptor during inflammatory responses.
Other chemokine receptors have been reported to mark Th1 or Treg subsets of CD4+ T cells,22,23,24 but CD4+ T cells from CCR1−/− mice produced less of both the Th1 cytokine IFN-γ and the Th2 cytokines IL-4 and IL-13 on restimulation with antigen. Furthermore, intracellular cytokine staining of lymph node T cells from wild-type animals revealed that CCR1 is associated with CD4+ T cells producing IFN-γ, IL-4, and/or IL-13. These data have several implications. Firstly, whereas several studies have demonstrated a link between removal of the CCR1 gene and decreased IL-13 expression, none have shown which cell types might be contributing to pathology through IL-13 production in CCR1+/+ mice.2,3,4,5 Here we demonstrate that CCR1+ CD4+ T cells produce more IL-13 than the bulk population of CD4+ cells. However, CCR1+ CD4+ T cells also produce more IFN-γ. Thus, a second implication from these data are CCR1 may not be restricted to either the Th1 or Th2 cell subset but instead may be expressed by both during T-cell activation. We have found that a percentage of both CCR1+ CD8+ and CD4+ T cells express the very early activation marker CD69, again suggesting that CCR1 is a marker associated with activation (data not shown). Although much research has been done on the effect of CCR1 deficiency in models that promote a Th2 phenotype, one study has also shown that CCR1−/− mice have lower IFN-γ production during Th1 granuloma formation.25 Additionally, several chemokines that bind to CCR1 are produced during respiratory viral infection, which classically elicits a Th1 response.26,27
We have shown that CCR1+ T cells are recruited to the lung during viral infection. Initiating an allergic response during the viral infection further increased the number of CCR1+ T cells in both the lung and the lymph node, likely attributable to increased production of CCR1 ligands induced during the exacerbated response. Whereas the number of T cells in the lungs of CCR1−/− CRA/RSV mice remained similar to wild-type CRA/RSV mice throughout the time course of allergen challenge, the number of T cells in the lymph node was significantly different between wild-type and CCR1−/− CRA/RSV mice. These data, and the fact that T cells from CCR1−/− mice do not migrate to the lung or lymph node as efficiently as wild-type T cells, suggest that CCR1 is an important T-cell receptor during the progression of severe inflammation. Whereas other studies have shown that the absence of CCR1 can protect against acute lung injury1,28 or severe sepsis,29 both of these effects were attributed to innate immune responses. One study demonstrating that CCR1−/− mice were protective in a murine model of cornea transplantation found less infiltrating CD3+ cells and an impaired delayed type hypersensitivity response.30 This latter research concurs with our own findings that CCR1+ T cells are important during inflammatory processes.
These data demonstrated that T cells from the draining lymph nodes of exacerbated mice could respond to both allergen and viral antigens, a novel finding that may help to explain why the exacerbation response is so severe. It is our contention that the accumulation of T cells in wild-type animals is attributable to an influx of a combination of allergen-specific and virus-specific cells that are responding to CCR1 ligands produced as a result of viral infection. Allergen-responsive T cells may share a similar chemokine receptor profile as T cells recruited to eliminate virus. The enhanced expression of CCR1 ligands during viral infection could cause increased AHR and cytokine production by migration of allergen- and virus-specific T cells into the lung and lymph nodes. Previous studies using influenza virus and memory T cells specific for OVA have demonstrated increased OVA-specific CD4+ and CD8+ T-cell recruitment to the lung and BAL occurs in the presence of viral infection,31,32 further implicating that a viral response can attract nonviral responsive cells to the site of inflammation. However, for exacerbation to occur, allergen must be present to elicit a response from allergen-specific T cells. Two human studies examining the relationship between exacerbated responses and the presence of allergen found this to be the case.10,33
Collectively, the above studies suggest that CCR1 has differential roles on CD4+ and CD8+ T cells. Whereas CCR1+ CD4+ T cells display enhanced inflammatory cytokine production, we observed no defect in the cytokine production of CD8+ T cells from CCR1−/− CRA/RSV mice in response to either allergen or viral antigen. However, there was a significant reduction in the number of donor CCR1−/− CD8+ T cells recruited to the lymph node when compared to wild type. Furthermore, we observed a significant difference in the ability of CCR1−/− CD8+ T cells to proliferate in response to allergen when compared to wild-type CD8+ T cells, suggesting that CCR1+ CD8+ T cells contribute to allergic responses. Intracellular cytokine staining of wild-type CD8+ T cells from the lymph nodes of CRA/RSV mice suggests that CCR1 is associated with IL-13-producing CD8+ T cells. Furthermore, we observed a decrease in IFN-γ+ cells in the CCR1+ CD8+ T-cell population as compared to total CD8+ T-cell population. Altogether, these data suggest that CCR1+ T cells may contribute to the outcome of allergic responses.
In conclusion, these data indicate that CCR1 is involved in the exacerbation of allergen-induced response and is expressed by distinct subsets of CD4+ and CD8+ T cells. This effect may not be specific to RSV and may account for exacerbation of asthma by other respiratory viruses as well. Several respiratory viruses have been shown to cause production of CCL3 and CCL5 in humans.12,34 CCR1 and its ligands could provide a viable target for future therapies to regulate T-cell-mediated inflammation, especially in virally exacerbated diseases.
Acknowledgments
We thank Dr. Craig Gerard for his gift of CCR1−/− mice.
Footnotes
Address reprint requests to Matthew Schaller, University of Michigan, 109 Zina Pitcher Pl., Ann Arbor, MI 48109. E-mail: mschalle@umich.edu.
Supported by the National Institutes of Health (grant AIO36302).
References
- Gerard C, Frossard JL, Bhatia M, Saluja A, Gerard NP, Lu B, Steer M. Targeted disruption of the beta-chemokine receptor CCR1 protects against pancreatitis-associated lung injury. J Clin Invest. 1997;100:2022–2027. doi: 10.1172/JCI119734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blease K, Mehrad B, Standiford TJ, Lukacs NW, Kunkel SL, Chensue SW, Lu B, Gerard CJ, Hogaboam CM. Airway remodeling is absent in CCR1−/− mice during chronic fungal allergic airway disease. J Immunol. 2000;165:1564–1572. doi: 10.4049/jimmunol.165.3.1564. [DOI] [PubMed] [Google Scholar]
- Miller AL, Gerard C, Schaller M, Gruber AD, Humbles AA, Lukacs NW. Deletion of CCR1 attenuates pathophysiologic responses during respiratory syncytial virus infection. J Immunol. 2006;176:2562–2567. doi: 10.4049/jimmunol.176.4.2562. [DOI] [PubMed] [Google Scholar]
- John AE, Gerard CJ, Schaller M, Miller AL, Berlin AA, Humbles AA, Lukacs NW. Respiratory syncytial virus-induced exaggeration of allergic airway disease is dependent upon CCR1-associated immune responses. Eur J Immunol. 2005;35:108–116. doi: 10.1002/eji.200425439. [DOI] [PubMed] [Google Scholar]
- Ma B, Zhu Z, Homer RJ, Gerard C, Strieter R, Elias JA. The C10/CCL6 chemokine and CCR1 play critical roles in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol. 2004;172:1872–1881. doi: 10.4049/jimmunol.172.3.1872. [DOI] [PubMed] [Google Scholar]
- Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- Sato K, Kawasaki H, Morimoto C, Yamashima N, Matsuyama T. An abortive ligand-induced activation of CCR1-mediated downstream signaling event and a deficiency of CCR5 expression are associated with the hyporesponsiveness of human naive CD4+ T cells to CCL3 and CCL5. J Immunol. 2002;168:6263–6272. doi: 10.4049/jimmunol.168.12.6263. [DOI] [PubMed] [Google Scholar]
- Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- Weber C, Weber KS, Klier C, Gu S, Wank R, Horuk R, Nelson PJ. Specialized roles of the chemokine receptors CCR1 and CCR5 in the recruitment of monocytes and T(H)1-like/CD45RO(+) T cells. Blood. 2001;97:1144–1146. doi: 10.1182/blood.v97.4.1144. [DOI] [PubMed] [Google Scholar]
- Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ. 2002;324:763–766. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga K, Yanagisawa S, Ichikawa T, Ueshima K, Akamatsu K, Hirano T, Nakanishi M, Yamagata T, Minakata Y, Ichinose M. Airway cytokine expression measured by means of protein array in exhaled breath condensate: correlation with physiologic properties in asthmatic patients. J Allergy Clin Immunol. 2006;118:84–90. doi: 10.1016/j.jaci.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Bonville CA, Rosenberg HF, Domachowske JB. Macrophage inflammatory protein-1alpha and RANTES are present in nasal secretions during ongoing upper respiratory tract infection. Pediatr Allergy Immunol. 1999;10:39–44. doi: 10.1034/j.1399-3038.1999.101005.x. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Lamm WJ, Strieter RM, Albert RK. Airway hyperreactivity is associated with specific leukocyte subset infiltration in a mouse model of allergic airway inflammation. Pathobiology. 1996;64:308–313. doi: 10.1159/000164065. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Warmington K, Lincoln P, Chensue SW, Kunkel SL. Differential recruitment of leukocyte populations and alteration of airway hyperreactivity by C-C family chemokines in allergic airway inflammation. J Immunol. 1997;158:4398–4404. [PubMed] [Google Scholar]
- Herlocher ML, Ewasyshyn M, Sambhara S, Gharaee-Kermani M, Cho D, Lai J, Klein M, Maassab HF. Immunological properties of plaque purified strains of live attenuated respiratory syncytial virus (RSV) for human vaccine. Vaccine. 1999;17:172–181. doi: 10.1016/s0264-410x(98)00155-8. [DOI] [PubMed] [Google Scholar]
- Miller AL, Bowlin TL, Lukacs NW. Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J Infect Dis. 2004;189:1419–1430. doi: 10.1086/382958. [DOI] [PubMed] [Google Scholar]
- John AE, Berlin AA, Lukacs NW. Respiratory syncytial virus-induced CCL5/RANTES contributes to exacerbation of allergic airway inflammation. Eur J Immunol. 2003;33:1677–1685. doi: 10.1002/eji.200323930. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Tekkanat KK, Berlin A, Hogaboam CM, Miller A, Evanoff H, Lincoln P, Maassab H. Respiratory syncytial virus predisposes mice to augmented allergic airway responses via IL-13-mediated mechanisms. J Immunol. 2001;167:1060–1065. doi: 10.4049/jimmunol.167.2.1060. [DOI] [PubMed] [Google Scholar]
- Marsland BJ, Scanga CB, Kopf M, Le Gros G. Allergic airway inflammation is exacerbated during acute influenza infection and correlates with increased allergen presentation and recruitment of allergen-specific T-helper type 2 cells. Clin Exp Allergy. 2004;34:1299–1306. doi: 10.1111/j.1365-2222.2004.02021.x. [DOI] [PubMed] [Google Scholar]
- Peebles RS, Jr, Sheller JR, Collins RD, Jarzecka K, Mitchell DB, Graham BS. Respiratory syncytial virus (RSV)-induced airway hyperresponsiveness in allergically sensitized mice is inhibited by live RSV and exacerbated by formalin-inactivated RSV. J Infect Dis. 2000;182:671–677. doi: 10.1086/315783. [DOI] [PubMed] [Google Scholar]
- Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol. 2004;5:337–343. doi: 10.1038/ni1041. [DOI] [PubMed] [Google Scholar]
- Freeman CM, Chiu BC, Stolberg VR, Hu J, Zeibecoglou K, Lukacs NW, Lira SA, Kunkel SL, Chensue SW. CCR8 is expressed by antigen-elicited, IL-10-producing CD4+CD25+ T cells, which regulate Th2-mediated granuloma formation in mice. J Immunol. 2005;174:1962–1970. doi: 10.4049/jimmunol.174.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Adachi Y, Onoue Y, Adachi YS, Okabe Y, Itazawa T, Toyoda M, Seki T, Morohashi M, Matsushima K, Miyawaki T. Differential expression of the chemokine receptors by the Th1- and Th2-type effector populations within circulating CD4+ T cells. J Leukoc Biol. 2000;68:568–574. [PubMed] [Google Scholar]
- Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang X, Qiu B, Frait KA, Hu JS, Sonstein J, Curtis JL, Lu B, Gerard C, Chensue SW. Chemokine receptor 1 knockout abrogates natural killer cell recruitment and impairs type-1 cytokines in lymphoid tissue during pulmonary granuloma formation. Am J Pathol. 2000;157:2055–2063. doi: 10.1016/S0002-9440(10)64844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Reed W, Henderson FW, Noah TL. RSV infection of human airway epithelial cells causes production of the beta-chemokine RANTES. Am J Physiol. 1997;272:L512–L520. doi: 10.1152/ajplung.1997.272.3.L512. [DOI] [PubMed] [Google Scholar]
- Haeberle HA, Kuziel WA, Dieterich HJ, Casola A, Gatalica Z, Garofalo RP. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1alpha in lung pathology. J Virol. 2001;75:878–890. doi: 10.1128/JVI.75.2.878-890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Horuk R, Bhatia M. Treatment with BX471, a nonpeptide CCR1 antagonist, protects mice against acute pancreatitis-associated lung injury by modulating neutrophil recruitment. Pancreas. 2007;34:233–241. doi: 10.1097/mpa.0b013e31802e7598. [DOI] [PubMed] [Google Scholar]
- Ness TL, Carpenter KJ, Ewing JL, Gerard CJ, Hogaboam CM, Kunkel SL. CCR1 and CC chemokine ligand 5 interactions exacerbate innate immune responses during sepsis. J Immunol. 2004;173:6938–6948. doi: 10.4049/jimmunol.173.11.6938. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Yamagami S, Liu Y, Zhang Q, Vora SS, Lu B, Gerard CJ, Dana MR. Deletion of the chemokine receptor CCR1 prolongs corneal allograft survival. Invest Ophthalmol Vis Sci. 2007;48:1228–1236. doi: 10.1167/iovs.05-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham DJ, Castrucci MR, Wingo FS, Belz GT, Doherty PC. The role of antigen in the localization of naive, acutely activated, and memory CD8(+) T cells to the lung during influenza pneumonia. J Immunol. 2001;167:6983–6990. doi: 10.4049/jimmunol.167.12.6983. [DOI] [PubMed] [Google Scholar]
- Chapman TJ, Castrucci MR, Padrick RC, Bradley LM, Topham DJ. Antigen-specific and non-specific CD4(+) T cell recruitment and proliferation during influenza infection. Virology. 2005;340:296–306. doi: 10.1016/j.virol.2005.06.023. [DOI] [PubMed] [Google Scholar]
- de Kluijver J, Evertse CE, Sont JK, Schrumpf JA, van Zeijl-van der Ham CJ, Dick CR, Rabe KF, Hiemstra PS, Sterk PJ. Are rhinovirus-induced airway responses in asthma aggravated by chronic allergen exposure? Am J Respir Crit Care Med. 2003;168:1174–1180. doi: 10.1164/rccm.200212-1520OC. [DOI] [PubMed] [Google Scholar]
- Garofalo RP, Hintz KH, Hill V, Patti J, Ogra PL, Welliver RC., Sr A comparison of epidemiologic and immunologic features of bronchiolitis caused by influenza virus and respiratory syncytial virus. J Med Virol. 2005;75:282–289. doi: 10.1002/jmv.20268. [DOI] [PubMed] [Google Scholar]