Abstract
Cytokines, such as granulocyte macrophage-colony stimulating factor (GM-CSF) and interleukin (IL)-8 attract neutrophils into inflammatory sites. During emigration from the blood neutrophils interact with extracellular matrix proteins such as fibronectin. Fibronectin provides β2-integrin co-stimulation, allowing GM-CSF and IL-8 to activate nuclear factor (NF)-κB, an effect that does not occur in suspension. We tested the hypothesis that exposure of mice to fever-like temperatures abrogates neutrophil recruitment and NF-κB activation in a mouse model of skin inflammation. Mice that were exposed to 40°C for 1 hour showed strongly reduced GM-CSF- and IL-8-induced neutrophilic skin inflammation. In vitro heat exposure did not interfere with neutrophil adhesion or spreading on fibronectin but strongly inhibited migration toward both cytokines. Using specific inhibitors, we found that PI3-K/Akt was pivotal for neutrophil migration and that heat down-regulated this pathway. Furthermore, neutrophils on fibronectin showed abrogated NF-κB activation in response to GM-CSF and IL-8 after heat. In vivo heat exposure of mice followed by ex vivo stimulation of isolated bone marrow neutrophils confirmed these results. Finally, less NF-κB activation was seen in the inflammatory lesions of mice exposed to fever-like temperatures as demonstrated by in situ hybridization for IκBα mRNA. These new findings suggest that heat may have anti-inflammatory effects in neutrophil-dependent inflammation.
During sepsis or inflammation neutrophils are attracted to inflammatory sites by chemokines. Within this ambience neutrophils are also exposed to increased temperatures either systemically and or locally. Sepsis leads to enhanced leukocyte migration,1 and granulocyte macrophage-colony stimulating factor (GM-CSF) and interleukin (IL)-8 are important chemokines that recruit and prime neutrophils during inflammation.2,3,4,5 Conceivably, increased temperatures down-regulate inflammatory responses thereby limiting collateral damage. For example, in vitro heat shock application in cultured rat hepatocytes leads to the inhibition of cytokine-induced nitric oxide and nuclear factor (NF)-κB expression.6 Furthermore, endotoxin-stimulated murine macrophages revealed reduced tumor necrosis factor (TNF)-α production after heat shock.7 Whole body hyperthermia improved survival in a mouse endotoxin model,8 and a better outcome was observed in endotoxemic mice after heat shock with reduced NF-κB activity in the liver.9 Finally, even in noninflammatory conditions, hyperthermia in Sprague-Dawley rats suppressed angiotensin II-induced blood pressure elevation, reduced myocardial interstitial fibrosis, and inhibited the up-regulation of NF-κB in the heart.10 The significance of heat exposure on neutrophil functions is poorly investigated. We have studied the effect of fever-like temperatures in an in vivo model of a neutrophil-specific skin inflammation responding to GM-CSF and IL-8 and suggest that moderate heat has anti-inflammatory effects by inhibiting neutrophil recruitment and NF-κB activation. At an initial step in neutrophil emigration, circulating neutrophils adhere to and interact with cells and extracellular matrix components of the vessel wall. Several investigators have shown that adhesion to extracellular matrix via β2-integrins accelerates intracellular signaling pathways and biological functions.11,12,13,14,15,16,17 We have found previously that GM-CSF and IL-8 cannot activate NF-κB signaling in suspended neutrophils but do so after receiving a co-stimulatory signal via β2-integrins.18 Thus, chemokine-activated neutrophils that migrate into inflamed tissue provide a source for various NF-κB-dependent molecules. NF-κB is a transcription factor that controls gene expression during inflammation, immunity, cell proliferation, stress response, and apoptosis.19,20,21,22,23 NF-κB is therefore a potent upstream modulator of the proinflammatory response. Our data question the generous use of antipyretic drugs and physical maneuvers to lower body temperature during inflammation.24
Materials and Methods
Human GM-CSF and IL-8 were obtained from R&D Systems (Wiesbaden-Nordenstedt, Germany). Murine GM-CSF, fmlp, corticosterone, and Ficoll-Hypaque were from Sigma (Deisenhofen, Germany). Dextran was purchased from Amersham Pharmacia (Amsterdam, The Netherlands), the α-actin antibody (C-2), the polyclonal rabbit antibody against IκBα (C-21), the monoclonal mouse antibody against c-Jun kinase (JNK, G-7), and the horseradish peroxidase-labeled goat anti-mouse IgG were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-specific antibodies to p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK), and Akt (S473) were from Cell Signaling (Frankfurt, Germany). Inhibitors LY294002, SB202190, SP600125, and PD98059 as well as recombinant annexin I were purchased from Calbiochem (Bad Soden, Germany). A mouse antibody against annexin I and the IgG1 isotype control were from BD Biosciences (Heidelberg, Germany). Horseradish peroxidase-labeled donkey anti-rabbit IgG was from Amersham (Braunschweig, Germany). Hanks balanced salt solution (HBSS), phosphate-buffered saline (PBS), RPMI, Dulbecco’s modified medium, fetal calf serum, glutamine, penicillin/streptomycin, and trypan blue were from Biochrom (Berlin, Germany). Fluorescein isothiocyanate (FITC)-labeled antibodies to CD18 and the isotype control were purchased from Immunotech (Marseille, France). Antibodies for GM-CSF- and IL-8-receptor were from Santa Cruz, FITC-labeled goat anti-mouse antibodies were from DAKO (Hamburg, Germany). Fibronectin was from Roche (Mannheim, Germany). Endotoxin-free reagents and plastic disposables were used in all experiments.
In Vivo Hyperthermia, Bone Marrow Neutrophil Isolation, and Skin Inflammation
Female, 8- to 12-week-old, C57BL/6 mice were anesthetized with an isoflurane gas (Abbott, Wiesbaden, Germany) using a Univentor Anaesthesia unit (Univentor Ltd., Zejtan, Malta) and placed on a heating pad (Effenberger, Pfaffing, Germany). Core body temperature was monitored using a rectal probe and was maintained at 40 ± 0.5°C for 60 minutes. The control group was kept at 37.0 ± 0.5°C. To prevent dehydration, mice received 1 ml of sterile 0.9% saline subcutaneously between the scapulae after anesthesia. Thereafter mice were allowed to recover for 1 hour, to eat and drink ad libitum; they behaved normally after manipulation. For the isolation of bone marrow neutrophils mice were sacrificed, femurs and tibias were dissected, and the bone marrow was flushed with ice-cold sterile PBS without calcium and magnesium. Neutrophils were further isolated by Ficoll-Hypaque density gradient centrifugation and red blood cell lysis. The cell viability was >99% according to trypan blue exclusion. The neutrophil isolation was >95% by Wright-Giemsa staining. For the induction of skin inflammation, 500 ng of murine GM-CSF or human IL-8 were injected intracutaneously into the right flank of mice after hyperthermia versus normal temperature control, the opposite site of the flank was used for the injection of sterile PBS as a control. After 3 hours all mice were sacrificed, and the infiltrated skin was removed and further processed for immunohistochemical analysis. The Berlin Animal Review Board (reg. 0261/02) approved all protocols.
Histological Assessment of Skin Inflammation and in Situ Hybridization
For the assessment of neutrophil infiltration into the skin, 3-μm paraffin sections were dewaxed, stained for naphthol AS-D chloracetatesterase (ASD), and counterstained with hematoxylin and eosin. The total number of infiltrating neutrophils was assessed quantitatively by counting the number of neutrophils in 10 randomly selected ×40 fields of five different tissue sections. To determine mast cells, tissue sections were stained for 15 minutes with toluidine blue (4% formaldehyde, 0.1% toluidine blue, and 1% acetic acid, pH 2.8).
In situ hybridization was performed on 3-μm paraffin sections, and samples were rehydrated, postfixed in 4% paraformaldehyde/PBS, bleached, and treated with proteinase K (Roche). Hybridization with digoxigenin- labeled antisense RNA probes was performed according to the manufacturer’s protocol (Roche). The following mouse cDNA was used: IκBα (nucleotides 1 to 1091, U36277/NM010907). After hybridization, the slides were washed as follows: 1) 60°C for 5 minutes in 5× standard saline citrate (SSC) (0.75 mol/L NaCl, 75 mmol/L sodium citrate, pH 7.0) and 50% formamide; 2) 60°C for 10 minutes in 2× SSC and 50% formamide; 3) room temperature for 10 minutes in 2× SSC, 50% formamide, 1:1 in TES buffer (0.5 mol/L NaCl, 10 mmol/L Tris-HCl, pH 8.0, 1 mmol/L ethylenediaminetetraacetic acid); 4) 37°C for 2 minutes in TES buffer; 5) two high stringency washes at 60°C for 15 minutes in 1× SSC followed by 30 minutes in 0.2× SSC. The digoxigenin-labeled probe was detected with an anti-digoxigenin alkaline phosphatase-coupled Fab fragment (Roche) and subsequent BM-purple (Roche) treatment. Sections were counterstained with 0.01% PyroninG and mounted with Entellan (Merck, Darmstadt, Germany) after dehydration. All pictures were taken with an Axioplan 2 imaging microscope/Axiophot camera (Zeiss, Jena, Germany).
Preparation of Human Neutrophils and Cell Culture Conditions
Heparinized whole blood was drawn from healthy human donors after due written informed consent was obtained according to the requirements of our internal review board (AA3/00/44). Neutrophils were isolated by red blood cell sedimentation with dextran 1%, followed by Ficoll-Hypaque density gradient centrifugation. Erythrocytes were lysed by incubation with hypotonic saline for 15 seconds, and neutrophils were spun down (1050 rpm, 10 minutes) and reconstituted in HBSS with calcium and magnesium or in RPMI supplemented with 10% fetal calf serum when cultured for 20 hours. The final cell concentration was 5 × 106 cells per ml. The cell viability was detected in every cell preparation by trypan blue exclusion and found to be greater than 99%. The percentage of neutrophils after isolation was >95% by Wright-Giemsa staining and by light microscopy. The endothelial cell line EA.hy926 was kindly provided by Dr. C.-J. Edgell (University of North Carolina, Chapel Hill, NC). Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 1% glutamine, and 1% penicillin/streptomycin and subcultured twice weekly.
In Vitro Heat Exposure
Isolated neutrophils at 107 cells/ml HBSS were incubated for 90 minutes at 37°C or were exposed to 40°C. Thermo blocks from Eppendorf (Hamburg, Germany) were used, and the temperature was checked with a digital thermometer (Testo 900; Testo, Lenzkirch, Germany). Thereafter, cells were adjusted for 15 minutes at 37°C before further testing. Cell viability was always >95% as determined by trypan blue exclusion.
Spreading, Adhesion, and Transmigration Assay
We used 96-well plates coated with (10 μg/cm2) fibronectin for the adhesion assay. Neutrophils (1 × 105) in 100 μl of HBSS++ were either left untreated or were treated with 5 ng/ml of GM-CSF or 100 nmol/L IL-8. Plates were incubated at 37°C in 5% CO2 for 60 minutes. Wells were flicked dry and washed three times with PBS, and adherent cells were estimated using the MPO assay. Briefly, adherent cells were lysed in 100 μl of 0.5% Triton X-100 for 10 minutes. One hundred μl of substrate (2.2′-azino-bis 3-ethylbenzthiazoline-6-sulfonicacid; Sigma) were added, and OD was read after 10 minutes at 450 nm with a microtiter plate reader. Each experiment was done in triplicate. ODs of the experimental sample were compared to a standard curve that showed an excellent correlation between OD and cell number. The standard curve was established throughout a range of 1 × 104 to 1 × 105 cells and showed an R2 value 0.96.
For estimation of cell spreading, neutrophils were cultured in 12-well plates as described above. At the indicated time points, nonadherent cells were discarded, and the percentage of spread cells was assessed. At least 100 cells were counted using phase contrast microscopy and those that were phase dark, enlarged, and irregular were considered to be spread. The ability of neutrophils to migrate through extracellular matrix components was tested with 20 μg/ml fibronectin-coated transwells (3.0-μm pore size, 6.5 mm diameter from Dow Corning, Corning, NY). Neutrophils (1.5 × 106), if indicated, were pretreated with inhibitors for 30 minutes or with 40°C for 90 minutes. GM-CSF or IL-8 was added to the lower well, and transmigration of neutrophils toward the lower well was assessed at 37°C. Every 60 minutes the upper chamber of the transwell device was moved to a new well filled with the chemokine in HBSS++, transmigrated cells were quantified by MPO assay after 3 hours as described previously.25 In migration experiments studying the role of annexin I, fibronectin-coated transwells were seeded with endothelial cells (EA.hy926, density 1 × 105/ml). Cells were allowed to grow to confluence before use in the experiments.26
Western Blot
Samples were incubated for 5 minutes at 95°C in loading buffer (250 mmol/L Tris-HCl, pH 6.8, with 4% sodium dodecyl sulfate, 20% glycerol, 0.01% bromphenol blue, 10% β-mercaptoethanol). Five to twenty μg of protein was loaded per lane, electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide gel, and transferred to a nitrocellulose membrane. The membrane was blocked with TBS-T/5% skim milk for 1 hour and incubated overnight with the indicated antibodies. Membranes were washed and incubated with a horseradish peroxidase-labeled secondary Ab. The blot was developed by incubation in a chemiluminescence substrate (ECL, Amersham) and exposed to X-ray film. We confirmed equal protein loading in parallel experiments using α-actin or total ERK antibodies.
Flow Cytometry to Assess GM-CSF-, IL-8 Receptor Expression, β2-Integrin Expression, Intracellular Annexin I, and Neutrophil Apoptosis
Flow cytometry was used to evaluate the membrane expression of CD18 and of the GM-CSF and IL-8 receptor. Immunostaining was done as described previously.27 Cells were incubated with FITC-labeled antibodies against CD18 (7E4), GM-CSF, or IL-8 receptor. To measure intracellular annexin I content, EA.hy926 cells were grown to confluence in 96-wells. Neutrophils (1.5 × 106) were added to the wells and incubated with 10−6 mol/L corticosterone or dimethyl sulfoxide control for 30 minutes. The supernatant was removed and further conferred to the fraction of nonadherent PMNs, whereas the remaining endothelial cells with adherent PMNs were removed with PBS/5 mmol/L ethylenediaminetetraacetic acid. Both fractions were adjusted to equal neutrophil numbers fixed with 0.5% paraformaldehyde for 30 minutes, followed by cell permeabilization with 0.015% saponin/PBS/1% bovine serum albumin. Immunostaining was done with annexin I antibody or an isotype control at a final concentration of 5 μg/ml for 30 minutes followed by a secondary FITC-conjugated F(ab)2-fragment of goat anti-mouse IgG for another 30 minutes. To assess neutrophil apoptosis, 2 × 106 cells were washed, pelleted, and resuspended in 200 μl of buffer with 2 μl of FITC-labeled annexin V. After incubation in the dark at room temperature for 10 minutes, cells were subjected to flow cytometry analysis. Flow cytometry was performed using a FACSort (Becton Dickinson, Heidelberg, Germany), and 10,000 events per sample were collected.
Nuclear Extract Preparation
Nuclear extracts were prepared as described previously.28 Briefly, after centrifugation of the harvested cells, the pellets were resuspended with a hypotonic buffer containing an anti-protease cocktail. Cells were kept on ice for 10 minutes, vortexed, and spun down at 1000 × g to pellet the nuclei and to remove intracellular granules that may cause nuclear degradation. The pellet was washed again, and the hypertonic, high-salt buffer was added together with the protease inhibitor cocktail. After centrifugation, the supernatant was collected and referred to as the nuclear fraction. Protein measurements were done using the Bio-Rad assay (Bio-Rad, Munich, Germany).
Electrophoretic Mobility Shift Assay
Electrophoretic mobility shift assay was performed as previously described.28 Briefly, nuclear extracts (5 μg of protein) were incubated with 20,000 cpm of a 32P-labeled H2K probe. Incubations were performed for 30 minutes at room temperature in the presence of poly/dI-dC and 20 mmol/L HEPES, containing 60 mmol/L KCl, 4% Ficoll, 5 mmol/L dithiothreitol, and 0.5 μg/μl nuclease-free bovine serum albumin. Probes were subjected to electrophoresis on native 5% polyacrylamide gels and autoradiographed.
Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNAs were isolated according to a Qiagen (Hilden, Germany) protocol including DNase treatment. Quantitative RT-PCR was performed as described previously using TaqMan technology (Applied Biosystems, Weiterstadt, Germany).29 Reverse transcription was performed according to the Superscript protocol (Invitrogen, De Schelp, The Netherlands). TaqMan RT-PCR was performed using the Master Mix (Applied Biosystems). The following oligonucleotides were used for TNF-α: forward primer 5′-GGTGCTTGTTCC TCAGCCTC-3′, reverse primer 5′-CAGGCAGAAGAGCGTGGTG-3′ and IκBα: forward primer 5′-CCCTGTAATGGCCGGACTG-3′, reverse primer 5′-AGGAGTGACACCAGGTCAGGA-3′ and the probe Fam 5′-CCTTCACCTCGCAGTGGACCTGC-3′ Tamra. The quantification was checked for each sample using probes for GAPDH mRNA. Results were imported in an Excel spreadsheet and analyzed according to the standard curve method.
Statistical Analysis
Results are given as mean ± SEM. Comparisons between two groups were done using paired t-tests. Comparisons between multiple groups were done using one- or two-way analysis of variance as indicated. Specific differences between multiple groups were then determined by use of a Bonferroni posthoc test. Differences were considered significant if P < 0.05 and are indicated by asterisks.
Results
Fever-Like Temperatures Reduced GM-CSF- and IL-8-Induced Neutrophilic Skin Inflammation in Mice
We first investigated the effect of fever-like temperatures in a neutrophilic skin inflammation model. Whole-body temperature of 8- to 12-week-old B6 mice was increased to 40°C for 60 minutes or left at 37°C. After 1 hour of recovery, skin inflammation was induced by intradermal injection of PBS, 500 ng of murine GM-CSF, or 500 ng of human IL-8. After 3 hours mice were sacrificed, and neutrophil migration into the site of inflammation was assessed by histology. PBS-injected control animals did not show neutrophil infiltrates under either temperature condition (Figure 1, A and B). In contrast, numerous neutrophils migrated into the skin after IL-8 (Figure 1C) or GM-CSF (not depicted) challenge. This neutrophil infiltrate was strongly reduced in mice exposed to prior heat (Figure 1D). Because of the nature of the staining procedure, mast cells are also detected with ASD in the histological slides. However, mast cells are clearly larger than neutrophils, have a more rounded nucleus, and stain with a metachromatic mast cell-specific dye (Figure 1C, inset). The number of migrated neutrophils into the skin was assessed by counting ASD-positive cells in 10 randomly selected fields (×40) of five different tissue sections. The quantitative results for both GM-CSF and IL-8 (Figure 1E) indicated that exposure of mice to moderate heat significantly inhibited neutrophilic infiltration.
Figure 1.
In vivo hyperthermia reduced neutrophil infiltration in an inflammatory skin model. Whole-body temperature of mice was increased to 40°C or left at 37°C for 60 minutes. After 1 hour of recovery intradermal injections of 500 ng of murine GM-CSF or 500 ng of human IL-8 were given in one shaved flank (n = 6 for heat-exposed versus n = 6 kept at normal temperature, three of the animals from each group were challenged with GM-CSF and three with IL-8). PBS injections were used as a solvent control in the opposite flank of each mouse. After 3 hours, mice were sacrificed, the skin was excised, and neutrophil infiltration (small dots denoted by red arrows, most prominent in C) was assessed by counting ASD-positive cells in 10 randomly selected ×40 fields of five different tissue sections. PBS injection did not result in neutrophil recruitment at either 37°C (A) or 40°C (B). To clarify that the larger ASD-positive cells (black arrow) are mast cells, mast cell-specific metachromatic staining was done as illustrated in the inset in A. Note the typical dye of the mast cell granula. C: IL-8 injection led to a strong increase in skin neutrophils in mice exposed to 37°C. Typical segmented nuclei of ASD-positive neutrophils (red arrows) can be appreciated in the inset in C. D: IL-8-mediated neutrophil infiltration was strongly inhibited after increasing whole body temperature to 40°C. E: A statistical analysis of neutrophil infiltration in GM-CSF- and IL-8-induced skin inflammation with (gray columns) or without (black columns) hyperthermia is provided (n = 6 for 37 versus 40°C, n = 3 for GM-CSF versus PBS, n = 3 for IL-8 versus PBS). The mean number of cells per microscopic field (×40 HPF) is indicated. *P < 0.05, **P < 0.01. Original magnifications, ×40.
In Vitro Heat Exposure Had No Effect on GM-CSF- and IL-8-Induced Neutrophil Adhesion or Spreading on Fibronectin, but Inhibited Migration
To understand the underlying mechanisms leading to heat-abrogated neutrophil emigration into the skin, we first investigated whether or not adhesion, spreading, and migration of neutrophils to fibronectin are impaired by prior heat exposure. All three functions are essential for neutrophil recruitment. For assessment of adhesion and spreading, cells were incubated for 90 minutes at 37°C or 40°C, respectively. After adjusting at 37°C for 15 minutes, samples were stimulated with GM-CSF or IL-8 on fibronectin. The results demonstrate that short-term heat had no effect on neutrophil adhesion and spreading (Figure 2, A and B). In contrast, when we studied migration toward GM-CSF and IL-8 in transwell experiments, we observed significant inhibition by prior heat (Figure 2C). Although 69.3 ± 15.4 × 104 neutrophils migrated toward GM-CSF after 3 hours during 37°C conditions, only 21.0 ± 5.2 × 104 cells did so after prior heat (P < 0.05). For IL-8 the numbers were 19.8 ± 3.9 × 104 for 37°C and 6.4 ± 2.8 × 104 after heat exposure (P < 0.01).
Figure 2.
Short-term heat exposure influenced neither neutrophil (=neutr.) adherence nor spreading but inhibited GM-CSF- and IL-8-induced migration. Cells were either exposed to 37°C or 40°C (black versus gray columns) for 90 minutes, followed by the stimulation with buffer control (CTRL), 5 ng/ml GM-CSF, or 100 nmol/L IL-8 for 30 minutes on fibronectin. A: Adhesion to fibronectin was estimated using the MPO assay (n = 3). B: Neutrophil spreading after GM-CSF or IL-8 activation was analyzed at 60 minutes with a phase contrast microscope (n = 3). The ability of neutrophils to migrate toward GM-CSF or IL-8 was investigated using transwell experiments after heat (C, n = 5) or after pretreatment with 5 μmol/L annexin I (A-I) or 10−6 mol/L corticosterone (CORT) (D, n = 5). The number of experiments reflects independent experiments performed in duplicates. The number of migrated neutrophils was quantified by the MPO assay after 3 hours. *P < 0.05, **P < 0.01.
We were interested in whether or not glucocorticoids per se or annexin I would mimic the inhibitory heat effect in vitro. Glucocorticoid levels increase during in vivo stress, and glucocorticoids as well as adhesion to endothelial cells trigger annexin I release from neutrophils.30 Accordingly, we observed that annexin I was released when neutrophils were incubated on endothelial cells for 30 minutes. Annexin I release was assessed as a decrease in intracellular annexin I shown by fluorescence-activated cell sorting analysis (1028 ± 235 MFI nonadherent versus 403 ± 74 MFI after adherence to endothelial cells, n = 3). However, neither corticosterone nor annexin I inhibited neutrophil migration in response to GM-CSF or IL-8 (Figure 2D), migration to GM-CSF was 77 ± 9 neutrophils × 104 with buffer versus 76 ± 11 × 104 with 5 μmol/L annexin and 68 ± 9 × 104 with 10−6 mol/L corticosterone, migration to IL-8 was 21 ± 8 × 104 with buffer versus 23 ± 17 × 104 with annexin and 21 ± 13 × 104 with corticosterone. In parallel, annexin I had a small but significant inhibitory effect on fmlp-induced migration (migration to 10−9 fmlp was 62 ± 24 × 104 with buffer versus 42 ± 18 × 104 with annexin, n = 5, P < 0.05). To provide a positive control for corticosterone, we confirmed in parallel the apoptosis-delaying effect after 20 hours. We detected 56 ± 5% apoptotic neutrophils with buffer control versus 44 ± 5% with corticosterone, P < 0.01, n = 5). We obtained similar migration and apoptosis data when dexamethasone was used instead of corticosterone (data not shown).
Inhibition of Migration by Heat Exposure Is Not a Consequence of Cytokine Receptor or β2-Integrin Shedding
Because cytokine receptors and β2-integrins are necessary for both recruitment and signal transduction, we studied the effect of heat exposure on the expression of these receptors. After exposure to 37°C, 40°C, and 42°C, cells were stained with receptor-specific antibodies and assayed by flow cytometry. Expression of GM-CSF and IL-8 receptors and of CD18 was not affected by heat and a typical experiment at 42°C is depicted in Figure 3. Therefore, these experiments exclude that the inhibitory effects by heat are merely a consequence of cytokine receptor or β2-integrin shedding.
Figure 3.
Heat exposure of neutrophils did not alter the receptor expression of GM-CSF and IL-8 or the expression of β2-integrin CD18. After exposure of neutrophils to either 37°C or 42°C for 90 minutes, cells were stained with specific antibodies to either receptors or CD18 (bold) or an isotype control (regular) and assessed by flow cytometry (n = 3).
In Vitro Heat Exposure Diminished GM-CSF- and IL-8-Mediated Neutrophil Migration Mainly via Decreasing PI3-K/Akt Activation
In the next set of experiments we used specific pharmacological compounds to identify which signaling pathways controlled GM-CSF- and IL-8-mediated neutrophil migration and whether or not heat showed inhibitory effects. Cells were pretreated with 10 μmol/L SB202190 as a specific p38 MAPK inhibitor, with 10 μmol/L SP600125 for JNK, with 25 μmol/L PD98059 for ERK, and 10 μmol/L LY294002 for PI3-K/Akt inhibition. After 30 minutes the samples were subjected to transwells for migration toward GM-CSF (Figure 4, top) and IL-8 (Figure 4, bottom) at 37°C. PD98059 and LY294002 significantly inhibited cytokine-induced migration. Thus, our data indicate that PI3-K/Akt and ERK, but not p38 MAPK or JNK, control neutrophil migration toward GM-CSF and IL-8.
Figure 4.
Short-term heat exposure inhibited GM-CSF- and IL-8-induced neutrophil migration via ERK and PI3-K/Akt. To test which signaling pathways control neutrophil migration at 37°C conditions, cells were pretreated with dimethyl sulfoxide control (CTRL = buffer, black columns), 10 μmol/L SB202190 (SB), 10 μmol/L SP600125 (SP), 25 μmol/L PD98059 (PD), or 10 μmol/L LY294002 (LY) for 30 minutes (all inhibitors in white columns). Thereafter migration toward 5 ng/ml GM-CSF (top, n = 5) or 100 nmol/L IL-8 (bottom, n = 5) was measured. The number of experiments reflects independent experiments performed in duplicates. Data are mean ± SEM. *P < 0.05, **P < 0.01.
Having shown that PI3-K/Akt and ERK signals control GM-CSF- and IL-8-induced neutrophil migration, we investigated the effect of prior heat exposure on both pathways. After exposure to either 37°C or 40°C, cells were stimulated with GM-CSF (Figure 5A) and IL-8 (Figure 5B). Activation of the PI3-K/Akt and ERK pathway was assessed at the indicated time points by Western blotting using phospho-specific antibodies. Both cytokines resulted in strong phosphorylation of Akt and ERK. However, only Akt phosphorylation was significantly inhibited by prior heat exposure. The corresponding OD measurements are provided in the lower panels. We also performed dose-response experiments for both cytokines, testing increasing concentrations without and with prior exposure to 40°C. The results indicate that heat inhibits Akt phosphorylation in response to lower GM-CSF concentrations (1, 5, and marginally at 10 ng/ml). In contrast, heat did not affect Akt phosphorylation in neutrophils stimulated with 20 ng/ml of GM-CSF (Figure 5C). Moreover, heat-mediated inhibition of Akt phosphorylation was observed with all IL-8 concentrations (Figure 5D). Together, these experiments indicate that inhibition of PI3-K/Akt by heat is, at least in part, responsible for the inhibitory effect of heat on neutrophil migration.
Figure 5.
Short-term heat exposure inhibits activation of PI3-K/Akt, whereas it has no significant effect on ERK. After heat exposure at 37°C versus 40°C for 90 minutes, suspension neutrophils were stimulated with 5 ng/ml GM-CSF (A) or 100 nmol/L IL-8 (B). Cells were harvested at the indicated time points and subjected to Western blot experiments using phospho-specific antibodies to Akt and ERK. Equal loading was confirmed using total ERK. OD measurements are provided in the bottom panels. Data are given in mean ± SEM from five experiments. **P < 0.01, n.s. not significant. Additional dose-response studies were performed to assess heat effects on increasing cytokine concentrations. After 40°C exposure, cells were stimulated with the indicated concentrations of GM-CSF for 5 minutes or with IL-8 for 1 minute and subjected to Western blot as described above. A typical of three independent experiments is depicted in C and D.
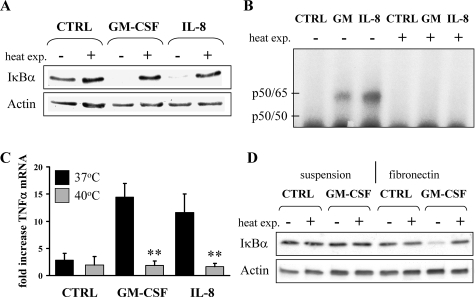
In Vitro Heat Exposure Abrogates NF-κB Activation in GM-CSF- and IL-8-Treated Neutrophils on Fibronectin
We next tested whether or not heat exposure would affect NF-κB activation in GM-CSF- and IL-8-treated neutrophils on fibronectin. Cells were exposed to 37°C or 40°C for 90 minutes before cytokine stimulation. Samples were assayed for NF-κB activation using different independent methods (Figure 6). The data unequivocally demonstrate that short-term heat abrogated GM-CSF- and IL-8-induced IκBα degradation as shown by Western blotting (Figure 6A) and NF-κB DNA-binding activity (Figure 6B). We performed quantitative RT-PCR experiments to assess whether or not GM-CSF- and IL-8-activated neutrophils initiate transcription of TNF-α, a gene that itself is under the control of NF-κB. Short-term heat exposure (Figure 6C) completely blocked GM-CSF- and IL-8-mediated up-regulation of TNF-α mRNA in neutrophils on fibronectin (for GM-CSF, 14.4 ± 3.0-fold increase of TNF-α mRNA at 37°C versus 1.8 ± 0.5 at 40°C; for IL-8 11.6 ± 3.9-fold increase at 37°C versus 1.7 ± 0.6 at 40°C). Along the same lines, we found that the up-regulation of IκBα mRNA, itself controlled by NF-κB, was blocked by prior heat (data not shown).
Figure 6.
Hyperthermia leads to the abrogation of NF-κB activation in vitro and after fever induction of mice in ex vivo stimulated bone marrow-derived neutrophils on fibronectin. Human neutrophils were exposed to 37°C or 40°C for 90 minutes, followed by the stimulation with buffer control (CTRL), 5 ng/ml GM-CSF, or 100 nmol/L IL-8 on fibronectin for 30 minutes. For the assessment of NF-κB activation cells were harvested and subjected to Western blot experiments using an IκBα antibody (A, n = 3) or nuclear extracts were analyzed by electrophoretic mobility shift assay using an H2K binding site probe for NF-κB (B, n = 3). C: Up-regulation of TNF-α mRNA was assessed by quantitative RT-PCR (n = 3). Total RNAs were isolated according to a Qiagen protocol. D: Body temperatures of mice were increased to 40°C or left at 37°C for 60 minutes. After 1 hour of recovery, mouse bone marrow neutrophils were isolated and stimulated with 5 ng/ml of murine GM-CSF for 30 minutes in suspension or on fibronectin, respectively. Western blot experiments were performed for IκBα (n = 7). Equal protein loading was confirmed using an α-actin antibody. The data are given in mean ± SEM. **P < 0.01.
We then tested whether or not the inhibition of NF-κB by heat affected GM-CSF- and IL-8-delayed neutrophil apoptosis. Constitutive apoptosis was 60 ± 5% without and 61 ± 5% with heat. GM-CSF significantly delayed apoptosis to 32 ± 3% without and to 32 ± 2% with prior heat. For IL-8 the numbers were 29 ± 7% apoptotic cells without and 30 ± 7% with heat exposure (n = 3). The data suggest that heat-mediated blockade of antiapoptotic NF-κB-mediated signals and proapoptotic signals neutralize each other.
Bone Marrow Neutrophils and Skin Infiltrates from Mice Exposed to 40°C Heat Revealed Abrogated Chemokine-Induced NF-κB Activation
We next investigated whether or not in vivo heat exposure of mice would also prevent NF-κB activation. Mice were exposed to 40°C for 60 minutes. After 1 hour of recovery, bone marrow neutrophils were isolated and stimulated with murine GM-CSF in suspension versus on fibronectin. As expected, we observed no NF-κB activation in suspension neutrophils. In contrast, strong activation was observed in neutrophils on fibronectin. Exposure of mice to short-term fever-like temperatures resulted in inhibition of GM-CSF-induced NF-κB activation as shown by IκBα degradation (Figure 6D).
Finally, we assayed IκBα mRNA expression in the GM-CSF- or IL-8-induced skin infiltrate by in situ hybridization (Figure 7). Infiltrated neutrophils primarily expressed IκBα mRNA in mice that were cytokine challenged at 37°C, indicating increased NF-κB activity. IκBα mRNA detection was strongly reduced in mice that were subjected to increased whole-body temperature.
Figure 7.
Hyperthermia-mediated reduction of neutrophilic skin infiltration is accompanied by reduced NF-κB activation as assessed by in situ hybridization for IκBα mRNA. In contrast to PBS control injections, in mice kept at 37°C (A) or heated to 40°C (B) intense NF-κB activation was observed in the IL-8-induced skin inflammation (C). Note that cells expressing IκBα mRNA are stained as blue dots (black arrows) not present in any other panel. The larger prominent circular structures are hair follicles within the skin and should not be mistaken for cells. D: The NF-κB activation was strongly reduced with prior hyperthermia as evidenced by absent blue dots. Hybridization with digoxigenin-labeled antisense RNA probe was performed followed by microscopy. A representative of two independent experiments is given. Original magnifications, ×40.
Discussion
The novel findings of our study are that short-term increase of whole-body temperature strongly inhibited neutrophilic skin inflammation in vivo. Investigating underlying mechanisms, we observed that short-term heat inhibited neutrophil migration toward GM-CSF and IL-8 in vitro by down-regulating PI3-K/Akt. Moreover, in vitro and in vivo exposure to fever-like temperatures abrogated NF-κB-dependent gene transcription in cytokine-stimulated neutrophils interacting with fibronectin. These data may have implications for defense mechanisms in inflammatory conditions and the role of fever as a regulatory process.
Our experiments indicate that fever-like temperatures abrogated inflammation in an animal model of neutrophil-mediated skin inflammation. Other investigators provide evidence from animal models, and even from clinical studies in patients, that fever improved survival.31,32 Failure to reach a febrile response within the first 24 hours was associated with increased mortality.33 Local and systemic hyperthermia, with temperatures of 40 to 43°C is even used as an adjunctive therapy with various established cancer treatments such as radiotherapy and chemotherapy.34
We studied neutrophils because these cells are pivotal for inflammatory responses. GM-CSF and IL-8 are chemokines involved in neutrophil recruitment and activation. Evidence has been provided for functional roles of both cytokines during skin inflammation. Endothelial cells synthesize IL-8 at inflammatory sites after exposure to IL-1, TNF-α, or lipopolysaccharide.4,35 T cells also release IL-8 and GM-CSF with subsequent neutrophil-mediated inflammation.36 Furthermore, activated keratinocytes are a common source of GM-CSF in patients with atopic dermatitis.37 Based on our in vivo observation that chemokine-induced neutrophil-specific skin inflammation was inhibited in mice exposed to heat, we focused on two aspects namely neutrophil recruitment, and activation of the proinflammatory NF-κΒ. Although mice lack murine IL-8, a homologue of the human IL-8 receptor (mIL-8RH) has been described.38,39 Instead of IL-8, several other α chemokines with proinflammatory activity were identified in animals such as mouse MIP-2, KC, GCP-2, and rat CINC.40,41,42,43 In our mouse experiments, we used human IL-8 for the induction of neutrophilic skin inflammation. Rot44 showed that human IL-8 induces murine neutrophil chemotaxis in vitro. Moreover, human IL-8 was used in mouse inflammatory models with a neutrophil influx peaking at 3 hours.45
Our data indicate that short-term exposure to fever-like temperatures did not affect adhesion and spreading of GM-CSF- and IL-8-treated neutrophils to fibronectin but inhibited neutrophil migration. In accordance with our data, Frohlich and colleagues46 demonstrated that heat did not prevent neutrophil adhesion to IL-1β-activated endothelial cells. Similarly, we observed no heat effect in a previous study using TNF-α to stimulate neutrophil adhesion to fibronectin.29 Our inhibitor studies revealed that migration in response to both chemokines was mediated by PI3-K/Akt and ERK, but not by JNK or p38 MAPK, and that short-term heat exposure inhibited PI3-K/Akt with a marginal effect on ERK activation. These experiments support the notion that heat-mediated inhibition of migration was, at least in part, a consequence of PI3-K/Akt abrogation. Other investigators also reported the fact that PI3-K/Akt controls neutrophil migration in response to IL-8 or GM-CSF.2,47,48 However, whether or not ERK is important for migration is still a matter of controversy. ERK inhibitors blocked CCL3-induced migration of GM-CSF-primed neutrophils in a study done by Ottonello and colleagues,49 but Coffer and colleagues48 found PI3-K dependence in GM-CSF-induced migration whereas inhibition of ERK with PD98059 had no effect. Fuhler and colleagues47 showed that specific inhibitors for ERK1/2 abrogated neutrophil migration toward IL-8, similar to our own observation. However, in our experiments PI3-K/Akt, but not ERK activation was inhibited by prior heat. Therefore, abrogation of PI3-K/Akt rather than ERK is responsible for reduced neutrophil migration after heat exposure. Heat per se activates JNK in several cell types. However, this effect occurred at rather high temperatures between 42°C and 45°C, and neutrophils were not studied.50,51,52 In agreement with these data, we did not see JNK phosphorylation at 40°C in our experimental settings, whereas a weak signal was detected with 43°C (data not shown).
Glucocorticoids are up-regulated during stressful events in vivo. Therefore heat stress might act via the increase of glucocorticoids or indirectly via glucocorticoid-induced up-regulation of anti-inflammatory annexin I.30 Moreover, Rhee and colleagues53 suggested that annexin I is directly induced by heat stress. However, we found that neither corticosterone nor dexamethasone nor annexin I inhibited GM-CSF- or IL-8-induced neutrophil migration in vitro.
We reported earlier that GM-CSF and IL-8 do not activate the transcription factor NF-κB in suspended neutrophils but do so when an additional β2-integrin signal is provided.18 We found that exposure to fever-like temperatures prevented NF-κB activation in GM-CSF- or IL-8-stimulated human neutrophils on fibronectin. As a consequence, NF-κB-mediated transcription was blocked, as demonstrated for TNF-α. Conceivably, the prevention of TNF-α generation is strongly anti-inflammatory, for example, by limiting the activation of neighboring cells and by preventing TNF-α-related effects on the neutrophil itself. Interestingly, we found recently that heat was not able to block NF-κB activation of TNF-α-stimulated neutrophils on fibronectin, underscoring the importance of preventing TNF-α generation upstream.29 When we used more complex settings by exposing mice to 37°C or 40°C for 60 minutes followed by ex vivo stimulation of isolated bone marrow neutrophils with GM-CSF on fibronectin, we observed strong NF-κB activation with 37°C and significant inhibition with exposure to 40°C. Thus, heat exposure in cell culture experiments and exposure of the whole animal produced similar results with respect to NF-κB inhibition.
Wong and colleagues54 showed stress-induced NF-κB inhibition mediated by IκBα mRNA up-regulation. This IκΒα mRNA increase was even accelerated after combined treatment with heat and TNF-α. The authors assumed that this IκBα mRNA up-regulation in combination with stabilization of the IκBα-NF-κB complex by stress proteins led to the observed NF-κB inhibition. However, under our experimental conditions we observed no IκBα mRNA increase after heat in human neutrophils. In fact, our data indicate that heat actually blocked the induction of IκBα mRNA by IL-8 and GM-CSF. We found in an earlier neutrophil study that heat caused dissociation of HSP90 from the IKK complex resulting in NF-κB inhibition.55 Conceivably, such cell-specific effects are at work here. In contrast to Wong and colleagues,54 we used neutrophils instead of A549 cells and exposed the cells to 40°C instead of 43°C. Furthermore, we applied heat for 90 minutes instead of 4 to 20 hours.
Finally, NF-κB activation was primarily abrogated in the skin model of neutrophilic inflammation. Temperature-mediated inhibitory effects on NF-κB activation were also shown in other cell types, where angiotensin II, IL-1β, or lipopolysaccharide demonstrated a reduced capability to activate NF-κB after heat.56,57 However, none of these studies investigated heat effects on neutrophils, a cell type that is pivotal for host defense mechanisms and that dominates the early inflammatory infiltrate in many infections. Moreover, several studies on heat effects applied high temperatures that exceed values measured during infections. A mouse macrophage cell line (RAW 264.7) was exposed to 43°C for up to 90 minutes58; in HeLa cells investigators even heated cells up to 45°C.59 In vivo hyperthermia in mice or rats is frequently done at 42°C for 15 to 20 minutes.10,60 In addition, cells in culture were frequently exposed to heat throughout rather prolonged time periods, for example up to 6 hours.6,61 Under these conditions, heat shock proteins were implicated in NF-κB inhibition.62 In contrast, we used 40°C heat exposure for 60 minutes, thereby mimicking short-term fever spikes that are commonly observed in patients with systemic infections. Previously, we found no evidence that under these conditions neutrophils up-regulated expression of heat shock proteins, even when assayed up to 4 hours (eg, HSP70 and HSP90).55 However, in this study we demonstrated that NF-κB inhibition involved dissociation of HSP90 from the IKK complex.
In summary, we used a skin model to demonstrate that exposure to fever-like temperatures inhibits neutrophil-mediated inflammation. We observed abrogated neutrophil recruitment, at least in part by preventing PI3-K/Akt activation, and down-regulated proinflammatory NF-κB signaling. However, the overall effect of heat in a more complex in vivo situation may be beneficial or detrimental depending on the time of occurrence. Conceivably, short-term temperature spikes help by limiting an inflammatory response but also have the potential to abrogate a necessary inflammatory response. Interestingly, with infectious diseases, this is the fever pattern generally observed, as opposed to the Pel-Epstein fever pattern observed in Hodgkin’s disease. We still do not understand the nature and purpose of fever. However, the phenomenon viewed as man’s nemesis for centuries, deserves further investigations.
Acknowledgments
We thank Baerbel Kuhlmann and Karin Ganzel for excellent technical assistance.
Footnotes
Address reprint requests to Ralph Kettritz, HELIOS Klinikum Berlin-Buch, Schwanebecker Chaussee 50, 13125 Berlin, Germany. E-mail: kettritz@charite.de.
References
- Guo RF, Riedemann NC, Laudes IJ, Sarma VJ, Kunkel RG, Dilley KA, Paulauskis JD, Ward PA. Altered neutrophil trafficking during sepsis. J Immunol. 2002;169:307–314. doi: 10.4049/jimmunol.169.1.307. [DOI] [PubMed] [Google Scholar]
- Gomez-Cambronero J, Horn J, Paul CC, Baumann MA. Granulocyte-macrophage colony-stimulating factor is a chemoattractant cytokine for human neutrophils: involvement of the ribosomal p70 S6 kinase signaling pathway. J Immunol. 2003;171:6846–6855. doi: 10.4049/jimmunol.171.12.6846. [DOI] [PubMed] [Google Scholar]
- Li FK, Davenport A, Robson RL, Loetscher P, Rothlein R, Williams JD, Topley N. Leukocyte migration across human peritoneal mesothelial cells is dependent on directed chemokine secretion and ICAM-1 expression. Kidney Int. 1998;54:2170–2183. doi: 10.1046/j.1523-1755.1998.00174.x. [DOI] [PubMed] [Google Scholar]
- Smart SJ, Casale TB. TNF-alpha-induced transendothelial neutrophil migration is IL-8 dependent. Am J Physiol. 1994;266:L238–L245. doi: 10.1152/ajplung.1994.266.3.L238. [DOI] [PubMed] [Google Scholar]
- Yong KL, Rowles PM, Patterson KG, Linch DC. Granulocyte-macrophage colony-stimulating factor induces neutrophil adhesion to pulmonary vascular endothelium in vivo: role of beta 2 integrins. Blood. 1992;80:1565–1575. [PubMed] [Google Scholar]
- de Vera ME, Kim YM, Wong HR, Wang Q, Billiar TR, Geller DA. Heat shock response inhibits cytokine-inducible nitric oxide synthase expression in rat hepatocytes. Hepatology. 1996;24:1238–1245. doi: 10.1002/hep.510240542. [DOI] [PubMed] [Google Scholar]
- Snyder YM, Guthrie L, Evans GF, Zuckerman SH. Transcriptional inhibition of endotoxin-induced monokine synthesis following heat shock in murine peritoneal macrophages. J Leukoc Biol. 1992;51:181–187. doi: 10.1002/jlb.51.2.181. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R, Nunnally I, Lindquist S, Taulien J, Perdrizet G, Karl I. Hyperthermia protects mice against the lethal effects of endotoxin. Am J Physiol. 1993;265:R1447–R1457. doi: 10.1152/ajpregu.1993.265.6.R1447. [DOI] [PubMed] [Google Scholar]
- Pritts TA, Wang Q, Sun X, Fischer DR, Hungness ES, Fischer JE, Wong HR, Hasselgren PO. The stress response decreases NF-kappaB activation in liver of endotoxemic mice. Shock. 2002;18:33–37. doi: 10.1097/00024382-200207000-00007. [DOI] [PubMed] [Google Scholar]
- Chen Y, Arrigo AP, Currie RW. Heat shock treatment suppresses angiotensin II-induced activation of NF-kappaB pathway and heart inflammation: a role for IKK depletion by heat shock? Am J Physiol. 2004;287:H1104–H1114. doi: 10.1152/ajpheart.00102.2004. [DOI] [PubMed] [Google Scholar]
- Dransfield I, Stocks SC, Haslett C. Regulation of cell adhesion molecule expression and function associated with neutrophil apoptosis. Blood. 1995;85:3264–3273. [PubMed] [Google Scholar]
- Evangelista V, Pamuklar Z, Piccoli A, Manarini S, Dell’elba G, Pecce R, Martelli N, Federico L, Rojas M, Berton G, Lowell CA, Totani L, Smyth SS. Src family kinases mediate neutrophil adhesion to adherent platelets. Blood. 2007;109:2461–2469. doi: 10.1182/blood-2006-06-029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenei V, Deevi RK, Adams CA, Axelsson L, Hirst DG, Andersson T, Dib K. Nitric oxide produced in response to engagement of beta2 integrins on human neutrophils activates the monomeric GTPases Rap1 and Rap2 and promotes adhesion. J Biol Chem. 2006;281:35008–35020. doi: 10.1074/jbc.M601335200. [DOI] [PubMed] [Google Scholar]
- Liles WC, Ledbetter JA, Waltersdorph AW, Klebanoff SJ. Cross-linking of CD18 primes human neutrophils for activation of the respiratory burst in response to specific stimuli: implications for adhesion-dependent physiological responses in neutrophils. J Leukoc Biol. 1995;58:690–697. doi: 10.1002/jlb.58.6.690. [DOI] [PubMed] [Google Scholar]
- Schnitzler N, Haase G, Podbielski A, Lutticken R, Schweizer KG. A co-stimulatory signal through ICAM-beta2 integrin-binding potentiates neutrophil phagocytosis. Nat Med. 1999;5:231–235. doi: 10.1038/5597. [DOI] [PubMed] [Google Scholar]
- Shappell SB, Toman C, Anderson DC, Taylor AA, Entman ML, Smith CW. Mac-1 (CD11b/CD18) mediates adherence-dependent hydrogen peroxide production by human and canine neutrophils. J Immunol. 1990;144:2702–2711. [PubMed] [Google Scholar]
- Fuortes M, Jin WW, Nathan C. Adhesion-dependent protein tyrosine phosphorylation in neutrophils treated with tumor necrosis factor. J Cell Biol. 1993;120:777–784. doi: 10.1083/jcb.120.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettritz R, Choi M, Rolle S, Wellner M, Luft FC. Integrins and cytokines activate nuclear transcription factor-kappaB in human neutrophils. J Biol Chem. 2004;279:2657–2665. doi: 10.1074/jbc.M309778200. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Karin M. The NF-kappa B activation pathway: its regulation and role in inflammation and cell survival. Cancer J Sci Am. 1998;4(Suppl 1):S92–S99. [PubMed] [Google Scholar]
- Ward C, Chilvers ER, Lawson MF, Pryde JG, Fujihara S, Farrow SN, Haslett C, Rossi AG. NF-kappaB activation is a critical regulator of human granulocyte apoptosis in vitro. J Biol Chem. 1999;274:4309–4318. doi: 10.1074/jbc.274.7.4309. [DOI] [PubMed] [Google Scholar]
- Wulczyn FG, Krappmann D, Scheidereit C. The NF-kappa B/Rel and I kappa B gene families: mediators of immune response and inflammation. J Mol Med. 1996;74:749–769. doi: 10.1007/s001090050078. [DOI] [PubMed] [Google Scholar]
- Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304–315. doi: 10.1016/s0002-9343(01)00834-8. [DOI] [PubMed] [Google Scholar]
- Parkos CA, Colgan SP, Liang TW, Nusrat A, Bacarra AE, Carnes DK, Madara JL. CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J Cell Biol. 1996;132:437–450. doi: 10.1083/jcb.132.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, Flower RJ. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med. 1996;2:1259–1262. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- Kettritz R, Schreiber A, Luft FC, Haller H. Role of mitogen-activated protein kinases in activation of human neutrophils by antineutrophil cytoplasmic antibodies. J Am Soc Nephrol. 2001;12:37–46. doi: 10.1681/ASN.V12137. [DOI] [PubMed] [Google Scholar]
- Choi M, Rolle S, Wellner M, Cardoso MC, Scheidereit C, Luft FC, Kettritz R. Inhibition of NF-kappaB by a TAT-NEMO-binding domain peptide accelerates constitutive apoptosis and abrogates LPS-delayed neutrophil apoptosis. Blood. 2003;102:2259–2267. doi: 10.1182/blood-2002-09-2960. [DOI] [PubMed] [Google Scholar]
- Salanova B, Choi M, Rolle S, Wellner M, Scheidereit C, Luft FC, Kettritz R. The effect of fever-like temperatures on neutrophil signaling. FASEB J. 2005;19:816–818. doi: 10.1096/fj.04-2983fje. [DOI] [PubMed] [Google Scholar]
- Perretti M, Flower RJ. Annexin 1 and the biology of the neutrophil. J Leukoc Biol. 2004;76:25–29. doi: 10.1189/jlb.1103552. [DOI] [PubMed] [Google Scholar]
- Bryant RE, Hood AF, Hood CE, Koenig MG. Factors affecting mortality of gram-negative rod bacteremia. Arch Intern Med. 1971;127:120–128. [PubMed] [Google Scholar]
- Van Dissel JT, Numan SC, Van’t Wout JW. Chills in ‘early sepsis’: good for you? J Intern Med. 2005;257:469–472. doi: 10.1111/j.1365-2796.2005.01498.x. [DOI] [PubMed] [Google Scholar]
- Kreger BE, Craven DE, McCabe WR. Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am J Med. 1980;68:344–355. doi: 10.1016/0002-9343(80)90102-3. [DOI] [PubMed] [Google Scholar]
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3:487–497. doi: 10.1016/s1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- Huber AR, Kunkel SL, Todd RF, III, Weiss SJ. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- Schaerli P, Britschgi M, Keller M, Steiner UC, Steinmann LS, Moser B, Pichler WJ. Characterization of human T cells that regulate neutrophilic skin inflammation. J Immunol. 2004;173:2151–2158. doi: 10.4049/jimmunol.173.3.2151. [DOI] [PubMed] [Google Scholar]
- Pastore S, Fanales-Belasio E, Albanesi C, Chinni LM, Giannetti A, Girolomoni G. Granulocyte macrophage colony-stimulating factor is overproduced by keratinocytes in atopic dermatitis. Implications for sustained dendritic cell activation in the skin. J Clin Invest. 1997;99:3009–3017. doi: 10.1172/JCI119496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI. Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- Tekamp-Olson P, Gallegos C, Bauer D, McClain J, Sherry B, Fabre M, van Deventer S, Cerami A. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J Exp Med. 1990;172:911–919. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo P, Alberta J, Wen DZ, Graycar JL, Derynck R, Stiles CD. The platelet-derived growth factor-inducible KC gene encodes a secretory protein related to platelet alpha-granule proteins. J Biol Chem. 1989;264:4133–4137. [PubMed] [Google Scholar]
- Watanabe K, Konishi K, Fujioka M, Kinoshita S, Nakagawa H. The neutrophil chemoattractant produced by the rat kidney epithelioid cell line NRK-52E is a protein related to the KC/gro protein. J Biol Chem. 1989;264:19559–19563. [PubMed] [Google Scholar]
- Singh UP, Singh S, Boyaka PN, McGhee JR, Lillard JW., Jr Granulocyte chemotactic protein-2 mediates adaptive immunity in part through IL-8Rbeta interactions. J Leukoc Biol. 2004;76:1240–1247. doi: 10.1189/jlb.0903444. [DOI] [PubMed] [Google Scholar]
- Rot A. Chemotactic potency of recombinant human neutrophil attractant/activation protein-1 (interleukin-8) for polymorphonuclear leukocytes of different species. Cytokine. 1991;3:21–27. doi: 10.1016/1043-4666(91)90006-y. [DOI] [PubMed] [Google Scholar]
- Furuta R, Yamagishi J, Kotani H, Sakamoto F, Fukui T, Matsui Y, Sohmura Y, Yamada M, Yoshimura T, Larsen CG, Oppenheim JJ, Matsushima K. Production and characterization of recombinant human neutrophil chemotactic factor. J Biochem (Tokyo) 1989;106:436–441. doi: 10.1093/oxfordjournals.jbchem.a122870. [DOI] [PubMed] [Google Scholar]
- Fröhlich D, Wittmann S, Rothe G, Sessler DI, Vogel P, Taeger K. Mild hyperthermia down-regulates receptor-dependent neutrophil function. Anesth Analg. 2004;99:284–292. doi: 10.1213/01.ane.0000117142.28174.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhler GM, Knol GJ, Drayer AL, Vellenga E. Impaired interleukin-8- and GROalpha-induced phosphorylation of extracellular signal-regulated kinase result in decreased migration of neutrophils from patients with myelodysplasia. J Leukoc Biol. 2005;77:257–266. doi: 10.1189/jlb.0504306. [DOI] [PubMed] [Google Scholar]
- Coffer PJ, Geijsen N, M’Rabet L, Schweizer RC, Maikoe T, Raaijmakers JA, Lammers JW, Koenderman L. Comparison of the roles of mitogen-activated protein kinase kinase and phosphatidylinositol 3-kinase signal transduction in neutrophil effector function. Biochem J. 1998;329:121–130. doi: 10.1042/bj3290121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottonello L, Montecucco F, Bertolotto M, Arduino N, Mancini M, Corcione A, Pistoia V, Dallegri F. CCL3 (MIP-1alpha) induces in vitro migration of GM-CSF-primed human neutrophils via CCR5-dependent activation of ERK 1/2. Cell Signal. 2005;17:355–363. doi: 10.1016/j.cellsig.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Wang X, Tokuda H, Hirade K, Kozawa O. Stress-activated protein kinase/c-Jun N-terminal kinase (JNK) plays a part in endothelin-1-induced vascular endothelial growth factor synthesis in osteoblasts. J Cell Biochem. 2002;87:417–423. doi: 10.1002/jcb.10323. [DOI] [PubMed] [Google Scholar]
- Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Volloch V, Gabai VL, Rits S, Force T, Sherman MY. HSP72 can protect cells from heat-induced apoptosis by accelerating the inactivation of stress kinase JNK. Cell Stress Chaperones. 2000;5:139–147. doi: 10.1379/1466-1268(2000)005<0139:hcpcfh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee HJ, Kim GY, Huh JW, Kim SW, Na DS. Annexin I is a stress protein induced by heat, oxidative stress and a sulfhydryl-reactive agent. Eur J Biochem. 2000;267:3220–3225. doi: 10.1046/j.1432-1327.2000.01345.x. [DOI] [PubMed] [Google Scholar]
- Wong HR, Ryan M, Wispe JR. Stress response decreases NF-kappaB nuclear translocation and increases I-kappaBalpha expression in A549 cells. J Clin Invest. 1997;99:2423–2428. doi: 10.1172/JCI119425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettritz R, Choi M, Salanova B, Wellner M, Rolle S, Luft FC. Fever-like temperatures affect neutrophil NF-kappaB signaling, apoptosis, and ANCA-antigen expression. J Am Soc Nephrol. 2006;17:1345–1353. doi: 10.1681/ASN.2005090948. [DOI] [PubMed] [Google Scholar]
- Yoo CG, Lee S, Lee CT, Kim YW, Han SK, Shim YS. Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J Immunol. 2000;164:5416–5423. doi: 10.4049/jimmunol.164.10.5416. [DOI] [PubMed] [Google Scholar]
- Chen Y, Currie RW. Small interfering RNA knocks down heat shock factor-1 (HSF-1) and exacerbates pro-inflammatory activation of NF-kappaB and AP-1 in vascular smooth muscle cells. Cardiovasc Res. 2006;69:66–75. doi: 10.1016/j.cardiores.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Schell MT, Spitzer AL, Johnson JA, Lee D, Harris HW. Heat shock inhibits NF-kB activation in a dose- and time-dependent manner. J Surg Res. 2005;129:90–93. doi: 10.1016/j.jss.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Curry HA, Clemens RA, Shah S, Bradbury CM, Botero A, Goswami P, Gius D. Heat shock inhibits radiation-induced activation of NF-kappaB via inhibition of I-kappaB kinase. J Biol Chem. 1999;274:23061–23067. doi: 10.1074/jbc.274.33.23061. [DOI] [PubMed] [Google Scholar]
- Chan JY, Ou CC, Wang LL, Chan SH. Heat shock protein 70 confers cardiovascular protection during endotoxemia via inhibition of nuclear factor-kappaB activation and inducible nitric oxide synthase expression in the rostral ventrolateral medulla. Circulation. 2004;110:3560–3566. doi: 10.1161/01.CIR.0000143082.63063.33. [DOI] [PubMed] [Google Scholar]
- Ostberg JR, Taylor SL, Baumann H, Repasky EA. Regulatory effects of fever-range whole-body hyperthermia on the LPS-induced acute inflammatory response. J Leukoc Biol. 2000;68:815–820. [PubMed] [Google Scholar]
- Wirth D, Bureau F, Melotte D, Christians E, Gustin P. Evidence for a role of heat shock factor 1 in inhibition of NF-kappaB pathway during heat shock response-mediated lung protection. Am J Physiol. 2004;287:L953–L961. doi: 10.1152/ajplung.00184.2003. [DOI] [PubMed] [Google Scholar]