Abstract
Endosialin (Tem1) has been identified by two independent experimental approaches as an antigen of tumor-associated endothelial cells, and it has been claimed to be the most abundantly expressed tumor endothelial antigen, making it a prime candidate for vascular targeting purposes. Recent experiments have challenged the endothelial expression of endosialin and suggested an expression by activated fibroblasts and pericytes. Thus, clarification of the controversial cellular expression of endosialin is critically important for an understanding of its role during tumor progression and its validation as a potential therapeutic target. We have therefore performed extensive expression profiling analyses of endosialin. The experiments unambiguously demonstrate that endosialin is expressed by tumor-associated myofibroblasts and mural cells and not by endothelial cells. Endosialin expression is barely detectable in normal human tissues with moderate expression only detectable in the stroma of the colon and the prostate. Corresponding cellular experiments confirmed endosialin expression by mesenchymal cells and indicated that it may in fact be a marker of mesenchymal stem cells. Silencing endosialin expression in fibroblasts strongly inhibited migration and proliferation. Collectively, the experiments validate endosialin as a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. The data warrant further functional analysis of endosialin during tumor progression and its exploitation as marker of tumor vessel-associated mural cells, expression of which may reflect the non-normalized phenotype of the tumor vasculature.
Tumor progression and metastasis are critically dependent on the tumor stroma consisting of fibrous connective tissue, newly formed blood vessels, extracellular matrix, and recruited inflammatory cells. Tumor stromal fibroblasts (activated fibroblasts or myofibroblasts) acquire a specific phenotype that differentiates them from normal resting fibroblasts. They express smooth muscle actin (SMA), and isolated myofibroblasts have higher proliferation rates than their normal counterparts.1 The importance of stromal mesenchymal interactions with adjacent epithelial cells during development is well characterized. Proliferation of mesenchymal cells is tightly controlled by soluble paracrine factors. Conversely, differentiated fibroblasts do not proliferate and acquire a quiescent phenotype.2 In contrast, the complex paracrine regulatory mechanisms between the mesenchymal tumor stroma compartment and the tumor compartment are far from being understood. Coinjection of activated fibroblasts and tumor cells has been shown to enhance the invasiveness of colon tumors.3 Tumor-associated fibroblasts affect migration and invasion of tumor cells by secreting extracellular matrix proteins, matrix metalloproteinases, and growth factors.4,5 Paracrine-acting factors affecting tumor and stromal cell interactions include fibroblast growth factors, hepatocyte growth factor, and transforming growth factor-β. Correspondingly, elevated plasma levels of transforming growth factor-β have been shown to predict early metastasis.6 Lastly, activated fibroblasts exert important immune modulatory roles and affect inflammatory leukocyte recruitment by secreting factors such as interferon-γ, interleukin-6, and tumor necrosis factor-α.7 The search for markers of activated fibroblasts has led to the production of the FB5 antibody, which was generated by immunizing mice with fetal mesenchymal fibroblasts.8 Subsequent immunohistochemical analyses revealed that the FB5 antigen is not detectable in normal tissues. Surprisingly though, intense expression was detected in blood vessels of different tumors, including carcinomas, sarcomas and neuroectodermal tumors.8 As such, the FB5 antigen was the first marker molecule of activated, tumor-associated neovessels, a fact that was widely ignored throughout the 1990s. It was not until 2000 that a detailed serial analysis of gene expression analysis of tumor-associated endothelial cells identified the FB5 antigen as Tem1, the most abundantly expressed tumor endothelial marker. The Tem nomenclature was established by numbering serial analysis of gene expression hits according to frequency.9 The FB5 antigen Tem1 was at the same time affinity-purified and cloned as the highly sialylated transmembrane molecule endosialin. Endosialin consists of a 90-kDa core protein with an N-terminal C-type lectin domain followed by a Sushi domain and three EGF-like domains.10 Its structure suggests functions as an adhesion molecule, and its extensive negative charges correspond to the observation that angiogenic endothelial cells are strongly hypersialylated11 and preferentially bind cationic liposomes.12,13 However, no function has been ascribed to endosialin thus far. Endosialin-null mice develop normally, are fertile, and have physiological wound healing.14 However, abdominal implantation of tumor fragments in endosialin-null mice led to reduced tumor growth, invasiveness, and metastasis compared to wild-type mice, suggesting a mechanistically hitherto unexplained role of endosialin in tumor progression and metastasis.14 The experiments performed thus far appear to have solidly established endosialin as an important marker of (activated) tumor-associated endothelial cells. However, MacFadyen and co-workers have recently challenged the endothelial expression of endosialin and claimed rather that it is expressed by periendothelial mural cells (ie, pericytes) and activated tumor fibroblasts.15 We consequently designed experiments to definitely determine the expression of endosialin in normal and neoplastically transformed tissues. The experiments clarified that endosialin is not an endothelial cell antigen but rather is expressed by tumor stromal fibroblasts. More importantly, they solidly establish endosialin as a marker molecule of (activated) tumor-associated pericytes.
Materials and Methods
Primary Cells and Cell Lines
LA1-5s neuroblastoma cells were cultured in RPMI 1640 medium (PAA, Pasching, Austria) with 10% fetal calf serum and 1% penicillin/streptavidin (PAA). HeLa and A375 cells were cultured in Dulbecco’s modified Eagle’s medium (PAA) with 10% fetal calf serum and 1% penicillin/streptavidin (PAA). Smooth muscle cells, fibroblasts, human dermal microvascular endothelial cells, human umbilical vein endothelial cells, human umbilical artery endothelial cells (PromoCell, Heidelberg, Germany), and pericytes (ScienCell, San Diego, CA) were cultured according to the manufacturers’ protocols. Mesenchymal stem cells were isolated as previously described.16
siRNA
Control siRNA or human endosialin siRNA (Ambion, Austin, TX) were transfected using Oligofectamine reagent (Invitrogen, Carlsbad, CA).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Endosialin expression in endothelial cells and other cell types was analyzed by RT-PCR using 1 μg of total cellular RNA for reverse transcription. Endosialin and the expression of the housekeeping gene TBP were analyzed using the following primers: endosialin forward, 5′-AACCAGACCTCACCCATCAG-3′; endosialin reverse, 5′-GTTCTGTTGGGCTCTTGCTC-3′; TBP forward, 5′-ATGGATCAGAACAACAGCCTG-3′; TBP reverse, 5′-CCCTGTGTTGCCTGCTGGGA-3′.
Western Blot Analysis
Cell extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes for 1 hour at 100 V. The membranes were blocked overnight at 4°C with 5% milk powder in Tris-buffered saline/Tween 20 (140 mmol/L NaCl, 10 mmol/L Tris-HCl, pH 7.4, and 0.05% Tween 20) and incubated with primary antibody (0.5 to 2 μg/ml in Tris-buffered saline/Tween 20) for 1 hour at room temperature. Following extensive washings, the membranes were incubated with peroxidase-coupled secondary antibody. Bound antibody was detected with enhanced chemiluminescence (Amersham Biosciences, Uppsala, Sweden) and exposure to Biomax MR.
Zymography
Endosialin or control siRNA-transfected cells were cultured in basal medium. After 24 hours and 48 hours, the supernatant was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis/0.1% gelatin. The gel was incubated in renaturing buffer (2.5% Triton X-100, v/v, in H2O) for 30 minutes at room temperature, 30 minutes at room temperature in zymogram developing buffer (50 mmol/L Tris, 0.2 mol/L NaCl, 5 mmol/L CaCl2, 0.02% Brij), and overnight at 37°C in zymogram developing buffer. The gel was stained with Coomassie Blue R-250.
MTT Proliferation Assay
Endosialin and control siRNA-transfected cells were seeded into a 96-well tissue culture plate (1000 cells/well), and the proliferation assay was performed 24 hours, 48 hours, and 72 hours after seeding using the Cell Proliferation Kit I (Roche, Mannheim, Germany) according to the manufacturer’s protocol.
Cell Migration Assay
Endosialin or control siRNA-treated human fibroblasts (50,000 cells/well) were plated in Dulbecco’s modified Eagle’s medium/2% fetal calf serum into the upper chamber of gelatin-coated Transwells (Vitaris AG, Baar, Switzerland). Cells were stimulated with 50 ng/ml platelet-derived growth factor-BB (Peprotech Inc., Rodey Hill, NJ) in Dulbecco’s modified Eagle’s medium/10% fetal calf serum, which was added to the lower chamber. After 2 hours of incubation, cells were fixed with methanol and stained with Hoechst dye. Migrated cells were counted after removal of the cells on the upper side of the Transwell membrane using a cotton swab.
Immunocytochemistry and Immunohistochemistry
Cells were methanol-fixed, blocked for 1 hour with 10% goat serum, and incubated for 1 hour with mouse monoclonal anti-endosialin antibody FB5 (10 μg/ml) or ChromPure mouse IgG (Dianova, Hamburg, Germany) as negative control. Following extensive washings, bound antibody was detected after incubation with the adequate fluorescently labeled secondary antibody by confocal fluorescence microscopy. Nuclei were counterstained with propidium iodide (14 μg/ml) for 1 hour. Cryosections of normal and malignant human tissues (NCT, Heidelberg, Germany; BioCat, Heidelberg, Germany) were acetone-fixed, blocked with methanol/3% H2O2 for 30 minutes, avidin/biotin for 10 minutes each, and goat serum for 20 minutes before incubating with 20 μg/ml mouse monoclonal anti-endosialin antibody FB5 for 1 hour. ChromPure mouse IgG (Dianova) was used as negative control. Bound antibody was detected after treatment with EnVision + Dual Link HRP secondary antibody (Dako, Glostrup, Denmark) and diaminobenzidine solution (Dako) by light microscopy. Nuclei were stained with Mayer’s hemalaun. For immunofluorescence stainings, cryosections were fixed in acetone and blocked with avidin/biotin and 10% goat serum. After incubation with 20 μg/ml mouse monoclonal anti-endosialin antibody FB5, 20 μg/ml rabbit polyclonal anti-LYVE-1 antibody (ReliaTech, Braunschweig, Germany) or polyclonal rabbit anti-podoplanin serum (1:200; kindly provided by D. Kerjaschki, Medical University of Vienna, Vienna, Austria), slides were washed extensively. Bound antibodies were detected using a biotin-labeled anti-mouse antibody (Zymed, San Francisco, CA) and following treatment with corresponding streptavidin Alexa Fluor 488 (Invitrogen) or anti-rabbit-Cy3 antibody (Dianova). Nuclei were counterstained with Hoechst dye. For SMA/endosialin and CD31/endosialin fluorescence double stainings, the endosialin staining was accomplished as described above. Following detection of bound mouse monoclonal anti-endosialin antibody FB5 by Alexa Fluor 488-labeled streptavidin, slides were extensively washed and incubated with 3 μg/ml Cy3-labeled mouse monoclonal anti-α-SMA antibody or blocked with 10% donkey serum and incubated with 7.5 μg/ml sheep polyclonal anti-CD31 (PECAM-1) antibody for 1 hour. Bound anti-CD31 (PECAM-1) antibody was detected by incubation with Alexa Fluor 546-labeled donkey anti-sheep antibody (Invitrogen). Nuclei were counterstained with Hoechst dye.
Results
Endosialin (Tem1) Is Expressed by Fibroblasts and Mural Cells in Vitro and in Vivo
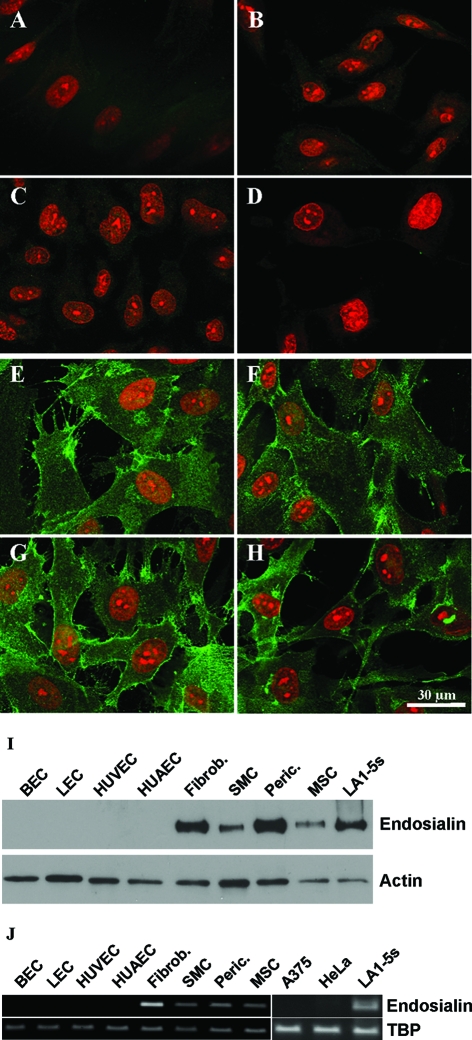
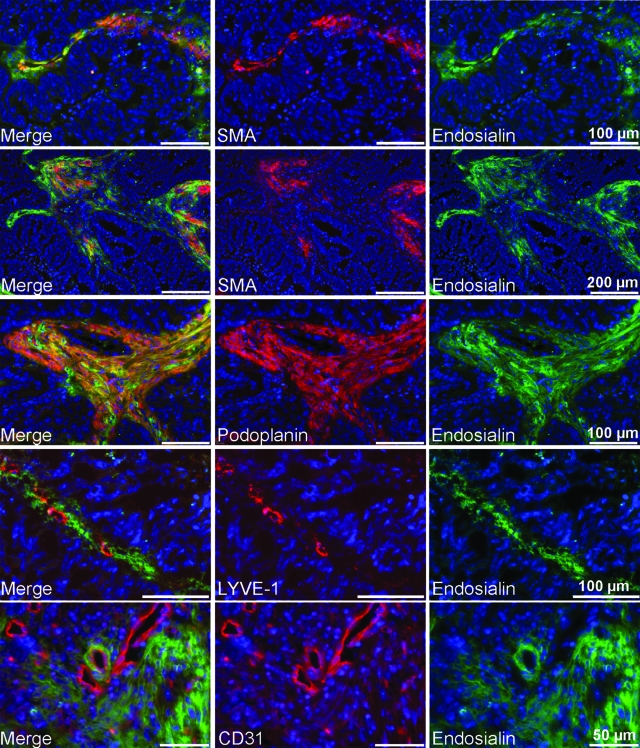
Immunocytochemistry experiments using the monoclonal antibody FB5 were performed on cultured cells to unambiguously define distinct stromal cell populations that express endosialin. These experiments revealed expression of endosialin by cultured primary fibroblasts, pericytes, and smooth muscle cells but not by human umbilical vein endothelial cells, human umbilical artery endothelial cells, primary blood endothelial cells, or lymphatic endothelial cells that were isolated by sorting human dermal microvascular endothelial cells for the lymphatic marker podoplanin (Figure 1, A–G). All endosialin-positive primary cells expressed the 165-kDa endosialin protein (Figure 1I). As all of the endosialin-expressing primary cells were of mesenchymal origin, we analyzed endosialin expression in mesenchymal stem cells isolated from human fat tissue. As shown in Figure 1H, endosialin is also expressed by mesenchymal stem cells, suggesting that it is an activation/stem cell marker of the mesenchymal lineage. To exclude the possibility that the specific expression of endosialin may be due to the monoclonal antibody FB5 recognizing a specific modification on the endosialin protein, we used RT-PCR to compare endosialin expression in different primary human cells and tumor cell lines (Figure 1J). Endosialin mRNA was strongly expressed by human fibroblasts, smooth muscle cells, pericytes, mesenchymal stem cells, and the neuroblastoma cell line LA1–5s but not by blood endothelial cells, lymphatic endothelial cells, human umbilical vein endothelial cells, human umbilical artery endothelial cells, the amelanotic melanoma cell line A375, or the cervical cancer cell line HeLa. To extend the expression analysis, we performed double-staining experiments of human colon carcinomas in vivo using the monoclonal antibody FB5 and antibodies against the pan-endothelial marker CD31 and the lymphatic marker LYVE-1, as well as against the mural cell marker SMA and the stromal marker podoplanin. As shown in Figure 2, endosialin (Tem1) was found strongly expressed in different compartments of the tumor stroma. It did not colocalize with CD31- or LYVE1-positive cells, but it colocalized with SMA-positive vessel-associated mural cells and podoplanin/SMA-positive strands of the tumor stroma. Since podoplanin and SMA have been shown to be expressed by activated fibroblasts (ie, myofibroblasts), these results confirmed the in vitro results of endosialin being expressed by mural cells and activated fibroblasts.
Figure 1.
Analysis of endosialin expression in cultured cells. Different human primary cells were stained for endosialin. No endosialin expression was detectable in primary blood endothelial cells (A), lymphatic endothelial cells (B), human umbilical vein endothelial cells (C) or human umbilical artery endothelial cells (D). In contrast, fibroblasts (E), smooth muscle cells (F), pericytes (G), and mesenchymal stem cells (H) expressed endosialin. Western blot analysis using monoclonal antibody FB5 detected a 165-kDa protein in fibroblasts, smooth muscle cells, pericytes, and mesenchymal stem cells but not in blood endothelial cells, lymphatic endothelial cells, human umbilical vein endothelial cells, or human umbilical artery endothelial cells. Human actin was used as loading control (I). Expression of endosialin was confirmed by RT-PCR. TBP was used as loading control (J).
Figure 2.
Endosialin expression in human tissues. Cryosections of human colon carcinomas were double stained using the monoclonal antibody FB5 and antibodies against SMA, podoplanin, LYVE-1, or CD31. Endosialin was found to be expressed by smooth muscle actin-positive vessel-like structures as well as in podoplanin/SMA-positive strands of fibroblasts. Endosialin did not colocalize with the lymphatic marker LYVE-1 or the blood endothelial marker CD31.
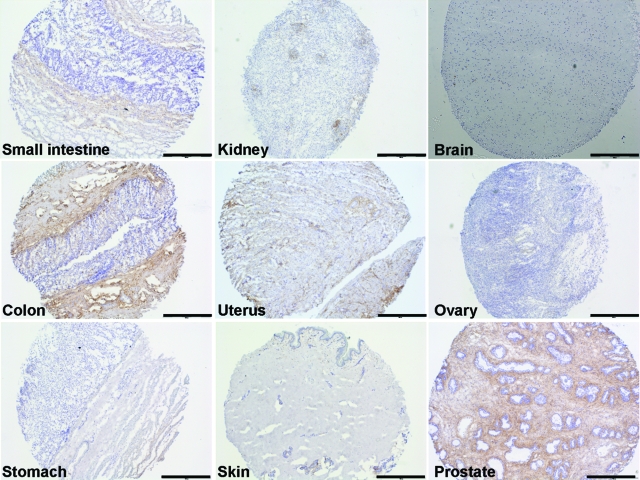
Endosialin Is Weakly Expressed in Normal Human Tissues and Strongly Up-Regulated in Tumors
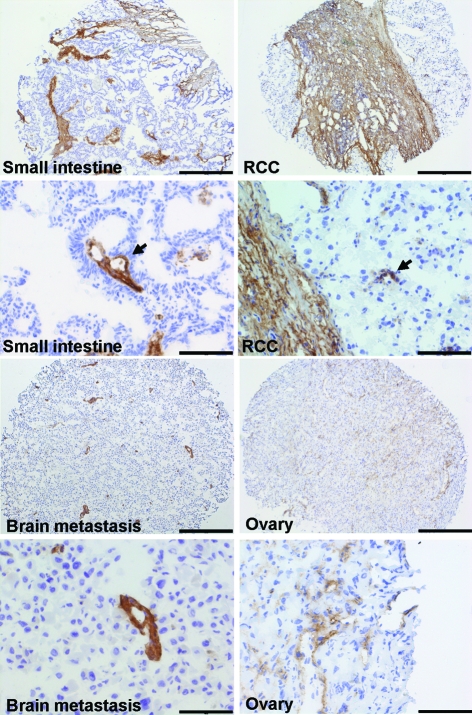
To extend the expression profiling to different human tissues in vivo, we performed immunohistochemistry on a range of normal human tissues (Figure 3). In most of the tissues tested, endosialin was either not detectable (brain, ovary, stomach, skin) or only weakly expressed (small intestine, kidney, uterus). Intense expression of the endosialin protein was detectable in the smooth muscle cell layer of normal colon and in normal prostate. The expression of endosialin was in all samples restricted to mesenchymal tissue structures and completely absent in epithelial cell layers. Likewise, no endosialin-positive endothelial cells or periendothelial cells (pericytes) could be detected in normal tissues. We continued our analysis of endosialin expression to a range of human tumors. In none of the analyzed tumors was endosialin expression detectable in the tumor cell compartment itself. Instead, expression was strictly restricted to the host-derived tumor stroma in which it could be detected in most tested malignant tissues (Figure 4). Only prostate tumors exhibited a different behavior with detectable expression in normal prostate tissue and virtually no expression in corresponding tumors (Figure 3 and data not shown). The intensity of endosialin expression in the tumor stroma varied considerably and appeared to reflect tumor-type specific differences in the recruitment of host-derived tumor stroma. Carcinomas of the small intestine and the kidney expressed endosialin in wide strands of activated fibroblasts that represented a major percentage of total tumor volume (Figure 4, small intestine and RCC). In addition, expression was detectable in tumor neovessel-associated mural cells (Figure 4, arrow). Similar results were obtained for carcinomas of the colon and the uterus (data not shown). In other tumor types, the tumor stroma represented only a smaller fraction of total tumor volume. For example, mainly vascular pericytes/smooth muscle cells and only a few activated fibroblasts were positive for the monoclonal antibody FB5 in a melanoma metastasis to the brain (Figure 4, brain metastasis) and tumors of the ovary (Figure 4, ovary).
Figure 3.
Expression of endosialin in normal tissues. Immunohistochemical analysis of endosialin expression on different normal human tissues (small intestine, kidney, brain, colon, uterus, ovary, stomach, skin, prostate) revealed no or very weak expression of endosialin in normal tissues with the exceptions of prostate and of smooth muscle cells in the colon. Scale bars = 500 μm.
Figure 4.
Endosialin expression in human tumors. The immunohistochemical analysis of human tumors [small intestine, renal cell carcinoma (RCC), melanoma metastasis to the brain, adenocarcinoma of the ovary] identified intense endosialin expression in the host-derived tumor stroma. Scale bars: 500 μm in the upper panels and 100 μm in the lower panels.
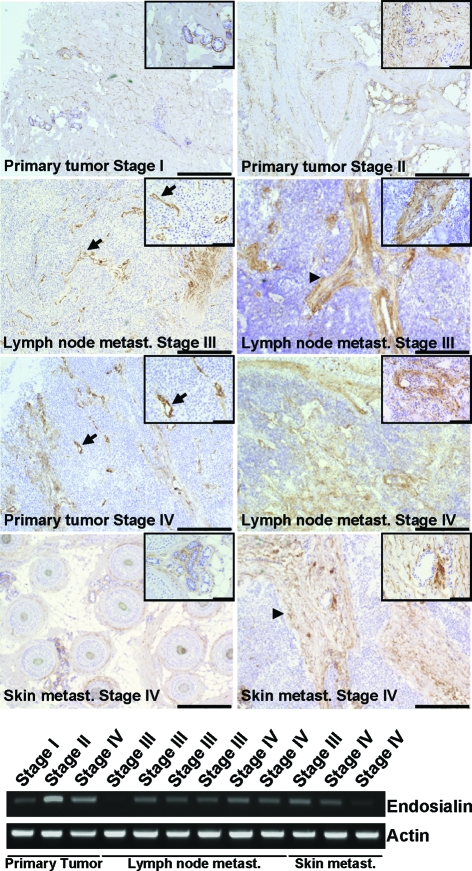
Endosialin Expression by Myofibroblasts Is Enhanced during Melanoma Progression
As endosialin was found only weakly expressed in normal human tissues and strongly up-regulated in malignant tissues, we analyzed gradual changes of endosialin expression during human melanoma progression. Toward this end, serial comparisons of endosialin expression in primary tumors, lymph node metastases and distant skin metastases were examined. Endosialin-positive structures were detectable in all analyzed samples (Figure 5). Endosialin expression did not change during disease progression as determined by RT-PCR (Figure 5). Interestingly, endosialin-positive structures varied between different melanoma samples with some tumors expressing endosialin predominately in vessel-associated mural cells (Figure 5, arrow) and some tumors expressing endosialin primarily in strands of myofibroblasts (Figure 5, arrowhead). Endosialin-positive myofibroblasts were clearly more abundant in melanoma metastases (stages III and IV) compared to primary tumors (stages I and II).
Figure 5.
Endosialin expression during melanoma progression. The immunohistochemical analysis of human melanoma samples originating from primary tumors, lymph node, and distant skin metastases of different malignant stages (I to IV) using the monoclonal anti-endosialin antibody FB5 identified endosialin expression in vessel-associated mural cells (arrow) and stromal fibroblasts (arrowhead). Scale bars represent 500 μm and 100 μm, respectively. Endosialin expression was compared on the mRNA level by RT-PCR. Actin was used as loading control.
Endosialin Expression Affects Migration and Proliferation of Primary Human Fibroblasts
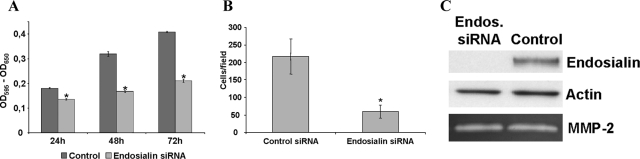
The experiments performed thus far had solidly established endosialin as a marker of the tumor-associated connective tissue consisting of activated fibroblasts. We consequently performed siRNA-based loss-of-function experiments to study whether endosialin might play a role in cellular functions related to the recruitment of fibroblasts to tumors. Transfection of human fibroblasts with endosialin siRNA led to robustly reduced steady-state levels of endosialin protein expression (Figure 6C). Comparative analysis of endosialin-silenced and control siRNA-transfected fibroblasts revealed a major role in controlling fibroblast migration and proliferation. Silencing of endosialin expression inhibited platelet-derived growth factor-BB-induced fibroblast transmigration by almost 80% (Figure 6B). Likewise, endosialin down-regulation significantly reduced fibroblast proliferation (Figure 6A). The effects of endosialin silencing on fibroblast migration and proliferation were not due to a difference in the secretion or activation of matrix metalloproteinases as evidenced by an unaltered zymogram of supernatants from control-transfected and endosialin siRNA-transfected fibroblasts (Figure 6C).
Figure 6.
Cellular loss-of-function experiments by siRNA-mediated silencing of endosialin expression. MTT proliferation assay comparing endosialin-silenced and control siRNA-transfected fibroblasts revealed a pronounced reduction of cell proliferation after silencing of endosialin expression. The graphs show the mean value ± SD of three experiments; *P < 0.001 (A). Down-regulation of endosialin leads to a decrease in cell migration. Transwell migration assay comparing endosialin-silenced and control siRNA-transfected fibroblasts revealed a strong reduction in cell migration after down-regulation of endosialin. The graphs show the mean value ± SD of three independent experiments; *P < 0.001 (B). Robust down-regulation of endosialin protein expression after siRNA transfection was verified by Western blot analysis using monoclonal antibody FB5 and anti-actin as loading control. Expression of matrix metalloproteinase-2 was not changed as determined by zymography. Active forms of other matrix metalloproteinases were not detectable (C).
Discussion
We have identified endosialin as a marker of activated mesenchymal cells/mesenchymal stem cells in vitro and in the tumor stroma in vivo where it is expressed by activated fibroblasts (ie, myofibroblasts) and neovessel mural cells (ie, pericytes and smooth muscle cells). Furthermore, we have obtained the first experimental evidence that endosialin controls cellular functions of activated fibroblasts by affecting their migration and proliferation. Endosialin was first described as a marker of tumor endothelial cells,8 which was confirmed years later by an independent experimental approach.9 Recently, endosialin has again been claimed to be an endothelial cell marker molecule.17 In turn, MacFadyen and co-workers have challenged the endothelial expression of endosialin and reported endosialin expression in fibroblasts and pericytes in the stroma of different breast tumors and in pericytes of the developing brain.15 However, a detailed expression analysis of endosialin in the stroma of different human tumors and their corresponding normal tissues has not been performed thus far. However, a detailed analysis of the cell types expressing endosialin is also timely and important in view of the recently reported site-specific tumor progression and metastasis phenotype of endosialin-deficient mice.14 We found endosialin expression by activated fibroblasts and mural cells in the stroma of human colon carcinomas. An extensive analysis of different tumors and the corresponding normal tissue identified endosialin as a protein that is strongly up-regulated in the tumor tissue with the exception of human prostate. The expression pattern on activated mesenchymal cells was confirmed by expression studies in vitro, where endosialin was shown to be expressed by primary human pericytes, smooth muscle cells, and fibroblasts. Interestingly, endosialin is also expressed by mesenchymal stem cells isolated from human fat, indicating that the protein may be a more general marker for activated mesenchymal cells.18 This point is strengthened by the observation by Rettig and colleagues that neuroblastoma cells (LA1-5s) are commonly positive when tested for endosialin expression.8 Neuroblastomas are the most common malignant childhood tumor. They originate from undifferentiated cells of the neural crest, which has also been shown to be the source for mesenchymal stem cells during development.19
Endosialin is widely considered as an abundantly expressed marker of tumor endothelial cells.8,9,17 We found no expression of the protein on blood or lymphatic endothelial cells in vitro and in vivo. Interestingly, the monoclonal antibody FB5 that was used for the identification and purification of endosialin was generated against human embryonic fibroblasts, strengthening the point of endosialin expression by activated mesenchymal cells. The serial analysis of gene expression analysis that has led to the tumor endothelial identification of endosialin was based on the use of the anti-CD146 monoclonal antibody P1H12.9 The specific expression of the antigen recognized by P1H12 is controversial as it has been shown either to be specific for endothelial cells or to detect endothelial cells and activated mural cells.15,20 Thus, the validation of endosialin as a mural and not endothelial cell marker also questions the endothelial specificity of the P1H12 antibody and likely suggests a careful reconsideration of the Tem nomenclature. Endosialin-deficient mice have been reported to develop normally. They also display physiological wound healing. Tumor cells injected subcutaneously into these mice grow normally with unaltered tumor angiogenesis. Yet, tumor fragments implanted into an abdominal site displayed a reduction in tumor growth and invasion compared to wild-type mice.14 The authors describe this effect as an organ-specific role of endosialin during tumor progression. However, as no detailed analyses of stroma recruitment for the different tumor models were performed, it may be more likely that subcutaneously growing tumors have different stromal fibroblast recruitment properties as primary tumors or tumors grown in the abdominal cavity. Thus, the study of tumor/activated fibroblast interactions during tumor progression likely requires improved animal models that better mimic the growth and progression of human tumors. The promigratory and proinvasive phenotype of tumors grown in wild-type mice compared to endosialin-null mice also suggests a role of endosialin in cell migration and/or matrix remodeling.21 Our cellular loss-of-function experiments showed no differences in matrix metalloproteinase secretion but a strong effect on cell migration and proliferation. Factors and microenvironmental conditions regulating endosialin expression have not yet been defined.
Our data show that activated mesenchymal cells (fibroblasts, pericytes, and smooth muscle cells) are recruited to the stroma of human tumors. Endosialin is a specific marker for these activated mesenchymal stroma cells and controls their migration and proliferation. Our results strongly suggest that the tumor stroma influences tumor cells to generate a proinvasive phenotype. As the expression of endosialin correlates with a proproliferative, promigratory phenotype, targeting stromally expressed endosialin may provide an attractive target to interfere therapeutically with stroma recruitment, proliferation, and metastasis.22 The identification of endosialin as an activation marker for activated fibroblast and activated pericytes has important implications for tumor biology as well as for antitumor therapies. Thus far, only few activation markers for the tumor stroma compartment have been described. This is particularly relevant for tumor vessel-associated pericytes for which the regulator of G-protein signaling-5 (RGS-5) is hitherto the only identified activation marker during neovascularization.23,24 However, in contrast to endosialin, RGS-5 expression is described not only for pericytes but also for cytokine-activated endothelial cells in models of atherosclerosis and during capillary tube formation.25,26,27 Therefore, endosialin is an attractive target for the development of novel antiangiogenic therapies. Immature blood vessels are not covered by mural cells.28 As such, they are more responsive to antiangiogenic therapies. Pruning of immature vessels during antiangiogenesis leads to tumor vessel normalization.28 As such, mural cell coverage defines the therapeutic window of antiangiogenic therapies. The combinatorial targeting of both endothelial cells and pericytes is a very promising approach for improved antiangiogenic therapies.29 Consequently, the unambiguous clarification of the cellular expression of endosialin more than resolves an important controversial scientific issue. Its characterization as a marker of tumor-associated mural cells also opens novel avenues for basic tumor progression as well as translational antiangiogenesis research.
Footnotes
Address reprint requests to Dr. Hellmut G. Augustin, Joint Research Division Vascular Biology, Medical Faculty Mannheim, University of Heidelberg, Im Neuenheimer Feld 581, D-69120 Heidelberg, Germany. E-mail: augustin@angiogenese.de.
Supported by grant SPP1190 “The Tumor-Vessel Interface” (Au83/9-1) from the Deutsche Forschungsgemeinschaft and grant LSHG-CT-2004-503573 from the European Union.
S.C. and R.W. contributed equally to this work.
References
- Tsukada T, Tippens D, Gordon D, Ross R, Gown AM. HHF35, a muscle-actinspecific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987;126:51–60. [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimanche-Boitrel MT, Vakaet L, Jr, Pujuguet P, Chauffert B, Martin MS, Hammann A, Van RF, Mareel M, Martin F. In vivo and in vitro invasiveness of a rat colon-cancer cell line maintaining E-cadherin expression: an enhancing role of tumor-associated myofibroblasts. Int J Cancer. 1994;56:512–521. doi: 10.1002/ijc.2910560410. [DOI] [PubMed] [Google Scholar]
- Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Yang F, Tuxhorn JA, Ressler SJ, McAlhany SJ, Dang TD, Rowley DR. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005;65:8887–8895. doi: 10.1158/0008-5472.CAN-05-1702. [DOI] [PubMed] [Google Scholar]
- Tsushima H, Ito N, Tamura S, Matsuda Y, Inada M, Yabuuchi I, Imai Y, Nagashima R, Misawa H, Takeda H, Matsuzawa Y, Kawata S. Circulating transforming growth factor beta 1 as a predictor of liver metastasis after resection in colorectal cancer. Clin Cancer Res. 2001;7:1258–1262. [PubMed] [Google Scholar]
- Silzle T, Randolph GJ, Kreutz M, Kunz-Schughart LA. The fibroblast: sentinel cell and local immune modulator in tumor tissue. Int J Cancer. 2004;108:173–180. doi: 10.1002/ijc.11542. [DOI] [PubMed] [Google Scholar]
- Rettig WJ, Garin-Chesa P, Healey JH, Su SL, Jaffe EA, Old LJ. Identification of endosialin, a cell surface glycoprotein of vascular endothelial cells in human cancer. Proc Natl Acad Sci USA. 1992;89:10832–10836. doi: 10.1073/pnas.89.22.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Christian S, Ahorn H, Koehler A, Eisenhaber F, Rodi HP, Garin-Chesa P, Park JE, Rettig WJ, Lenter MC. Molecular cloning and characterization of endosialin, a C-type lectin-like cell surface receptor of tumor endothelium. J Biol Chem. 2001;276:7408–7414. doi: 10.1074/jbc.M009604200. [DOI] [PubMed] [Google Scholar]
- Augustin HG, Braun K, Telemenakis I, Modlich U, Kuhn W. Ovarian angiogenesis: phenotypic characterization of endothelial cells in a physiological model of blood vessel growth and regression. Am J Pathol. 1995;147:339–351. [PMC free article] [PubMed] [Google Scholar]
- McLean JW, Fox EA, Baluk P, Bolton PB, Haskell A, Pearlman R, Thurston G, Umemoto EY, McDonald DM. Organ-specific endothelial cell uptake of cationic liposome-DNA complexes in mice. Am J Physiol. 1997;273:H387–H404. doi: 10.1152/ajpheart.1997.273.1.H387. [DOI] [PubMed] [Google Scholar]
- Thurston G, McLean JW, Rizen M, Baluk P, Haskell A, Murphy TJ, Hanahan D, McDonald DM. Cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in mice. J Clin Invest. 1998;101:1401–1413. doi: 10.1172/JCI965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda A, Karim B, Peng Z, Liu G, Qiu W, Gan C, Vogelstein B, St Croix B, Kinzler KW, Huso DL. Tumor endothelial marker 1 (Tem1) functions in the growth and progression of abdominal tumors. Proc Natl Acad Sci USA. 2006;103:335–13356. doi: 10.1073/pnas.0511306103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFadyen JR, Haworth O, Roberston D, Hardie D, Webster MT, Morris HR, Panico M, Sutton-Smith M, Dell A, van der GP, Wienke D, Buckley CD, Isacke CM. Endosialin (TEM1. CD248) is a marker of stromal fibroblasts and is not selectively expressed on tumour endothelium. FEBS Lett. 2005;579:2569–2575. doi: 10.1016/j.febslet.2005.03.071. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Karlsson M, Pertoft H, Pettersson P, Sjostrom L, Smith U. Isolation and characterization of cells from rat adipose tissue developing into adipocytes. J Lipid Res. 1978;19:316–324. [PubMed] [Google Scholar]
- Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11:539–554. doi: 10.1016/j.ccr.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker A, Le BK, Astrom G, van H, V, Gotherstrom C, Blomqvist L, Arner P, Ryden M. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res. 2005;308:283–290. doi: 10.1016/j.yexcr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Riley RD, Heney D, Jones DR, Sutton AJ, Lambert PC, Abrams KR, Young B, Wailoo AJ, Burchill SA. A systematic review of molecular and biological tumor markers in neuroblastoma. Clin Cancer Res. 2004;10:4–12. doi: 10.1158/1078-0432.ccr-1051-2. [DOI] [PubMed] [Google Scholar]
- Bagley RG, Weber W, Rouleau C, Teicher BA. Pericytes and endothelial precursor cells: cellular interactions and contributions to malignancy. Cancer Res. 2005;65:9741–9750. doi: 10.1158/0008-5472.CAN-04-4337. [DOI] [PubMed] [Google Scholar]
- Stefanidakis M, Koivunen E. Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression. Blood. 2006;108:1441–1450. doi: 10.1182/blood-2006-02-005363. [DOI] [PubMed] [Google Scholar]
- Teicher BA. Newer vascular targets: endosialin (review). Int J Oncol. 2007;30:305–312. [PubMed] [Google Scholar]
- Berger M, Bergers G, Arnold B, Hammerling GJ, Ganss R. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood. 2005;105:1094–1101. doi: 10.1182/blood-2004-06-2315. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114:2755–2773. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- Horrevoets AJ, Fontijn RD, van Zonneveld AJ, de Vries CJ, ten Cate JW, Pannekoek H. Vascular endothelial genes that are responsive to tumor necrosis factor-alpha in vitro are expressed in atherosclerotic lesions, including inhibitor of apoptosis protein-1, stannin, and two novel genes. Blood. 1999;93:3418–3431. [PubMed] [Google Scholar]
- Bondjers C, Kalen M, Hellstrom M, Scheidl SJ, Abramsson A, Renner O, Lindahl P, Cho H, Kehrl J, Betsholtz C. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol. 2003;162:721–729. doi: 10.1016/S0002-9440(10)63868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]