Abstract
Podocyte dysfunction plays an essential role in the pathogenesis of proteinuria and glomerulosclerosis. However, the mechanism underlying podocyte dysfunction in many common forms of chronic kidney diseases remains poorly understood. Here we tested the hypothesis that podocytes may undergo epithelial-to-mesenchymal transition after injury. Conditionally immortalized mouse podocytes were incubated with transforming growth factor (TGF)-β1, a potent fibrogenic cytokine that is up-regulated in the diseased kidney. TGF-β1 suppressed the slit diaphragm-associated protein P-cadherin, zonula occludens-1, and nephrin, a change consistent with loss of the epithelial feature. Meanwhile, TGF-β1 induced the expression of the intermediate filament protein desmin and interstitial matrix components fibronectin and collagen I. Furthermore, TGF-β1 promoted the expression and secretion of matrix metalloproteinase-9 by podocytes. Functionally, TGF-β1 increased albumin permeability across podocyte monolayers, as demonstrated by a paracellular albumin influx assay. The expression of Snail, a key transcriptional factor that has been implicated in initiating epithelial-to-mesenchymal transition, was induced by TGF-β1, and ectopic expression of Snail suppressed P-cadherin and nephrin in podocytes. In vivo, in addition to loss of nephrin and zonula occludens-1, mesenchymal markers such as desmin, fibroblast-specific protein-1, and matrix metalloproteinase-9 could be observed in glomerular podocytes of diabetic nephropathy. These results suggest that podocyte dedifferentiation and mesenchymal transition could be a potential pathway leading to their dysfunction, thereby playing a role in the genesis of proteinuria.
Proteinuria, the clinical manifestation of structural and functional defects in glomerular filtration barrier, occurs often in the early stage of many forms of primary glomerular diseases. A large body of evidence suggests that the podocyte foot processes and slit diaphragm are pivotal components of the glomerular filter, and disruption of their integrity is a critical event in the development of proteinuria and nephrotic syndrome in a variety of inherited and acquired glomerular disorders.1,2,3 Many genetic studies have underscored that podocyte slit diaphragm-associated proteins, such as nephrin and podocin, play an essential role in establishing the size-selective filtration barrier of the kidney, and mutations or deletions of the genes encoding these proteins are consequently associated with the development of proteinuria in both animal models and patients.4,5,6,7 However, mutations in the slit diaphragm-associated proteins are rare in most common forms of chronic kidney diseases such as diabetic nephropathy.8,9,10 In this regard, the pathogenesis of podocyte dysfunction in the vast majority of acquired proteinuric kidney diseases remains to be elucidated.
Podocytes are specialized, terminally differentiated visceral epithelial cells that reside on the glomerular basement membrane (GBM) outside the glomerular capillaries.1,3 In response to injurious stimuli, they often undergo a range of adaptive changes, including hypertrophy, dedifferentiation, detachment, and apoptosis, depending on the severity and duration of the injury.11,12,13 Because of their limited proliferative capacity, podocyte detachment from GBM and apoptosis will inevitably lead to cell depletion or drop out, which could reduce podocyte density, results in an impaired glomerular filtration, and causes proteinuria.14,15,16,17 However, podocyte depletion often takes place in the advanced stage of chronic kidney disease in which proteinuria is already prominent. Recent experimental evidence also demonstrates that podocyte detachment and apoptosis significantly lag behind the onset of proteinuria, arguing against a causative role of podocyte loss in the genesis of proteinuria.18,19 In this context, it is conceivable to speculate that the aberrant regulation of podocyte differentiation or function, rather than podocyte depletion, could be an initial cause of proteinuria in many clinical circumstances.
Similar to the cells in most parts of the nephron, podocytes are developmentally derived from the metanephric mesenchyme through mesenchymal-to-epithelial transdifferentiation. This raises an interesting possibility that the podocyte may undergo epithelial-to-mesenchymal transition (EMT), a process of reverse embryogenesis that occurs in diseased kidney as well as in many other organs under pathological conditions.20,21 It is widely recognized that renal tubular epithelial cells are able to undergo EMT after chronic injury22,23,24; a process that is believed to play a critical role in generating matrix-producing fibrogenic cells and in causing tubular atrophy and dysfunction.25,26 In analogy to tubular EMT, we hypothesized that mesenchymal transition of podocytes after injury may play a vital role in causing podocyte dysfunction that ultimately leads to a defective glomerular filtration.
In this study, we have investigated the possibility of podocyte EMT in a conditionally immortalized mouse podocyte cell line by incubating with transforming growth factor (TGF)-β1, a potent EMT inducer that is up-regulated in diseased kidneys.27 Our results suggest that EMT could be a potential pathway leading to podocyte dysfunction and proteinuria under pathological conditions.
Materials and Methods
Cell Culture and Treatment
The conditionally immortalized mouse podocyte cell line was kindly provided by Dr. Peter Mundel (Mount Sinai School of Medicine, New York, NY).28 To propagate podocytes, cells were cultured at 33°C in RPMI 1640 medium supplemented with 10% fetal bovine serum and 10 U/ml mouse recombinant interferon-γ (R&D Systems, Minneapolis, MN) to enhance the expression of a thermosensitive T antigen. To induce differentiation, podocytes were grown under nonpermissive conditions at 37°C in the absence of interferon-γ for 14 days. Podocytes were treated under differentiating condition with recombinant TGF-β1 at the concentration of 2 ng/ml, unless otherwise indicated. For some experiments, podocytes were kept in RPMI 1640 medium supplemented with 100 nmol/L 1,25-dihydroxyvitamin D3 and 1 μmol/L all-trans retinoic acid (Sigma, St. Louis, MO) to induce nephrin expression, as recently described elsewhere.29 Cells were then collected at different time points for subsequent analyses. For some studies, podocytes were transfected with Snail expression vector (pHA-Snail; provided by A. Garcia de Herreros, Universitat Pompeu Fabra, Barcelona, Spain) by using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA).
Animal and Human Tissue Samples
Mouse kidney tissues were prepared from a uninephrectomized diabetic model, as described previously.30 Briefly, male CD-1 mice underwent uninephrectomy 1 week before intravenous injection of streptozotocin (Sigma) at 150 mg/kg body weight. A group of mice that underwent sham operation and received no streptozotocin injection served as normal control. Three months after injection of streptozotocin, mice were sacrificed, and the kidney was analyzed as described previously.30 Human diabetic kidney specimens were obtained from diagnostic renal biopsy performed at the University of Pittsburgh Medical Center. As normal controls, nontumor kidney tissue from the patients who had renal cell carcinoma and underwent nephrectomy was used. All studies involving animal model and human tissues were approved by the Institutional Animal Care and Use Committee and the Institutional Review Board, respectively, at the University of Pittsburgh.
Western Blot Analysis
Western blot analysis for specific protein expression was performed essentially according to an established procedure.31,32 The primary antibodies used were as follows: rat monoclonal anti-P-cadherin (R&D Systems), rabbit polyclonal anti-desmin (MP Biomedicals, Solon, OH), anti-α-tubulin, and anti-matrix metalloproteinase (MMP)-9 (Sigma), and anti-fibronectin (sc-9068; Santa Cruz Biotechnology, Santa Cruz, CA). Quantification was performed by measuring the intensity of the bands with the use of the National Institutes of Health Image analysis software.
Immunostaining
Indirect immunofluorescence staining was performed using an established procedure.31,33 Briefly, cells cultured on coverslips were fixed with cold methanol:acetone (1:1) for 10 minutes at −20°C, followed by blocking with 20% normal donkey serum in phosphate-buffered saline (PBS) for 30 minutes at room temperature. Cells were incubated with the specific primary antibodies against P-cadherin, desmin, fibronectin, and MMP-9, as described above, and rabbit polyclonal anti-ZO-1 (61-7300, Invitrogen). To visualize the primary antibodies, cells were stained with cyanine Cy2-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). As a negative control, the primary antibody was replaced with nonimmune IgG, and no staining occurred. Cells were double stained with 4′,6-diamidino-2-phenylindole HCl to visualize the nuclei. Kidney sections from paraffin-embedded tissues were prepared at 4-μm thickness and stained for WT-1 and TGF-β type I receptor (Santa Cruz Biotechnology), desmin, MMP-9, ZO-1, nephrin (Progen, Heidelberg, Germany) and fibroblast-specific protein-1 (FspI, also known as S100A4; DAKO, Carpinteria, CA), respectively, using a routine procedure.33 Stained slides were viewed under an Eclipse E600 Epi-fluorescence microscope equipped with a digital camera (Nikon, Melville, NY).
Gelatin Zymographic Analysis
Zymographic analysis of MMP proteolytic activity in the supernatant of cultured cells was performed according to the method described previously.22,32 Briefly, a constant amount of protein from the conditioned media (15 μg) was loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel containing 1 mg/ml of gelatin (Bio-Rad Laboratories, Hercules, CA). After electrophoresis, the gel was incubated at 37°C for 16 to 36 hours in a developing buffer containing 50 mmol/L Tris-HCl, pH 7.6, 0.2 mol/L NaCl, 5 mmol/L CaCl2, and 0.02% Brij 35. The gel was then stained with a solution of 30% methanol, 10% glacial acetic acid, and 0.5% Coomassie blue G250, followed by destaining in the same solution without dye. Proteinase activity was detected as unstained bands on a blue background representing areas of gelatin digestion.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
Total RNA was prepared using a TRIzol RNA isolation system according to the instructions specified by the manufacturer (Invitrogen). The first strand of cDNA was synthesized using 2 μg of RNA in 20 μl of reaction buffer using AMV-RT (Promega, Madison, WI) and random primers at 42°C for 30 minutes. PCR was performed using a standard PCR kit on 1-μl aliquots of cDNA and HotStarTaq polymerase (Qiagen Inc., Valencia, CA) with specific primer pairs. The sequences of primer pairs are shown in Table 1. The PCR products were size fractionated on a 1.0% agarose gel and detected by ethidium bromide staining. No detectable signal was found in a parallel control tube without RT (data not shown).
Table 1.
The Sequences of the PCR Primer Pairs Used in This Study
| Gene | GenBank accession number | Sense | Antisense | Product size |
|---|---|---|---|---|
| ZO-1 | NM_009386 | 5′-TAGCACGGACAGTAGACACA-3′ | 5′-ATGGAAGTTGGGGTTCATAG-3′ | 635 (α+) |
| Fibronectin | NM_010233 | 5′-CGAGGTGACAGAGACCACAA-3′ | 5′-CTGGAGTCAAGCCAGACACA-3′ | 149 |
| Collagen I | NM_007742 | 5′-ATCTCCTGGTGCTGATGGAC-3′ | 5′-ACCTTGTTTGCCAGGTTCAC-3′ | 154 |
| Desmin | NM_010043 | 5′-TGCAGCCACTCTAGCTCGTA-3′ | 5′-GACATGTCCATCTCCACCTG-3′ | 150 |
| MMP-9 | NM_013599 | 5′-CACCACCACAACTGAACCAC-3′ | 5′-CTCAGAAGAGCCCGCAGTAG-3′ | 199 |
| MMP-2 | NM_008610 | 5′-AAGGGGATCCAGGAGCTCTA-3′ | 5′-GCTTGTCACGTGGTGTCAC-3′ | 199 |
| Snail | NM_011427 | 5′-AGCCCAACTATAGCGAGCTG-3′ | 5′-CCAGGAGAGAGTCCCAGATG-3′ | 150 |
| β-Actin | NM_007393 | 5′-CAGCTGAGAGGGAAATCGTG-3′ | 5′-CGTTGCCAATAGTGATGACC-3′ | 150 |
| P-cadherin | X06340 | 5′-GATTTTGAGGCTCAGGACCA-3′ | 5′-GACCTTGGAAGGTGGAACAA-3′ | 150 |
| Nephrin | NM_019459 | 5′-CCCAGGTACACAGAGCACAA-3′ | 5′-CTCACGCTCACAACCTTCAG-3′ | 200 |
Albumin Influx Assay
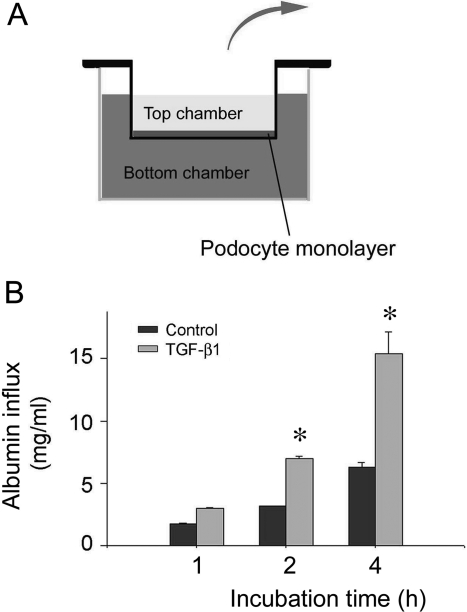
A simple albumin influx assay was used to evaluate the filtration barrier function of podocyte monolayer, as described previously.34 Briefly, podocytes (5 × 103) were seeded onto the collagen-coated transwell filters (3-μm pore; Corning, New York, NY) in the top chamber and cultured under differentiating conditions. After 10 days, podocytes were serum-starved overnight and treated without or with 2 ng/ml of TGF-β1 for 48 hours. Cells were washed twice with PBS supplemented with 1 mmol/L MgCl2 and 1 mmol/L CaCl2 to preserve the cadherin-based junctions. The top chamber was then refilled with 0.15 ml of RPMI 1640 and the bottom chamber with 1 ml of RPMI 1640 supplemented with 40 mg/ml of bovine serum albumin and incubated at 37°C. A small aliquot of media from the top chamber was collected at different time points and the albumin concentration was determined using a bicinchoninic acid protein assay kit (Sigma).
Statistical Analysis
All data examined were expressed as mean ± SEM. Statistical analysis of the data were performed using SigmaStat software (Jandel Scientific Software, San Rafael CA). Comparison between groups was made using one-way analysis of variance, followed by Student-Newman-Keuls test. A P value of less than 0.05 was considered significant.
Results
TGF-β1 Suppresses Epithelial P-Cadherin and ZO-1 Expression in Podocytes
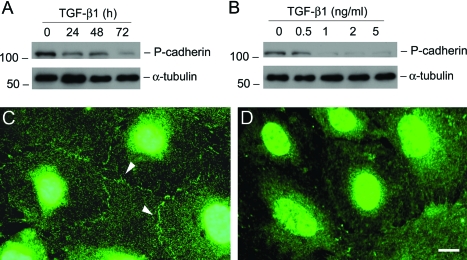
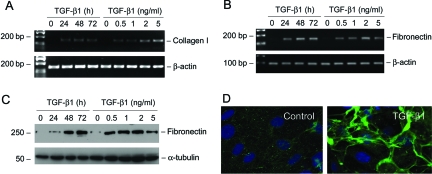
Podocyte slit diaphragm, a modified P-cadherin-containing adherens junction, is an essential component of the glomerular filtration barrier. We first examined the expression of P-cadherin in podocytes after TGF-β1 treatment. As shown in Figure 1A, treatment of podocytes with TGF-β1 at a concentration of 2 ng/ml markedly suppressed P-cadherin protein expression in a time-dependent manner. Western blot analysis revealed that P-cadherin started to decrease as early as 24 hours and almost completely disappeared at 72 hours after TGF-β1 treatment. The suppression of P-cadherin expression by TGF-β1 in podocytes was also dose-dependent (Figure 1B). Immunofluorescence staining exhibited that P-cadherin was primarily localized in the cell-cell junctional sites of the differentiated podocytes (Figure 1C), and its staining primarily disappeared after TGF-β1 treatment (Figure 1D).
Figure 1.
TGF-β1 inhibits P-cadherin expression in podocytes. A and B: Western blot analyses show that TGF-β1 inhibited P-cadherin protein expression in a time- and dose-dependent manner. Mouse podocytes were incubated with either the same concentration of TGF-β1 (2 ng/ml) for various periods of time as indicated (A), or increasing amounts of TGF-β1 for 72 hours (B). Cell lysates were immunoblotted with antibodies against P-cadherin and α-tubulin, respectively. C and D: Immunofluorescence staining shows the localization of P-cadherin in control (C) or TGF-β1-treated podocytes (D). Arrowheads indicate the positive P-cadherin staining. Scale bar = 10 μm.
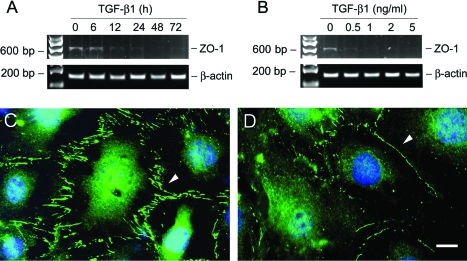
Zonula occludens-1 (ZO-1), a tight junction-associated protein, locates at the slit diaphragm and is linked through catenin intermediates to the transmembrane proteins Neph1 and P-cadherin.35 RT-PCR showed that TGF-β1 inhibited ZO-1 mRNA expression as early as 12 hours (Figure 2A). This suppression of ZO-1 by TGF-β1 was also dose-dependent (Figure 2B). Immunofluorescence staining showed abundant ZO-1 at the sites of cell-cell contacts, with a characteristic zipper-like pattern between the interdigitating processes of podocytes (Figure 2C). After TGF-β1 treatment, the overall density of ZO-1 staining was markedly decreased, and its zipper-like staining pattern was changed to a continuous belt along the cell borders (Figure 2D).
Figure 2.
TGF-β1 suppresses ZO-1 expression in podocytes. A and B: RT-PCR demonstrates that TGF-β1 inhibited ZO-1 mRNA expression in podocytes. Podocytes were incubated with either the same concentration of TGF-β1 (2 ng/ml) for various periods of time as indicated (A), or increasing amounts of TGF-β1 for 72 hours (B). Shown is the PCR product of ZO-1 α+ isoform with 635 bp in size. RT-PCR amplification of housekeeping β-actin was performed in an identical manner to serve as controls. C and D: Immunofluorescence staining shows the localization of ZO-1 in control (C) or TGF-β1-treated podocytes (D). Arrowheads indicate the positive ZO-1 staining. Scale bar = 10 μm.
TGF-β1 Inhibits Expression of the Slit-Diaphragm Protein Nephrin in Podocytes
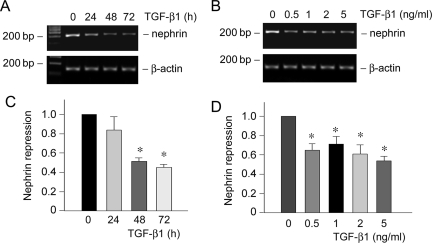
Nephrin is a slit diaphragm protein that plays an essential role in establishing an effective glomerular filtration. Mutations or down-regulation of nephrin is associated with a defective filtration barrier, causing proteinuria.6 Cultured mouse podocytes expressed little nephrin under normal differentiating conditions; however, significant nephrin expression was observed when they were incubated in the medium containing active vitamin D and retinoic acid, as recently reported.29 Using this culture system, we examined the effect of TGF-β1 on nephrin expression in podocytes. As shown in Figure 3, A and C, TGF-β1 at a concentration of 2 ng/ml repressed nephrin mRNA expression in a time-dependent manner. Similarly, TGF-β1 could inhibit nephrin expression in different concentrations ranging from 0.5 to 5 ng/ml (Figure 3, B and D). Together with the data presented above, these results suggest that TGF-β1 is able to induce podocyte dedifferentiation by suppressing P-cadherin, ZO-1, and nephrin expression.
Figure 3.
TGF-β1 inhibits nephrin expression in podocytes. A and B: RT-PCR demonstrates that TGF-β1 inhibited nephrin mRNA expression in podocytes. Podocytes were cultured in RPMI 1640 medium supplemented with 100 nmol/L 1,25-dihydroxyvitamin D3 and 1 μmol/L all-trans retinoic acid, followed by treating with either the same concentration of TGF-β1 (2 ng/ml) for various periods of time as indicated (A) or increasing amounts of TGF-β1 for 72 hours (B). RT-PCR amplification of housekeeping β-actin was performed in an identical manner to serve as controls. C and D: Quantitative determination of nephrin mRNA abundance after normalization with β-actin. Data are presented as mean ± SEM of three experiments. *P < 0.05 versus controls.
TGF-β1 Induces Mesenchymal Markers and Interstitial Matrix in Podocytes
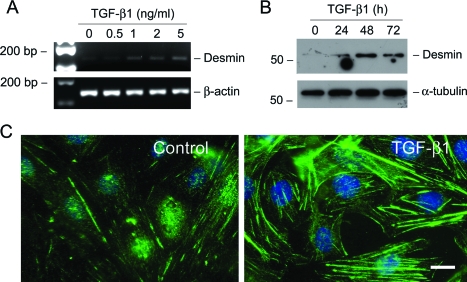
We next investigated whether TGF-β1 induces a mesenchymal conversion of podocytes. To this end, the expression of desmin, an intermediate filament protein, was examined in podocytes after TGF-β1 treatment. As illustrated in Figure 4, incubation of podocytes with TGF-β1 induced desmin mRNA and protein expression in a time- and dose-dependent manner. Immunostaining also demonstrated clearly the induction of desmin-positive intermediate filaments in the cytoplasm of podocytes after TGF-β1 treatment (Figure 4C).
Figure 4.
TGF-β1 induces desmin expression in podocytes. A: RT-PCR analysis demonstrates that TGF-β1 stimulated desmin mRNA expression in podocytes. RT-PCR amplification of housekeeping β-actin was performed in an identical manner to serve as controls. B: Western blot analysis shows marked induction of desmin protein in podocytes by TGF-β1. Podocytes were incubated with TGF-β1 (2 ng/ml) for various periods of time as indicated. Cell lysates were immunoblotted with antibodies against desmin and α-tubulin, respectively. C: Representative photographs of desmin visualized by indirect immunofluorescence staining in the control and TGF-β1-treated podocytes. Scale bar = 10 μm.
We also examined the expression of interstitial matrix components in podocytes on TGF-β1 stimulation. As shown in Figure 5, A and B, incubation of podocytes with TGF-β1 induced de novo expression of interstitial matrix components. Podocytes at basal conditions barely expressed interstitial type I collagen and fibronectin. However, after TGF-β1 stimulation, podocytes began to express an impressive amount of type I collagen and fibronectin mRNA. Western blot analysis and immunofluorescence staining also revealed a remarkable induction of fibronectin protein, which was assembled and deposited in the extracellular compartment (Figure 5, C and D).
Figure 5.
TGF-β1 induces interstitial matrix type I collagen and fibronectin expression in podocytes. A and B: RT-PCR analysis demonstrates that TGF-β1-stimulated type I collagen (A) and fibronectin (B) mRNA expression in podocytes. C: Western blot analysis shows that TGF-β1 induced fibronectin protein expression in a time- and dose-dependent manner. D: Immunofluorescence staining demonstrates the deposition and extracellular assembly of fibronectin by the control and TGF-β1-treated podocytes.
TGF-β1 Stimulates MMP-9 Expression and Secretion by Podocytes
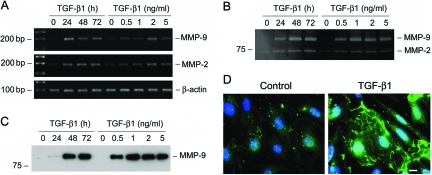
We further investigated the MMP-9 and MMP-2 expression in podocytes after TGF-β1 treatment by a wide variety of approaches, including RT-PCR, zymographic analysis, Western blot, and immunostaining. As shown in Figure 6A, MMP-9 mRNA was dramatically induced by TGF-β1 in podocytes. Zymographic analysis of the conditioned media demonstrated that TGF-β1 induced a marked increase in MMP-9 protein expression and secretion, and such MMP-9 induction was both time- and dose-dependent (Figure 6B). The striking induction of MMP-9 by TGF-β1 in podocytes was independently confirmed by Western blot analysis of the conditioned media (Figure 6C). TGF-β1 also marginally induced MMP-2 mRNA and increased MMP-2 secretion (Figure 6, A and B). Of interest, MMP-9 protein was apparently aggregated on the surface of podocytes after TGF-β1 treatment, as shown by immunofluorescence staining (Figure 6D). This pattern of MMP-9 distribution suggests a high local concentration of its protein in certain areas of cell surface.
Figure 6.
TGF-β1 induces MMP-9 production and secretion by podocytes. A: RT-PCR analysis demonstrates that TGF-β1 induced MMP-9 and MMP-2 mRNA expression in podocytes. Mouse podocytes were incubated with TGF-β1 (2 ng/ml) for various periods of time or with increasing amounts of TGF-β1 for 72 hours. B: Zymographic analysis of the conditioned media derived from podocytes treated without (control) or with TGF-β1 as indicated. Samples equalized for protein content were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel containing 0.1% gelatin. The locations of bands corresponding to MMP-9 and MMP-2 are indicated. C: Western blot analysis of the conditioned media from podocytes treated without (control) or with TGF-β1 as indicated. D: Immunofluorescence staining demonstrates the distribution of MMP-9 in the control and TGF-β1-treated podocytes. Scale bar = 10 μm.
TGF-β1 Impairs the Filtration Barrier Function of Podocytes
To assess the functional consequence of podocyte EMT, we examined the filtration barrier function of podocyte by using a paracellular permeability influx assay.34 As depicted in Figure 7A, this simple assay measured the albumin flux rate across the differentiated podocyte monolayer. Differentiated podocytes were incubated with TGF-β1 for 48 hours to induce podocyte EMT, and then subjected to albumin influx assay. As shown in Figure 7B, compared with the controls, TGF-β1 treatment resulted in a greater albumin influx across the podocyte monolayer. These results indicate that the filtration barrier function of podocytes is severely impaired after the phenotypic conversion triggered by TGF-β1.
Figure 7.
TGF-β1 impairs the filtration barrier function of podocyte monolayer. A: Schematic depiction of the paracellular permeability influx assay. Podocyte monolayer on collagen-coated Transwell filters was incubated without or with TGF-β1 for 48 hours, and albumin permeability across podocyte monolayer was then determined. B: Graphic presentation of the albumin influx across podocyte monolayer. Duration of albumin incubation is shown on x-axis. Data are presented as means ± SEM, n = 6. *P < 0.05 versus control.
Snail Mediates the TGF-β1-Triggered P-Cadherin and Nephrin Suppression in Podocytes
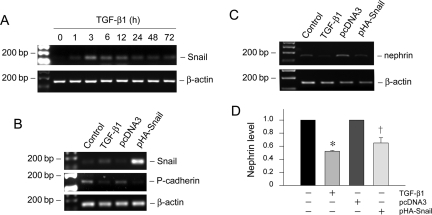
Snail transcription factor has been shown to play a critical role in mediating E-cadherin suppression during EMT in various model systems.36,37 To investigate the potential involvement of Snail in podocyte EMT, we examined the expression of Snail in podocytes after TGF-β1 treatment. As shown in Figure 8A, Snail mRNA began to increase as early as 1 hour, reached the peak at 3 hours, and sustained at least to 72 hours. These results demonstrate an early, marked induction of Snail expression in the podocyte EMT induced by TGF-β1.
Figure 8.
Snail mediates the TGF-β1-triggered P-cadherin and nephrin suppression in podocytes. A: RT-PCR shows that TGF-β1 induced Snail mRNA expression in a time-dependent manner. Podocytes were incubated with TGF-β1 (2 ng/ml) for various periods of time as indicated. B: Forced expression of Snail suppresses P-cadherin expression in podocytes. Podocytes were transiently transfected with Snail expression vector (pHA-Snail) for 72 hours. Snail, P-cadherin, and β-actin expression was assessed by RT-PCR. Podocytes transiently transfected with empty vector pcDNA3 plasmid were used as negative control, whereas podocytes treated with TGF-β1 served as a positive control. C and D: Ectopic expression of Snail inhibits nephrin expression in podocytes. C: Nephrin mRNA expression was assessed by RT-PCR. Quantification of nephrin mRNA abundance was performed after normalization with β-actin. Data are presented as mean ± SEM of three experiments. *P < 0.05 versus controls. †P < 0.05 versus pcDNA3 control.
To examine the role of Snail induction in podocyte EMT, we investigated the P-cadherin expression in podocyte overexpressing Snail. As demonstrated in Figure 8B, ectopic expression of exogenous Snail markedly suppressed the cell-cell adhesion receptor P-cadherin. Similarly, Snail overexpression also caused nephrin suppression (Figure 8, C and D). These results indicate that Snail induction is relevant to EMT and mediates the TGF-β1-triggered P-cadherin and nephrin suppression in podocytes.
Podocyte EMT in Vivo
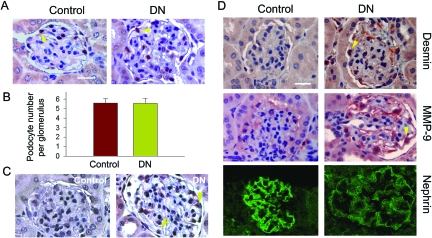
To assess the possibility of podocyte EMT in vivo, we examined the expression of key EMT markers in the glomeruli of diabetic nephropathy. A mouse uninephrectomized-streptozotocin diabetic model was used, as described previously.30 At 3 months after injection of streptozotocin, mice developed diabetic nephropathy, as demonstrated by glomerular hypertrophy, mesangial expansion, and matrix deposition.30 Heavy albuminuria and proteinuria developed at this time point, as reported previously.30 However, the WT-1-positive podocytes were completely preserved (Figure 9, A and B), suggesting that podocyte loss may not be the initial cause of albuminuria. Podocytes in diabetic kidney expressed an increased TGF-β type I receptor (TβR-I) (Figure 9C), underscoring that these cells are susceptible to TGF-β1 stimulation in vivo. Immunohistochemical studies showed an induction of desmin in the glomeruli of diabetic kidneys (Figure 9D), which was specifically localized in glomerular podocytes. Similarly, MMP-9 expression was also substantially up-regulated in podocytes in the diabetic kidneys. Conversely, the expression of the slit diaphragm protein nephrin was down-regulated (Figure 9D). Hence, it appears that podocytes undergo a mesenchymal transition after injury in vivo.
Figure 9.
De novo expression of desmin and MMP-9 and suppression of nephrin in podocytes of diabetic kidney in vivo. A and B: Determination of podocyte numbers of diabetic kidney at 3 months after STZ injection in uninephrectomized mice. A: Kidney sections from the control and diabetic mice were stained immunohistochemically for WT-1. The WT-1-positive podocytes were counted in 10 randomly chosen glomeruli and the averages of podocyte numbers per glomerulus were calculated. B: Data are expressed as mean ± SEM of five animals per group. C: Specific up-regulation of TGF-β type I receptor (TβR-I) in the glomerular podocytes of diabetic kidney. Kidney sections from the control and diabetic mice were stained with anti-TβR-I antibody. D: De novo expression of desmin and MMP-9 and suppression of nephrin in podocytes of diabetic kidney. Kidney sections from the control and diabetic mice were stained with specific antibodies against desmin, MMP-9, and nephrin, respectively. Representative micrographs are shown. Arrowheads indicate positive staining. Scale bars = 25 μm.
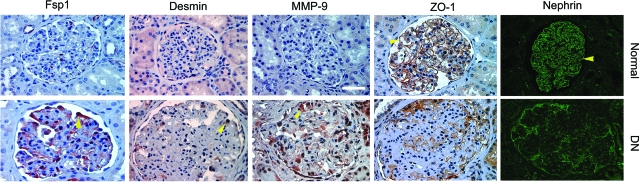
We also studied several EMT-related markers by immunohistochemical staining in human kidney samples. As shown in Figure 10, compared to normal controls, ZO-1 and nephrin largely disappeared in the glomeruli of human diabetic kidneys. In contrast, FspI, also known as S100A4, a cytoskeleton-associated, calcium-binding protein that is normally expressed in fibroblasts but not epithelia,38 was specifically induced in glomerular podocytes of diabetic kidney. Similarly, desmin and MMP-9 were also induced markedly in human glomerular podocytes under pathological conditions (Figure 10). Of note, these patients developed different degrees of proteinuria ranging from 1.3 to 11 g per 24 hours. These results suggest that podocyte EMT occurs in vivo as well and presumably leads to podocyte dysfunction.
Figure 10.
Podocyte EMT in human diabetic nephropathy. Representative micrographs show the immunohistochemical staining for mesenchymal markers FspI, desmin, and MMP-9, as well as epithelial markers ZO-1 and nephrin, in human kidney sections. Loss of ZO-1 and nephrin and acquisition of FspI, desmin, and MMP-9 were evident in the podocytes of human diabetic kidney, comparing with normal controls. Arrowheads indicate positive staining. Scale bar = 40 μm.
Discussion
Podocyte dysfunction is a principal feature of many proteinuric kidney diseases.11,39 Despite the obvious importance of the podocyte in establishing the glomerular filtration barrier, exactly how various injurious stimuli cause the podocyte to behave differently in pathological states remains ambiguous. The results presented in this study demonstrate that podocytes are capable of undergoing EMT after injury, and this phenotypic conversion causes the podocyte to lose its specialized epithelial features and to acquire new mesenchymal markers. It is conceivable that podocytes after EMT will abandon their complex morphological architecture and relinquish their highly specialized functions, which undoubtedly impairs the integrity of glomerular filtration barrier, leading to the onset of proteinuria. Therefore, our present findings provide a novel and rational illumination of some cellular events leading to podocyte dysfunction and proteinuria in response to injury.
The observation that podocytes undergo EMT suggests an incredible plasticity of these terminally differentiated cells in certain pathological conditions. Given that podocyte and tubular epithelial cells are developmentally derived from the same origin,40 it comes as little surprise that podocytes, similar to tubular epithelial cells, undergo a phenotypic conversion after TGF-β1 stimulation. Podocyte EMT is supported by the loss of epithelial P-cadherin, ZO-1, and nephrin, and the acquisition of mesenchymal FspI, desmin, collagen I, and fibronectin. Because P-cadherin, ZO-1, and nephrin are important components of the slit diaphragm cell adhesion complexes,3,11 their loss will certainly lead to podocyte dedifferentiation and impair the integrity of the slit diaphragm. It is conceivable to speculate that the zipper-like cell-cell junctions of podocytes, as illustrated in ZO-1 staining (Figure 2C), could be the in vitro equivalent of the primitive interdigitating secondary foot processes. Therefore, loss of this zipper-like junction after TGF-β1 treatment may, to some degree, imitate the foot process effacement observed in vivo. This speculation is experimentally corroborated by a functional paracellular albumin flux assay, in which the podocyte monolayer after TGF-β1 treatment displays increased albumin permeability. Therefore, our observations support the notion that podocyte dedifferentiation, as manifested by the loss of P-cadherin, ZO-1, and nephrin, could lead to a defective filtration barrier function.
It is important to emphasize that the loss of podocyte epithelial features after TGF-β1 stimulation is accompanied by the induction of mesenchymal markers, such as desmin, FspI, fibronectin, and type I collagen, underscoring a phenotypic conversion. Desmin, an intermediate filament protein, has long been suggested as a podocyte injury indicator the expression of which is often up-regulated in various glomerular diseases in which podocyte damage is involved.41,42 Although little is known about its function in podocytes, induction of desmin is a common feature of the mesenchymal cell activation in different organs. In this context, de novo expression of desmin could be a reliable, pathophysiologically relevant marker for podocyte EMT. Similarly, FspI, a fibroblast-specific protein that is absent in epithelial cells,38 could serve as a valuable marker for podocyte EMT. Podocytes normally produce type IV collagen and laminin, the major components of the GBM on which they reside. By producing the interstitial matrix components type I collagen and fibronectin, podocytes have adapted a mesenchymal phenotype after injury, which could profoundly change their functions. Of note, podocytes may not undergo EMT in a synchronized fashion after injury, and therefore a whole spectrum of podocytopathies that reflect different stage of podocyte EMT could be envisioned in vivo. It is also worthwhile to point out that in many circumstances podocytes could only endure a tendency of EMT after injury, and they may seldom undergo a complete phenotypic conversion that results in the cells resembling typical fibroblasts.
One interesting observation in the present study is the striking induction of MMP-9 expression after TGF-β1 stimulation in podocytes. Increased MMP-9 mRNA and protein expression by podocytes would lead to an augmented secretion of this MMP, as demonstrated by gelatin zymographic analysis. Because the specific substrates for MMP-9 are type IV collagen and laminin,43 the major components of GBM, elevation of the secreted MMP-9 would inevitably cause the remodeling of GBM, thereby altering its composition and impairing its structural and functional integrity. Disruption of the delicate balance of GBM composition, per se, has been shown to cause the onset of proteinuria, as reported in genetic Alport syndrome and in laminin β2-deficient mice.44 In this context, podocyte dysfunction after injury also indirectly impairs the glomerular filtration barrier by altering GBM composition through elevated MMP-9.
Increased MMP-9 protein tends to aggregate on the surface of podocytes after TGF-β1 stimulation, as revealed by immunostaining. This novel finding is somewhat unexpected because MMP-9 is a secreted, soluble protein. Aggregation of MMP-9 protein on podocyte surface would not only prevent it from being washed away by glomerular filtrate but also drastically increase the local concentration of this protease, enabling it to efficiently degrade its specific substrates. The podocyte surface has been divided into apical, basal, and junctional membrane domains, with different locations, protein compositions, and functions.45 Depending on where MMP-9 aggregate is formed, it would have a profound impact on podocyte functions through an assortment of feedback mechanisms. Because the basal domain is responsible for anchoring the podocyte to the underlying GBM, deposition of MMP-9 aggregates there would selectively degrade type IV collagen and laminin, leading to an altered GBM composition. This will in turn adversely affect podocyte behavior via outside-in integrin signaling. If MMP-9 protein aggregates on the junctional membrane domain of podocyte surface, it could impair the integrity of the cell-cell adhesion slit diaphragm that comprises numerous trans-membrane proteins such as nephrin, Neph-1, Neph-2, and P-cadherin. Along this line, it is of interest to note that Neph-2 has recently been shown to be cleaved from podocyte by metalloproteinases.46 Finally, podocyte apical domain proteins such as podocalyxin are often detectable in the urine under pathological conditions,47 suggesting a potential role of the proteases in shedding these proteins. Together, increased MMP-9 could exacerbate podocyte functions by multiple mechanisms.
It appears clear that TGF-β1 is a potent trigger for podocyte EMT. This is in harmony with many observations that TGF-β1 triggers tubular EMT, and its expression is up-regulated in virtually every type of chronic kidney disease.22,48 Overexpression of TGF-β1 in transgenic mice causes the development of proteinuria and glomerulosclerosis.14 The importance of TGF-β1 in podocyte dysfunction is also highlighted by the fact that it can integrate the actions of other pathogenic mediators such as high glucose and angiotensin II. Notably, TGF-β type I receptor, which determines the target specificity of TGF-β action, is specifically up-regulated in glomerular podocytes of diabetic kidney (Figure 9C), suggesting that podocytes are susceptible to TGF-β1 stimulation under pathological conditions. Although it remains elusive as to the mechanism underlying TGF-β1 action, the observation that it induces Snail expression underscores that it is capable of targeting the key EMT-regulatory genes. It should be noted that podocytes may respond differently to TGF-β stimulation according to its specific concentrations, and a high concentration of TGF-β1 causes podocyte apoptosis.49 However, as shown in this study, TGF-β1 is able to induce phenotypic conversion at a concentration as low as 0.5 ng/ml in which significant apoptosis is not evident.49 This suggests that EMT perhaps is an early and predominant response of podocytes in most pathophysiological conditions.
The notion of podocyte EMT offers a completely new explanation for how injury causes podocyte dysfunction, which leads to the impairment of glomerular filtration barrier and proteinuria. At present, the prevalent view on the pathogenesis of podocyte lesions leading to proteinuria in many common glomerular diseases is emphasized on podocyte loss. Podocyte depletion undoubtedly contributes to a defect of the glomerular filtration, since reduction of podocyte numbers in otherwise healthy kidney, per se, in experimental animal models causes proteinuria.16,17 However, many studies indicate that proteinuria is an early event that precedes podocyte depletion in most circumstances.18 In this study, we show that when albuminuria was eminent in a mouse model of diabetic nephropathy,30 podocyte numbers per glomerulus are unaltered (Figure 8). This is consistent with a recent observation that in aging nephropathy podocytes are well preserved when proteinuria develops.50 Therefore, podocyte loss may not be the initial cause of proteinuria. This view is in harmony with clinical observations demonstrating that proteinuria is a reversible process, at least in the early stage, that can recover spontaneously or after therapeutic intervention.
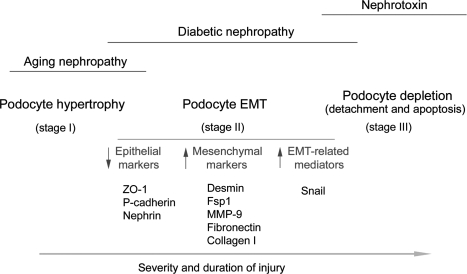
Based on the data presented, it is tempting to propose that EMT is a potential pathway leading to podocyte dysfunction and represents an early cellular event causing a defective glomerular filtration and proteinuria, although we cannot exclude the role of podocyte foot process effacement in this process. Of interest, foot process effacement, per se, could be viewed as a consequence of podocyte dedifferentiation, thereby representing a part of the EMT process. Our EMT hypothesis emphasizes the phenotypic alterations of podocytes, rather than their loss, as the primary cause of podocyte dysfunction leading to proteinuria in many common glomerular diseases. As depicted in Figure 11, we speculate that podocytes respond to injurious stimuli in different ways, depending on the severity and duration of the injury. The initial response of podocytes to an injury may be cell hypertrophy (stage I), an adaptive change in cell size in an attempt to compensate for any lost function. At this stage, no proteinuria occurs. However, if the injury is progressive and prolonged, podocytes will undergo EMT (stage II), a phenotypic conversion that results in the loss of highly specialized podocyte features and gain of new mesenchymal markers. This leads to an impaired glomerular filtration barrier, thereby ensuring the onset of proteinuria. More severe and/or longer injury would induce podocyte detachment from GBM and/or apoptosis, resulting in podocyte loss (stage III), which certainly aggravates proteinuria and leads to glomerulosclerosis. The involvement and relative contribution of these podocyte responses in a given disease model may be different. For instance, podocyte hypertrophy may be a predominant feature in aging nephropathy, whereas cell depletion could be a major finding in nephrotoxin-induced proteinuric glomerular diseases. It is conceivable that for many common glomerular diseases such as diabetic nephropathy, EMT could be a primary pathway leading to podocyte dysfunction, proteinuria, and glomerulosclerosis.
Figure 11.
Schematic presentation of podocyte responses after injury. Depending on the severity and duration of the injury, podocytes may respond to injurious stimuli in different ways, including hypertrophy, dedifferentiation and mesenchymal transition, detachment, and apoptosis. EMT could be a primary pathway leading to podocyte dysfunction, proteinuria, and glomerulosclerosis in the vast majority of chronic kidney diseases.
Acknowledgments
We thank Dr. A. Garcia de Herreros for kindly providing the Snail expression vector.
Footnotes
Address reprint requests to Youhua Liu, Ph.D., Department of Pathology, University of Pittsburgh, S-405 Biomedical Science Tower, 200 Lothrop St., Pittsburgh, PA 15261. E-mail: liuy@upmc.edu.
Supported by the National Institutes of Health (grants DK061408, DK064005, and DK071040).
References
- Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- Tryggvason K, Wartiovaara J. Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens. 2001;10:543–549. doi: 10.1097/00041552-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Benzing T. Signaling at the slit diaphragm. J Am Soc Nephrol. 2004;15:1382–1391. doi: 10.1097/01.asn.0000130167.30769.55. [DOI] [PubMed] [Google Scholar]
- Roselli S, Heidet L, Sich M, Henger A, Kretzler M, Gubler MC, Antignac C. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol. 2004;24:550–560. doi: 10.1128/MCB.24.2.550-560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 2003;300:1298–1300. doi: 10.1126/science.1081068. [DOI] [PubMed] [Google Scholar]
- Tryggvason K. Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J Am Soc Nephrol. 1999;10:2440–2445. doi: 10.1681/ASN.V10112440. [DOI] [PubMed] [Google Scholar]
- Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 2001;108:1621–1629. doi: 10.1172/JCI12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson-Fernholm K, Forsblom C, Perola M, Groop PH. Polymorphisms in the nephrin gene and diabetic nephropathy in type 1 diabetic patients. Kidney Int. 2003;63:1205–1210. doi: 10.1046/j.1523-1755.2003.00855.x. [DOI] [PubMed] [Google Scholar]
- Sako M, Nakanishi K, Obana M, Yata N, Hoshii S, Takahashi S, Wada N, Takahashi Y, Kaku Y, Satomura K, Ikeda M, Honda M, Iijima K, Yoshikawa N. Analysis of NPHS1. NPHS2, ACTN4, and WT1 in Japanese patients with congenital nephrotic syndrome. Kidney Int. 2005;67:1248–1255. doi: 10.1111/j.1523-1755.2005.00202.x. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Finn LS, Kowalewska J, McDonald RA, Eddy AA. Expression of nephrin in acquired forms of nephrotic syndrome in childhood. Pediatr Nephrol. 2004;19:300–305. doi: 10.1007/s00467-003-1346-3. [DOI] [PubMed] [Google Scholar]
- Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- Durvasula RV, Shankland SJ. Podocyte injury and targeting therapy: an update. Curr Opin Nephrol Hypertens. 2006;15:1–7. doi: 10.1097/01.mnh.0000199012.79670.0b. [DOI] [PubMed] [Google Scholar]
- Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- Schiffer M, Bitzer M, Roberts IS, Kopp JB, ten Dijke P, Mundel P, Bottinger EP. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- Macconi D, Bonomelli M, Benigni A, Plati T, Sangalli F, Longaretti L, Conti S, Kawachi H, Hill P, Remuzzi G, Remuzzi A. Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol. 2006;168:42–54. doi: 10.2353/ajpath.2006.050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, Dedhar S, Liu Y. Essential role of integrin-linked kinase in podocyte biology: bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol. 2006;17:2164–2175. doi: 10.1681/ASN.2006010033. [DOI] [PubMed] [Google Scholar]
- El-Aouni C, Herbach N, Blattner SM, Henger A, Rastaldi MP, Jarad G, Miner JH, Moeller MJ, St-Arnaud R, Dedhar S, Holzman LB, Wanke R, Kretzler M. Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol. 2006;17:1334–1344. doi: 10.1681/ASN.2005090921. [DOI] [PubMed] [Google Scholar]
- Xia JL, Dai C, Michalopoulos GK, Liu Y. Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am J Pathol. 2006;168:1500–1512. doi: 10.2353/ajpath.2006.050747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc. 2006;3:377–382. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutz F, Zeisberg M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol. 2006;17:2992–2998. doi: 10.1681/ASN.2006050420. [DOI] [PubMed] [Google Scholar]
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- Takano Y, Yamauchi K, Hiramatsu N, Kasai A, Hayakawa K, Yokouchi M, Yao J, Kitamura M. Recovery and maintenance of nephrin expression in cultured podocytes and identification of HGF as a repressor of nephrin. Am J Physiol. 2007;292:F1573–F1582. doi: 10.1152/ajprenal.00423.2006. [DOI] [PubMed] [Google Scholar]
- Dai C, Yang J, Bastacky S, Xia J, Li Y, Liu Y. Intravenous administration of hepatocyte growth factor gene ameliorates diabetic nephropathy in mice. J Am Soc Nephrol. 2004;15:2637–2647. doi: 10.1097/01.ASN.0000139479.09658.EE. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang J, Dai C, Wu C, Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112:503–516. doi: 10.1172/JCI17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Shultz RW, Mars WM, Wegner RE, Li Y, Dai C, Nejak K, Liu Y. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest. 2002;110:1525–1538. doi: 10.1172/JCI16219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol. 2002;13:96–107. doi: 10.1681/ASN.V13196. [DOI] [PubMed] [Google Scholar]
- Rico M, Mukherjee A, Konieczkowski M, Bruggeman LA, Miller RT, Khan S, Schelling JR, Sedor JR. WT1-interacting protein and ZO-1 translocate into podocyte nuclei after puromycin aminonucleoside treatment. Am J Physiol. 2005;289:F431–F441. doi: 10.1152/ajprenal.00389.2004. [DOI] [PubMed] [Google Scholar]
- Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11:1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2006;17:3382–3393. doi: 10.1681/ASN.2006050520. [DOI] [PubMed] [Google Scholar]
- Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- Horster MF, Braun GS, Huber SM. Embryonic renal epithelia: induction, nephrogenesis, and cell differentiation. Physiol Rev. 1999;79:1157–1191. doi: 10.1152/physrev.1999.79.4.1157. [DOI] [PubMed] [Google Scholar]
- Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, Ritz E, Amann K. 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol. 2004;286:F526–F533. doi: 10.1152/ajprenal.00316.2003. [DOI] [PubMed] [Google Scholar]
- Zou J, Yaoita E, Watanabe Y, Yoshida Y, Nameta M, Li H, Qu Z, Yamamoto T. Upregulation of nestin, vimentin, and desmin in rat podocytes in response to injury. Virchows Arch. 2006;448:485–492. doi: 10.1007/s00428-005-0134-9. [DOI] [PubMed] [Google Scholar]
- Lenz O, Elliot SJ, Stetler-Stevenson WG. Matrix metalloproteinases in renal development and disease. J Am Soc Nephrol. 2000;11:574–581. doi: 10.1681/ASN.V113574. [DOI] [PubMed] [Google Scholar]
- Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities in Lamb2−/− mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest. 2006;116:2272–2279. doi: 10.1172/JCI28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest. 2001;108:1583–1587. doi: 10.1172/JCI14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke P, Sellin L, Kretz O, Petraschka D, Zentgraf H, Benzing T, Walz G. NEPH2 is located at the glomerular slit diaphragm, interacts with nephrin and is cleaved from podocytes by metalloproteinases. J Am Soc Nephrol. 2005;16:1693–1702. doi: 10.1681/ASN.2004060439. [DOI] [PubMed] [Google Scholar]
- Hara M, Yanagihara T, Kihara I, Higashi K, Fujimoto K, Kajita T. Apical cell membranes are shed into urine from injured podocytes: a novel phenomenon of podocyte injury. J Am Soc Nephrol. 2005;16:408–416. doi: 10.1681/ASN.2004070564. [DOI] [PubMed] [Google Scholar]
- Böttinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- Wu DT, Bitzer M, Ju W, Mundel P, Bottinger EP. TGF-beta concentration specifies differential signaling profiles of growth arrest/differentiation and apoptosis in podocytes. J Am Soc Nephrol. 2005;16:3211–3221. doi: 10.1681/ASN.2004121055. [DOI] [PubMed] [Google Scholar]
- Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005;16:2953–2966. doi: 10.1681/ASN.2005050488. [DOI] [PubMed] [Google Scholar]