Abstract
The Gram-positive Streptococcus pneumoniae is the leading cause of community-acquired pneumonia worldwide, resulting in high mortality. Our in vivo studies show that galectin-3−/− mice develop more severe pneumonia after infection with S. pneumoniae, as demonstrated by increased bacteremia and lung damage compared to wild-type mice and that galectin-3 reduces the severity of pneumococcal pneumonia in part by augmenting neutrophil function. Specifically, we show that 1) galectin-3 directly acts as a neutrophil-activating agent and potentiates the effect of fMLP, 2) exogenous galectin-3 augments neutrophil phagocytosis of bacteria and delays neutrophil apoptosis, 3) phagocytosis of apoptotic neutrophils by galectin-3−/− macrophages is less efficient compared to wild type, and 4) galectin-3 demonstrates bacteriostatic properties against S. pneumoniae in vitro. Furthermore, ad-back of recombinant galectin-3 in vivo protects galectin-3-deficient mice from developing severe pneumonia. Together, these results demonstrate that galectin-3 is a key molecule in the host defense against pneumococcal infection. Therapeutic strategies designed to augment galectin-3 activity may both enhance inflammatory cell function (by directly affecting neutrophil responsiveness and prolonging neutrophil longevity) and have direct bacteriostatic activity, improving clinical outcomes after severe pneumococcal infection.
Despite improvements in therapy and intensive-care support, mortality attributable to respiratory tract infections is ever increasing. The Gram-positive bacterium Streptococcus pneumoniae is the most common cause of community-acquired pneumonia. Mortality is high, especially in developing countries.1 Limitations of conventional therapies and emerging drug resistance among strains of S. pneumoniae to antibiotics, such as penicillin2 and vancomycin,3 necessitates continued study into the mechanisms involved in the pathogenesis of pneumococcal disease and the host immune response against pneumococcal invasion.
Alveolar macrophages and recruited macrophages and neutrophils play a key role in the clearance and killing of invading pathogens. In the lung, resident alveolar macrophages are the first line of cellular defense and play a phagocytic role during the early stages of infection. Interaction of these cells with pneumococcus provokes an inflammatory response inducing recruitment of the more efficient polymorphonuclear leukocytes (or neutrophils) and increased concentrations of serum opsonins at the site of infection. Polymorphonuclear leukocytes are major effector cells in host defense against pneumococcal pneumonia as they phagocytose pneumococci and synthesize a variety of cytotoxic products, including active oxygen metabolites and defensins.4 Although these products kill the invading pathogens, they can also severely damage the lung tissue itself.4 Nonresident macrophages are subsequently recruited and contribute to the resolution of inflammation by phagocytosing cell debris and apoptotic neutrophils. Therefore, the mechanisms that orchestrate neutrophil and macrophage function are important for the clearance of bacteria and resolution of pneumonic infection.
Galectin-3 is a unique member of the growing family of β-galactoside-binding lectins.5,6,7 Galectin-3 contains a single carboxy-terminal domain and a glycine-rich amino-terminal domain through which it forms oligomers and functions to cross-link both carbohydrate and noncarbohydrate ligands. Galectin-3 has been implicated in many facets of the inflammatory response including neutrophil adhesion and activation,8 chemoattraction of monocytes/macrophages,9 and activation of mast cells10 and lymphocytes.11 Galectin-3 is abundantly expressed and secreted by macrophages.12 However, mouse neutrophils do not express galectin-3.13 Secreted galectin-3 can cross-link surface glycoproteins and activate pathways involved in several innate immune responses such as the oxidative burst in neutrophils11,14 and degranulation in mast cells.10 Galectin-3 also contributes to chemotaxis by mediating cell-cell and cell-substratum adhesion.15,16
Galectin-3 has been shown to play a role in apoptosis. Extracellular galectin-3 induces T-cell apoptosis17 whereas intracellular galectin-3 results in an inhibition of apoptosis.18 Furthermore, peritoneal macrophages taken from galectin-3−/− mice are more prone to undergo apoptosis than wild-type (WT) macrophages.19 However, minimal data exist regarding the role of galectin-3 in neutrophil apoptosis.
Galectin-3 has been shown to play a critical role in phagocytosis of opsonized red blood cells20; however, its role in the phagocytic clearance of microorganisms and apoptotic neutrophils has not been elucidated. Clearance of apoptotic neutrophils by macrophages is a key step in the resolution of inflammation. Without this step, apoptotic neutrophils will undergo secondary necrosis resulting in the release of damaging toxic products. Removal of these potentially toxic apoptotic neutrophils results in the release of anti-inflammatory and reparative cytokines such as transforming growth factor-β1. These clearance and resolution phases help to limit the degree of tissue injury.
Galectin-3 has recently been demonstrated to have antimicrobial activity toward the pathogenic fungus Candida albicans.21 This antimicrobial activity may also be relevant for other pathogens, thus revealing an interesting therapeutic use of this galectin. Sato and colleagues13 demonstrated that after pneumococcal infection of the lungs, galectin-3 accumulates in the alveolar space, and this correlates with the onset of neutrophil extravasation. However, although neutrophils were actively recruited into Escherichia coli pneumonia-infected lungs, there was no increase in galectin-3 expression. Furthermore galectin-3 was released by alveolar macrophages on incubation with S. pneumoniae membrane fraction.13 In addition, lipopolysaccharide expressed on E. coli has been shown to down-regulate galectin-3 expression.12,22 The relevance of these observations to the mechanistic role of galectin-3 in the host immune response to S. pneumoniae infection has not been examined. We therefore studied pneumococcal pneumonia in mutant mice lacking the galectin-3 gene and demonstrate that reconstituting galectin-3 in a deficient mouse can reduce the severity of infection.
Materials and Methods
Materials
Tissue culture reagents were purchased from PAA (The Cell Culture Company, Somerset, UK). Recombinant mouse and human galectin-3 were prepared using the baculovirus expression system (Invitrogen, Paisley, UK). All other reagents were purchased from Sigma-Aldrich (Poole, UK) unless otherwise stated.
Animals
Generation of galectin-3−/− mice in a 129sv and C57/B6 background by gene targeting technology has been described.23 As controls, age- and sex-matched WT littermate mice were used. All procedures were undertaken with approved license from the Animal Scientific Procedure Division of the Home Office (London, UK).
Bacteria
S. pneumoniae type 3 was used in this study (American Type Culture Collection, Rockville, MD). After overnight incubation on 5% sheep blood agar plates (BD Biosciences, Oxford, UK), freshly grown colonies were suspended in heart infusion broth (BD Biosciences) with 10% heat-treated horse serum (HIB-S) (Invitrogen) at an OD550 of 0.17. The bacterial suspension was diluted 100-fold with fresh HIB-S and was incubated for 4 hours at 37°C with shaking, corresponding to a point compatible with logarithmic growth. The suspension was centrifuged at 3000 rpm for 10 minutes at room temperature. The supernatant was discarded, and the pellet was resuspended with the same volume of phosphate-buffered saline (PBS). Serial dilutions were used for determination of exact bacterial concentration. Fluorescein isothiocyanate (FITC)-labeled S. pneumoniae were prepared as described previously.24
In Vivo Pneumonia Model
Under anesthesia (avertin 10 μl/g body weight), WT or galectin-3−/− mice were intratracheally inoculated with 1 × 105 colony-forming units (CFU) of S. pneumoniae. Fifteen hours after bacterial administration, mice were sacrificed, blood was collected aseptically, and bronchoalveolar lavage (BAL) was performed with two separate aliquots of 400 μl of sterile PBS. Half of the lung was stored in 2 ml of PBS for bacterial counts. The other half was fixed in 10% formalin (Sigma-Aldrich), paraffin-embedded, cut into 3-μm-thick sections, and stained with hematoxylin and eosin (H&E). Blood was inoculated on a 5% sheep blood agar plate (BD Biosciences) and incubated at 37°C overnight. The BAL fluid was centrifuged at 3000 rpm for 10 minutes at 4°C. Protein concentration in BAL was determined by the BCA protein assay reagent (Pierce Biotechnology, Northumberland, UK) according to the manufacturer’s instructions, and cytokines [interleukin (IL)-6 and tumor necrosis factor (TNF)-α] were measured using the Mouse Inflammation Cytometric Bead Array kit (BD Biosciences) according to the manufacturer’s instructions. The supernatant was stored at −20°C until further use. The pellet was resuspended in 300 μl of PBS, and total cell number was determined with a hemocytometer. For neutrophil and macrophage cell counts, cytospins were prepared (280 rpm, 3 minutes at room temperature), and cells were stained with Diff-Quik to enable the morphological discrimination between mononuclear cells and polymorphonuclear cells. The lung was homogenized in 2 ml of PBS, and the homogenates were serially diluted 10-fold with PBS, inoculated on 5% sheep blood agar plates, and incubated at 37°C overnight. Colonies formed from lung homogenates were counted, and blood plates were examined for the presence (sepsis) or absence of colonies. Myeloperoxidase activity in lung homogenates was assayed using 3,3′,5,5′-tetramethylbenzidine (Sigma-Aldrich) as a substrate. Myeloperoxidase activity/mg lung homogenate was determined. Western blot analysis for galectin-3 was performed on neat BAL and 1:10 dilution of lung homogenate using the antibody anti-galectin-3 clone A3A12 (Alexis Biochemicals, San Diego, CA). The galectin-3 ad-back experiment was performed by administration of 5 μg of recombinant mouse galectin-3 intratracheally at the time of infection.
Tissue Culture
Bone marrow-derived macrophages (BMDMs) and bone marrow-derived neutrophils were prepared from WT and galectin-3−/− mouse femurs and tibias. BMDMs were prepared by maturing bone marrow cells in Dulbecco’s modified Eagle’s medium-F12 containing 10% heat inactivated fetal bovine serum (FBS), 1% penicillin and streptomycin, and 10% L929 conditioned media as a source of granulocyte-macrophage colony-stimulating factor (GM-CSF), for 7 to 9 days. L929-conditioned media was prepared by growing L929 cells to confluency and sterile-filtering the conditioned media before adding to the Dulbecco’s modified Eagle’s medium-F12.
Bone marrow-derived neutrophils were prepared from WT and galectin-3−/− mouse femurs and tibias. This technique has previously been shown to yield greater than 90% neutrophil purity.25 Furthermore, studies have reported that mouse bone marrow-derived neutrophils show very similar morphology and function to neutrophils isolated from mouse whole blood.26 Bone marrow-derived neutrophils were prepared by Percoll gradient centrifugation as described previously.25 To assess neutrophil purity, bone marrow-derived neutrophils were incubated with 1:200 phycoerythrin (PE)-conjugated LY-6G and LY-6C (GR-1, clone RB6-8C5) monoclonal antibody (BD Pharmingen, Oxford, UK) for 30 minutes at 4°C and analyzed by fluorescence-activated cell sorting (FACS) analysis using a BD FACSCalibur flow cytometer. When needed, mouse neutrophils were made apoptotic by culturing in suspension at 5 × 106/ml in RPMI supplemented with 10% heat-inactivated FBS, 1% penicillin and streptomycin, and 1% l-glutamine for the indicated duration. To measure neutrophil apoptosis, neutrophils were incubated with 1:200 PE-conjugated LY-6G and LY-6C (GR-1, clone RB6-8C5) monoclonal antibody for 30 minutes at 4°C followed by incubation with 1:500 Annexin-V-FITC (Roche, Hertfordshire, UK) in Annexin-V bind buffer (Hanks plus 5 mmol/L CaCl2) for 10 minutes at 4°C. Samples were treated with 1:10,000 ToPro-3 (Invitrogen) and immediately analyzed by FACS analysis using a BD FACSCalibur flow cytometer.
Human peripheral blood neutrophils were prepared as described previously.27 Apoptotic human neutrophils were prepared by culturing neutrophils for up to 24 hours in Iscove’s modified Dulbecco’s medium containing 10% FBS and treated as indicated. Human neutrophil apoptosis was assessed after 18 hours by identifying morphological changes of Diff-Quik-stained cells followed by cell counting. Binding of Annexin V was also assessed by incubating neutrophils with 1:500 Annexin-V-FITC (Roche) in Annexin-V bind buffer (HANKS plus 5 mmol/L CaCl2) for 10 minutes at 4°C. Samples were treated with 1:10,000 ToPro-3 (Invitrogen) and immediately analyzed by FACS analysis using a BD FACSCalibur flow cytometer.
Baculovirus Expression System
The Invitrogen baculovirus expression system was used to produce endotoxin-free recombinant human or mouse galectin-3. Detailed protocols are given in the Bac-to-Bac Baculovirus Expression System manual (Invitrogen 10359-016). Briefly, mouse or human galectin-3 was cloned out of pcDNA 3.1 or pGEM-T Easy vectors, respectively, and cloned into a pFastBac HT donor plasmid. Recombinant pFastBac vectors were amplified and transformed into DH10Bac E. coli containing a baculovirus shuttle vector (bacmid). Recombinant bacmid containing the galectin-3 sequence was produced on transposition of the pFastBac construct and purified using the S.N.A.P midi-prep kit (Invitrogen). Recombinant bacmid was transfected into Sf9 insect cells and amplified using the method given in the manual. Galectin-3 protein was purified using the Ni-NTA His bind resin (Novagen, San Diego, CA) according to the manufacturer’s instructions. Galectin-3 was verified using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting using 1:2000 anti-galectin-3 antibody (clone A3A12; Affinity Bioreagents, Nottingham, UK) (data not shown).
Phagocytosis Assays
Phagocytosis of S. pneumoniae by bone marrow-derived macrophages or neutrophils was performed as follows. FITC-S. pneumoniae were opsonized with 10% mouse serum for 1 hour at 37°C. Bone marrow neutrophils were incubated with a 10:1 ratio of FITC-S. pneumoniae to neutrophils for the indicated time points. Excess bacteria were washed off, and neutrophils were incubated with 1:200 PE-conjugated LY-6G and LY-6C (GR-1, clone RB6-8C5) antibody for 30 minutes. Samples were treated with 1:10,000 ToPro-3 (Invitrogen) and immediately analyzed by FACS analysis using a BD FACSCalibur flow cytometer. In some experiments, bone marrow neutrophils were treated with 10 μg/ml of recombinant mouse galectin-3 for 1 hour at 37°C before phagocytosis. Bone marrow-derived macrophages were incubated with a 10:1 ratio of FITC-S. pneumoniae to macrophages for the indicated duration and analyzed by FACS analysis using a BD FACSCalibur flow cytometer.
Phagocytosis of apoptotic human neutrophils by BMDMs was performed as follows. BMDMs were seeded in 48-well plates, stained with 1 μg/ml Cell Tracker Orange (CMTMR, Invitrogen) and incubated with 10:1 ratio of 1 μg/ml Cell Tracker Green (CMFDA, Invitrogen) labeled day 1 apoptotic human neutrophils for the indicated time points at 37°C. Excess neutrophils were washed off, and BMDMs were removed using 5 mmol/L ice-cold ethylenediaminetetraacetic acid with gentle scraping. Percentage of phagocytosis was determined by FACS analysis using a BD FACSCalibur flow cytometer.
Neutrophil Activation Assays
Three assays were used to examine neutrophil activation in response to galectin-3. On activation, neutrophils release reactive oxygen species (ROS) and up-regulate CD11b expression and shed l-selectin (CD62L). Priming of neutrophils induces shape change without a noticeable increase in activation. ROS release was assessed by two methods, cytochrome c reduction, which measures the O2− metabolite,28 and dihydrorhodamine (DHR) fluorescence, which measures the H2O2 metabolite.29 1) Neutrophils isolated from human peripheral blood were incubated with varying concentrations of recombinant human galectin-3 (0.1 to 10 μg/ml) or 1 μmol/L PAF for 15 minutes followed by 15 minutes of incubation with 0.1 μmol/L fMLP in 1 mg/ml cytochrome c in Hanks’ media. Color change in supernatants (an indication of O2− release) was detected using a scanning spectrophotometer 500 to 600 nm. Absorbance was converted to concentration using the calculation A = Σ.l.c. 2) Neutrophils isolated from human peripheral blood were incubated with 0.1 mmol/L DHR for 5 minutes at 37°C and incubated with 1 μmol/L PAF or 10 μg/ml of recombinant human (Hu) or mouse (Ms) galectin-3 for 15 minutes at 37°C. Cells were incubated with 0.1 μmol/L fMLP or PBS for 15 minutes and DHR fluorescence was measured as mean FL-1 fluorescence on the BD FACSCalibur flow cytometer. 3) Shape change of isolated human neutrophils in response to varying concentrations of galectin-3 (0.25, 2.5, 10, and 20 μg/ml) or 1 μmol/L PAF alone was performed by measuring change in forward scatter by FACS analysis. 4) CD11b and l-selectin expression on neutrophils from human whole blood and CD11b expression on neutrophils from WT and galectin-3−/− mouse whole blood were measured by FACS analysis. Heparinized human whole blood was incubated with 0.1 μmol/L fMLP, 100 ng/ml lipopolysaccharide, or varying concentrations of galectin-3 (0.25 to 25 μg/ml) for 30 minutes. Blood was incubated with anti-human CD11b-APC (clone ICRF44) and anti-human CD62L-PE (clone DREG-56) for 30 minutes at 4°C. Samples were treated with 1 ml of 1× FACS lysing solution (BD Biosciences) for 5 minutes at room temperature to lyse the red blood cells. The cell pellet was washed and resuspended in 300 μl of 1% PFA in PBS containing 0.1% bovine serum albumin. Samples were analyzed using a BD FACSCalibur flow cytometer, and neutrophils were identified by forward and side scatter properties.
Mouse whole blood was collected from the vena cava of mice anesthetized with avertin. Whole blood was incubated with 1:200 PE-conjugated LY-6G and LY-6C (GR-1, clone RB6-8C5) antibody and analyzed by FACS analysis to confirm the neutrophil population in the mouse whole blood. Mouse whole blood was incubated with 30 μg/ml of recombinant mouse galectin-3 followed by anti-mouse CD11b-APC (clone M1/70) (1:66.7) antibody. Samples (gated on the GR-1-positive population) were analyzed using a BD FACSCalibur flow cytometer.
Antibacterial Assay
S. pneumoniae was incubated overnight on a blood agar plate at 37°C. Ten ml of 100% heart infusion broth (BD Biosciences) was inoculated with one colony of S. pneumoniae and incubated overnight at 37°C with gentle shaking. Cultures were centrifuged at 3000 rpm for 20 minutes, washed with broth, and centrifuged again. The culture was resuspended in broth to an OD600 = 0.1 and diluted 1:100. Seventy-five μl of the bacterial reaction mix was incubated with 75 μl of antibiotic or galectin-3 at 37°C for 2 hours at 800 rpm on the thermomixer. Antibiotics used included recombinant mouse galectin-3 (0.3 and 15 μg) and ampicillin (20 μg/ml). A reaction was prepared for t = 0, which was plated before the 2-hour incubation. At time 0 and after 2 hours, the reactions were serially diluted into PBS, and 100 μl of each dilution was plated onto blood agar plates. Plates were incubated overnight and colonies counted the next day.
Statistical Analysis
Results are presented as means ± SEM. Significance of the differences between means was assessed using one-way analysis of variance or two-tailed Student’s t-test. Values of P < 0.05 were considered significant.
Results
Galectin-3 Is a Critical Regulator of the Severity of an Acute Pneumococcal Infection in the Lung in Vivo
WT and galectin-3−/− mice were intratracheally inoculated with 1 × 105 CFU S. pneumoniae, and animals were sacrificed after 15 hours (Figure 1). Initial dosing experiments performed throughout a 24-hour period resulted in high mortality of the galectin-3-null mice. This was most likely attributable to the inadequate host response to pneumococcal infection in the galectin-3-null mouse. A shorter duration of 6 hours did not prove long enough to show a prominent difference in the severity of disease outcome in WT and galectin-3−/− mice. We therefore monitored pneumonia for 15 hours with 1 × 105 CFU because this time point and dose achieve measurable disease with no mortality.
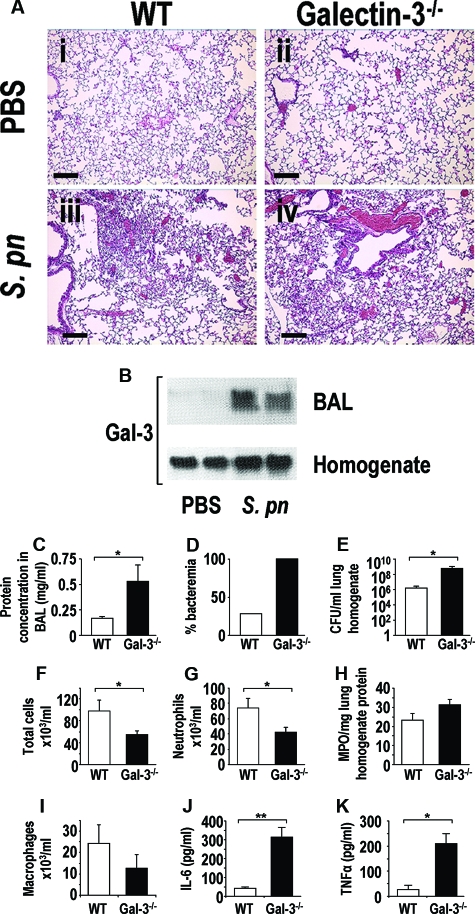
Figure 1.
Galectin-3 plays a critical role in the clearance of an acute pneumococcal infection. Mice were inoculated intratracheally with 1 × 105 CFU S. pneumoniae for 15 hours (n = 10 mice in each group). A: H&E staining of WT and galectin-3−/− lung tissue from control (i and ii) (PBS instilled intratracheally) and S. pneumoniae pneumonia (iii and iv) (S. pneumoniae inoculated intratracheally) at 15 hours after instillation. B: Representative galectin-3 Western blots showing galectin-3 levels in BAL (neat) and homogenates (1:10) of WT mice inoculated with S. pneumoniae compared to PBS control. C–K: WT (white bars) and galectin-3−/− (black bars). C: Protein concentration was significantly higher in lavage fluid from galectin-3−/− mice compared with WT (*P < 0.05 compared to WT). D: Blood from galectin-3−/− and WT mice was plated on blood agar plates and the percentage of plates with bacterial growth is expressed as percent bacteremia. E: Lungs from galectin-3−/− and WT mice were homogenized, serially diluted, plated on blood agar plates, and bacterial counts determined. Galectin-3−/− lung homogenate demonstrated higher bacterial counts compared to WT (*P < 0.05 compared to WT). Total cell recruitment (F) and neutrophil recruitment (G) into the alveolar space was reduced in galectin-3−/− mice compared to WT mice after S. pneumoniae infection (*P < 0.05 compared to WT). H: Myeloperoxidase activity in lung homogenate was similar between the two groups. I: Macrophage recruitment into the alveolar space of WT and galectin-3−/− mice was similar. IL-6 (J) and TNF-α (K) concentration in BAL was determined by CBA and demonstrated to be higher in galectin-3-deficient animals (**P < 0.01 compared to WT and **P < 0.05 compared to WT). Scale bars = 100 μm.
Figure 1A depicts representative H&E staining of lungs from WT and galectin-3−/− mice inoculated with S. pneumoniae or PBS as a control demonstrating increased cell infiltrate in the lungs of infected mice. Galectin-3−/− mice demonstrated more severe pneumonia with increased lung injury and septicemia. Expression of galectin-3 in lung homogenates and BAL was increased in WT mice inoculated with S. pneumoniae compared to PBS controls (Figure 1B). The concentration of galectin-3 in the BAL after infection was ∼50 μg/ml. Overall protein concentration was significantly higher in BAL fluid from galectin-3−/− mice compared to WT mice (Figure 1C), indicating a greater amount of leakage from the vasculature and therefore a higher degree of tissue injury. One hundred percent of culture plates inoculated with galectin-3−/− blood produced colonies indicating that all galectin-3−/− mice were bacteremic after S. pneumoniae infection compared to 30% of WT mice (Figure 1D). Culture plates of lung homogenate from galectin-3−/− mice demonstrated ∼450-fold greater bacterial load than culture plates inoculated with WT lung homogenate (8.6 × 108 CFU/ml in galectin-3−/− compared to 1.9 × 106 CFU/ml in WT) (Figure 1E) indicating a clearance defect of S. pneumoniae in galectin-3−/− mice. Total cell counts were significantly reduced in galectin-3−/− mice after pneumococcal pneumonia infection compared to WT (Figure 1F). In addition, differential cell counts revealed reduced neutrophil recruitment in galectin-3−/− mice compared to WT (Figure 1G). However, myeloperoxidase activity was similar in lung homogenates from WT and galectin-3−/− mice after S. pneumoniae infection (Figure 1H). This would suggest that neutrophils accumulate in the interstitial lung tissue during pneumonia in galectin-3−/− mice but are hindered from transmigrating into the alveolar space in the absence of galectin-3. Although galectin-3−/− mice demonstrated reduced macrophage numbers in lavage fluid compared to WT, this was not significant (Figure 1I). Increased severity of pneumococcal infection in galectin-3−/− mice correlates with greater concentrations of IL-6 and TNF-α in BAL compared to WT mice (Figure 1, J and K). These proinflammatory cytokines can have pathological consequences on host tissue and may contribute to increased leakage from the vasculature and sepsis in the galectin-3-deficient mouse. These results demonstrate that galectin-3 is a critical molecule regulating the severity of a pneumococcal pneumonia infection in vivo through its ability to enhance the clearance of pneumococcal pneumonia and to resolve inflammation.
Expression of Galectin-3 in Macrophages Does Not Enhance Phagocytosis of S. pneumoniae but Does Augment Phagocytosis of Apoptotic Neutrophils
Previous studies have shown that galectin-3−/− macrophages exhibit reduced phagocytosis of IgG-opsonized erythrocytes and apoptotic thymocytes.20 Galectin-3-deficient macrophages demonstrated no phagocytic defect toward S. pneumoniae compared to WT (Figure 2, A and B). However, galectin-3−/− BMDMs demonstrated reduced phagocytosis of apoptotic human neutrophils (Figure 2C). Phagocytosis of apoptotic cells is crucial for the resolution of inflammation by protecting tissues from excess exposure to inflammatory and immunogenic components of dying cells.30 This result may partially explain our in vivo findings of increased lung damage leading to septicemia in the galectin-3-deficient mouse. The different phagocytic responses toward pneumococcus and apoptotic cells may be attributable to alternate mechanisms regulating pathogen recognition by macrophages.
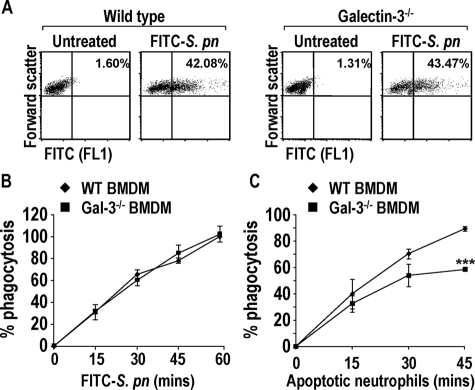
Figure 2.
Expression of galectin-3 in macrophages does not enhance phagocytosis of S. pneumoniae but endogenous galectin-3 augments macrophage phagocytosis of apoptotic human neutrophils. A: Representative forward scatter versus FL1 dot blots of WT and galectin-3−/− BMDMs treated for 60 minutes with 10:1 ratio of FITC-S. pneumoniae. Gated on macrophages according to forward and side scatter properties. Percent in upper right quadrant indicates macrophages that have phagocytosed FITC-labeled bacteria. B: Phagocytosis of a 10:1 ratio of opsonized FITC-S. pneumoniae for up to 1 hour by WT (♦) and galectin-3−/− (▪) BMDMs measured using FACS analysis. Results represent the mean percentage phagocytosis (compared to WT 60 minutes) (n = 3). C: Phagocytosis of a 10:1 ratio of apoptotic human neutrophils for up to 45 minutes by WT (♦) and galectin-3−/− (▪) BMDMs measured using FACS analysis. Results represent the mean percent phagocytosis (n = 3) (***P < 0.0001 compared to WT 45 minutes).
Exogenously Added Recombinant Galectin-3 Causes Human and Mouse Neutrophil Activation
Despite reduced neutrophil transmigration from the lung interstitia into the alveolar space after S. pneumoniae infection in galectin-3−/− mice compared to WT, recruitment is not completely abolished. We therefore examined the effect of galectin-3 on neutrophil function because neutrophils that are successfully recruited in the galectin-3-deficient mouse may demonstrate reduced activity. Mouse neutrophils do not express galectin-3 (our own observations, data not shown),13 and it is widely believed that the action of galectin-3 on neutrophils is extracellular where it oligomerizes on the cell surface to exert its effects.31
On activation, neutrophils release ROS and up-regulate CD11b expression and shed l-selectin (CD62L). Priming of neutrophils induces shape change without a noticeable increase in activation. Isolated human neutrophils preincubated with recombinant human galectin-3 at 3 and 10 μg/ml followed by fMLP produced significantly more superoxide as measured by cytochrome c reduction compared with neutrophils treated with fMLP alone (Figure 3A). Incubation of neutrophils with galectin-3 alone (3, 10, and 15 μg/ml) also resulted in increased superoxide release compared to untreated or cells treated only with PAF (Figure 3A). When treated with either recombinant mouse (Ms) or human (Hu) galectin-3 (10 μg/ml) followed by fMLP, the level of free radical generation, measured by DHR activation also exceeded that of neutrophils treated with fMLP alone (Figure 3B). This assay also demonstrated that galectin-3 alone (10 μg/ml) is sufficient to elicit ROS release from neutrophils (Figure 3B). Figure 3C shows representative histogram overlays of neutrophils. Human neutrophils pretreated with mouse or human recombinant galectin-3 (solid line) before fMLP treatment produced more H2O2 compared to neutrophils treated with fMLP alone (dashed line). At concentrations less than those that directly activate isolated human neutrophils, galectin-3 alone does not significantly alter shape change. Higher concentrations of galectin-3 (>2.5 μg/ml) produced a small increase in shape change but not to the magnitude as that observed with PAF (Figure 3D), suggesting that the predominant effect of galectin-3 is on neutrophil activation.
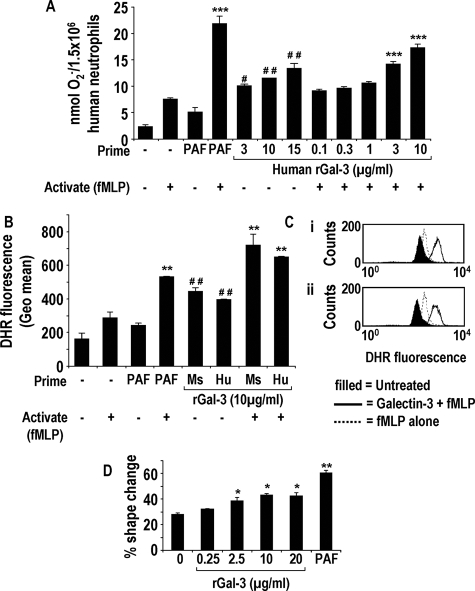
Figure 3.
Exogenously added recombinant galectin-3 causes human neutrophil activation and the generation of ROS. Free radical generation from human neutrophils treated with PAF (1 μmol/L) or human galectin-3 (0.1 to 15 μg/ml) for 15 minutes followed by fMLP (0.1 μmol/L) or PBS for 15 minutes was measured by cytochrome c reduction (A) (n = 6) (***P < 0.0001 compared to treatment with fMLP alone, #P < 0.05 and ##P < 0.01 compared to treatment with PAF alone) and DHR fluorescence using FACS analysis (B and C) (n = 3) (**P < 0.01 compared to treatment with fMLP alone and ##P < 0.01 compared to treatment with PAF alone). C: Representative histogram showing DHR fluorescence in response to fMLP pretreated with either 10 μg/ml mouse (i) or human recombinant galectin-3 (ii). D: Percentage of shape change of human neutrophils treated with galectin-3 (0.25 to 20 μg/ml) or 1 μmol/L PAF for 15 minutes (n = 3) (*P < 0.05 and **P < 0.01 compared with untreated).
Activation of whole blood human neutrophils with fMLP, lipopolysaccharide, and galectin-3 caused l-selectin shedding and CD11b up-regulation (Figure 4, A and B, respectively). A dot blot (Figure 4A, inset) shows forward and side scatter of human peripheral blood neutrophils. Together these experiments show that galectin-3 can cause direct activation of neutrophils in whole blood and can both directly activate isolated neutrophils and prime neutrophils to subsequent activation by fMLP.
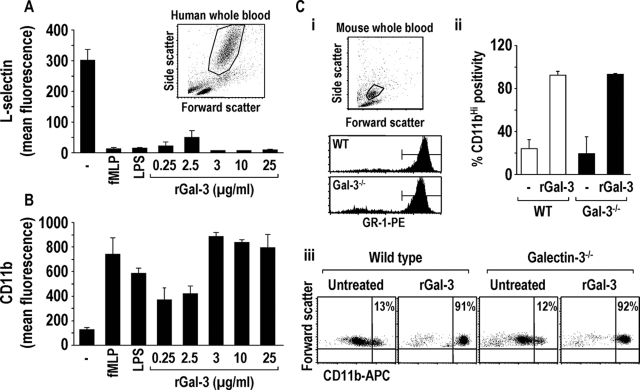
Figure 4.
Exogenously added recombinant galectin-3 causes l-selectin shedding and CD11b up-regulation on human whole blood neutrophils and CD11b up-regulation on both WT and galectin-3−/− mouse neutrophils. l-selectin shedding (A) and CD11b up-regulation (B) on human neutrophils after a 30-minute incubation with fMLP (0.1 μmol/L), lipopolysaccharide (100 ng/ml), or human galectin-3 (0.25 to 25 μg/ml) was assessed by FACS analysis (n = 6) (for all treatments P < 0.01 compared to untreated). A, inset: Representative dot blot showing forward and side scatter properties of human whole blood, gated on neutrophils. C: i: Representative dot blot showing forward and side scatter properties of WT mouse whole blood gated on GR-1-positive neutrophils. ii and iii: Mouse whole blood was treated with 30 μg/ml of mouse galectin-3 for 30 minutes and incubated with anti-CD11b-APC and anti-GR-1-PE. Galectin-3-treated WT (white bars) and galectin-3−/− (black bars) whole blood demonstrated increased CD11b expression (CD11bHI) on GR-1-positive neutrophils as measured by FACS analysis (n = 3).
Galectin-3−/− mouse whole blood shows the same forward and side scatter properties as WT (data not shown). WT and galectin-3−/− mouse whole blood was treated with 30 μg/ml of recombinant mouse galectin-3 and high CD11b expression (CD11bHI) on neutrophils was assessed by FACS analysis. Samples were gated on GR-1-positive neutrophils (Figure 4Ci). CD11bHI expression on neutrophils in galectin-3-treated mouse whole blood was compared to those of untreated controls. Both WT and galectin-3−/− neutrophils demonstrated more than 90% CD11bHI expression when treated with galectin-3 compared to just 20% of untreated neutrophils demonstrating CD11bHI expression (Figure 4C, ii and iii). Together these data demonstrate that exogenously added galectin-3 can activate both human and mouse neutrophils. Furthermore, exogenous galectin-3 increases CD11b expression on both WT and galectin-3−/− neutrophils to similar levels.
Exogenous Galectin-3 Enhances Phagocytosis of Bacteria by Both WT and Galectin-3−/− Neutrophils
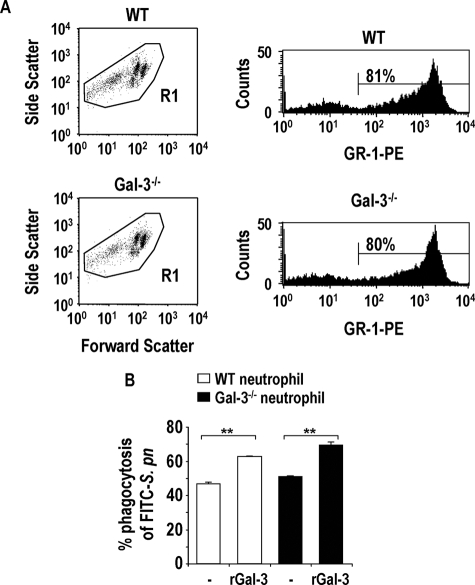
WT and galectin-3−/− mouse bone marrow neutrophils were isolated as described previously.25 After an infection, this fully competent neutrophil reservoir in the bone marrow is required to rapidly supplement peripheral neutrophils at times of increased demand.26 Figure 5A illustrates forward and side scatter properties of a bone marrow neutrophil preparation from WT and galectin-3−/− mice. The various cell populations observed most likely demonstrate neutrophils that are matured to different extents. However, when all cell populations are taken into account (gate R1), the percentage of GR-1-positive cells is ∼80%.
Figure 5.
Exogenous galectin-3 enhances phagocytosis of S. pneumoniae by both WT and galectin-3−/− neutrophils. A: Representative dot blots from WT and galectin-3−/− mouse bone marrow neutrophil preparations gated on all populations (R1). Representative histograms demonstrate ∼80% GR-1 positivity within the R1 gate. B: WT (white bars) and galectin-3−/− (black bars) bone marrow neutrophils were prepared as described in the Materials and Methods. Neutrophils were incubated with 30 μg/ml of recombinant mouse galectin-3 or PBS (control) for 1 hour at 37°C then incubated with a 10:1 ratio of opsonized FITC-S. pneumoniae for 1 hour at 37°C. Samples were gated on GR-1-positive cells and analyzed using a BD FACSCalibur (n = 3) (**P < 0.01 compared to untreated).
Untreated WT and galectin-3−/− GR-1-positive mouse bone marrow neutrophils incubated with opsonized FITC-labeled S. pneumoniae showed similar levels of phagocytosis. However, after a 1-hour incubation with 10 μg/ml of recombinant mouse galectin-3, both WT and galectin-3−/− bone marrow neutrophils enhanced their phagocytic capability (Figure 5B). This indicates that exogenous galectin-3 can stimulate phagocytosis of S. pneumoniae by neutrophils from WT and galectin-3−/− mice.
Neutrophils from WT and Galectin-3−/− Mice Undergo Apoptosis at the Same Rate in Vitro, but Exogenous Galectin-3 Prolongs Neutrophil Survival
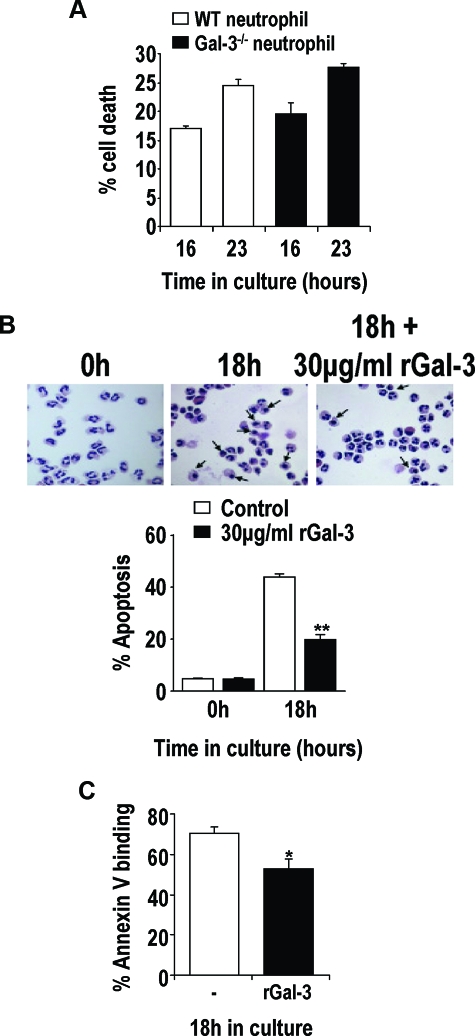
Previous reports have demonstrated an important role for galectin-3 during apoptosis.17,18,19 We therefore examined the rate of spontaneous apoptosis of neutrophils from WT or galectin-3−/− mice in vitro. Figure 6A shows that neutrophils from galectin-3−/− mice aged in vitro demonstrate a similar rate of cell death compared to WT neutrophils. However, in keeping with its ability to activate neutrophils, incubation with galectin-3 delays spontaneous apoptosis in human peripheral blood neutrophils aged for 18 hours in culture assessed by both nuclear morphology and cell surface changes as measured by annexin V binding (Figure 6, B and C). The different rates of apoptosis demonstrated in Figure 6, B and C, reflect the techniques used to assess neutrophil apoptosis.
Figure 6.
WT and galectin-3−/− mouse neutrophils undergo apoptosis at similar rates in culture, and galectin-3 protects human neutrophils from apoptosis. A: Mouse bone marrow neutrophils were cultured for the indicated time points and incubated with PE-conjugated anti-GR-1 antibody followed by annexin-V-FITC. ToPro-3 was added to each sample before FACS analysis. Apoptotic and necrotic neutrophils were identified as those demonstrating GR-1 and annexin-V/ToPro-3 positivity (n = 3). B: Human peripheral blood neutrophils were cultured for 18 hours in Iscove’s modified Dulbecco’s medium containing 10% FBS and 30 μg/ml of human recombinant galectin-3 or PBS. Cytospin preparations were prepared, and neutrophil apoptosis was determined by morphology. Arrows show apoptotic human neutrophils. Percent apoptosis was calculated from total cell number of five fields of each slide (n = 3) (**P < 0.01 compared to untreated). C: Human peripheral blood neutrophils were cultured for 18 hours in Iscove’s modified Dulbecco’s medium containing 10% FBS and 20 μg/ml of human recombinant galectin-3. Neutrophils were incubated with annexin-V-FITC, and ToPro-3 was added to each sample before FACS analysis. Annexin-V-positive and ToPro-3-negative neutrophils were classed as apoptotic (n = 3) (*P < 0.05 compared to untreated).
In addition to the above findings suggesting that galectin-3-deficient macrophages are less able to phagocytose apoptotic neutrophils than their WT counterparts (Figure 2C), galectin-3 delays spontaneous human neutrophil apoptosis. Thus after S. pneumoniae infection in vivo, the greater accumulation of apoptotic neutrophils in the galectin-3−/− mouse may contribute to tissue damage and subsequent bacteremia. These results suggest that the increased vasculature damage and bacteremia observed in galectin-3−/− mice is in part attributable to ineffective clearance of apoptotic cells by galectin-3−/− macrophages.
Galectin-3 Is Bacteriostatic Toward S. pneumoniae
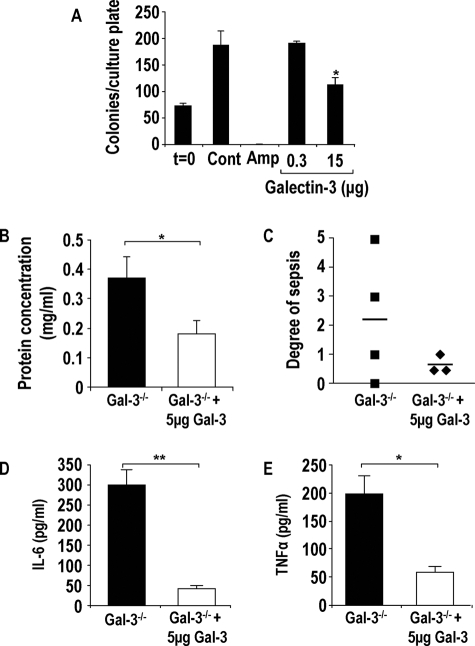
Galectin-3 has recently been shown to have antimicrobial activity toward the pathogenic fungus Candida albicans.21 We therefore investigated whether galectin-3 had a direct antimicrobial effect on S. pneumoniae. The same strain of S. pneumoniae used in the in vivo experiments was incubated with varying concentrations of galectin-3 or ampicillin as a positive control as described in the Materials and Methods. Incubation of 15 μg of galectin-3 with S. pneumoniae significantly inhibited bacterial growth (Figure 7A), suggesting that galectin-3 is bacteriostatic toward S. pneumoniae. Growth inhibition of S. pneumoniae by galectin-3 may further explain our in vivo findings that galectin-3−/− mice demonstrate more severe S. pneumoniae infection. This may have important implications as to the function of galectin-3 in the lungs of infected mice.
Figure 7.
Galectin-3 is bacteriostatic, and delivery of recombinant galectin-3 into the lungs of galectin-3−/− mice reduces severity of pneumonia. A: S. pneumoniae cultures were incubated with galectin-3 (0.3 and 15 μg) or ampicillin (20 μg/ml) for 2 hours, diluted 1:10, and plated onto blood agar plates. Colonies were counted the following day (n = 3) (*P < 0.05 compared to control). Galectin-3-deficient mice were intratracheally inoculated with 1 ×105 CFU S. pneumoniae in the presence or absence of 5 μg of recombinant mouse galectin-3 for 15 hours. B: Protein concentration was significantly higher in lavage fluid from galectin-3−/− (black bars) mice compared with galectin-3−/− mice treated with galectin-3 (white bars) (*P < 0.05 compared to galectin-3−/−). C: Blood from galectin-3−/− and WT mice was plated on blood agar plates, and the degree of bacterial presence per plate was scored according to the arbitrary scale 0 to 5 (0, no bacterial growth; 5, significant bacterial growth). Galectin-3−/− mice treated with galectin-3 demonstrated reduced sepsis compared to untreated galectin-3−/− mice. IL-6 (D) and TNF-α (E) concentration in BAL was determined by CBA and demonstrated to be lower in galectin-3-treated galectin-3−/− mice (**P < 0.01 compared to galectin-3−/− and *P < 0.05 compared to galectin-3−/−).
Addition of Recombinant Galectin-3 into the Lungs of Galectin-3−/− Mice Reduces Severity of Pneumonia
To expand our in vivo work, we inoculated galectin-3−/− mice with S. pneumoniae in the presence or absence of 5 μg of galectin-3. Sepsis and protein content in the BAL were assessed as indicators of lung injury and degree of pneumonic severity. Inoculation of galectin-3-deficient mice with S. pneumoniae and galectin-3 reduced the protein content in lavage indicating reduced lung injury (Figure 7B). Furthermore, severity of bacteremia was reduced in galectin-3-treated mice (Figure 7C) and IL-6 and TNF-α levels in the BAL were reduced in mice treated with galectin-3 (Figure 7, D and E). These results reveal the potential therapeutic use of galectin-3 toward infection.
Discussion
The Gram-positive Streptococcus pneumoniae is the leading cause of community-acquired pneumonia worldwide, resulting in high mortality. The increasing prevalence of antibiotic resistance means that novel treatment strategies to combat pneumonia are urgently required. Previous studies have demonstrated that galectin-3 is increased in the lung after infection with S. pneumoniae, and this correlates with the onset of neutrophil extravasation.13 However, the relevance of these observations to the mechanistic role of galectin-3 in the host immune response to S. pneumoniae infection has not been examined. We therefore studied pneumococcal pneumonia in mutant mice lacking the galectin-3 gene. Our in vivo studies show that galectin-3−/− mice develop more severe pneumonia after infection with S. pneumoniae, as demonstrated by increased septicemia and lung damage compared to WT mice. Neutrophil recruitment to the alveolar space was reduced in galectin-3−/− mice; however, myeloperoxidase activity in lung homogenates was not reduced in these mice compared to WT. This would suggest that neutrophils accumulate in the interstitial lung tissue during pneumonia in galectin-3−/− mice but are hindered from transmigrating into the alveolar space in the absence of galectin-3.
Galectin-3 is highly expressed and secreted from activated macrophages and acts as a powerful proinflammatory signal.12 Previous studies have shown that galectin-3 promotes the respiratory burst in neutrophils (and our observations),11,32 increases CD66 expression on the surface of neutrophils,33 and binds to CD66a and CD66b,34 the receptors most likely responsible for inducing neutrophil NADPH oxidase activation. Galectin-3 promotes the adhesion of neutrophils to laminin8 and enhances phagocytic activity of opsonized erythrocytes.33 However, the role of galectin-3 with regard to neutrophil function in response to infection is not well characterized.
Neutrophils play a critical role in the host immune defense against infection. Neutrophils are recruited early after infection, and their ability to phagocytose bacteria and secrete cytotoxic mediators makes them an important innate defense mechanism. Therefore, augmenting their function is critical to their antibacterial properties after infection. However, their removal from the site of infection during resolution is equally important because this limits host cell damage. Neutrophil apoptosis occurs as part of the normal resolution process, rendering neutrophils unresponsive to further stimulation and allowing noninflammatory recognition by tissue macrophages and their removal by phagocytosis. Factors that increase neutrophil activation and prolong neutrophil survival at sites of infection will have an important bearing on the host response to infection.
Here we demonstrate that galectin-3 can directly activate both human and mouse neutrophils and potentiate the effect of fMLP. We have revealed that whole blood neutrophils can be directly activated by concentrations of galectin-3 lower than those that directly activate isolated neutrophils. This suggests that in whole blood there may be factors present in the serum (eg, GM-CSF) functioning to prime circulating neutrophils.
Although mouse neutrophils express very low levels of endogenous galectin-3 (our own observations),13 they can be activated by extracellular galectin-3, which is up-regulated in the surrounding tissue environment after infection. However, in galectin-3-null mice this galectin-3 up-regulation does not occur, resulting in reduced neutrophil recruitment into the alveolar spaces, activation, and phagocytosis.
Galectin-3 expression is up-regulated after pneumococcal infection (our own observations),13 and macrophages are capable of secreting large amounts of galectin-3.12,13 We show that longevity of human neutrophils is increased after incubation with exogenous galectin-3, thus delaying spontaneous apoptosis in vitro. Furthermore, galectin-3−/− macrophages displayed reduced phagocytosis of apoptotic human neutrophils compared to WT. The resultant accumulation of apoptotic neutrophils in the lungs of galectin-3−/− mice after infection would cause considerable damage to lung tissue, thus allowing bacteria to traverse the lung epithelia and enter the blood stream resulting in septicemia.
During S. pneumoniae infection the galectin-3−/− mouse mounts a greater Th1 response demonstrated by increased IL-6 and TNF-α cytokine levels compared to WT mice or galectin-3−/− mice treated with recombinant galectin-3. This increased Th1 response may contribute to lung damage and subsequent septicemia. We have shown that galectin-3−/− macrophages demonstrate a deficit in their ability to adopt an anti-inflammatory alternative (M2) phenotype.35 We therefore propose that, in addition to reduced neutrophil activation and apoptotic neutrophil clearance by macrophages in the galectin-3−/− mice, these mice are less able to dampen down the excessive inflammation and destructive potential of pneumococcal infection.
We propose that galectin-3 released from resident alveolar macrophages in response to pneumococcal pneumonia infection can activate neutrophils and enhance their bacterial killing and phagocytic capability, aiding pathogen clearance. In addition, we show that galectin-3 may play a further role in host defense by a direct bacteriostatic effect on S. pneumoniae. Galectin-3 (15 μg) dramatically reduced S. pneumoniae growth in vitro. Sato and colleagues13 demonstrated that the galectin-3 concentration in the lung is increased in mice after S. pneumoniae infection. We observed very high concentrations of galectin-3 in lung homogenate and BAL after S. pneumoniae infection (concentration in BAL >50 μg/ml). This suggests that bacteriostatic concentrations of galectin-3 are achieved in the lung and may play an important role in the defense against S. pneumoniae infection.
Our study has shown that galectin-3 protects against pneumococcal pneumonia through a variety of mechanisms including augmentation of neutrophil function and a direct bacteriostatic role. Strategies designed to augment galectin-3 expression in the lung may result in the development of novel treatments for pneumococcal pneumonia.
Acknowledgments
We thank Dr. Simon Hart for helpful discussions.
Footnotes
Address reprint requests to Tariq Sethi, Centre for Inflammation Research, The Queen’s Medical Research Institute, University of Edinburgh, 47 Little France Crescent, Edinburgh EH16 4TJ, UK. E-mail: t.sethi@ed.ac.uk.
Supported by the Medical Research Council, UK (Ph.D. studentship to S.L.F.); and the Wellcome Trust, UK (clinical training fellowship to N.C.H. and senior research leave fellowship to T.S.).
S.L.F. and N.C.H. contributed equally.
References
- Paterson GK, Blue CE, Mitchell TJ. Role of interleukin-18 in experimental infections with Streptococcus pneumoniae. J Med Microbiol. 2005;54:323–326. doi: 10.1099/jmm.0.45873-0. [DOI] [PubMed] [Google Scholar]
- Feldman C. Clinical relevance of antimicrobial resistance in the management of pneumococcal community-acquired pneumonia. J Lab Clin Med. 2004;143:269–283. doi: 10.1016/j.lab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Novak R, Henriques B, Charpentier E, Normark S, Tuomanen E. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature. 1999;399:590–593. doi: 10.1038/21202. [DOI] [PubMed] [Google Scholar]
- Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990;141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- Hughes RC. The galectin family of mammalian carbohydrate-binding molecules. Biochem Soc Trans. 1997;25:1194–1198. doi: 10.1042/bst0251194. [DOI] [PubMed] [Google Scholar]
- Cooper DN. Galectinomics: finding themes in complexity. Biochim Biophys Acta. 2002;1572:209–231. doi: 10.1016/s0304-4165(02)00310-0. [DOI] [PubMed] [Google Scholar]
- Kuwabara I, Liu FT. Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol. 1996;156:3939–3944. [PubMed] [Google Scholar]
- Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara I, Yamanaka T, Hirashima M, Liu FT. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol. 2000;165:2156–2164. doi: 10.4049/jimmunol.165.4.2156. [DOI] [PubMed] [Google Scholar]
- Frigeri LG, Zuberi RI, Liu FT. Epsilon BP, a beta-galactoside-binding animal lectin, recognizes IgE receptor (Fc epsilon RI) and activates mast cells. Biochemistry. 1993;32:7644–7649. doi: 10.1021/bi00081a007. [DOI] [PubMed] [Google Scholar]
- Yamaoka A, Kuwabara I, Frigeri LG, Liu FT. A human lectin, galectin-3 (epsilon bp/Mac-2), stimulates superoxide production by neutrophils. J Immunol. 1995;154:3479–3487. [PubMed] [Google Scholar]
- Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- Sato S, Ouellet N, Pelletier I, Simard M, Rancourt A, Bergeron MG. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J Immunol. 2002;168:1813–1822. doi: 10.4049/jimmunol.168.4.1813. [DOI] [PubMed] [Google Scholar]
- Nieminen J, St Pierre C, Sato S. Galectin-3 interacts with naive and primed neutrophils, inducing innate immune responses. J Leukoc Biol. 2005;78:1127–1135. doi: 10.1189/jlb.1204702. [DOI] [PubMed] [Google Scholar]
- Lotan R, Belloni PN, Tressler RJ, Lotan D, Xu XC, Nicolson GL. Expression of galectins on microvessel endothelial cells and their involvement in tumour cell adhesion. Glycoconj J. 1994;11:462–468. doi: 10.1007/BF00731282. [DOI] [PubMed] [Google Scholar]
- Dumic J, Dabelic S, Flogel M. Galectin-3: An open-ended story. Biochim Biophys Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Fukumori T, Takenaka Y, Yoshii T, Kim HR, Hogan V, Inohara H, Kagawa S, Raz A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63:8302–8311. [PubMed] [Google Scholar]
- Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA. 1996;93:6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, Liu FT. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–1083. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, Izui S, Liu FT. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest. 2003;112:389–397. doi: 10.1172/JCI17592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohatsu L, Hsu DK, Jegalian AG, Liu FT, Baum LG. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J Immunol. 2006;177:4718–4726. doi: 10.4049/jimmunol.177.7.4718. [DOI] [PubMed] [Google Scholar]
- Sato S, Hughes RC. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem. 1994;269:4424–4430. [PubMed] [Google Scholar]
- Colnot C, Ripoche MA, Milon G, Montagutelli X, Crocker PR, Poirier F. Maintenance of granulocyte numbers during acute peritonitis is defective in galectin-3-null mutant mice. Immunology. 1998;94:290–296. doi: 10.1046/j.1365-2567.1998.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoue A, Clatworthy MR, Smith P, Green S, Townsend MJ, Jolin HE, Smith KG, Fallon PG, McKenzie AN. SIGN-R1 contributes to protection against lethal pneumococcal infection in mice. J Exp Med. 2004;200:1383–1393. doi: 10.1084/jem.20040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney MR, Su X, Van Ziffle JA, Lowell CA, Matthay MA. Neutrophils and their Fc gamma receptors are essential in a mouse model of transfusion-related acute lung injury. J Clin Invest. 2006;116:1615–1623. doi: 10.1172/JCI27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol. 2004;75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- Dransfield I, Stocks SC, Haslett C. Regulation of cell adhesion molecule expression and function associated with neutrophil apoptosis. Blood. 1995;85:3264–3273. [PubMed] [Google Scholar]
- Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods. 1999;232:3–14. doi: 10.1016/s0022-1759(99)00146-5. [DOI] [PubMed] [Google Scholar]
- Walrand S, Valeix S, Rodriguez C, Ligot P, Chassagne J, Vasson MP. Flow cytometry study of polymorphonuclear neutrophil oxidative burst: a comparison of three fluorescent probes. Clin Chim Acta. 2003;331:103–110. doi: 10.1016/s0009-8981(03)00086-x. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Funakoshi N, Onizuka M, Yanagi K, Ohshima N, Tomoyasu M, Sato Y, Yamamoto T, Ishikawa S, Mitsui T. A new model of lung metastasis for intravital studies. Microvasc Res. 2000;59:361–367. doi: 10.1006/mvre.2000.2238. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood. 1998;91:3430–3438. [PubMed] [Google Scholar]
- Fernández GC, Ilarregui JM, Rubel CJ, Toscano MA, Gomez SA, Beigier BM, Isturiz MA, Rabinovich GA, Palermo MS. Galectin-3 and soluble fibrinogen act in concert to modulate neutrophil activation and survival: involvement of alternative MAPK pathways. Glycobiology. 2005;15:519–527. doi: 10.1093/glycob/cwi026. [DOI] [PubMed] [Google Scholar]
- Feuk-Lagerstedt E, Jordan ET, Leffler H, Dahlgren C, Karlsson A. Identification of CD66a and CD66b as the major galectin-3 receptor candidates in human neutrophils. J Immunol. 1999;163:5592–5598. [PubMed] [Google Scholar]
- MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T: Regulation of alternative macrophage activation by galectin-3. J Immunol 2008 (in press). [DOI] [PubMed] [Google Scholar]