Abstract
The cyclin-dependent kinase cdk5 is atypically active in postmitotic neurons and enigmatic among the kinases proposed as molecular actors in neurodegeneration. We generated transgenic mice to express p25, the N-terminally truncated p35 activator of cdk5, in forebrain under tetracycline control (TET-off). Neuronal expression of p25 (p25ON) caused high mortality postnatally and early in life. Mortality was completely prevented by administration of doxycycline in the drinking water of pregnant dams and litters until P42, allowing us to study the action of p25 in adult mouse forebrain. Neuronal p25 triggered neurodegeneration and also microgliosis, rapidly and intensely in hippocampus and cortex. Progressive neurodegeneration was severe with marked neuron loss, causing brain atrophy (40% loss at age 5 months) with nearly complete elimination of the hippocampus. Neurodegeneration did not involve phosphorylation of protein tau or generation of amyloid peptide. Degenerating neurons did not stain for terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling or activated caspase-3 but were marked by FluoroJadeB in early stages. Diseased neurons were always closely associated with activated microglia already very early in the disease process. Primary neurons derived from p25 embryos were more prone to apoptosis than wild-type neurons, and they activated microglial cells in co-culture. The inducible p25 mice present as a model for neurodegeneration in hippocampal sclerosis and neocortical degeneration, with important contributions of activated microglia.
Among the cyclin-dependent kinases, cdk5 is not typical because it is not directly involved in cell cycle control. Rather, cdk5 is specifically active in postmitotic neurons and can be regarded as negatively controlling or even blocking their cycling. The catalytic subunit of cdk5 is widely expressed, but its obligate activating subunits are expressed almost exclusively in brain in postmitotic neurons.1 Cdk5 is best known for its role in development in the cortical layering, which is disturbed in cdk5−/− mice, causing embryonic lethality,2 similar to reeler mice.3 On the other hand, perinatal abrogation of cdk5 reduces embryonic lethality although cortical layer defects remain, thereby dissociating both phenomena.4
Proper kinase activity of cdk5 requires heterodimer formation with neuronal activators p35 or p39, whereas in pathological circumstances, N-truncated derivatives p25 and p29 have been identified.5,6 Mice lacking p35 are viable with only minor problems of corticogenesis,7,8 whereas p39-deficient mice are normal.1 Combined p35/p39 deficiency was lethal similar to cdk5−/−, and the combined data demonstrate that p35 is the dominant activator of cdk5 in neurons.1
Besides these normal physiological functions in cortical development, pathological roles for cdk5 have been claimed in ageing and degenerating brain. Confusion reigns, however, with respect to the exact contributions of cdk5/p35 or p25 to neurodegeneration, whether in Alzheimer’s disease, in primary tauopathies, or in other brain disorders. The identification of cdk5 as tau kinase-II, besides glycogen synthase kinase-3β as tau kinase-I, based on in vitro experiments9 originated the hypothesis that both kinases contributed in concert to the phosphorylation of protein tau, leading to aggregation and tauopathy.2,10
On the other hand, triple transgenic mice that overexpressed cdk5 and its normal activating subunit p35 in addition to human tau were phenotypically normal and failed to produce tauopathy.11 Moreover, impaired cdk5 activity in p35-deficient mice led paradoxically to increased phosphorylation of protein tau and other cytoskeletal proteins.8 These in vivo findings were supported by observations in a cell-biological system of tauopathy in humanized yeast cells, wherein lack of cdk5 activity increased the phosphorylation and aggregation of human protein tau to impair its binding to microtubules.12,13
Proteolysis of p35 by calpain generates p25 and thereby soluble cdk5/p25 kinase complexes, because p35 is N-glycine myristoylated and tethers active cdk5/p35 complexes to membranes.5,6 The presence of p25 in normal and diseased human brain remains debated, mainly because postmortem proteolysis cannot be excluded completely, whereas its mode of action and its targets provoke discussion. In this respect, the relation to the synapse and the N-methyl-d-aspartate receptors in particular might prove central in physiological functions of cdk5/p35 in memory and learning and in pathological roles of cdk5/p25 (Refs. 14,15,16,17,18,19 and refs. therein).
Expression of p25 in brain of transgenic mice causes neurodegeneration and cognitive problems, proposed to result from phosphorylation of protein tau although no overt tauopathy,20,21 which was eventually obtained by constitutional coexpression of p25 and tau-P301L.22 Surprisingly, mild or transient overexpression of p25 was beneficial for cognition.23,24
Inflammation is the third important pathological hallmark in Alzheimer’s disease, besides neurofibrillary tangles and amyloid plaques. Persistent inflammation, purportedly triggered by amyloid aggregates and factors originating from “sick” neurons could play an essential role in the disease process by fuelling and maintaining a vicious circle that eventually leads to the severe brain atrophy typical for the late phase in Alzheimer’s disease. Neuroinflammation is hardly explored in relation to cdk5, and we are aware of only one study that relates induction of inflammation by lipopolysaccharide to increased tauopathy in a transgenic model for Alzheimer’s disease.25 Moreover, the proposed involvement of cdk5 was indirect and, based on inhibition with roscovitine, not a specific inhibitor of cdk5.26 On the other hand, the innate immune system is increasingly recognized as essential in the normal functioning brain, and conversely, its loss of control contributes to neurodegeneration.27,28,29,30
Here, we generated mice with neuronal expression of p25 controlled by tetracycline to analyze and test directly and independently the role of p25 in neurodegeneration. The p25ON mice showed extensive neurodegeneration, resulting in dramatic loss of neurons in hippocampus and cortex and in severe brain atrophy. Neuronal death did not involve apoptosis or increase phosphorylation of protein tau even in progressed stages. On the other hand, neuroinflammation appeared important by the activation of microglia in close spatial and temporal association with p25-expressing neurons. The hippocampal pathology recapitulates several aspects of the human condition known as hippocampal sclerosis (Refs. 31,32,33,34, and refs. therein), which is due to unknown mechanisms and for which the p25 mice are believed to offer an interesting experimental model.
Materials and Methods
Transgenic Mice
Human p25 cDNA11 was subcloned in both sites of the pBI-Tet vector (Clontech, Worcester, MA) under control of the bi-directional tetracycline-responsive promoter element.35 The cDNA inserts of the final pBI.p25 construct were sequenced to control their orientation and exclude mutations eventually caused by the molecular cloning procedures. The pBI.p25 construct was linearized by restriction with AseI, and a 4.4-kb restriction fragment was purified for microinjection into 0.5-day-old prenuclear mouse embryos by standard procedures.11,36,37 Transgenic founders were selected by genotyping using Southern blotting and PCR on tail DNA. Newly generated pBI.p25 transgenic founders were crossed with CaMKIIα.tTA transgenic mice in the C57Bl background.38
The actual p25 line used in the current experiments was selected among four founder lines for its consistent and high expression of p25 in neurons (see Results). The line was back-crossed into the C57Bl6 background for six generations and maintained by inbreeding. Crossing with the heterozygous CaMKII-tTA mice resulted in bigenic F1 offspring that were heterozygous for both transgenes, besides littermates that contained only the pBI.p25 transgene, which were used as age- and gender-matched controls (see Results). Routine genotyping of offspring was by two independent PCRs with primers for the CaMKII-tTA transgene (5′-GTGATTAACAGCGCATTAGAGC-3′ and 5′-GAAGGCTGGCTCTGACCTTGGTG-3′) and for the p25 transgene (5′-AATGCCGACCCACACTAC-3′ and 5′-CCCACACCTCCCCCTGAA-3′).
Analysis of Brain Pathology by Immunohistochemistry and Histology
Mice were anesthetized with pentobarbital (120 mg/kg i.p.), and brain was isolated after transcardiac perfusion with ice-cold saline. Brain hemispheres were quickly prelevated, and the left hemisphere was fixed in cold 4% paraformaldehyde. After washing in PBS, brain tissue was stored in PBS with 0.1% sodium azide until processing. Sagital vibratome sections of 40 μm were cut and stored in PBS/0.1% sodium azide before analysis by immunohistochemistry (IHC), performed as described previously37 with the antibodies listed in Table 1. Histological and immunohistochemical staining was also performed on brain sections of 6 μm cut from brains imbedded in paraffin by standard techniques.
Table 1.
List of Primary Antibodies Used in This Study
| Antibody | Antigen/epitope | Source | Dilution
|
|
|---|---|---|---|---|
| IHC | Western blot | |||
| C19 | p35/p25 (C-terminal) | Santa Cruz Biotechnology (Santa Cruz, CA) | 1/1000 | 1/500 |
| NeuN | Neuronal marker | Chemicon (Temecula, CA) | 1/25,000 | — |
| Cdk5 | cdk5 (C-terminal) | Santa Cruz Biotechnology | — | 1/1000 |
| Caspase-3 | caspase-3 (activated) | Cell Signaling Technology (Danvers, MA) | 1/500 | — |
| CD11b | Complement type 3 receptor (microglia) | Serotec (Oxford, UK) | 1/10,000 | — |
| CD45 | Protein tyrosine phosphatase (microglia) | Pharmingen (San Diego, CA) | 1/5000 | — |
| MHCII | Major histocompatibility complex (class II) | Pharmingen | 1/10,000 | — |
| GFAP | Glial fibrillary acidic protein (astroglia) | Dako (Carpinteria, CA) | 1/50,000 | — |
| Tau5 | Protein tau (human/mouse) | Pharmingen | — | 1/1000 |
| AT8 | tau phosphorylated at S202/T205 | Innogenetics (Zwÿnderecht, Antwerp, Belgium) | 1/1000 | 1/200 |
| AD2 | tau phosphorylated at S396/S404 | Bio-Rad (Hercules, CA) | 1/100,000 | 1/20,000 |
| AT270 | tau phosphorylated at T181 | Innogenetics | 1/5000 | 1/500 |
| Rb | Retinoblastoma protein | Santa Cruz Biotechnology | 1/500 | 1/500 |
| pRb | Rb phosphorylated at S807/S811 | Cell Signaling | 1/500 | 1/500 |
| Tubulin | α-Tubulin | Amersham | — | 1/2000 |
Staining with FluoroJadeB was performed to monitor neurodegeneration.39,40 Free-floating sections were mounted on gelatin-coated glass-slides and air-dried. Dehydration was by passage through a graded series of ethanol (100, 75, 50, and 25%; 5 minutes each) before incubation with 0.0004% FluoroJadeB solution.39,40 After three washes of 2 minutes each in water, slides were dried for 10 minutes at 40°C before mounting with Depex as described previously.37
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining for apoptotic neurons was performed according the instructions of the manufacturer as described previously.41
Cresyl violet staining (Nissl) was optimized for free-floating vibratome sections to stain neurons. A solution of 0.1% Cresyl violet in water was left for 2 weeks at room temperature before being filtered and adjusting the pH to 3.1 with glacial acid. Vibratome sections (40 μm) were mounted on gelatin-coated glass slides, air dried at 50°C for 2 hours, and incubated overnight in 70% ethanol to reduce background. All of the following washing steps lasted 5 minutes. The sections were washed twice with 70% ethanol, twice with 100% ethanol, 15 minutes in chloroform, twice in 100% ethanol, twice in 95% ethanol, and once in 70% ethanol in water. After incubation for 4 minutes in 0.1% Cresyl violet, sections were washed twice for 2 minutes in water; washed once in 95% ethanol, twice in 100% ethanol, and twice in xylol; and mounted and enclosed with Depex as above.
Ultrastructural evaluation was performed on 300-μm vibratome sections that were postfixed for 60 minutes in 1% osmium tetroxide, dehydrated, and impregnated with Agar100 resin. Ultrathin sections (80 nm) were cut and stained with uranyl acetate and lead citrate by standard procedures, and digital images were obtained with a transmission electron microscope at 160kV (JEM-2100; Jeol, Tokyo, Japan).
Biochemical Analysis of Brain Protein Extracts by Western Blotting
The right hemisphere was frozen in liquid nitrogen immediately after dissection and stored at −70° until biochemical analysis by Western blotting. Tissue was homogenized in a Potter-type mechanical homogenizer with teflon pestle, in 10 volumes of ice-cold Tris/phosphatase-inhibitor buffer, containing 20 mmol/L Tris-HCl (pH 8.5), 20 mmol/L ethylenediaminetetraacetic acid, 10 mmol/L ortho-phenantroline, 20 mmol/L sodium fluoride, 200 μmol/L sodium vanadate, 1 μmol/L okadaic acid, 1 mmol/L phenylmethylsulfonyl fluoride, and a cocktail of proteinase inhibitors (Roche Diagnostics, Brussels, Belgium). Total protein extracts were diluted appropriately in sample buffer and analyzed in SDS-polyacrylamide gel electrophoresis on 4 to 20% or 8% polyacrylamide gels (Novex, San Diego, CA). Proteins were transferred to nitrocellulose membranes (Hybond-ECL) at 70 mA overnight in 25 mmol/L Tris-HCl (pH 8.6), 190 mmol/L glycine, and 20% methanol. Nonspecific binding was prevented by incubation of the blots in 5% low-fat milk, before incubating for 2 hours with the primary antibody as indicated (Table 1). Subsequently, blots were washed four times for 10 minutes each in Tris-buffered saline-Tween 0.1% before incubation with the appropriate secondary antibody, ie, either goat anti-mouse or anti-rabbit IgG, labeled with horseradish peroxidase. Blots were washed five times in Tris-buffered saline-Tween 0.1%, and immune reactions were visualized by chemiluminescence (ECL; Amersham, Little Chalfont, Buckinghamshire, UK). Blots were exposed to photographic film, and films were developed mechanically. Subsequently all blots were reincubated with anti-tubulin and developed similarly, as a loading control. Chemiluminescence was recorded on film with different and appropriate exposure times, allowing scanning and quantification by densitometry as described previously.37,41
Primary Hippocampal Neuron Culture
Primary cultures of embryonic hippocampal neurons were prepared from p25 transgenic or nontransgenic embryonal day 17.5 mouse embryos according to standard procedures.42,43 The p25 genotype was confirmed by PCR. Cells derived from single embryos were seeded on polylysine-coated glass coverslips at a density of 200,000 cells per 60-mm dish and cultured for up to 12 days (12 DIC) in neurobasal medium with B-27 supplement (Invitrogen, Gent, Belgium) in the presence of astroglial feeder cells. In some experiments, as indicated, neurons were co-cultured with N11 microglial cells for the terminal 24 hours in a filter-based compartmentalized culture system. N11 is an immortalized microglial cell line derived from embryonic mouse brain cultures.44
For cytochemical analysis, cells were fixed with 4% paraformaldehyde in PBS. TUNEL staining (Promega, Leiden, The Netherlands) was performed as described previously.37,41 Immunocytochemical analysis for NeuN, CD45, and p35/p25 was performed with specific antibodies diluted 1:1000 in 10% fetal calf serum/PBS, and reaction was revealed by secondary goat anti-mouse and goat anti-rabbit antibodies labeled with Alexa Fluor 594 (diluted 1:1000) and fluorescence microscopy. Cell nuclei were counterstained with Hoechst fluorescent dye, and slides were mounted in diaminobenzidine/Mowiol.
For Western blotting, cells were lysed with 1% Triton X-100, 1 mmol/L NaF, and 0.05 mmol/L orthovanadate in PBS, with addition of the complete protease inhibitor mix (Roche Diagnostics). Proteins were separated by SDS-polyacrylamide gel electrophoresis on 4 to 20% Tris-glycine gels (Novex, Gent, Belgium) and analyzed by Western blotting with the antibodies as indicated and as described above. All experiments were repeated three or more times as indicated, on independent cell preparations. Statistical analysis was performed using the Mann-Whitney test.
Results
Generation of Inducible p25 Mice: Early Lethality and Initial Characterization
We generated inducible p25 transgenic mice using the tetracycline-controlled transactivator (tTA) system,35 which itself is under control of the mammalian CaMKIIα gene promoter to drive expression specifically to neurons in the forebrain.38,45 We generated transgenic pBI-p25 mice and crossed them with available CaMKII-tTA mice (see Materials and Methods).38 For the sake of clarity, we denote the pBI-p25 × CaMKII-tTA double transgenic mice as p25.T mice, whereas the term “control mice” refers to the single transgenic pBI-p25 mice that contain the pBI.p25 transgene but not the CaMKII-tTA transgene, preventing any expression of p25. We further refer by p25OFF and p25ON to the conditions without and with expression of p25, respectively, as obtained by suppression or not of the transgene by adding doxycycline to the sweetened drinking water of the mice.
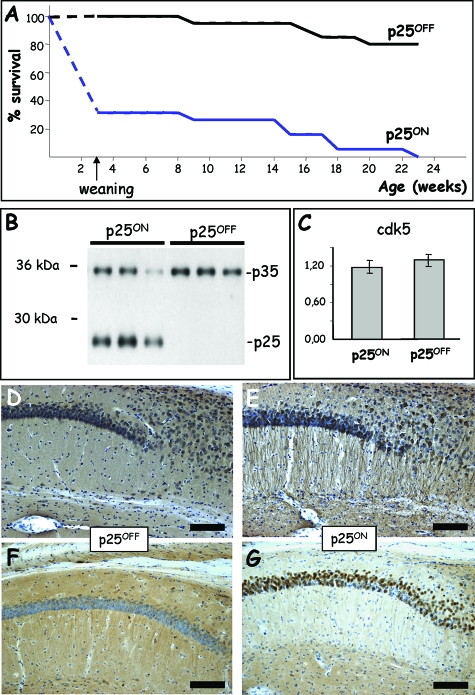
Genotyping of the initial offspring of pBI-p25 × CaMKII-tTA crosses indicated that the number of bigenic mice with combined presence of the CaMKII-tTA and the pBI-p25 transgene was much lower than expected from Mendelian inheritance (Figure 1A). The neuronal expression of human p25 caused high mortality of p25.T pups before weaning at P21, which is our routine time point for genotyping transgenic offspring. The mortality remained high during puberty and early adulthood because p25ON mice that never received doxycycline did not survive for more than 6 months (Figure 1A).
Figure 1.
Expression of p25 induces early mortality that is alleviated by doxycycline. A: Early severe mortality of p25ON transgenic mice without administration of doxycycline was nearly completely alleviated by the tetracycline. Because pups were genotyped at weaning (P21 or age 3 weeks), the exact survival or time of death was not exactly defined before that age. The broken lines indicate extrapolation to 100% as the expected number of offspring at birth based on normal Mendelian transmission of the transgenes. B: Western blotting for p35 and p25 in brain extracts of p25 mice (5 months old) with and without administration of doxycycline. C: Protein levels of the catalytic subunit cdk5 measured by Western blotting were unaffected by p25 expression in brain of p25ON mice at age of 2 months (mean ± SEM, n = 6). D and E: Immunohistochemistry for p35/p25 in the hippocampus of p25 mice (2 months old) without (D) and with (E) expression of p25. Note the different subcellular localization of p25 in apical dendrites and the disturbed architecture in the stratum radiatum of p25ON mice. Scale bar = 100 μm. F and G: Immunohistochemistry for phospho-retinoblastoma protein in the hippocampus of p25 mice (2 months old) with (F) and without (G) administration of doxycycline. Note the intense staining for phospho-retinoblastoma protein in CA1 pyramidal neurons. Scale bars = 100 μm.
Conversely, administration of doxycycline to the drinking water of pregnant dams and their offspring nearly completely alleviated the early lethality, yielding the expected number of p25OFF mice when genotyped at weaning (Figure 1A). Moreover, continued treatment with doxycycline prevented almost all fatalities, and although interesting, we refrained from attempting to define the cause of early death because our main interest was to study in adult brain the consequences of neuronal expression of human p25. Therefore, the p25.T mice were raised in p25OFF mode by administration of doxycycline in the drinking water of pregnant dams and their offspring until postnatal day (P) 42 (age 6 weeks). Under these conditions, p25OFF mice were breeding normally, appeared healthy and behaved normally in their home cage and in various behavioral test conditions (data not shown).
Less than a week after imposing p25ON mode by omission of doxycycline from the drinking water at P42, the expression of p25 was evident by Western blotting of forebrain protein extracts (Figure 1B) and by IHC (Figure 1, D and E). Western blotting demonstrated the complete absence of p25 in the brain of p25OFF mice (Figure 1B) and wild-type mice (results not shown). The p25 levels in brain of p25ON mice were higher than those of endogenous p35, detected in parallel on the same Western blots, and levels were comparable with endogenous murine p35 in p25OFF mice (Figure 1B). Obviously, expression of the human p25 transgene lowered the levels of endogenous murine p35 subunits, whereas cdk5 catalytic subunit levels were not affected (Figure 1C). The turnover of p35 is faster than that of p25,5 and it appears further increased by the coexpression of p25, likely by competitive displacement from the cdk5/p35 complex.
Moreover, IHC for p35/p25 demonstrated expression of p25 in a different subcellular localization than endogenous p35 and revealed important structural changes in the hippocampus of p25ON mice (Figure 1E). Particularly striking was the marked staining of apical dendrites of pyramidal neurons in CA1 (Figure 1E), but p25 was detected in practically all cellular compartments, including neuronal somata and dendrites. This delocalization can be ascribed to the fact that p25 lacks the N-terminal membrane-tethering domain of p35 (see the introduction).5
Finally, indications for cell-cycle re-entry claimed in p25 neuroblastoma cells46 were also evident in this novel model by increased phosphorylation of the retinoblastoma protein (Figure 1, F and G). Nevertheless, of several other and typical cell-cycle markers that we have tested, none was markedly increased in p25-expressing neurons in vivo, and this line of analysis was not further pursued.
Progressive Forebrain Atrophy Due to Neurodegeneration in p25ON Mice
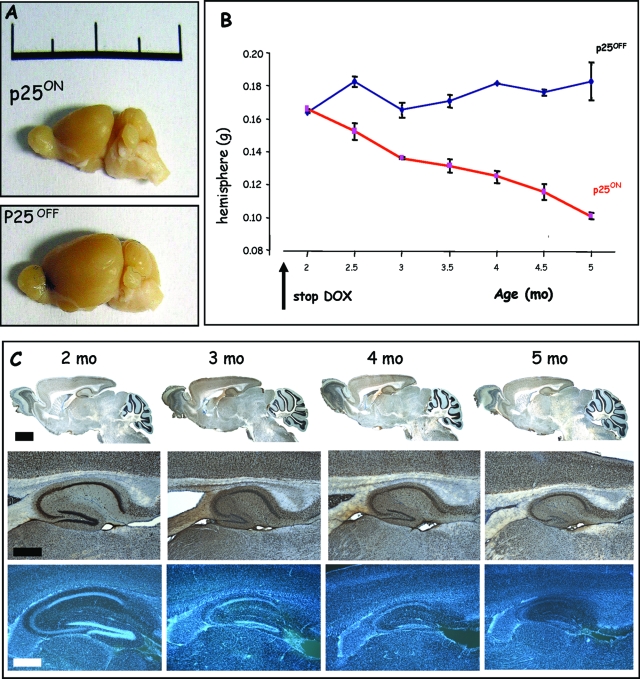
Groups of p25 mice in p25ON and p25OFF modes were first analyzed at 5 months of age, ie, 20 to 21 weeks after onset of p25 expression at P42. The size and weight of the forebrain of p25ON mice (Figure 2A) was reduced significantly: from 175 ± 8 mg per hemisphere in control mice to 98 ± 7 mg in p25ON mice (P < 0.01, n = 6). Moreover, the hippocampal region of the p25ON mice was completely deteriorated, as demonstrated by IHC for the neuronal marker NeuN (see below).
Figure 2.
Neuronal expression of p25 causes progressive neurodegeneration. A: Reduction in the size of the brain of p25ON mice at 5 months of age, ie, after 3.5 months of p25 expression. Note that the bulbus olfactorius and the cerebellum maintained their normal size as in p25OFF mice, because expression of p25 is absent in those brain regions. Scale is 2 cm. B: Neurodegeneration produced a progressive reduction in cerebrum weight with age in p25ON mice (mean ± SEM, n = 4–6 at each time point). C: Progressive deterioration of the hippocampus of p25ON mice at the ages indicated. Top panels are IHC for the neuronal marker NeuN, and bottom panels are stained with Hoechst 33342. Scale bars = 500 μm and 200 μm for top and bottom panels, respectively.
These pathological defects were unexpected because the p25ON mice were only 5 months old with 3.5 months of p25.ON expression, appeared in good health, and behaved normally in their home cages and in the lab vivarium after transfer for experiments. Brief evaluation by general observation before euthanasia and pathological analysis and examination by different simple tests, including clasping, walking, and rotarod, revealed no major clinical problems at 5 months of age (results not shown).
We went on to study the timeline of brain deterioration systematically in groups of p25ON mice, analyzed with 2-week intervals after the withdrawal of doxycycline at P42, marking start of p25 expression. Histological and immunohistochemical analysis demonstrated progressive deterioration of the forebrain in general (Figure 1, B and C), but particularly dramatic in the hippocampus, which nearly completely degenerated by sustained p25 expression over a period of 3 months (Figure 2C). The strata of neurons in the CA regions became progressively thinner, even to a point in some p25ON mice that the typical hippocampal morphology was hardly visible at 5 months of age (Figure 2C; Supplemental Figure 1, see http://ajp.amjpathol.org). The progressive neurodegeneration was paralleled by a monotonous decrease in brain weight of p25ON mice, in sharp contrast to p25OFF mice that carry exactly the same transgenes in the same genetic background but were treated with doxycycline to effectively suppress p25 expression (Figure 2B).
The brain atrophy of p25ON mice was due to the loss of neurons as inferred from the specificity of the CaMKIIα gene promoter that steers expression of the transgene exclusively to forebrain neurons (this study).21,23,38,45 Olfactory bulbs and cerebellum of p25ON mice did not show any signs of atrophy at any age, fully concordant with the absence of expression of the p25 transgene (Figure 2C; data not shown). The neuronal layering of the neocortex of p25ON mice was affected in as far that all layers became progressively thinner with progressing neurodegeneration (data not shown).
We conclude that expression of p25 in adult forebrain neurons induced a pathogenic process that led to severe degeneration and loss of neurons in all hippocampal subregions and neocortex, resulting in severe, progressive brain atrophy. The time-line analysis demonstrated that neurodegeneration sets in rapidly, ie, less than 2 weeks after induction of p25 expression, whereas clinical repercussions appeared minimal.
Mechanism of Neurodegeneration in p25ON Mice
The dramatic loss of neurons in the p25ON mice was analyzed by IHC for active caspase-3 and by TUNEL assay, well known markers for apoptotic cell death. Neither active caspase-3- nor TUNEL-positive neurons were observed in any of the brain sections of p25ON mice at any age (Supplemental Figure 2, see http://ajp.amjpathol. org; results not shown), indicating that other mechanisms were responsible for the neurodegeneration in the p25ON mice.
To eventually gather information on the processes and mechanisms that were ongoing and responsible for the neurodegeneration, we analyzed histologically stained brain sections at higher magnification and performed ultrastructural analysis on the brain of p25ON mice at different ages, in comparison with age-matched wild-type and control p25 mice (Supplemental Figure 3, see http://ajp.amjpathol.org). The data illustrate important changes in the cyto-architecture of degenerating neurons in p25ON mice in the cortex and hippocampus (Supplemental Figure 3; data not shown). Different features of degeneration were evident, mainly cells with enlarged nuclei containing clearer, nonstaining material, occasionally surrounded by clearer cytoplasm. Many degenerating neurons presented with vacuolated cytoplasm, albeit to different degrees at any given age, as can be expected from on ongoing degeneration process (Supplemental Figure 3). The combined features do not typically fit an excitotoxic or a necrotic mechanism of degeneration, and we believe that a combination of both is ongoing in the p25ON mice.
Given the claimed relation of cdk5 to tauopathy as a major tau kinase and in the light of reported data in other p25 transgenic mice,20,21,22 we painstakingly analyzed the phosphorylation status of endogenous protein tau in p25ON mice by Western blotting and by IHC. We used the large panel of monoclonal antibodies directed against specified phospho-epitopes of protein tau, as used in the analysis of our Tau-4R, Tau-P301L, and Tau-KOKI mice (Refs. 36,37,38,47,48, and refs. therein). No marked increase in any phospho-epitope of protein tau was observed in p25ON mice relative to p25OFF or to wild-type or control mice at any age of the timeline (Figure 2; Supplemental Figure 2; results not shown).
We must conclude that tauopathy is not contributing appreciably to the neurodegeneration in our p25ON mouse model. In addition, we searched for intracellular or extracellular formation or deposition of amyloid peptides by IHC and Western blotting by procedures effectively used in our amyloid transgenic mice48,49,50 but failed to detect signs of amyloid pathology in p25ON mice at any age (Supplemental Figure 2; results not shown).
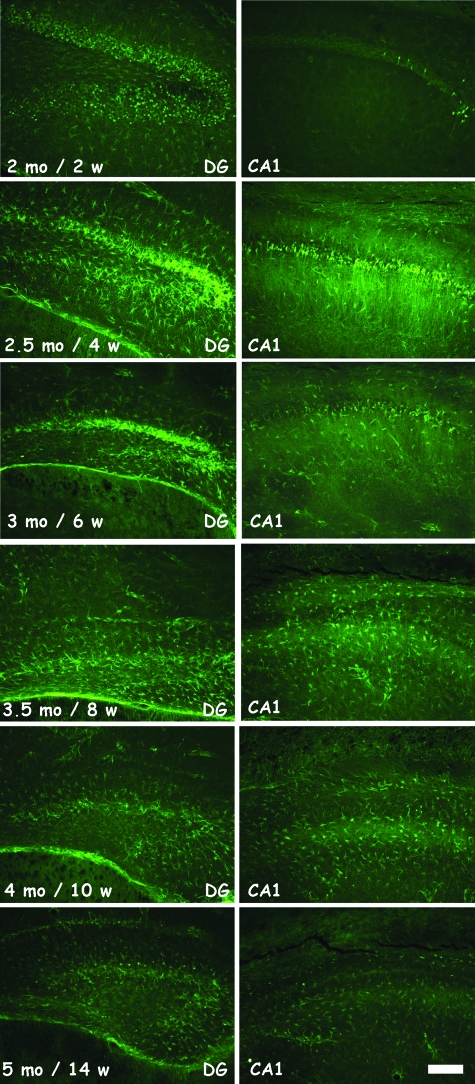
Conversely, brain sections of p25ON mice stained intensely with FluoroJadeB (FJB), a marker for neurons that undergo cell death presumably by exitotoxicity.39,40 Many neurons in forebrain of p25ON mice reacted with FJB already at 2 months of age, ie, after only 2 weeks of p25 induction, particularly and first in the dentate gyrus and later in the CA regions of the hippocampus (Figure 3; Supplemental Figure 4, see http://ajp.amjpathol.org). Reaction with FJB did not surpass background in p25OFF mice nor in wild-type or control p25 mice at any age (results not shown). The cells that were stained histologically by FJB changed progressively with time of p25 expression, ie, from the typical aspect of pyramidal neurons in dendate gyrus and cornus ammonis regions of the hippocampus to the more filigreed network of processes reminiscent of activated glial cells (Figure 3, compare ages 2.5 and 3.5 months, corresponding to 4 and 8 weeks of 25ON). This was confirmed on parallel sections stained with Hoechst 33342 revealing abundant punctated nuclei representing activated inflammatory cells (results not shown).
Figure 3.
Progressive neurodegeneration revealed by FluoroJadeB staining. Histological staining with FluoroJadeB, which demonstrated the only positive marker for the neurodegeneration. Representative pictures of DG and CA1 of p25ON mice, shown in 2-week intervals, beginning at age 2 months, ie, 2 weeks after induction of p25 expression. Degenerating neurons are particularly evident at ages 2 to 3 months, whereas at later time points also activated glial cells become evident as stained by this method. See text for details. Scale bar = 100 μm.
The combined results demonstrated that the progressive neuronal loss caused by neuronal expression of p25 was not due to apoptotic mechanisms nor was it dependent on tauopathy or amyloid formation, which could have been anticipated from the presumed relation of cdk5 to primary and secondary tauopathies, including Alzheimer’s disease. The experimental observations combined with growing evidence for the contribution of inflammation to neurodegenerative disorders led us to analyze the inflammatory aspects in this novel mouse model for hippocampal degeneration.
Progressive Microgliosis and Astrogliosis in p25ON Mice
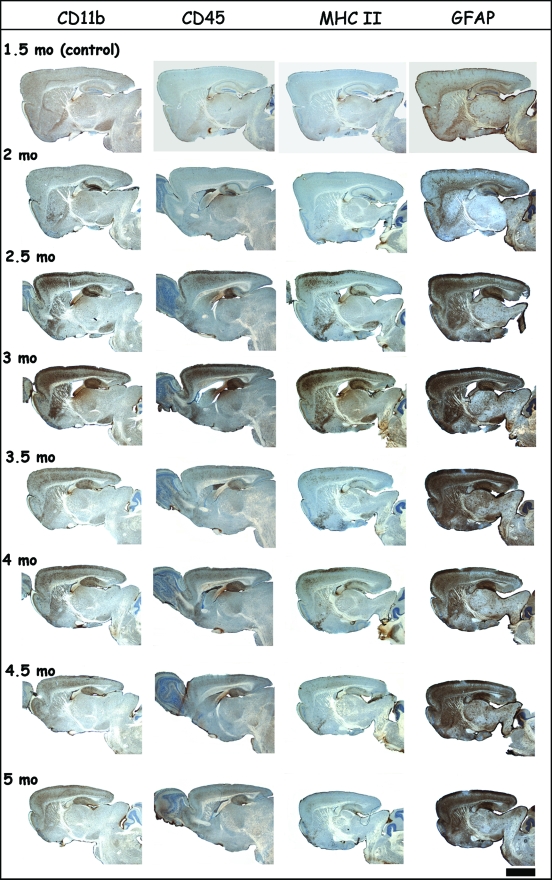
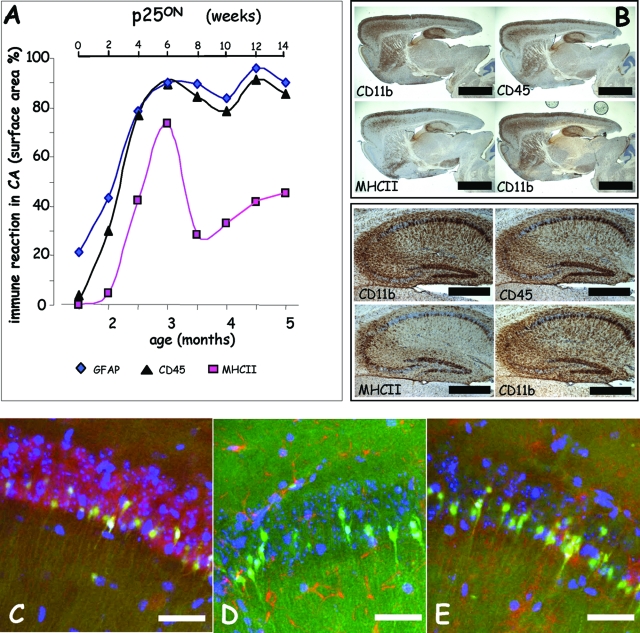
In addition to FJB staining (Figure 3) and IHC for the neuronal marker NeuN, we performed IHC for microglial markers CD11b, CD45, and MHCII and for astroglial marker glial fibrillary acidic protein (GFAP) on the same mice of the timeline analysis of neurodegeneration described in previous sections (Figures 2 and 3).
The inflammation markers were already detectable on brain sections of 2-month-old p25ON mice, ie, barely 2 weeks after onset of p25 expression (Figures 4 and 5) and the time point at which neurodegeneration became first detectable by FJB staining (Figure 3). Detailed analysis demonstrated the presence of activated microglia first in the dentate gyrus, immediately followed by other hippocampal subregions and neo-cortex (Figures 4 and 5).
Figure 4.
Progressive neuroinflammation associated with neurodegeneration. Representative brain sections of p25 mice stained by IHC for inflammatory markers of microglial (CD11b, CD45, and MHCII) and astroglial (GFAP) cells at the indicated ages. The first time point at age 1.5 months is taken as control, because P42 is the time point when administration of doxycycline was stopped to initiate expression of the p25 transgene. Note the microglial activation already at age 2 months in the DG of the hippocampus and more widespread in the cortex. Microglial activation, revealed especially by the MHCII marker, peaked at age 3 months and later subsided (see also Figure 5), whereas astroglia remained activated throughout the p25ON expression and observation period. Scale bar = 2 mm.
Figure 5.
Microglial activation is closely associated with progressive neurodegeneration. A: Quantification of microglial markers shows reactions marked on the one hand by MHCII (squares, pink line) and on the other hand by CD45 (triangles, black line) and CD11b (not shown) comparable with the time course of the astrocytic GFAP marker (diamonds, blue line). B: IHC for CD11b, CD45, MHCII, and CD11b again of four serial brain sections from p25ON mouse at 3 months of age, ie, at the peak of microgliosis. Note the close correlation of the three markers with each with cells in the strata but also with degenerating neurons in the CA regions. Scale bar = 2 mm (top panels) and 500 μm (bottom panels). C–E: Double fluorescent staining of degenerating neurons in CA1 marked by FluoroJadeB, in combination with neuronal marker NeuN (C), with astroglial marker GFAP (D), and with microglial marker CD11b (E). Note the closer spatial correlation of degenerating neurons with microglia (E) than with astroglia (D). Scale bars = 20 μm.
Gliosis markers peaked at 3 months of age, somewhat later than neurodegeneration marker FJB, whereas the transient pattern of microglial marker MHCII deviated from the other microglial markers and from the astroglia marker (Figure 5A). Activated astroglia were evident throughout the brain (Figure 4), but their localization did not correlate well with neurons that expressed p25 and were marked by FJB staining (Figures 1 and 3; Supplemental Figure 1; results not shown). Microgliosis on the other hand was temporally early and spatially closely associated with degenerating neurons in hippocampus (Figures 4 and 5E).
Double and triple fluorescent staining for these markers in different combinations and analysis by confocal microscopy performed on p25ON mice revealed the expected overlap of FJB with NeuN (Figure 5C) and total lack of overlap with GFAP (Figure 5D). Thereby, the degenerating cells reacting with FJB were confirmed as neurons in the early degeneration process in p25ON mice (2.5 to 3.5 months old). The extensive coincident staining of FJB with microglial marker CD11b further underlined the close association of degenerating neurons and activated microglia in p25ON mice (Figure 5E).
Several typical inflammation markers were also analyzed by RT-PCR in extracts of forebrain mRNA of p25ON mice at age 2.5 months. As expected, mRNA levels of interleukin-1β and interleukin-6 were up-regulated intensely by p25 expression (results not shown), corroborating their role as pro-inflammatory cytokines. The mRNA coding for chemokines MCP1 and RANTES were also up-regulated and probably function as chemo-attractants for immune-competent cells from the periphery. Nevertheless, the mRNA levels of these factors and of others analyzed (results not shown) varied very importantly in individual p25ON mice and thereby did not correlate well with the other parameters analyzed.
We conclude that the extensive neurodegeneration in p25ON mice was closely paralleled by intense inflammatory activation of glial cells, whereby particularly microgliosis was closely related, temporally and spatially, with degenerating neurons.
Switching Off p25 Expression Stops Neurodegeneration and Inflammation
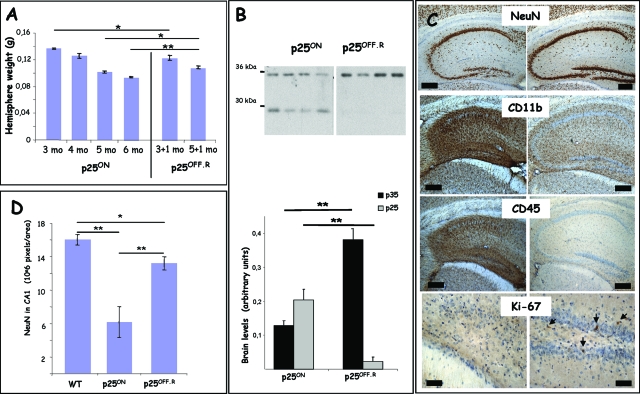
In three chronic experiments on different groups of p25.T mice, we defined that expression of p25 could effectively be stopped by re-administration of doxycycline in the drinking water of the p25ON mice, and we analyzed the effects on neurodegeneration and inflammation. Doxycycline (2 mg/ml) was re-administered for 1 month to groups of p25ON mice aged 3 or 5 months (n = 6 per group). These time points were chosen on the one hand at the peak of microglial activation at age 3 months and on the other hand for the severe neurodegeneration with almost annihilation of the hippocampus at age 5 months. We assessed further, by using p25OFF.R mice, the mice in this condition for reversal or suppression of p25 by doxycycline administration for 1 month after the period of p25ON expression.
Treatment with doxycycline for 1 month reversed and reduced p25 protein levels dramatically although not completely in p25OFF.R mice (Figure 6B) at either age tested. We ascribe the traces of residual p25 protein to biological factors related to p35/p25 rather than to technical or molecular aspects of the TET-off system.35 Although the half-life of p25 is not known in vivo, in cells, it is much longer than that of p35.5 In addition, we believe that the neurodegenerative disease process itself is likely to generate p25 from endogenous p35, given the lower p35 levels in p25ON mice relative to p25OFF mice (Figures 1B and 6B).
Figure 6.
Suppression of p25 expression by doxycycline stops neurodegeneration and inflammation. A: Reversal of p25 expression by re-administration of doxycycline for 1 month starting at either 3 or 5 months of age, resulting in final ages of 4 and 6 months of the p25OFF.R mice when analyzed. The p25ON mice used here as control continued to express p25 from P42 onwards because they did not receive doxycycline. Data are mean ± SEM, n = 6, *P < 0.05, **P < 0.01. B: Biochemical analysis of protein levels of p35/p25 in total brain homogenates of p25ON and p25OFF.R mice, all age 6 months. Some residual p25 expression was evident in p25OFF.R mice after 1 month of suppression by doxycycline (see text for details). The p35 levels in p25OFF.R mice were similar to those in brain of wild-type or p25OFF mice (see text and Figure 1B) (mean ± SEM; **P < 0.01). C: IHC for neuronal marker NeuN, for microglial inflammation markers CD11b and CD45, and for cell-cycle marker Ki-67 in p25ON (left panels) and in p25OFF.R (right panels) after 1 month of reversal of p25 expression (4 months of age). Note that treatment with doxycycline for 1 month in the p25OFF.R mice, neurodegeneration was halted (NeuN marker) whereas inflammation nearly completely subsided (CD11b and CD45 markers). Only some cells stained for the proliferation marker Ki-67 in the p25OFF.R mice (arrows), indicating that regeneration by cell proliferation was minimal. Scale bars = 200 μm, 50 μm for Ki-67. D: Quantification of NeuN signal in CA1 of the hippocampus demonstrated that the neurodegeneration was halted in p25OFF.R mice compared with age-matched p25ON mice (age 6 months) after 1 month of reversal by doxycycline administration (mean ± SEM; *P < 0.05, **P < 0.01). Scale bars = 200 μm.
Treatment with doxycycline halted the neurodegeneration in p25OFF.R mice, even when given late in the disease process at 5 months of age when the hippocampus was already severely deteriorated (Figure 6, A and C). The forebrain hemispheres of p25OFF.R mice at age 6 months, ie, after 1 month of doxycycline, weighed significantly more than that of age-matched p25ON mice that continued to express p25 (Figure 6A). This was, however, not observed in the p25OFF.R mice reversed at 3 months and analyzed at 4 months (Figure 6A), which might be related to the much more important contribution of microgliosis at 3 than at 5 months of age (Figures 3 to 5). Nevertheless, microgliosis nearly completely subsided in all p25OFF.R mice analyzed in the three reversal experiments whether suppression of p25 expression by doxycycline was started at 3 or at 5 months of age (Figure 6C).
Despite indications for recovery of neuronal cells and hippocampal cytoarchitecture by the suppression of p25 expression in p25OFF.R mice, no significant neuronal regeneration was evident in the groups of p25OFF.R mice compared with age-matched p25ON mice (Figure 6; Supplemental Figure 3). The CA1 area that stained for neuronal marker NeuN was considerably greater in p25OFF.R mice than in age-matched p25ON mice but remained significantly smaller than in control mice (Figure 6D). These data demonstrated that the neurodegeneration was halted by suppression of p25 expression but failed to reveal regeneration. Similar analysis of all p25OFF.R mice in the three reversal experiments showed an insufficient trend toward neuro-regeneration to warrant analysis of neuronal density by unbiased stereology.
The absence of neuro-regeneration on suppression of p25 expression was underlined by analysis for the Ki-67 proliferation marker or by BrdU injection, as performed in our protein Tau mouse models (Figure 6D).41,47 Whereas somewhat more cells labeled for the Ki-67 marker in p25OFF.R mice, neither the Ki-67 nor the BrdU indices showed major differences between the number of labeled cells in the hippocampus of p25OFF.R mice relative to age-matched p25ON mice (results not shown).
We conclude that suppression of the expression of the p25 transgene with the tetracycline doxycycline, which inhibits the reverse transactivator component of the transgenic construction, effectively stopped the neurodegeneration process and thereby apparently also the microglial activation.
Primary Hippocampal Neurons Activate Microglia in Culture
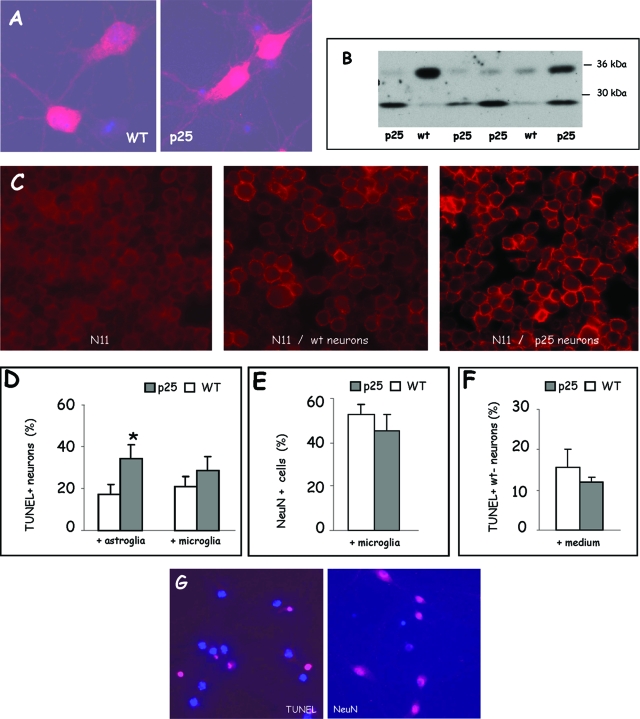
The mechanism of neurodegeneration and microglial activation triggered by p25 was investigated in primary neuronal cell cultures derived from individual p25 transgenic mouse embryos (E17.5) and compared with cultures from nontransgenic mouse embryos (E17.5). The expression of p35/p25 in genotypically authenticated p25 transgenic embryos was confirmed by immunocytochemistry and Western blotting (Figure 7, A and B). Because no specific antibody to p25 is available, endogenous p35 and transgenic p25 are visualized in 12 DIC primary cultures. Relatively old primary cultures were used for analysis because the CaMKIIα gene promoter is a “late” promoter38,45 similar to the mouse Thy1-gene promoter.41,47,48,49,50
Figure 7.
Analysis of primary hippocampal neurons derived from p25 mice. A: Immunocytochemical staining for p35/p25 of primary hippocampal cultures derived from p25 transgenic and wild-type (WT) mouse embryos (E17.5) analyzed at 12 DIC. B: Western blotting for p35/p25 of different primary hippocampal cultures derived from p25 transgenic and nontransgenic (wt) mouse embryos (E17.5). The relative fraction of neuronal cells expressing p25 depended on the days in culture, and 12 DIC was found most suitable. C: Immunocytochemical analysis of the microglial activation marker CD45 on N11 cells (left panel) and on N11 cells co-cultured for 24 hours with primary hippocampal neurons (12 DIC in total) derived from wild-type embryos (middle panel) or from transgenic p25 embryos (right panel). Note the marked staining for CD45 of the microglial cell membrane when co-cultured with p25 neurons (right panel). D: Quantification of TUNEL assay on primary hippocampal neurons derived from wild-type (WT) and p25 embryos, grown in co-culture with either astroglia or N11 microglia as indicated and analyzed at 12 DIC (mean ± SEM, n = 3). E: Cytochemical analysis for NeuN as marker for established neurons in primary hippocampal cultures derived from wild-type (WT) and p25 transgenic embryos (E17.5) analyzed at 12 DIC after co-cultured with N11 microglia for 24 hours (mean ± SEM, n = 3). F: Quantification of TUNEL-positive cells in primary cultures derived from nontransgenic (wt) mouse embryos, challenged with conditioned spent media from wild-type (wt) or p25 cultures (mean ± SEM, n = 3). G: Representative examples of TUNEL and NeuN staining of primary hippocampal cultures derived from p25 transgenic embryos (E17.5) analyzed at 12 DIC, counterstained with Hoechst 33342 as nuclear marker.
In p25 primary cultures overall, more neurons stained more brightly for p35/p25 than in nontransgenic neuronal cultures (Figure 7A), although some variability was evident at the cellular level in each culture and from one culture to another. Western blotting for p35/p25 of separate cultures confirmed the presence of both isoforms (Figure 7B) and also demonstrated that at the later time points, ie, at 12 DIC, the expression of p25 was least variable (results not shown).
To demonstrate that p25-expressing neurons were capable of activating microglia, we used a filter-based co-culture system of primary neurons with N11 microglia, an immortalized cell line.44 After normal co-culture with astroglia for 11 DIC, the primary cultures were transferred for 24 hours in co-culture with N11 cells. The N11 cells were subsequently stained for the microglial activation marker CD45, whereas the neurons were analyzed by TUNEL staining. In some experiments, co-culture conditioned media were saved and transferred to nontransgenic neuron cultures to test for potential re-conditioning.
We observed that N11 microglial cells co-cultured with p25 primary cultures stained more strongly for CD45 than N11 microglia cultured alone or in co-culture with nontransgenic primary cultures (Figure 7C). Clearly, p25-expressing neurons activated the N11 microglial cells, and because no direct cell-cell contact was possible between the primary neurons and the microglia, soluble factors must be involved. Nevertheless, transfer of conditioned culture medium from p25 primary cultures did not markedly affect nontransgenic primary cultures, as judged by TUNEL staining, by morphology, or by survival (Figure 7F; results not shown).
The p25 primary cultures but also nontransgenic primary cultures contained TUNEL-positive cells even when cultured normally in the presence of astroglia (Figure 7D). This is at variance with the in vivo situation in which TUNEL-positive neurons were not detectable in p25ON mice at any stage (results described in previous sections; Supplemental Figure 2). Similar observations were recently reported in a different but related model system.51 Significantly more p25-expressing neurons were TUNEL-positive compared with wild-type neurons, when co-cultured with astroglia (Figure 7D, left histogram). Surprisingly, the fraction of TUNEL-positive p25 neurons was lower when co-cultured with N11 microglia than with astroglia and not significantly different from wild-type neurons (Figure 7D, right histogram). Staining for the neuronal marker NeuN did not reveal a difference in the number of positive neurons (Figure 7E).
We conclude that p25-expressing neurons activated microglial cells in co-culture more than wild-type neurons, most likely by secreting soluble factor(s) that appear to be labile. Moreover, the data argue that the evoked microglial and neuronal responses could be beneficial rather than harmful, although the mechanism of cell death of p25 primary neurons in culture appears different from that in vivo, as judged by the positive and negative TUNEL reactions, respectively (Figure 7; Supplemental Figure 2).
Discussion
The major pathological characteristic of the inducible p25 mouse model presented here is the dramatic neurodegeneration closely associated with intense neuroinflammation. Over a period of less then 3 months of effective neuronal expression of p25, the atrophy of the forebrain exceeded 40% of its weight. At the relatively young age of 5 months, most neurons were lost from the hippocampus CA and DG subregions, which were reduced to remnant strata of pyramidal neurons. The neocortex became progressively thinner, and the typical multilayered neuron-body architecture in the neocortex became hardly recognizable. Amazingly, the impact on the clinical condition of the p25 mice was minimal, as they continued to behave normally until age 6 months, actually the oldest p25ON mice we have analyzed to date. Obviously, these observations need follow up and analysis particularly of learning and memory capacities of the p25 mice.
Besides the chronic pathology, more acute changes were also remarkable. Less than two weeks after initiation of p25 expression in the young adult p25 mice (age 2 months), distinct morphological changes were evident in the hippocampus. Degeneration of neurons affected first the dentate gyrus, immediately followed by CA regions before spreading to the neocortex. This reflects the expression pattern of the CaMKII gene and its promoter used in the construction of these p25 mice.38,45 Expression was absent or very low in olfactory bulbs, cerebellum, and spinal cord also observed in mice expressing p25 from the CaMKII gene promoter in a constitutional23 or in a conditional mode.21 The major advantage of the nearly exclusive expression in forebrain is the complete absence of motor problems in our p25 mice as opposed to the axonopathy and motor defects in mice expressing p25 more widely and constitutionally by the neuron-specific enolase or platelet-derived growth factor gene promoters.20,52
Microgliosis
Particularly interesting was the microgliosis closely associated with degenerating p25 neurons, so that we could not separate them spatially or temporally by immunohistochemical analysis. Because the expression of p25 is restricted to neurons, the signal that activates the microglia must originate in diseased p25-expressing neurons. Evidence by RT-PCR demonstrated the mRNA of several pro-inflammatory cytokines and chemokines to be up-regulated in the brain of the p25ON mice (results not shown). Nevertheless, we believe that these are more likely consequence and not primary triggers originating from “sick” p25-expressing neurons and rather are produced by activated glia as part of the innate immune reaction in the central nervous system.
The activation of microglia subsided and even disappeared in the course of the disease process in parallel with the progressive loss of neurons from forebrain. We take this as corroborating the hypothesis that sick p25 neurons activate microglia by producing factors with levels that will decrease in the brain as fewer neurons remain to produce them. A final indication is provided by the reversal experiments wherein re-administration of doxycycline after 6 or 14 weeks of p25ON mode effectively suppressed the expression of p25. Significantly, thereby the microgliosis was also alleviated in p25OFF.R mice, not only late in the disease process (at 5 months of age) but importantly also at 3 months of age, ie, at the apex of microgliosis in p25ON mice. Thereby, microgliosis is in all experimental conditions subordinate and subsequent to neuronal expression of p25.
Whether intimate neuron-microglia cell-cell contact is involved in their mutual activation could not be resolved in vivo and was approached in the cell-based experiments. Activation of N11 microglial cells in co-cultures with primary hippocampal neurons derived from p25 mouse embryos clearly pointed to the involvement of soluble factors stemming from the neurons.
Conversely, this does not exclude that cell-cell contacts are important for effective neuron-microglia activation in vivo. In fact, the mechanism of neurodegeneration in culture could be different from that in vivo, because in primary cultures, neurons died by an apoptotic process involving DNA breakage as indicated by TUNEL staining, which was not observed in vivo in brain of the p25ON mice. A similar situation was recently observed in a different but related model system,51 reiterating the question of the relevance of cellular models for in vivo phenomena.
Other major questions remain unanswered, ie, whether the microgliosis was beneficial or contributed negatively to the neurodegeneration, and what mechanism is causing p25-expressing neurons to degenerate in vivo? The first question needs to be tackled experimentally by treating the p25 mice with anti-inflammatory drugs, whereas the latter question is addressed in the next section.
Type and Mechanism of Neurodegeneration
The mechanism underlying the progressive forebrain atrophy was unrelated to activated caspase-3 or DNA fragmentation (TUNEL) because these markers for apoptotic neuronal death were negative. The only positive histological staining was with FluoroJadeB, which effectively marked degenerating neurons.39,40 In later stages, the compound also reacted with activated glia, similar to observations in unrelated mouse models (Ref. 53, and refs. therein). Unfortunately, the finding yields no further clues to the actual mechanism of neurodegeneration, because the molecular target or reaction partner of the FluoroJadeB compound in degenerating neurons remains unknown (L. Schmued, personal communication).
Besides the pathological characteristics discussed in the previous section, the current p25 mouse model presents some remarkable differences with other mouse models expressing p25. First, the dramatic mortality was not reported in any other p25 mouse model.20,21,23,52 We did not pursue the cause of the early mortality, not for lack of interest but because our research focus is on adult and ageing brain and the pathological consequences of neuronal expression of human p25. This goal was achieved by administration of doxycycline via the drinking water to pregnant dams and litters until P42, which completely alleviated the early mortality and allowed us to study p25-induced defects in adult mouse brain. Second and equally surprising was the lack of increased phosphorylation of protein tau, let alone tauopathy in the forebrain of p25ON mice, despite painstaking analysis at all stages of the pathology up to 5 months of age by proven methods of immunohistochemistry and Western blotting.36,37,41,47,48 Thereby, the p25 mice deviate from similar p25 mice generated using the tetracycline-controlled system in the OFF mode.21 A major molecular difference is the expression of a p25-EGFP fusion protein in that model as opposed to the untagged p25 in the model presented here, although also differences in genetic background might contribute. Tauopathy with neurofibrillary tangles is rarely observed in hippocampal sclerosis and cannot explain the loss of neurons,34 adding considerable weight to our observations and to the potential of this novel mouse model for studying hippocampal sclerosis and neocortical degeneration.
We did not observe a significant delocalization of p25 to the nucleus,54 neither by immunohistochemistry on brain sections nor by biochemical analysis of subcellular fractions from brain tissue of p25ON and p25OFF mice. We also analyzed immunohistochemically and biochemically by immunoprecipitation and Western blotting the phosphorylation status of N-methyl-d-aspartate receptors15 but could not find marked differences in p25ON mice compared with p25OFF mice (results not shown), which we ascribe mostly to technical issues and lack of suitable antibodies. We additionally analyzed several other potential candidate markers, eg, phosphorylated retinoblastoma protein46 and chromogranin,55 but did not observe consistent indications for their involvement in the neuropathology in our p25 mice.
Remarkably, the p25ON mice moved and behaved quite normally in their home cage and during handling by caretakers and researchers, and we have not observed major defects in a limited set of behavioral tests (rotarod and object recognition test; results not shown). Moreover, the alternating Y-maze test did not reveal a difference between groups of p25ON mice and p25OFF.R mice, ie, arresting the pathological process by suppressing p25 expression with doxycycline in the drinking water did not affect their performance in the Y-maze. Further detailed examination of the p25ON is needed and is currently ongoing by cognitive testing comprising water maze, cued and contextual fear conditioning, and novel object recognition paradigms with different delay times. Some preliminary results indicate minor defects in cognition that are, however, rather subtle and hardly match the extensive degeneration of the forebrain. Whether this is in any way related to the improved cognition of mildly overexpressing p25 mice16,23 remains a pertinent question to be answered by these and other follow-up experiments.
We have to conclude that the molecular mechanism responsible for the massive neuronal death in our p25ON mice remains unknown. We never observed any signs of seizures or fits or any other indications of epileptic problems in the p25ON mice, which would exclude epileptic activity as the cause of the hippocampal neurodegeneration. We believe that our data reported (and those mentioned but not shown) indicate that p25 does not act by one single mechanism in provoking the neuropathology in the p25 mice.
The severe neuronal loss accompanied by gliosis is typical for the human condition of hippocampal sclerosis, which is observed in several different clinical settings,31,32,33,34 including Alzheimer’s disease31,33,56 as originally observed by Alzheimer.31 Deregulation of cdk5/p25 was proposed in this condition based on human pathology,57,58 but these studies also explicitly stated the major unsolved problem of the underlying mechanisms explicitly as “ … that apoptosis, necrosis and excitotoxicity could all contribute to the neuronal loss in hippocampal sclerosis.”57 The novel p25 mouse model recapitulates robustly and controllably two pathological features, ie, neurodegeneration and inflammation, making the model relevant for human pathology, including hippocampal sclerosis and neocortical degeneration. Thereby, this mouse model will allow the definition of mechanisms of neurodegeneration in the absence of tauopathy but with the added contribution of micro- and astrogliosis.
Acknowledgments
We thank L. Buée, M. Hamdane, M. Heneka, P. Castagnoli, P. Davies, L. Schmued, and K. Jellinger for generously providing materials and advice. We thank the Cell Imaging Core facility (KULeuven) for help with confocal microscopy.
Footnotes
Address reprint requests to Fred Van Leuven, Experimental Genetics Group, Department of Human Genetics, KULeuven–Campus Gasthuisberg ON1-06.602, B-3000 Leuven, Belgium. E-mail: fred.vanleuven@med.kuleuven.be.
Supported by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen, by the KULeuven Research Fund (BOF-DOC), by the de Rooms-Fund, by EEC-Framework-Program 6, by the Instituut voor Wetenschappelijk en Technologisch onderzoek, and by NEURAD–EEC-Marie Curie Training Site (QLK6-CT-2005-020013 to A.K.). I.D.W. is a postdoctoral fellow at Fonds voor Wetenschappelijk Onderzoek-Vlaanderen. D.T. was a postdoctoral fellow at Instituut voor Wetenschappelijk en Technologisch onderzoek. K.S. was a postdoctoral fellow supported by EEC-Marie Curie (MEIF-CT-2005-010219).
Supplemental material for this article can be found on http://ajp. amjpathol.org.
References
- Ko J, Humbert S, Bronson RT, Takahashi S, Kulkarni AB, Li E, Tsai LH. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci. 2001;21:6758–6771. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Ohshima T, Takahashi S, Longenecker G, Honjo Y, Veeranna, Pant HC, Mikoshiba K, Brady RO, Kulkarni AB. Perinatal abrogation of Cdk5 expression in brain results in neuronal migration defects. Proc Natl Acad Sci USA. 2004;101:6249–6254. doi: 10.1073/pnas.0307322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Hallows JL, Chen K, DePinho RA, Vincent I. Decreased cyclin-dependent kinase 5 (cdk5) activity is accompanied by redistribution of cdk5 and cytoskeletal proteins and increased cytoskeletal protein phosphorylation in p35 null mice. J Neurosci. 2003;23:10633–10644. doi: 10.1523/JNEUROSCI.23-33-10633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K, Omori A, Sato K, Tomizawa K, Imahori K, Uchida T. A serine/threonine proline kinase activity is included in the tau protein kinase fraction forming a paired helical filament epitope. Neurosci Lett. 1991;128:195–198. doi: 10.1016/0304-3940(91)90259-v. [DOI] [PubMed] [Google Scholar]
- Camins A, Verdaguer E, Folch J, Canudas AM, Pallas M. The role of cdk5/p25 formation/inhibition in neurodegeneration. Drug News Perspect. 2006;19:453–460. doi: 10.1358/dnp.2006.19.8.1043961. [DOI] [PubMed] [Google Scholar]
- Van den Haute C, Spittaels K, Van Dorpe J, Lasrado R, Vandezande K, Laenen I, Geerts H, Van Leuven F. Coexpression of human cdk5 and its activator p35 with human protein tau in neurons in brain of triple transgenic mice. Neurobiol Dis. 2001;8:32–44. doi: 10.1006/nbdi.2000.0333. [DOI] [PubMed] [Google Scholar]
- Vandebroek T, Vanhelmont T, Terwel D, Windericxk J, Van Leuven F. Identification and isolation of a hyperphosphorylated, conformationally changed intermediate human protein tau expressed in yeast. Biochemistry. 2005;44:11466–11475. doi: 10.1021/bi0506775. [DOI] [PubMed] [Google Scholar]
- Vandebroek T, Terwel D, Vanhelmont T, Gysemans M, Van Haesendonck C, Engelborghs Y, Winderickx J, Van Leuven F. Microtubule binding and clustering of human Tau-4R and Tau-P301L proteins isolated from yeast deficient in orthologues of glycogen synthase kinase-3beta or cdk5. J Biol Chem. 2006;281:25388–25397. doi: 10.1074/jbc.M602792200. [DOI] [PubMed] [Google Scholar]
- Samuels BH, Tsai LA. Cdk5 is a dynamo at the synapse. Nat Cell Biol. 2003;5:689–690. doi: 10.1038/ncb0803-689. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu S, Fu Y, Wang JH, Lu Y. Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat Neurosci. 2003;6:1039–1047. doi: 10.1038/nn1119. [DOI] [PubMed] [Google Scholar]
- Giese KP, Ris L, Plattner F. Is there a role of the cyclin-dependent kinase 5 activator p25 in Alzheimer’s disease? Neuroreport. 2005;16:1725–1730. doi: 10.1097/01.wnr.0000185019.67434.d2. [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Fu AK, Ip NY. Synaptic roles of Cdk5: implications in higher cognitive functions and neurodegenerative diseases. Neuron. 2006;50:13–18. doi: 10.1016/j.neuron.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, Greengard P, Powell CM, Cooper DC, Bibb JA. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, McNeish JD. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci USA. 2000;97:2910–2915. doi: 10.1073/pnas.040577797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J, Gaynor K, LaFrancois J, Wang L, Kondo T, Davies P, Burns M, Nixon R, Dickson D, Matsuoka Y, Ahlijanian M, Lau LF, Duff K. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38:555–565. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- Angelo M, Plattner F, Irvine EE, Giese KP. Improved reversal learning and altered fear conditioning in transgenic mice with regionally restricted p25 expression. Eur J Neurosci. 2003;18:423–433. doi: 10.1046/j.1460-9568.2003.02746.x. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Mushynski WE, Julien JP. Cycling at the interface between neurodevelopment and neurodegeneration. Cell Death Differ. 2002;9:1294–1306. doi: 10.1038/sj.cdd.4401108. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Abbracchio MP. To be or not to be (inflamed): is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol Sci. 2005;26:517–525. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer’s disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Alzheimer A. Ueber eigenartige Krankheitsfaelle des spaeteren Alters. Zeitschrift fuer gesamte Neurologie und Psychiatrie. 1911;4:356–385. [Google Scholar]
- Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, Aronson MK, Crystal HA. Hippocampal sclerosis: a common pathological feature of dementia in very old humans. Acta Neuropathol. 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- Attems J, Jellinger KA. Hippocampal sclerosis in Alzheimer disease and other dementias. Neurology. 2006;5:775. doi: 10.1212/01.wnl.0000200959.50898.26. [DOI] [PubMed] [Google Scholar]
- Probst A, Taylor KI, Tolnay M. Hippocampal sclerosis dementia: a re-appraisal. Acta Neuropathol. 2007;114:335–345. doi: 10.1007/s00401-007-0262-1. [DOI] [PubMed] [Google Scholar]
- Bujard H. Controlling genes with tetracyclines. J Gene Med. 1999;1:372–374. doi: 10.1002/(SICI)1521-2254(199909/10)1:5<372::AID-JGM61>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Spittaels K, Van den Haute C, Van Dorpe J, Bruynseels K, Vandezande K, Laenen I, Geerts H, Mercken M, Sciot R, Van Lommel A, Loos R, Van Leuven F. Prominent axonopathy in the brain and spinal cord of transgenic mice overexpressing four-repeat human tau protein. Am J Pathol. 1999;155:2153–2165. doi: 10.1016/S0002-9440(10)65533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwel D, Lasrado R, Snauwaert J, Vanderweert E, Van Haesendonck C, Borghgraef P, Van Leuven F. Changed conformation of mutant tau-P301L underlies the moribund tauopathy, absent in progressive, non-lethal axonopathy of tau-4R/2N transgenic mice. J Biol Chem. 2005;280:3963–3973. doi: 10.1074/jbc.M409876200. [DOI] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Sennvik K, Boekhoorn K, Lasrado R, Terwel D, Verhaeghe S, Korr H, Schmitz C, Tomiyama T, Mori H, Krugers H, Joels M, Ramakers GJ, Lucassen PJ, Van Leuven F. Tau-4R suppresses proliferation and promotes neuronal differentiation in the hippocampus of tau knockin/knockout mice. FASEB J. 2007;21:2149–2161. doi: 10.1096/fj.06-7735com. [DOI] [PubMed] [Google Scholar]
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LL, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci. 2001;114:1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- Righi M, Pierani A, Boglia A, De Libero G, Mori L, Marini V, Ricciardi-Castagnoli P. Generation of new oncogenic murine retroviruses by cotransfection of cloned AKR and MH2 proviruses. Oncogene. 1989;4:223–230. [PubMed] [Google Scholar]
- Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC, Kelly PT. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990;10:1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdane M, Bretteville A, Sambo AV, Schindowski K, Begard S, Delacourte A, Bertrand P, Buée L. p25/Cdk5-mediated retinoblastoma phosphorylation is an early event in neuronal cell death. J Cell Sci. 2005;118:1291–1298. doi: 10.1242/jcs.01724. [DOI] [PubMed] [Google Scholar]
- Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis. 2006;24:1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Muyllaert D, Terwel D, Borghgraef P, Devijver H, Dewachter I, Van Leuven F. Transgenic mouse models for Alzheimer’s disease: the role of GSK-3B in combined amyloid and tau-pathology. Rev Neurol (Paris) 2006;162:903–907. doi: 10.1016/s0035-3787(06)75098-6. [DOI] [PubMed] [Google Scholar]
- Van Dorpe J, Smeijers L, Dewachter I, Nuyens D, Spittaels K, Van Den Haute C, Mercken M, Moechars D, Laenen I, Kuiperi C, Bruynseels K, Tesseur I, Loos R, Vanderstichele H, Checler F, Sciot R, Van Leuven F. Prominent cerebral amyloid angiopathy in transgenic mice overexpressing the London mutant of human APP in neurons. Am J Pathol. 2000;157:1283–1298. doi: 10.1016/S0002-9440(10)64644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewachter I, Reversé D, Caluwaerts N, Ris L, Kuipéri C, Van den Haute C, Spittaels K, Umans L, Serneels L, Thiry E, Moechars D, Mercken M, Godaux E, Van Leuven F. Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [V717I] transgenic mice. J Neurosci. 2002;22:3445–3453. doi: 10.1523/JNEUROSCI.22-09-03445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Oh SJ, Park SH, Kang HJ, Won MH, Kang TC, Park JB, Kim JI, Kim J, Lee JY. Neuronal loss in primary long-term cortical culture involves neurodegeneration-like cell death via calpain and p35 processing, but not developmental apoptosis or aging. Exp Mol Med. 2007;28:14–26. doi: 10.1038/emm.2007.3. [DOI] [PubMed] [Google Scholar]
- Bian F, Nath R, Sobocinski G, Booher RN, Lipinski WJ, Callahan MJ, Pack A, Wang KK, Walker LC. Axonopathy, tau abnormalities, and dyskinesia, but no neurofibrillary tangles in p25-transgenic mice. J Comp Neurol. 2002;446:257–266. doi: 10.1002/cne.10186. [DOI] [PubMed] [Google Scholar]
- Damjanac M, Rioux Bilan A, Barrier L, Pontcharraud R, Anne C, Hugon J, Page G. Fluoro-Jade B staining as useful tool to identify activated microglia and astrocytes in a mouse transgenic model of Alzheimer’s disease. Brain Res. 2007;1128:40–49. doi: 10.1016/j.brainres.2006.05.050. [DOI] [PubMed] [Google Scholar]
- O’Hare MJ, Kushwaha N, Zhang Y, Aleyasin H, Callaghan SM, Slack RS, Albert PR, Vincent I, Park DS. Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. J Neurosci. 2005;25:8954–8966. doi: 10.1523/JNEUROSCI.2899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marksteiner J, Lechner T, Kaufmann WA, Gurka P, Humpel C, Nowakowski C, Maier H, Jellinger KA. Distribution of chromogranin B-like immunoreactivity in the human hippocampus and its changes in Alzheimer’s disease. Acta Neuropathol. 2000;100:205–212. doi: 10.1007/s004010000239. [DOI] [PubMed] [Google Scholar]
- Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Thom M, Martinian L, Jacobs T, Nikolic M, Sisodiya SM. Deregulation of cdk5 in Hippocampal sclerosis. J Neuropathol Exp Neurol. 2006;65:55–66. doi: 10.1097/01.jnen.0000195940.48033.a2. [DOI] [PubMed] [Google Scholar]
- Sen A, Thom M, Martinian L, Yogarajah M, Nikolic M, Sisodiya SM. Increased immunoreactivity of cdk5 activators in hippocampal sclerosis. Neuroreport. 2007;18:511–516. doi: 10.1097/WNR.0b013e3280586879. [DOI] [PubMed] [Google Scholar]