Abstract
We previously identified overexpression of galectin-1 in activated tumor endothelium. Currently, the tumor vasculature is a target for therapeutic approaches. Little is known about galectin expression and regulation in the tumor vasculature. Here, we report the expression of galectin-1/-3/-8/-9 in the endothelium as determined by quantitative PCR, Western blot, flow cytometry, and immunohistochemistry. Galectin-2/-4/-12 were detectable at the mRNA level, albeit very low. Galectin-8 and -9 displayed alternative splicing. Immunohistochemistry of normal tissues revealed a broad but low expression of galectin-1 in the vasculature, whereas the expression levels and localization of the other galectins varied. Endothelial cell activation in vitro significantly increased the expression of galectin-1 (5.32 ± 1.97; P = 0.04) and decreased the expression of both galectin-8 (0.59 ± 0.12; P < 0.04) and galectin-9 (0.32 ± 0.06; P < 0.002). Galectin-3 expression was unaltered. Although a portion of these proteins is expressed intracellularly, the membrane protein level of galectin-1/-8/-9 was significantly increased on cell activation in vitro, 6-fold (P = 0.005), 3-fold (P = 0.002), and 1.4-fold (P = 0.04), respectively. Altered expression levels and cellular localization was also observed in vivo in the endothelium of human tumor tissue compared with normal tissue. These data show that endothelial cells express several members of the galectin family and that their expression and distribution changes on cell activation, resulting in a different profile in the tumor vasculature. This offers opportunities to develop therapeutic strategies that are independent of tumor type.
Galectins are a family of proteins that share a binding affinity for β-galactoside-containing carbohydrates. Several members of this family are emerging as targets for cancer therapy. Apart from a direct role in cell transformation, their main contribution to tumor progression involves modification of the antitumor immune response and enhancement of the metastatic potential of tumor cells.1 There are several reports showing altered galectin expression profiles in tumor cells of different origin,2,3,4 and compounds that interfere with galectin function in tumor cells are therefore considered for cancer therapy.
An attractive site for therapeutic applications is the endothelium, ie, the monolayer of endothelial cells lining blood vessels. Endothelial cells in the tumor vasculature are easily accessible and less prone to become drug resistant, and disrupting the tumor endothelium results in massive death of tumor cells.5 Moreover, tumor endothelial cells express molecules that allow specific targeting independent of the tumor type eg, integrin αVβ3, CD44v3, and CD105.6,7,8,9 Recently, we identified galectin-1 as a target molecule in the tumor endothelium. Activated tumor endothelial cells increase the expression of galectin-1, and ablation of its expression in vitro and in vivo results in impaired endothelial cell function and hampered tumor angiogenesis.10 Whether other galectins are also directly involved in endothelial cell biology and tumor angiogenesis is less well studied (for review, see Ref. 11). In the current study, we analyzed the expression of all known human galectins in the endothelium to get more insight in their possible function in endothelial cell biology. We show that multiple galectins and galectin isoforms are expressed by endothelial cells. Furthermore, the expression and distribution of endothelial galectins is altered on cell activation. These observations provide prospects for the development of therapies that target galectins in the tumor endothelium.
Materials and Methods
Cell Cultures
Primary human umbilical vein endothelial cells (HUVECs) were isolated from umbilical cords by infusion of trypsin into the vein and incubation for 20 minutes at 37°C. Cells were collected in HUVEC culture medium [RPMI (Invitrogen, Breda, the Netherlands) containing 10% fetal calf serum (Invitrogen) and 10% human serum and supplemented with l-glutamin (Invitrogen) and penicillin/streptomycin (Invitrogen)] by perfusion with PBS and divided over two T25 cultured flasks coated with 0.2% gelatin/PBS. After incubation for 1 hour at 37°C/5% CO2, unattached cells and erythrocytes were removed by washing three times with PBS. Next, the cells in one flask were directly collected and lysed for RNA isolation, Western blot, or fluorescence-activated cell sorting (FACS) analysis, and the other cells were passaged every 3 to 5 days and kept in HUVEC culture medium at 37°C and 5% CO2 up to three passages. For additional in vitro cell activation, HUVECs were cultured for 3 days in HUVEC culture medium supplemented with 10% culture medium of both colon carcinoma cell lines CaCo2 and LS174. The origin and culture conditions of all endothelial cell lines have been described previously.12
Human Tissues
All tissues, frozen or paraffin embedded, were obtained from the Maastricht Pathology Tissue Collection (Maastricht, the Netherlands). Collection, storage, and use of tissue and patient data were performed in agreement with the Code for Proper Secondary Use of Human Tissue in the Netherlands.
RNA Isolation and cDNA Synthesis
Total RNA was isolated using the RNeasy kit (QIAgen, Venlo, the Netherlands) according to the suppliers protocol from freshly isolated or cultured cells or frozen tissue sections (10- × 20-μm sections). All isolations were subjected to on-column DNase treatment (QIAgen) to remove any genomic DNA contaminations. The concentration and purity of the RNA was analyzed using the NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE). cDNA synthesis was performed with the iScript cDNA synthesis kit (Bio-Rad, Veenendaal, the Netherlands) on 100 ng of RNA according to the suppliers protocol. After cDNA synthesis, nuclease-free water was added up to a final volume of 50 μl.
Real-Time PCR
Real-time PCR was performed on the MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad) using a standard two-step amplification protocol (Ta at 60°C) followed by a melting curve analysis. For each reaction, 1.5 μl of cDNA was used in a total volume of 25 μl containing 1× iQ SYBR Green supermix (Bio-Rad) and 400 nmol/L of the appropriate forward and reverse primer. Each PCR was performed in duplicate on separate plates. Primers were designed to target specifically to human galectins as described previously.13 All primers were synthesized by Eurogentec (Liège, Belgium).
Western Blotting
For Western blotting, cultured cells were directly lysed in the culture flask using 2× sample buffer (100 mmol/L Tris, pH 6.8, 200 mmol/L dithiothreitol, 4% SDS, 0.2% bromophenol blue, and 20% glycerol). Samples were boiled for 5 minutes and quenched on ice. Subsequently, proteins were separated on a 15% polyacrylamide gel by electrophoresis and transferred onto nitrocellulose membranes (Schleicher & Schuell, Den Bosch, the Netherlands) according to standard procedures. Equal protein loading was confirmed by Ponceau red staining. Membranes were blocked with 5% nonfat dry milk (Bio-Rad) in 0.1% Tween 20/PBS and incubated overnight at 4°C with rabbit anti-galectin-1 antibody (dilution 1:500; kind gift of Dr. L.G. Baum, University of California, Los Angeles), goat anti-galectin-3 antibody (0.2 ng/ml; R&D Systems, Abingdon, UK), goat anti-galectin-8 antibody (0.2 ng/ml; R&D Systems), or goat anti-galectin-9 antibody (0.2 ng/ml; R&D Systems). After three washes with PBS for 5 minutes, the membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. Finally, membranes were washed three times for 5 minutes with PBS, and staining was visualized using 3′,3′-diaminobenzidine (0.5 mg/ml in 0.05 mol/L Tris-HCl, pH 7.6, supplemented with 0.03% H2O2).
FACS Analysis
For FACS analysis, freshly isolated, cultured, and tumor-conditioned HUVECs were harvested and fixed with 1% paraformaldehyde for 20 minutes at room temperature. Cells were washed in 0.1% BSA, 0.01% sodium azide, and PBS, incubated on ice with the appropriate anti-galectin antibody [rabbit anti-galectin-1, 1:1000 (gift from Dr. L.G. Baum); goat anti-galectin-3, 1:30 (R&D Systems); goat anti-galectin-8, 1:30 (R&D Systems); goat anti-galectin-9, 1:30 (R&D Systems)], and diluted in 0.1% BSA, 0.01% sodium azide, and PBS in the presence or absence of 0.05% Triton-100. Subsequently, cells were washed with PBS and incubated with an appropriate PE-labeled secondary antibody, washed with PBS, and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Breda, the Netherlands). All experiments were performed on five different HUVEC isolations.
Cytospin
For cytospins, cultured HUVECs were harvested and stained similar to FACS analysis. After the staining procedure, the cells were centrifuged for 5 minutes at 700 rpm onto standard glass microscope slides. Finally, the cells were mounted in Mowiol (Hoechst, Frankfurt, Germany) supplemented with 4′,6-diamidino-2-phenylindole (Invitrogen) for nuclear staining.
Immunohistochemistry
Immunohistochemistry was performed on paraffin-embedded tissue sections. Staining for CD31/34 and galectin-1 were performed as described previously.10 For galectin-3, -8, and -9 staining, sections were dewaxed and incubated in 0.3% H2O2/methanol. Next, the sections were microwave pretreated in citric acid and blocked with 1% BSA/PBS. Primary antibodies [goat anti-galectin-3, 1:30 (R&D Systems); goat anti-galectin-8, 1:30 (R&D Systems); and goat anti-galectin-9, 1:30 (R&D Systems)] were applied in 0.5% BSA/PBS. Finally, biotin-labeled secondary antibody was applied, and staining was performed using the StreptABComplex/horseradish peroxidase kit (Dako, Heverlee, Belgium). The sections were counterstained with hematoxylin (Merck, Haarlem, the Netherlands), dehydrated, and mounted in Entellan (Merck).
Statistical Analysis
Data of quantitative PCR (qPCR) are shown as mean values ± SD. FACS data are presented as mean values ± SEM. The Mann-Whitney rank sum test was used to calculate statistically significant differences in mRNA expression, protein expression, or protein localization on cell activation. P values <0.05 were considered statistically significant, and all calculations were performed in SPSS 12.0.1. (SPSS Inc., Gorinchem, the Netherlands).
Results
Quiescent Endothelial Cells Express Different Galectins and Multiple Galectin Isoforms
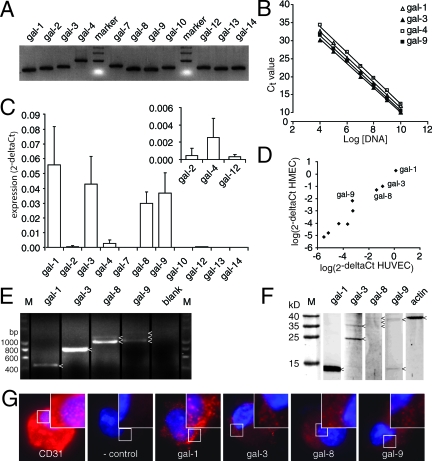
In this study, we examined galectin expression in endothelial cells. Up to now, 15 galectins have been described in literature, 11 of which are also expressed in humans (Table 1). Because little is known about their expression in endothelial cells, qPCR primers and full-length cDNA primers were designed for all 11 human galectin family members (Table 2). To validate the qPCR analysis, the primers were tested on available cDNA clones or on cDNA derived from tissues/cell lines that have been reported to express a specific galectin. The different amplicons were analyzed by gel electrophoresis and cloned into pCR2.1. To confirm primer specificity, the clones were sequenced, and PCR was performed with the appropriate primers (Figure 1A). Next, the cloned PCR fragments were used to determine primer sensitivity. For this, dilution series were generated with the cloned PCR fragments that covered a concentration range of at least six logscales (Figure 1B). All qPCR primers displayed an optimal amplification slope of approximately −3.3 and a broad cycle threshold range in which there was linear amplification (Table 3).
Table 1.
List of All Known Human Galectins
| Name | ENSEMBLE accession number | Type* |
|---|---|---|
| LGALS1 | ENSG00000100097 | Prototype |
| LGALS2 | ENSG00000100079 | Prototype |
| LGALS3 | ENSG00000131981 | Chimera |
| LGALS4 | ENSG00000171747 | Tandem repeat |
| LGALS7 | ENSG00000178934 | Prototype |
| LGALS8† | ENSG00000116977 | Tandem repeat |
| LGALS9† | ENSG00000168961 | Tandem repeat |
| LGALS10 | ENSG00000105205 | Prototype |
| LGALS12 | ENSG00000133317 | Tandem repeat |
| LGALS13 | ENSG00000105198 | Prototype |
| LGALS14† | ENSG00000006659 | Prototype |
Prototype galectins consist of a single carbohydrate recognition domain. Tandem repeat galectins are composed of two carbohydrate recognition domains connected by a linker peptide. Chimera galectins contain a proline/glycine rich tail at the N-terminus of a single carbohydrate recognition domain.
Splicing has been shown to occur in sequence encoding the linker peptide between the two carbohydrate recognition domains.
Table 2.
Galectin Primers Used for Full-Length PCR or qPCR
| Full-length primers | qPCR primers | ||
|---|---|---|---|
| gal-1 F* | 5′-ATGGCTTGTGGTCTGGTC-3′ | gal-1 F | 5′-TGCAACAGCAAGGACGGC-3′ |
| gal-1 R | 5′-TCAGTCAAAGGCCACACA-3′ | gal-1 R | 5′-CACCTCTGCAACACTTCCA-3′ |
| gal-2 F | 5′-ATGACGGGGGAACTTGAG-3′ | gal-2 F | 5′-GATGGCACTGATGGCTTTG-3′ |
| gal-2 R | 5′-TTATTCTTTTAACTTGAAAGAGGA-3′ | gal-2 R | 5′-AGACAATGGTGGATTCGCT-3′ |
| gal-3 F | 5′-ATGGCAGACAATTTTTCG-3′ | gal-3 F | 5′-CAGAATTGCTTTAGATTTCCAA-3′ |
| gal-3 R | 5′-TTATATCATGGTATATGAAGCAC-3′ | gal-3 R | 5′-TTATCCAGCTTTGTATTGCAA |
| gal-4 F | 5′-ATGGCCTATGTCCCCGCA-3′ | gal-4 F | 5′-CGAGGAGAAGAAGATCACCC-3′ |
| gal-4 R | 5′-TTAGATCTGGACATAGGACAAGG-3′ | gal-4 R | 5′-CTCTGGAAGGCCGAGAGG-3′ |
| gal-7 F | 5′-ATGTCCAACGTCCCCCAC-3′ | gal-7 F | 5′-CAGCAAGGAGCAAGGCTC-3′ |
| gal-7 R | 5′-TCAGAAGATCCTCACGGA-3′ | gal-7 R | 5′-AAGTGGTGGTACTGGGCG-3′ |
| gal-8 F | 5′-AGAATGATGTTGTCCTTAAAC-3′ | gal-8 F | 5′-CTTAGGCTGCCATTCGCT-3′ |
| gal-8 R | 5′-CTACCAGCTCCTTACTTCC-3′ | gal-8 R | 5′-AAGCTTTTGGCATTTGCA-3′ |
| gal-9 F | 5′-ATGGCCTTCAGCGGTTCC-3′ | gal-9 F | 5′-CTTTCATCACCACCATTCTG-3′ |
| gal-9 R | 5′-CTATGTCTGCACATGGGTCAG-3′ | gal-9 R | 5′-ATGTGGAACCTCTGAGCACTG-3′ |
| gal-10 F | 5′-ATGTCCCTGCTACCCGTG-3′ | gal-10 F | 5′-AGTGTGCTTTGGTCGTCGT-3′ |
| gal-10 R | 5′-TTATCTCTTTAAATAGCTGACAT-3′ | gal-10 R | 5′-ATGCTCAGTTCAAATTCTTGG-3′ |
| gal-12 F | 5′-ATGAGTCAGCCCAGTGGG-3′ | gal-12 F | 5′-TGTGAGCCTGAGGGACCA-3′ |
| gal-12 R | 5′-TCAGGAGTGGACACAGTAGAG-3′ | gal-12 R | 5′-GCTGAGATCAGTTTCTTCTGC-3′ |
| gal-13 F | 5′-ATGTCTTCTTTACCCGTG-3′ | gal-13 F | 5′-CTTTACCCGTGCCATACAA-3′ |
| gal-13 R | 5′-TCAATTGCAGACACACACT-3′ | gal-13 R | 5′-GTGGGTCATTGATAAAAGAGTG-3′ |
| gal-14 F | 5′-ATGTCCCTGACCCACAG-3′ | gal-14 F | 5′-CCTTGATGATTGTGGTACCAT-3′ |
| gal-14 R | 5′-TCAATCGCTGATAAGCACT-3′ | gal-14 R | 5′-GTGGGTCCTTGACAAAAGTG-3′ |
F, forward primer; R, reverse primer.
Figure 1.
Galectin expression profile in endothelial cells. A: Agarose gel electrophoresis showing the length of the different galectin PCR amplicons. All primers generated a single band of the expected size. B: Analysis of primer sensitivity. The graph shows linear amplification with a slope of ∼−3.3 over a broad dilution range. Only galectin-1, -3, -4, and -9 are shown, but similar results were obtained for all galectins (see also Table 3). C: The galectin expression profile in freshly isolated HUVECs. Apart from the predominantly expressed galectin-1, -3, -8, and -9, expression was also detected for galectin-2, -4, and -12 (see inset). D: Scatterplot showing a strong correlation (r = 0.967, P < 0.001) between galectin expression in HUVECs (x axis) and human microvascular endothelial cells (y axis). Galectin-1, -3, -8, and -9 were expressed most abundantly. E: Agarose gel electrophoresis following PCR with full-length primers on HUVEC cDNA. Different amplicons are indicated with an arrowhead. As blank no primers were added to the PCR mix. F: Western blot analysis of galectin protein expression in HUVECs. Protein bands are indicated with an arrowhead. Actin served as control. G: Fluorescent microscopic pictures of galectin staining (red) in HUVECs after cytospin. As positive control, a CD31 antibody was used. In the negative control, the primary antibody was omitted. Cells were counterstained with 4′,6-diamidino-2-phenylindole to visualize the nucleus (blue). Original magnification, ×1000.
Table 3.
Primer Sensitivity
| Target gene | Slope (SEM) | Lower limit (SEM) | Upper limit (SEM) |
|---|---|---|---|
| LGALS1 | 3.36 (0.08) | 14.67 (3.18) | 31.00 (1.15) |
| LGALS2 | 3.27 (0.03) | 16.67 (2.33) | 31.67 (1.67) |
| LGALS3 | 3.10 (0.16) | 10.33 (0.33) | 30.00 (0.58) |
| LGALS4 | 3.35 (0.11) | 12.67 (0.33) | 32.00 (1.53) |
| LGALS7 | 3.28 (0.15) | 13.00 (0.58) | 33.00 (2.31) |
| LGALS8 | 3.25 (0.07) | 14.33 (2.85) | 25.67 (1.86) |
| LGALS9 | 3.27 (0.15) | 13.33 (2.40) | 33.33 (1.33) |
| LGALS10 | 3.20 (0.12) | 10.33 (0.33) | 29.00 (2.31) |
| LGALS12 | 3.47 (0.10) | 13.00 (1.00) | 33.67 (0.88) |
| LGALS13 | 3.10 (0.15) | 13.67 (1.20) | 33.33 (0.88) |
| LGALS14 | 3.04 (0.04) | 13.33 (1.76) | 29.67 (0.33) |
To determine the galectin mRNA expression levels in endothelial cells, we next used the primers to perform qPCR on cDNA generated from quiescent HUVECs immediately after isolation from the umbilical vein. Results show that these endothelial cells mainly express galectin-1, -3, -8, and -9 (Figure 1C). In addition, faint expression of galectin-2, -4, and -12 could be detected (Figure 1C, inset). Galectin-7, -10, -13, and -14 all had cycle threshold values that were above the upper limit of linear amplification, and these galectins were therefore considered to be not expressed in quiescent endothelial cells. To compare the galectin expression profile in endothelial cells of different origin, we also performed qPCR on cDNA derived from a human microvascular endothelial cell line. There was a strong correlation (r = 0.967, P < 0.001) between galectin expression in cultured HUVECs and human microvascular endothelial cells (Figure 1D). Similar correlations were observed when expression in HUVECs was compared with expression in endothelial cell lines RF24 (r = 0.818, P = 0.004) and EVLc2 (r = 0.879, P = 0.001). These data indicate that cultured endothelial cells of different origin express a similar repertoire of galectins. For further analysis, we focused galectin-1, -3, -8, and -9, given the low expression of the other galectins in all endothelial cells tested. We used primers spanning the entire coding sequence to identify possible splice variants. For galectin-1 and -3, a single transcript was detected of the expected size, 450 and 750 bp, respectively. In contrast, multiple bands of different lengths were observed for galectin-8 (950 and 1150 bp) and galectin-9 (1050, 1200, and 1350 bp) (Figure 1E). Current research is focusing on the functional relevance of the different splice variants.
To confirm expression at the protein level, several approaches were followed. First, Western blotting was performed on total cell lysates. In line with the PCR data, protein expression could be detected for galectin-1, -3, -8, and -9 (Figure 1F). Galectin-1 showed an intense band at the expected molecular weight of 14 kDa. Galectin-3 displayed one prominent band at 25 kDa and another faint band at 35 kDa. Galectin-8 protein was hardly detectable, but three faint bands were visible at 34, 36, and 38 kDa. For galectin-9, one band was visible at 37 kDa together with a small band of 14 kDa. Cross-reactivity of the galectin-9 antibody with galectin-1 was excluded in a Western blot using recombinant human galectin-1 protein, which only displayed a 14-kDa band when the galectin-1 antibody was used (data not shown). Second, protein expression was detected using FACS. Again, galectin-1, -3, -8, and -9 were readily detectable (data shown below). Third, to get more insight in galectin protein localization, immunohistochemical staining was performed on cultured endothelial cells. Whereas the endothelial cell-specific marker CD31, which was used as positive control, showed strong staining, all four galectins showed a clear, dot-like staining pattern, further confirming their endothelial expression (Figure 1G). All of these results show that quiescent endothelial cells in vitro express a broad panel of galectins with galectin-1, -3, -8, and -9 as the most prominent ones.
Endothelial Galectin Expression Patterns in Vivo Differ between Tissue Types
To study the endothelial expression in vivo, immunohistochemical staining was performed on different human tissues. Vessels in these tissues were identified using an endothelial cell staining with anti-CD31/34 antibodies (Figure 2, A–D). Galectin-1 staining was observed in the endothelial cells of all tissues (Figure 2, E–H). In all tissues, faint staining could be detected throughout the cell (Figure 2, E–H). The staining of galectin-3 was less constant (Figure 2, I–L). Although no galectin-3 was detectable in the endothelial cells of liver (Figure 2J), faint staining could occasionally be observed in vessels of kidney and placenta both in the cytoplasm and in the nucleus (Figure 2, I and K). In colon, all endothelial cells displayed weak galectin-3 staining throughout the cell (Figure 2L). Endothelial galectin-8 staining was only rarely observed. Apart from colon (Figure 2P) and a sporadic faint staining in kidney (Figure 2O), galectin-8 was undetectable in the vessels of most tissues (Figure 2, M and N). Vascular staining was also undetectable for galectin-9 in kidney (Figure 2S). In placenta endothelium, sporadic nuclear staining could be observed (Figure 2Q). The same was true for liver (Figure 2R), whereas nuclear galectin-9 staining in endothelial cells was common in colon (Figure 2T). All of these data suggest that besides a common expression of galectin-1 in the endothelial cells of normal tissues, the expression of endothelial galectin-3, -8, and -9 protein is more variable and depends on the environment/tissue surrounding the endothelial cell.
Figure 2.
Galectin expression in the endothelium of different human tissues. Immunohistochemical staining of galectin expression in different human tissues, ie, placenta (A, E, I, M, and Q), liver (B, F, J, N, and R), kidney (C, G, K, O, and S), and colon (D, H, L, P, and T). As positive vessel staining, CD31/34 was used (A–D, brown staining). Staining was performed for galectin-1 (E–H), galectin-3 (I–L), galectin-8 (M–P), and galectin-9 (Q–T). Each inset shows the endothelial cells in more detail with asterisks marking the luminal site of the vessel. The lower inset in A–D shows the negative control in which the primary antibody was omitted.
Endothelial Cell Activation in Vitro Affects Galectin Expression and Cellular Localization
Because culturing endothelial cells under high serum conditions has been reported to increase galectin-1 expression,10 we next performed qPCR to compare the galectin mRNA expression between freshly isolated endothelial cells and cells cultured in the presence of 20% human serum. In agreement with our previous observations, cells cultured in 20% human serum significantly induce galectin-1 mRNA expression compared with quiescent cells (5.32 ± 1.97-fold; P = 0.04) (Figure 3A). In fact, galectin-1 was the only galectin of which the expression increased. Of the other abundantly expressed galectins, galectin-3 mRNA expression did not significantly change (1.91 ± 0.80; P = ns) whereas galectin-8 and -9 expression significantly decreased, respectively, 0.59 ± 0.12-fold (P < 0.04) and 0.32 ± 0.06-fold (P < 0.002). The expression of galectin-4, which was already low, further decreased (0.36 ± 0.20-fold, P = 0.04), and all of the other galectins were undetectable (data not shown). Additional activation by culturing the endothelial cells in the presence of basic fibroblast growth factor (data not shown) or tumor-conditioned medium (Figure 3B) did not further increase the expression of galectin-1 or affect the expression of galectin-3. The expression of galectin-8 and -9 was also not further decreased, suggesting that the changes in expression are an early event in endothelial cell activation.
Figure 3.
Galectin expression and localization after endothelial cell activation. A: Fold change in mRNA expression, measured by qPCR, of galectins in cultured HUVECs (black bars) compared with native HUVECs (white bars, set to 1). B: Effect of additional activation of cultured cells (black bars, set to 1) with tumor conditioned culture medium (gray bars) on mRNA expression, measured by qPCR. C: Analysis of total galectin protein levels (white bars) and extracellular, membrane-bound galectin protein (black bars) in native HUVEC measured by FACS. D: Effect of cell activation on total galectin protein expression levels. E: Effect of cell activation on extracellular, membrane-bound galectin protein levels. *P < 0.05 versus native cells. F: Relative increase in extracellular versus total galectin protein levels in response to cell activation. *P < 0.05 versus native cells.
To test whether the alterations in mRNA expression were also reflected at the protein level, quiescent, cultured, and tumor-activated endothelial cells were subjected to flow cytometry. To distinguish between total and membrane-bound galectin proteins, the paraformaldehyde-fixed cells were incubated with the primary antibody in the presence (total protein) or absence (membrane-bound protein) of the permeabilization agent Triton. Using this approach, it could be observed that approximately 30% of galectin-1 and -9 protein was located extracellularly (Figure 3C). For galectin-8, almost one-half of the total protein content was located at the outer surface of the cell, whereas galectin-3 appeared to be exclusively present at the cell membrane.
When the effects of cell activation on total galectin protein content were measured, a similar trend was observed as for mRNA expression, albeit not significant (Figure 3D). Only galectin-8 protein levels appeared to be unaltered in contrast to the significant decrease in mRNA expression. Interestingly, the amount of membrane-bound protein showed a more prominent increase, except for galectin-3 (Figure 3E). After culturing the cells in 20% serum, the fluorescence intensity of extracellular galectin-1 significantly increased almost sixfold (P = 0.005), galectin-8 increased threefold (P = 0.002), and even galectin-9 signals significantly increased (1.4-fold, P = 0.04) despite the trend toward decreased total protein levels. Again, additional activation with basic fibroblast growth factor or tumor-conditioned medium did not affect the expression, although the increase in galectin-8 was partially reversed. These observations suggested that on endothelial cell activation, galectin-1, -8, and -9 were translocated to the extracellular compartment. Calculation of the ratio of between extracellular and total galectin protein levels showed significantly increased membrane localization for galectin-1 and -9 (Figure 3F).
Tumor Endothelial Cells in Vivo Alter Their Galectin Expression Levels and Localization
The results above indicate that the endothelial expression and localization of galectins in vitro is altered on cell activation. We have previously shown that galectin-1 expression is increased in the activated endothelial cells of different human tumors, including colon and breast carcinoma as well as Ewing sarcoma.10 Thus, we next studied the expression of galectins in the endothelium of colon tumor tissue using immunohistochemical staining. Similar as described above, CD31/34 staining was used to identify the endothelial cells (Figure 4A). Again, a clear increase in galectin-1 staining intensity could be observed in the tumor endothelial cells (Figure 4B) compared with normal tissue (Figure 2H). Galectin-3 staining remained lightly positive and could be observed throughout the cell (Figure 4C). For galectin-8 and -9, more subtle changes could be observed. In normal tissue, the expression of both galectins was mainly detectable in the nuclei of endothelial cells. In tumor tissue, the number of positive cells appeared to decrease. Whereas the staining of galectin-9 in the positive cells was also detected more regularly in the cytoplasm, galectin-8 expression was only detectable in the nuclei (Figure 4, D and E). These data show that, similar to in vitro, the in vivo galectin expression pattern in endothelial cells of a specific tumor tissue differs from the endothelial expression pattern in normal tissue.
Figure 4.
Galectin expression in activated tumor endothelial cells. Immunohistochemical staining of galectin expression in human colon carcinoma tissue. Staining was performed for CD31/34 (A), galectin-1 (B), galectin-3 (C), galectin-8 (D), and galectin-9 (E). As negative control, the primary antibody was omitted (F). Bar = 50 um. Each inset shows a vessel in more detail with asterisks marking the luminal site of the vessel. The black arrows point toward positive stained endothelial cells. In F, the arrows point toward nuclei of unstained endothelial cells. Bar = 10 μm.
Discussion
This is the first study in which an extensive survey has been performed to gain insight in the endothelial expression regulation of all known human galectins. We and others have already shown that galectin-1 is expressed by endothelial cells and that cell activation induces the expression level in vitro and in vivo.10,14,15 Here, we confirm these findings and in addition show that in vitro, the increased expression of galectin-1 is accompanied by a translocation of the protein to the outer cell membrane. This is in agreement with a previous finding by Baum et al,14 who observed an increase in membrane-bound galectin-1 after activation of endothelial cells with MM-LDL in vitro. Given the role of galectin-1 in cell-matrix adhesion, it is likely that the extracellular galectin-1 is required for the attachment and migration of activated endothelial cells over the extracellular matrix.16 This also explains the inhibitory effects of galectin-1 inhibitors on migration and tube formation.10,15
Endothelial expression of galectin-3 has also been reported previously.15,17 Western blot revealed two galectin-3 bands in the lysate of endothelial cells. There are several reports in the literature showing multiple bands on galectin-3 staining. This is related to altered phosphorylation of the protein and/or proteolytic modifications.18,19,20 In fact, it has been shown that an approximately 27-kDa proteolytic fragment of galectin-3 binds with higher affinity to endothelial cells compared with the full-length protein.20 This is in agreement with our observation of a more intense band at 25 kDa.
Although we were able to measure expression in cultured HUVEC in vitro, we did not always observe galectin-3 expression in normal tissues in vivo. Although this might be due to low basal expression levels that are undetectable by immunohistochemistry, it is more likely that the endothelial galectin-3 expression depends on the specific microenvironment. This is supported by a study of Lotan et al21 who observed different galectin-3 expression levels in endothelial cells isolated from different tissues. In fact, they reported that hepatic sinusoidal endothelial cells were negative for galectin-3 expression, confirming our finding in human normal liver tissue.
In contrast to galectin-1, the response of endothelial cell activation on galectin-3 expression is also not as clear.11 It has been reported that endothelial cells do increase galectin-3 expression on interaction with tumor cells that present specific carbohydrates on their surface.22,23 This could actually increase interaction between tumor cells and the endothelium thereby facilitating the metastatic process. We did not see a significant change in galectin-3 expression or localization on activation in vitro. Possibly, direct heterotypic contact is required to increase and relocate the galectin-3 protein as also shown for neutrophil-endothelium interactions.24
As already suggested from the literature, we observed endothelial expression of galectin-9, although the mRNA levels were lower compared with galectin-1 and -3. However, in vivo protein expression was not always detectable. Similar to galectin-3, this could be related to specific microenvironmental conditions. Given the relative low expression of galectin-9 observed in vitro, it might also be possible that protein expression is undetectable in IHC. This also explains the detection of only a single isoform of galectin-9 in the Western blot, whereas full-length PCR identified three splice-variants of the galectin-9 mRNA. The latter observation is in agreement with the results of Spitzenberger et al.25 They also observed three constitutively expressed galectin-9 isoforms in HUVEC in which either exon 5 or exon 6 was alternatively spliced.25
Apart from the three known galectins described above, we also detected mRNA expression of galectin-2, -4, -8, and -12. The expression of the other known human galectins was outside the linear detection range of the qPCR primers, and they were therefore considered not to be expressed by endothelial cells. Furthermore, endothelial cells from different origin displayed comparable galectin expression patterns compared with HUVECs, confirming previous observations.12 Because the expression of galectin-2, -4, and -12 was too low to allow further analysis at the protein level, we did not further address the expression regulation and localization of these galectins. However, for future studies on the function of different galectins in endothelial cell biology, they should certainly be included.
Of all of the newly identified galectins, galectin-8 expression was detectable at the mRNA level and the protein level. This is the first study that reports on galectin-8 expression in endothelial cells. Galectin-8 is a tandem repeat galectin that can modulate cellular adhesion and intracellular signaling through interactions with integrins.16,26 It has been described that multiple isoforms of this galectin exist as a result of complex alternative mRNA splicing.4,27 This leads to deletions within the linker peptide that influence the biological function of the protein28,29 and might affect susceptibility to proteolysis.30 Because our main objective was to evaluate overall expression of galectins by endothelial cells, we did not focus on all different galectin-8 isoforms. Thus, the qPCR primers were designed to identify all known transcripts, whereas the full-length primers were targeted against the main coding region. Nevertheless, the latter could potentially identify alternative splicing in the linker region. The identification of more than one species after PCR and Western blot suggests that alternative splicing does occur in endothelial cells as well. Future analysis with splice-specific primers could provide more information regarding the exact composition and expression levels of these splice variants. Similar to galectin-9, the in vivo expression of galectin-8 was not always detectable. However, activation of endothelial cells in vitro as well as in vivo resulted in an altered galectin-8 expression pattern. This suggests that this galectin is also involved in endothelial cell activation, and future studies have to address the exact function of all different galectin-8 isoforms in endothelial cell biology and angiogenesis.
Apart from the observed alterations in galectin expression levels, it was also interesting to see that the cellular localization of several galectins was altered during endothelial cell activation. Apart from galectin-3, which already appeared to be mainly bound to the membrane, all other galectins were translocated to the outer surface of the cell on activation. Even extracellular galectin-9 protein levels increased, despite the overall decrease in galectin-9 expression. This could also explain the decreased frequency of nuclear staining and more regularly observed cytoplasmic staining in tumor endothelial cells compared with normal endothelial cells, which we observed in colon tissue. As a result of this redistribution over the entire cell, galectin-9 and galectin-8 might become less detectable. Although still speculative, it appears that activated endothelial cells require extracellular galectins for proper function. This has implications for therapeutic strategies, because exposure of galectins at the luminal site of tumor vessels makes them more easily accessible for drugs. It has already been shown that blocking endothelial galectins can inhibit tumor angiogenesis10,15,31 and tumor metastasis21,23,32,33,34 both in vitro and in vivo. The observation that several galectins are presented extracellularly by activated endothelial cells could facilitate the development of novel therapeutic or diagnostic applications.
In summary, galectins are promising targets for cancer therapy. Because the tumor endothelium provides an excellent site for tumor type-independent treatment strategies, we have evaluated the expression of the entire galectin family in human endothelial cells. Our results show that multiple galectins are expressed by endothelial cells. Furthermore, we provide evidence that the level and localization of galectin expression is affected by the activation status of the endothelial cell, both in vitro and in vivo. These observations suggest an important function for galectins in tumor angiogenesis. Together with their possible involvement in tumor immune escape and tumor metastasis,11 endothelial galectins might provide targets for therapy that is independent of tumor type.
Acknowledgments
We thank Dr. L. Baum for providing the rabbit polyclonal anti-galectin-1 antibody.
Footnotes
Address reprint requests to Arjan W. Griffioen, Ph.D., Department of Pathology, Angiogenesis Laboratory Maastricht, School for Oncology and Developmental Biology—GROW, Maastricht University and University Hospital Maastricht, P.O. Box 5800, 6202 AZ Maastricht, the Netherlands. E-mail: aw.griffioen@path.unimaas.nl.
References
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- van den Brûle F, Califice S, Castronovo V. Expression of galectins in cancer: a critical review. Glycoconj J. 2004;19:537–542. doi: 10.1023/B:GLYC.0000014083.48508.6a. [DOI] [PubMed] [Google Scholar]
- Huflejt ME, Leffler H. Galectin-4 in normal tissues and cancer. Glycoconj J. 2004;20:247–255. doi: 10.1023/B:GLYC.0000025819.54723.a0. [DOI] [PubMed] [Google Scholar]
- Bidon-Wagner N, Le Pennec JP. Human galectin-8 isoforms and cancer. Glycoconj J. 2004;19:557–563. doi: 10.1023/B:GLYC.0000014086.38343.98. [DOI] [PubMed] [Google Scholar]
- Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev. 2000;52:237–268. [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Thorpe PE, Burrows FJ. Antibody-directed targeting of the vasculature of solid tumors. Breast Cancer Res Treat. 1995;36:237–251. doi: 10.1007/BF00666044. [DOI] [PubMed] [Google Scholar]
- Griffioen AW, Coenen MJ, Damen CA, Hellwig SM, van Weering DH, Vooys W, Blijham GH, Groenewegen G. CD44 is involved in tumor angiogenesis: an activation antigen on human endothelial cells. Blood. 1997;90:1150–1159. [PubMed] [Google Scholar]
- van Beijnum JR, Dings RP, van der Linden E, Zwaans BM, Ramaekers FC, Mayo KH, Griffioen AW. Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood. 2006;108:2339–2348. doi: 10.1182/blood-2006-02-004291. [DOI] [PubMed] [Google Scholar]
- Thijssen VL, Postel R, Brandwijk RJ, Dings RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J, Mayo KH, Poirier F, Griffioen AW. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci USA. 2006;103:15975–15980. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen VL, Poirier F, Baum LG, Griffioen AW. Galectins in the tumor endothelium: opportunities for combined cancer therapy. Blood. 2007;110:2819–2827. doi: 10.1182/blood-2007-03-077792. [DOI] [PubMed] [Google Scholar]
- van Beijnum JR, van der Linden E, Griffioen AW. Angiogenic profiling and comparison of immortalized endothelial cells for functional genomics. Exp Cell Res. 2008;314:264–272. doi: 10.1016/j.yexcr.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Thijssen VL, Brandwijk RJ, Dings RP, Griffioen AW. Angiogenesis gene expression profiling in xenograft models to study cellular interactions. Exp Cell Res. 2004;299:286–293. doi: 10.1016/j.yexcr.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Baum LG, Seilhamer JJ, Pang M, Levine WB, Beynon D, Berliner JA. Synthesis of an endogeneous lectin, galectin-1, by human endothelial cells is up-regulated by endothelial cell activation. Glycoconj J. 1995;12:63–68. doi: 10.1007/BF00731870. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Cumashi A, Bianco GA, Ciavardelli D, Iurisci I, D’Egidio M, Piccolo E, Tinari N, Nifantiev N, Iacobelli S. Synthetic lactulose amines: novel class of anticancer agents that induce tumor-cell apoptosis and inhibit galectin-mediated homotypic cell aggregation and endothelial cell morphogenesis. Glycobiology. 2006;16:210–220. doi: 10.1093/glycob/cwj056. [DOI] [PubMed] [Google Scholar]
- Elola MT, Wolfenstein-Todel C, Troncoso P, Vasta GR, Rabinovich GA: Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol Life Sci 2007, (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol Biol Cell. 2004;15:3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huflejt ME, Turck CW, Lindstedt R, Barondes SH, Leffler H. L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. J Biol Chem. 1993;268:26712–26718. [PubMed] [Google Scholar]
- Cowles EA, Agrwal N, Anderson RL, Wang JL. Carbohydrate-binding protein 35: isoelectric points of the polypeptide and a phosphorylated derivative. J Biol Chem. 1990;265:17706–17712. [PubMed] [Google Scholar]
- Shekhar MP, Nangia-Makker P, Tait L, Miller F, Raz A. Alterations in galectin-3 expression and distribution correlate with breast cancer progression: functional analysis of galectin-3 in breast epithelial-endothelial interactions. Am J Pathol. 2004;165:1931–1941. doi: 10.1016/S0002-9440(10)63245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R, Belloni PN, Tressler RJ, Lotan D, Xu XC, Nicolson GL. Expression of galectins on microvessel endothelial cells and their involvement in tumour cell adhesion. Glycoconj J. 1994;11:462–468. doi: 10.1007/BF00731282. [DOI] [PubMed] [Google Scholar]
- Glinskii OV, Turk JR, Pienta KJ, Huxley VH, Glinsky VV. Evidence of porcine and human endothelium activation by cancer-associated carbohydrates expressed on glycoproteins and tumour cells. J Physiol. 2004;554:89–99. doi: 10.1113/jphysiol.2003.054783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinsky VV, Glinsky GV, Glinskii OV, Huxley VH, Turk JR, Mossine VV, Deutscher SL, Pienta KJ, Quinn TP. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–3811. [PubMed] [Google Scholar]
- Gil CD, La M, Perretti M, Oliani SM. Interaction of human neutrophils with endothelial cells regulates the expression of endogenous proteins annexin 1, galectin-1 and galectin-3. Cell Biol Int. 2006;30:338–344. doi: 10.1016/j.cellbi.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Spitzenberger F, Graessler J, Schroeder HE. Molecular and functional characterization of galectin 9 mRNA isoforms in porcine and human cells and tissues. Biochimie. 2001;83:851–862. doi: 10.1016/s0300-9084(01)01335-9. [DOI] [PubMed] [Google Scholar]
- Zick Y, Eisenstein M, Goren RA, Hadari YR, Levy Y, Ronen D. Role of galectin-8 as a modulator of cell adhesion and cell growth. Glycoconj J. 2004;19:517–526. doi: 10.1023/B:GLYC.0000014081.55445.af. [DOI] [PubMed] [Google Scholar]
- Bidon N, Brichory F, Hanash S, Bourguet P, Dazord L, Le Pennec JP. Two messenger RNAs and five isoforms for Po66-CBP, a galectin-8 homolog in a human lung carcinoma cell line. Gene. 2001;274:253–262. doi: 10.1016/s0378-1119(01)00598-4. [DOI] [PubMed] [Google Scholar]
- Carlsson S, Oberg CT, Carlsson MC, Sundin A, Nilsson UJ, Smith D, Cummings RD, Almkvist J, Karlsson A, Leffler H. Affinity of galectin-8 and its carbohydrate recognition domains for ligands in solution and at the cell surface. Glycobiology. 2007;17:663–676. doi: 10.1093/glycob/cwm026. [DOI] [PubMed] [Google Scholar]
- Levy Y, Auslender S, Eisenstein M, Vidavski RR, Ronen D, Bershadsky AD, Zick Y. It depends on the hinge: a structure-functional analysis of galectin-8, a tandem-repeat type lectin. Glycobiology. 2006;16:463–476. doi: 10.1093/glycob/cwj097. [DOI] [PubMed] [Google Scholar]
- Nishi N, Itoh A, Fujiyama A, Yoshida N, Araya S, Hirashima M, Shoji H, Nakamura T. Development of highly stable galectins: truncation of the linker peptide confers protease-resistance on tandem-repeat type galectins. FEBS Lett. 2005;579:2058–2064. doi: 10.1016/j.febslet.2005.02.054. [DOI] [PubMed] [Google Scholar]
- Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, Raz A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002;94:1854–1862. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- Clausse N, van den Brule F, Waltregny D, Garnier F, Castronovo V. Galectin-1 expression in prostate tumor-associated capillary endothelial cells is increased by prostate carcinoma cells and modulates heterotypic cell-cell adhesion. Angiogenesis. 1999;3:317–325. doi: 10.1023/a:1026584523789. [DOI] [PubMed] [Google Scholar]
- Glinskii OV, Huxley VH, Glinsky GV, Pienta KJ, Raz A, Glinsky VV. Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia. 2005;7:522–527. doi: 10.1593/neo.04646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Glinsky VV, Landon LA, Matthews L, Deutscher SL. Peptides specific to the galectin-3 carbohydrate recognition domain inhibit metastasis-associated cancer cell adhesion. Carcinogenesis. 2005;26:309–318. doi: 10.1093/carcin/bgh329. [DOI] [PubMed] [Google Scholar]