Abstract
Receptor signaling is mediated by direct protein interaction with various types of cytoskeletal, adapter, effector, and additional receptor molecules. In brain tissue and in cultured neurons, activation of dopamine D2 receptors (D2Rs) has been found to impact cellular calcium signaling. Using a yeast two-hybrid approach, we have uncovered a direct physical interaction between the D2R and the transient receptor potential channel (TRPC) subtypes 1, 4 and 5. The TRPC/D2R interaction was further validated by GST-pulldown assays and coimmunoprecipitation from mammalian brain. Ultrastructural analysis of TRPC1 and D2R expression indicates colocalization of the two proteins within the cell body and dendrites of cortical neurons. In cultured cells, expression of D2Rs was found to increase expression of TRPC1 at the cell surface by 50%. These findings shed new light on the constituents of the D2R signalplex, and support the involvement of D2Rs in cellular calcium signaling pathways via a novel link to TRPC channels.
Keywords: Dopamine receptor, Transient Receptor Potential, TRPC1, G-protein coupled receptor, interacting proteins, calcium signaling
1. Introduction
Dopamine is a catecholamine neurotransmitter that regulates important cognitive and behavioral functions in the mammalian brain including locomotion, memory, attention, and reward [1–3]. Dopamine exerts its effects through the activation of dopamine receptors (DRs) and their subsequent coupling to downstream effector molecules [4]. Alterations in dopaminergic neurotransmission have been implicated in a variety of neurological and neuropsychiatric conditions including schizophrenia [5–7], with the majority of antipsychotic drugs acting as antagonists at D2R sites in the brain [8, 9]. It has recently been suggested that dysregulation of dopaminergic signaling, a feature of disorders such as schizophrenia, may stem from alterations in expression or function of proteins that interact with and regulate DR-mediated signaling [10, 11].
The DR family, consisting of the D1-like and D2-like receptors, has been shown to associate with diverse intracellular signaling pathways in neurons. D2Rs are primary regulators of the inhibitory Gi/o pathways but can also activate calcium associated signaling pathways, including the canonical Gq/PLC pathway, and can mediate the mobilization of intracellular calcium stores and activation of calcium-dependent phosphatases [12–14]. A number of proteins have now been identified that interact with DRs (dopamine receptor interacting proteins; DRIPs) and regulate the life cycle and signaling properties of individual DR subtypes (reviewed in [15, 16]). For example, interaction of D2Rs with calcium binding proteins such as NCS-1 [17] and CAPS [18] plays an important role in D2R desensitization and dopamine secretion, respectively.
In the present study, we used the second intracellular loop of the D2R (D2IC2) as bait in a yeast two-hybrid screen and identified TRPC1 (transient receptor potential channel1) as a novel D2R interactor. In mammals, seven TRPC subtypes (TRPC1-7) have been identified based on sequence similarities to Drosophila TRP (reviewed in [19], and have been shown to function in a variety of physiological processes including Ca2+ and Na+ entry, receptor/phospholipase C (PLC) signaling, lipid raft integrity, cell volume regulation, and cell proliferation (reviewed in [20]). Homomeric TRPC1 has been reported to function as either a store-operated, receptor-operated, or DAG-activated ion channel [19, 21]. It has been suggested that TRPC1 may also represent a non-functional ion channel subunit [22]. Our data indicate that TRPC1 interacts with the D2R in native brain tissue, and that this interaction enhances the delivery of TRPC1 to the cell surface. Ultrastructural analysis of native TRPC1 and D2R proteins using electron microscopy shows that these proteins colocalize in postsynaptic compartments of cortical neurons in the primate cortex. These findings reveal a novel link between D2Rs and TRPC channels in neurons, and suggest a role for TRPC channels in neuropsychiatric disease.
2. Experimental Methods
2.1 DNA constructs and yeast two-hybrid assays
All constructs were generated by subcloning PCR amplification or restriction enzyme fragments into appropriate expression vectors, and each construct was verified by automated DNA sequencing. For the yeast two-hybrid screen, the second intracellular loop of the human D2R (D2IC2, amino acids 131–151) was subcloned into the yeast GAL4 DNA-binding domain vector pAS2-1 (BD Biosciences Clonetech) and used as bait to screen a human fetal brain cDNA library expressed in the GAL4 activation domain vector pACT2 (BD Biosciences Clonetech). Bait and prey constructs were simultaneously cotransformed into the yeast strain MaV103, and 1 X 106 independent clones were screened by selective growth on Leu-/Trp-/His-/Ade-plates as described previously [18]. Protein interaction was assayed for by β-galactosidase activity via the nitrocellulose filter lift method [23].
Sites within human DRs and TRPCs that contribute to the TRPC/DR interaction were mapped using a directed two-hybrid approach. The IC2 domain of the D2R (in pAS2-1) was assayed for interaction against truncation fragments of TRPC1, TRPC4, or TRPC5 (in pACT2). TRPC cDNAs were generously provided by Drs. Michael Schaefer (University of Berlin), Craig Montell (Johns Hopkins University), and David Beech (University of Leeds). Bait and prey plasmids were sequentially transformed into the yeast strain MaV103 and interactions were assayed as described above.
2.2 GST-pulldown and coimmunoprecipitation
Glutathione S-transferase (GST)-D2IC2 fusion protein (GST-D2IC2) and GST-D2LIC3 fusion protein (residues 211–373) were constructed in the bacterial expression vector pGEX-4T-1 (Amersham Pharmacia), while carboxyl terminal truncations of TRPC1 (residues 638–759), TRPC4 (residues 621–893), and TRPC5 (residues 619–973) were constructed in the pET30C vector (Amersham Pharmacia) to generate S-tagged protein fragments. All fusion proteins were induced in E. coli strain BL-21 (DE3). GST-D2IC2 and GST-D2LIC3 fusion proteins were purified using glutathione-sepharose (Amersham) according to the manufacturer’s instructions. GST pull-down assays were performed as described previously [23]. Eluted proteins were separated by SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) filter and probed with an anti-S-protein polyclonal antibody conjugated to horseradish peroxidase (1:5000 dilution; Novagen). Immunoreactivity was detected by enhanced chemiluminescence with an ECL Plus kit (Amersham).
For coimmunoprecipitation experiments, striatal and cortical tissue were isolated from 10-day-old Sprague-Dawley rats. Crude membranes were prepared and membrane proteins solubilized as previously described [24]. Immunoprecipitation of D2R complexes was performed using a goat polyclonal anti-D2R antibody (Santa Cruz Biotechnology). Immunocomplexes were separated by SDS-PAGE, transferred to a PVDF filter, and the filter sequentially probed with a monoclonal anti-TRPC1 antibody (generous gift from Dr. Leonidas Tsiokas, University of Oklahoma Health Sciences Center), a rabbit polyclonal anti-TRPC4 antibody (Chemicon), or a chicken polyclonal anti-NCS-1 antibody (Rockland Immunochemicals, Gilbertsville, PA). Peroxidase-conjugated secondary antibodies were from Jackson ImmunoResearch (West Grove, PA). Immunoreactivity was detected using an ECL Plus kit. The specificity of the monoclonal anti-TRPC1 antibody has been previously demonstrated [25]. In Western blot analysis of HEK293 cell membrane fractions and rat brain lysates, two different commercially available polyclonal anti-TRPC1 antibodies (Alomone; Sigma) identified a protein migrating with the same molecular mass as the protein detected with the monoclonal anti-TRPC1 antibody. Using this approach, the polyclonal anti-TRPC4 antibody (Chemicon) detected a protein at the predicted molecular weight of TRPC4, whereas the polyclonal anti-TRPC5 antibody (Chemicon) cross-reacted with an unknown protein of a smaller molecular mass than that predicted for TRPC5 [26].
2.3 Immunoelectron microscopy
Two adult rhesus monkeys (Macaca mulatta) were perfused, and brain tissue was prepared as described previously [27]. Affinity-purified polyclonal rabbit antibodies directed against human D2R (amino acids 284–311) and human TRPC1 (amino acids 557–571) were purchased from Chemicon (Temecula, CA). Sections of the dorsolateral prefrontal cortex (Walker’s area 46) were processed for electron microscopy as previously described [28].
For single gold-based immunocytochemistry, sections were incubated in either anti-D2R (diluted 1:500) or anti-TRPC1 (diluted 1:200) antibodies, and transferred to nanogold–anti-rabbit Fab′ (Nanoprobes, Yaphank, NY) or biotinylated anti-rabbit F(ab′)2 (Jackson ImmunoResearch) and nanogold–anti-biotin Fab (Nanoprobes), as previously described [27, 28]. Nanogold was enhanced with silver autometallographic developer (HQ Silver; Nanoprobes).
For dual gold-based immunocytochemistry, sections were incubated in anti-D2R (1:300 dilution) antibody, followed by nanogold–anti-rabbit Fab′ and gold enhancement (GoldEnhance; Nanoprobes). An excess of unconjugated goat anti-rabbit Fab (1:10–50; Jackson ImmunoResearch) and mild glutaraldehyde fixation was used to neutralize any remaining rabbit proteins. Sections were incubated in anti-TRPC1 (1:100 dilution) antibody and a second series of nanogold conjugates, followed by gold autometallography to enhance the TRPC-1-bound nanogold as previously described [29]. TRPC1 was labeled in the second series using biotinylated anti-rabbit F(ab′)2 followed by unconjugated anti-rabbit Fab and fixation. TRPC1 was visualized with peroxidase and diaminobenzidine chromogen, while D2Rs were visualized with nanogold as detailed above for single immunocytochemistry [28], and sampled for thin-sectioning and analysis under a JEM 1010 (Jeol, Tokyo, Japan) transmission electron microscope operated at 80kV.
2.4 Cell culture and cell surface biotinylation assay
Human embryonic kidney (HEK) 293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. HEK293 cells stably expressing FLAG-tagged D2L (long-splice isoform) dopamine receptors (HEK293-D2L) or FLAG-tagged μ-opiod receptors (HEK293-MOR) were provided by Dr. Mark von Zastrow (University of California San Francisco). HEK293-D2L and HEK293-MOR cell lines were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 300ug/ml Geneticin (Invitrogen, Grand Island, NY).
Cell surface labeling assays were performed using a modification of the cleavable biotin method described previously (Vickery and von Zastrow, 1999). Briefly, cells were labeled with 1mg/ml sulfo-NHS-SS-biotin (Pierce, Rockford IL) for 30 min at 4°C. Cells were washed three times with ice cold phosphate buffered saline (PBS) to remove unbound biotin, and crude membrane fractions prepared as described previously [30]. Biotin-labeled TRPC1 proteins were immunoprecipitated using polyclonal anti-TRPC1 antibodies (Alomone Labs). Complexes were washed repeatedly with ice cold PBS, resolved by SDS-PAGE, and transferred to a PVDF membrane. The biotinylated proteins were complexed with avidin conjugated horseradish peroxidase (Vectastain Elite ABC detection system; Vector Laboratories), then detected by enhanced chemiluminescence with an ECL Plus kit. Immunoblots were quantitated using a laser densitometer (Molecular Dynamics, Sunnyvale, CA) and analyzed using the Quantity One software package (PDI, Inc., Huntington Station, NY).
3. Results
3.1 Interaction between D2 dopamine receptors and TRPC proteins
We carried out a yeast two-hybrid screen using the 22 amino acid-long second intracellular domain of the D2R (D2IC2) as bait to screen a human brain cDNA library. The D2IC2 domain has previously been shown to interact with CAPS1 (calcium activated protein for secretion), a presynaptic calcium binding protein that links D2Rs with components of the exocytic machinery [18]. In control experiments, neither the D2IC2 construct nor any of the other bait constructs used in these studies was found to autoactivate β-galactosidase expression (data not shown). Using the D2IC2 construct as bait, a positive interacting cDNA clone was identified (Fig. 1A) that contained an ~200 base pair cDNA insert. Analysis of the predicted amino acid sequence revealed 100% identity with the carboxyl-terminal 65 residues of TRPC1 (transient receptor potential channel1). The interaction between TRPC1 and the D2IC2 appeared to be specific, as the TRPC1 cDNA failed to interact with the D2R IC3 domain, and the D2IC2 domain failed to interact with protein 4.1N (Fig. 1A). Protein 4.1N has previously been shown to interact with a region within the D2IC3 domain [31]. TRPC1 is one of seven (TRPC1-TRPC7) mammalian TRPC channels involved in agonist-stimulated Ca2+ influx (for reviews see [20, 32, 33] and store-operated Ca2+ release (reviewed in [20].
Figure 1. Interaction between the D2R and TRPC proteins.
(A) Results from a yeast two-hybrid screen showing interaction between TRPC1 and the D2IC2 domain (1) but not with an unrelated bait (D2IC3L; 3) or protein 4.1N (4). Interaction between CAPS and the D2IC2 domain (2) was included as a positive control. (B) The D2R associates with TRPC1. D2IC2-GST fusion protein pulled down S-tagged TRPC1 (residues 638–759) from a bacterial lysate. Only very faint bands were detected when the lysate was absorbed onto beads alone (control lane) or beads containing GST protein (GST lane). (C) The D2R also interacts with TRPC4 and TRPC5 proteins. The D2IC2 fusion protein pulled down S-tagged TRPC4 (residues 621–893; upper panel) and TRPC5 (residues 619–973; lower panel) from bacterial lysates. Only very faint immunoreactive bands were detected when lysates were absorbed onto beads containing GST protein (GST lane).
The TRPC1/D2R interaction was initially validated using pulldown techniques. A lysate prepared from bacteria expressing an S-tagged TRPC1 carboxyl-terminal cDNA fragment (residues 638–759) was tested for the ability to associate with a GST fusion protein containing the D2IC2 domain (D2IC2-GST). As shown in Fig. 1B, a Western blot containing lysate from bacteria expressing the S-tagged TRPC1 construct produced an immunoreactive band of ~18 kDa when probed with an anti-S-protein antibody (lane 1). This band corresponds to the expected size of the C-terminal TRPC1 fragment encoded by the cDNA construct. The same band was detected by pulldown after the bacterial lysate was incubated with the D2IC2-GST fusion protein (lane 4), but not when the lysate was absorbed onto beads alone (lane 2) or beads containing GST (lane 3). These results support the validity of the TRPC1/D2R interaction.
TRPC1 is most closely related in amino acid sequence to TRPC4 and TRPC5 and can form functional heteromeric channels with either of these TRPC subtypes [19]. Based on these considerations, we used the GST-pulldown assay to investigate whether the D2R might also interact with TRPC4 and/or TRPC5. As shown in Fig. 1C, Western blots containing lysates prepared from bacteria expressing S-tagged TRPC4 (residues 621–893; top panel) or TRPC5 (residues 619–973; bottom panel) C-terminal fragments were probed with anti-S-tag antibodies. Immunoreactive bands of the expected sizes were visualized in the bacterial lysates (lane 1) and after pulldown with the D2IC2-GST fusion protein (lane 2). No immunoreactive bands were visible when the lysates were incubated either with beads alone (data not shown) or with beads containing GST (lane 3). These results suggest that the IC2 of the D2R interacts directly with TRPC4 and TRPC5, as well as TRPC1.
3.2 Mapping protein interaction domains
Deletion mapping was performed to localize sites within TRPC1 that contribute to D2R interaction. A series of truncation fragments of the C-terminal domain of TRPC1 (Clone 44, amino acids 638–759) were tested for interaction with the D2IC2 domain in a directed yeast two-hybrid screen. As shown in Fig. 2B, TRPC truncation constructs A (residues 638–693) and B (residues 638–726) failed to interact with D2IC2 in the two-hybrid system, indicating that the D2R binding site resides within the C-terminal portion of Clone 44. In contrast, TRPC truncation constructs D (residues 638–759) and C (residues 638–745) tested positive with D2IC2 in the β-galactosidase assay. These mapping studies suggest that the region in TRPC1 spanned by residues 726–745 is required for D2R binding. The ability of construct E (residues 726–745) to directly interact with the D2R (Fig. 2B,D) provides very strong evidence that this region spans a portion of the D2R binding site.
Figure 2. Localization of the D2R binding site on TRPC1, TRPC4 and TRPC5.
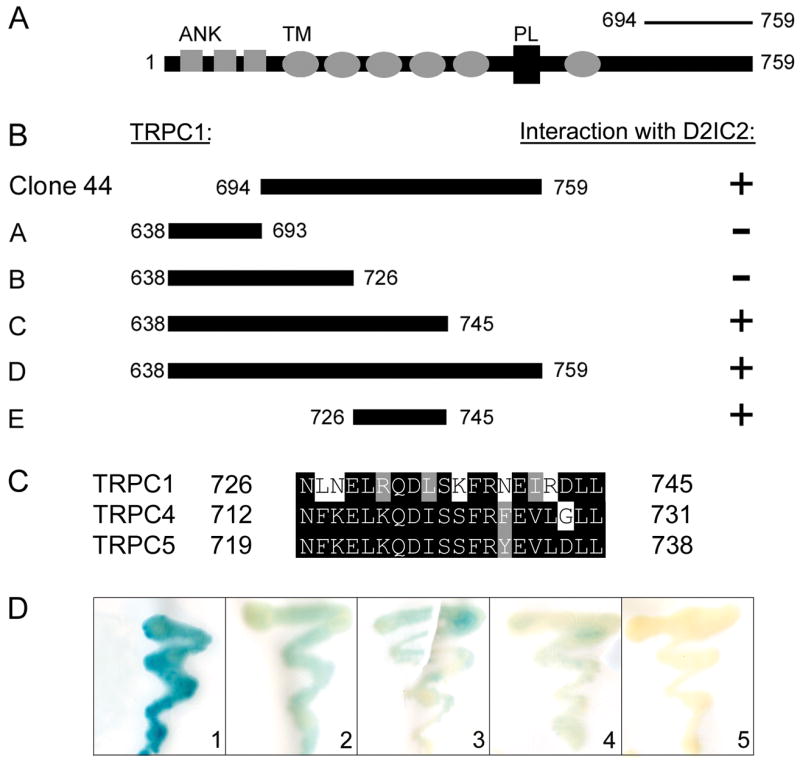
Schematic representation of constructs encoding truncations of TRPC1. (A) Full-length TRPC1 protein. Shaded boxes depict ankyrin repeats (ANK), shaded ovals represent transmembrane (TM) segments, and black box depicts the pore loop domain (PL). Residues 694–759 encompass the C-terminal fragment (Clone 44) identified in the original yeast two-hybrid screen. (B) TRPC1 truncation fragments A–E were tested for interaction with the D2IC2 domain in the two-hybrid assay. Interaction is indicated by the presence (+) or absence (−) of β-galactosidase activity. (C) Amino acid sequence alignment of TRPC1, TRPC4, and TRPC5 in the C-terminal region found to contain the D2IC2 binding site. Amino acids are numbered to the left and right of each line. Identical amino acids are highlighted in black, and conserved amino acids are highlighted in grey. (D) Representative β-galactosidase assays comparing the interaction of D2IC2 with Clone 44 (residues 694–759 of TRPC1; 1), TRPC1 (residues 726–745; 2), TRPC4 (residues 712–731; 3), and TRPC5 (residues 719–738; 4). Interaction between TRPC1 (residues 726–745) and the D2LIC3 is shown as a negative control (5).
Amino acid sequence alignments were used to predict potential D2R binding sites on TRPC4 and TRPC5 (Fig. 2C). Within the C-terminal domain of TRPC1, the region encompassing the D2R binding domain (residues 726–745) showed 70% amino acid similarity to the comparable region in TRPC4 (residues 735–754) and 75% amino acid similarity to the comparable region in TRPC5 (residues 742–761). We used a directed two-hybrid approach and tested for interaction between the D2IC2 and truncation fragments of TRPC4 and TRPC5 analogous to the D2R binding domain present in TRPC1. As shown in Fig. 2D, the TRPC4 and TRPC5 truncation fragments each displayed a positive interaction with the D2IC2 domain, and produced detectable, albeit lower, levels of β-galactosidase activity compared to the TRPC1/D2IC2 interaction. Taken together, these results support the view that TRPC1, TRPC4, and TRPC5 are all capable of interaction with the D2R, and that this interaction is mediated via a conserved binding domain located in the C-terminal tail of each of the three TRPC family members.
3.3 TRPCs interact with D2Rs in rat brain and are a component of the D2R signalplex
We examined the interaction between the D2R and TRPCs in rat brain by coimmunoprecipitation experiments. We tested several independent anti-TRPC1 antibodies to confirm the detection of native TRPC1 protein in cell lysates as well as rat cortex and striatum membrane preparations. All antibodies tested reacted with a polypeptide that migrated with the expected molecular mass of TRPC1 (between 75 kDa and 100 kDa – data not shown). This polypeptide was identified in HEK293T cells, HEK293-D2L cells, and rat cortex and striatum, indicating that TRPC1 protein is endogenously expressed in each of these cell lines and native rat tissues.
Crude membrane fractions from striatal and cortical rat tissue were immunoprecipitated using anti-D2R antibodies and immunocomplexes sequentially probed with anti-TRPC1 antibodies. As shown in Figure 3, two different TRPC1 antibodies were capable of detecting TRPC1 immunocomplexed with D2Rs. We were also able to immunoprecipitate the D2R with any of three independent commercially available anti-D2R antibodies (obtained from Abcam, Santa Cruz, or Calbiochem). Blots were stripped and re-probed to confirm the presence of additonal DRIPs. Immunoreactive bands corresponding in size to TRPC1 and TRPC4 were detected within the D2R-containing complexes (Fig. 4A, top and middle panels, respectively). In addition, D2R-containing immunocomplexes obtained from cortex and striatum also tested positive for the presence of the D2R interacting protein NCS-1 (data not shown) [17]. Although our two-hybrid and pulldown results indicate that TRPC5 and D2Rs also interact, we were unable to detect TRPC5 in D2R-containing immunocomplexes (data not shown). Failure to detect TRPC5 channels most likely reflects the poor quality of commercially available anti-TRPC5 antibodies [26].
Figure 3. TRPC1 Interacts with D2R in Rat Cortex and Striatal Tissue.

Anti-D2R antibodies were used to immunopreciptate D2Rs from rat cortex and striatum (ac-Abcam; sc-Santa Cruz; cb-Calbiochem). Blots containing immunocomplexes (IP) were sequentially probed with anti-TRPC1 antibodies (antibody used in top and middle panels was the monoclonal anti-TRPC1 antibody from Leonidas Tsiokas, University of Oklahoma; antibodies used in bottom panel were from Sigma). The position of TRPC1 endogenously expressed in cortical and striatal tissue is shown (lysate lane). No signal was observed in lysate sample adsorbed onto beads alone (Mock IP)
Figure 4. TRPCs interact with D2R and other components of the D2R signalplex.

(A) Anti-D2R antibody (Abcam) was used to immunopreciptate D2Rs from cortex and striatum of rat. Blots containing immunocomplexes were sequentially probed with anti-TRPC1 (monoclonal anti-TRPC1 antibody) and anti-TRPC4 (Chemicon) antibodies. The positions of TRPC1 and TRPC4 endogenously expressed in cortical and striatal tissue is shown (lysate lanes). No signal was seen in lysate adsorbed onto beads alone (Mock IP). (B) NCS-1-GST fusion protein was used to pull down S-tagged TRPC1 (residues 638–759) from a bacterial lysate. S-tagged TRPC1 was pulled down in the presence of NCS-1-GST, but not with GST or beads alone. The position of the TRPC truncation fragment is shown in the lysate lane.
Previous experiments we have carried out demonstrated a direct interaction between the DRIP, NCS-1, and TRPC5 [34]. We used pulldown techniques to examine whether TRPC1 and NCS-1 might also directly interact. A lysate prepared from bacteria expressing an Stagged TRPC1 C-terminal fragment (residues 638–759) was tested for the ability to associate with an NCS-1-GST fusion protein. As shown in Fig. 4B, the lysate produced an immunoreactive band of ~18 kDa, the expected size of the TRPC1 fragment, when probed with an anti-S-protein antibody. The same band was detected after the bacterial lysate was incubated with the NCS-1-GST fusion protein, but not when the lysate was absorbed onto either GST or beads alone. These results support the idea that TRPC1 interacts directly with the D2R as well as additional components of the D2R signalplex.
3.4 TRPC1 and D2Rs colocalize in postsynaptic compartments of cortical neurons
Immunohistochemical analysis of monkey prefrontal cortex (PFC) was used to gain insight into the subcellular distribution of TRPC1 and D2Rs. As shown in Fig. 5A, TRPC1 immunoreactivity was detected in both the cell body and the dendritic shafts of PFC pyramidal neurons, where immunoparticles were found in association with the trans-Golgi network and with the endoplasmic reticulum (Fig. 5A). A fraction of TRPC1 immunoreactivity was also localized at the plasma membrane, and often occurred at sites opposite smooth reticular endomembranes (Fig. 5A). Similarly, D2R immunolabeling was also found in the cell body (where it often decorated cisternae of the rough endoplasmic reticulum) and extrasynaptically in higher order dendrites (Fig. 5B). Consistent with earlier studies [27], D2Rs were found associated with the plasma membrane as well as intracellular compartments (Fig. 5B). In general, D2R expression within the PFC was considerably weaker than TRPC1 when comparing antibody reactivity.
Figure 5. Expression profile of TRPC1 and D2Rs in neurons of the PFC.

(A) Immunolabeling of TRPC1 subunits in the perikarya and dendrites of cortical neurons. TRPC1 immunolabeling was found in association with the (granular and agranular) endoplasmic reticulum and along the trans-Golgi axis (large inset). A limited plasmalemmal expression was also found at or near sites of juxtaposition of smooth endomembranes (small inset). (B) D2R expression was predominantly localized to (extrasynaptic ) membrane-bound compartments of high-order dendritic branches (den), and virtually absent in nearby axo-spinous (ax-sp) synapses. Scale bars: (A) 1 μm; (A-insets) 200 nm; (B) 400 nm.
Double immunoelectron microscopic analysis of monkey PFC was used to examine the subcellular colcoalization of TRPC1 and D2Rs. Peroxidase labeling of TRPC1 produced a diffuse precipitate that contrasted with the D2R immunogold particles (Fig. 6, A–D). TRPC1 labeling was found to colocalize with D2R expression within dendritic branches expressing both cytoplasmic and membrane bound D2Rs (Fig. 6B, C). In addition, TRPC1 labeling colocalized with D2R immunolabeling at the plasma membrane (Fig. 6A). Reversal of the immunocytochemical sequence (i.e., peroxidase-labeled D2R, gold-labeled TRPC1) corroborated these findings and demonstrated that the two immunoprobes clearly overlap on endoplasmic membranes (Fig. 6D).
Figure 6. Colocalization of D2R and TRPC1 in the cortex.

(A–C) TRPC1 labeled with immunoperoxidase is shown in dendritic branches expressing both cytoplasmic and plasmalemmal D2R-immunoparticles (arrowheads). Note that the diffuse TRPC1 labeling in A (indicated by bracket within frame) is restricted to a portion of the dendrite and overlaps with the expression of D2Rs at the plasma membrane (frame). Other profiles reactive for D2R alone are seen in A and C. To test for reagent and method selectivity, we reversed the immunocytochemical sequence. In D, D2R labeling with immunoperoxidase overlaps with TRPC1 immunoparticles (double arrowheads) within membranes of intracellular compartments of a primary dendrite (lower inset). In E and C, dual nanogold labeling for D2Rs (large particles; arrowheads) and TRPC1 (small particles; double arrowheads) shows that immunoprobes are often separated by fewer than 50 nm. (F) Spatial colocalization of D2R and TRPC1 immunoparticles strongly suggests that the two proteins are physically associated at the plasma membrane of dendrites. Asterisks mark a subsurface cistern; ax, axon; den, dendrite; sp, spine. Scale bars: 200 nm.
We next exploited the resolution power of postfixed nanogold to label TRPC1 and D2Rs on single sections of monkey PFC (Fig. 6E, F). TRPC1 and D2Rs exhibited comparable distribution patterns within individual subcellular compartments and organelles (Fig. 6E, F). Spatial co-expression of TRPC1 and D2R immunosignals (i.e. those cases where the two immunoprobes could be found separated by less than 20 nm as determined by the maximum linear dimension of both immunocomplexes) was often observed at extrasynaptic sites of the plasma membrane (Fig. 6F). The close spatial proximity of TRPC1 and D2R immunoreactivity demonstrates that the two proteins colocalize within neurons, and provides compelling evidence for a physical interaction between these binding partners in the primate cortex.
3.5 TRPC1/D2R interaction mediates cell surface expression of TRPC1 proteins
To help determine the functional significance of the TRPC1/D2R interaction, we used a cell surface biotinylation assay to analyze the effect of D2R expression on the levels of TRPC1 at the plasma membrane. We took advantage of the fact that wild-type HEK293 and HEK293-D2L cells endogenously express similar levels of TRPC1 (Fig. 7B), whereas only HEK293-D2L cells express D2Rs. HEK293 and HEK293-D2L cell surface proteins were biotinylated by incubation with NHS-SS-biotin. Biotinylated membrane proteins were immunoprecipitated using an anti-TRPC1 polyclonal antibody (Alomone) and TRPC1 proteins quantified by densitometric analysis. As shown in Fig. 7 (A and C), HEK293-D2L cells displayed a two-fold increase in the numbers of cell surface TRPC1 channels compared with wild-type HEK293 cells. Similar results were obtained with an independent (Sigma) anti-TRPC1 antibody [26].
Figure 7. TRPC1/D2R interaction mediates cell surface expression of TRPC1.
A cell surface biotinylation assay was used to examine the effect of the D2R on plasma membrane expression of TRPC1. HEK293, HEK293-D2L, and HEK293-MOR cell surface proteins were biotinylated by incubation with NHS-SS- biotin. Biotinylated membrane proteins were immunoprecipitated using an anti-TRPC1 antibody (Alomone) and TRPC1 proteins quantitated by densotimetric analysis. (A) Anti-TRPC1 antibody was used to immunoprecipitate TRPC1 proteins from HEK293 and HEK293-D2L cells. Immunoblots were probed for the presence of biotin to identify cell surfaced- expressed TRPC1. (B) Total expression of TRPC1 in crude membrane fractions prepared from HEK293 and HEK293-D2L cells. (C) Biotinylated TRPC1 proteins in A were quantitated by laser densitometry. (D) Anti-TRPC1 antibody was used to immunoprecipitate TRPC1 from HEK293-MOR and HEK293-D2L cells. (E) Total expression of TRPC1 in HEK293-D2L and HEK293-MOR cells. (F) Biotinylated TRPC1 proteins in D were quantitated by laser densitometry. HEK293-D2L cells stably expressing D2Rs show an approximately 50% increase in the level of cell surface TRPC1 proteins (Students two-tailed t test, n=6, *p< 0.05) compared to either HEK293 cells or HEK293-MOR cells (n=4. *p<0.05). (G) Anti-TRPC1 antibody was used to immunoprecipitate TRPC1 proteins from HEK293-D2L and HEK293-MOR cells. Blots containing TRPC1 immunocomplexes were probed with anti-FLAG antibodies to identify D2L-FLAG and MOR-FLAG proteins. D2R and TRPC1 coimmunoprecipitated from HEK293-D2L cells, whereas TRPC failed to coimmunoprecipitate with the MOR expressed in HEK293-MOR cells.
We also examined the cell surface expression of TRPC1 in HEK293 cells stably transfected with the μ-opioid receptor (HEK293-MOR cells). As shown in Fig. 7E, HEK293-D2L and HEK293-MOR cells endogenously express similar levels of TRPC1. However, HEK293-D2L cells exhibit an approximately two-fold increase in the numbers of cell surface TRPC1 channels compared with HEK293-MOR cells (Fig. 7D and F). In control experiments, we found that the D2R and TRPC1 coimmunoprecipitated from HEK293-D2L cells, whereas TRPC failed to coimmunoprecipitate with the MOR expressed in HEK293-MOR cells (Fig 7G). These results are consistent with the idea that the TRPC1/D2R interaction is required for the proper trafficking and/or expression of TRPC1 channels at the plasma membrane,
4. Discussion
TRPC channels are believed to play an important role in regulating calcium entry into cells [20, 22]. The presence of a signalplex containing D2Rs and TRPC1 suggests that D2Rs can impact cellular calcium signaling by coupling to TRPC channels. It is interesting to note that this is the first instance in which a direct interaction has been observed between a TRPC channel and a GPCR. Previous studies have demonstrated that TRPC channels are activated mainly by Gq-protein coupled receptor stimulation. Our results thus point to a novel mechanism of GPCR/TRPC channel regulation that may involve direct protein-protein interaction.
In this study, we present a variety of criteria establishing TRPC1, TRPC4, and possibly TRPC5, as bonafide DRIPs. Among the seven mammalian TRPC family members, TRPC1, TRPC4 and TRPC5 comprise a sequence-related subgroup in which TRPC1 can form heteromeric channels with either TRPC4 or TRPC5 (reviewed in [19, 33]). In GST-pulldown experiments, we detected interaction of D2R with the C-terminal segment of TRPC1, TRPC4 and TRPC5, a region known to interact with several TRPC binding partners including the IP3R and CaM [32, 33, 35–37]. Interaction of the D2R with TRPC1 and TRPC4 was validated by coimmunoprecipitation of the binding partners directly from rat brain. However, we could not confirm interaction of the D2R with TRPC5 by coimmunoprecipitation, suggesting that D2Rs maintain specificity in their interaction with TRPC channels.
Our biotinylation studies indicate an increase in cell surface expression of TRPC1 in cells expressing the D2R, suggesting that interaction between TRPC and the D2R regulates the trafficking/stability of TRPC1 at the plasma membrane. At present, it is not clear if the net increase in TRPC1 at the plasma membrane represents a net increase in TRPC1 trafficking to the cell surface or an increase in the stability of TRPC1 channels already at the cell surface. In this context, it is interesting to consider that treatment of cells with D2R agonists such as dopamine or quinpirole did not appear to have an effect on the cell surface expression of TRPC1 when compared to non-treated cells [26]. These results suggest that physical assembly of the TRPC1/D2R complex is sufficient for promoting TRPC1 trafficking to the plasma membrane, and may be a key determinant in the receptor-mediated activation of these channels.
In the future it will be important to determine whether the D2R/TRPC interaction can modulate the electrophysiological properties of cell surface-associated TRPC channels. Several recent studies point to a functional relationship between D2Rs and TRPC channels in the brain. It has been shown, for example, that TRP-like currents can be evoked via D2-like dopaminergic stimulation of rat dorsal raphe serotonin neurons [38]. Electrophysiological recording from rat brain slices of the dorsal raphe nucleus, a region known to express D2R [39, 40] as well as TRPC1 proteins [39–41], demonstrated the presence of a reversible slow membrane depolarization in response to stimulation with dopamine or quinpirole. This current was found to be abolished in the presence of the D2-like antagonist sulpride, and attenuated by the TRP channel inhibitors 2-APB and SKF-9636, suggesting that TRPC channels are responsible for the dopamine stimulated slow membrane depolarization current in dorsal raphe nucleus serotonin neurons [38].
Calcium entry through plasma membrane TRPC channels is believed to play an important role in a plethora of cellular events in both excitable and nonexcitable tissue [19, 20]. Moreover, it has been suggested that alterations in the calcium signaling properties of DRs contribute to several neuropathologies including schizophrenia and bipolar disorder [42]. Previous studies have shown that expression of NCS-1, a D2R interacting protein, is altered in patients with schizophrenia [10, 43] as well as in mice treated with antipsychotic drugs [44]. It is of particular interest that TRPC1 and TRPC5 also interact with NCS-1. In view of the critical roles assumed by TRPCs in regulating key neural functions, including neurite extension and growth cone guidance [45–47], it will be important to determine if alterations in expression or function of TRPC occur in diseases associated with dysregulation of dopamine neurotransmission. Alterations in TRPC protein expression or function could have a profound effect on the proper trafficking and/or signaling properties of the D2R-signalplex. In this context, it is interesting to consider that alterations in TRPC7 expression have recently been reported in patients with bipolar affective disorder [48].
Acknowledgments
We are grateful to Drs. Victor Canfield, Blaise Peterson, and Jamie Weiss for their helpful comments on the manuscript. This work was supported by an NIH Conte Center grant (MH 068789).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bertorello AM, Hopfield JF, Aperia A, Greengard P. Inhibition by dopamine of (Na(+)+K+)ATPase activity in neostriatal neurons through D1 and D2 dopamine receptor synergism. Nature. 1990;347:386–8. doi: 10.1038/347386a0. [DOI] [PubMed] [Google Scholar]
- 2.Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharmacol. 1998;42:707–11. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- 3.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 4.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 5.Civelli O, Bunzow JR, Grandy DK. Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol. 1993;33:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- 6.Sealfon SC, Olanow CW. Dopamine receptors: from structure to behavior. Trends Neurosci. 2000;23:S34–40. doi: 10.1016/s1471-1931(00)00025-2. [DOI] [PubMed] [Google Scholar]
- 7.Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61:641–4. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- 8.Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–9. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 9.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–3. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 10.Bai J, He F, Novikova SI, Undie AS, Dracheva S, Haroutunian V, Lidow MS. Abnormalities in the dopamine system in schizophrenia may lie in altered levels of dopamine receptor-interacting proteins. Biol Psychiatry. 2004;56:427–40. doi: 10.1016/j.biopsych.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Bergson C, Levenson R, Goldman-Rakic PS, Lidow MS. Dopamine receptor-interacting proteins: the Ca(2+) connection in dopamine signaling. Trends Pharmacol Sci. 2003;24:486–92. doi: 10.1016/S0165-6147(03)00232-3. [DOI] [PubMed] [Google Scholar]
- 12.Rasolonjanahary R, Gerard C, Dufour MN, Homburger V, Enjalbert A, Guillon G. Evidence for a direct negative coupling between dopamine-D2 receptors and PLC by heterotrimeric Gi1/2 proteins in rat anterior pituitary cell membranes. Endocrinology. 2002;143:747–54. doi: 10.1210/endo.143.3.8697. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J Neurosci. 2000;20:8987–95. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan Z, Feng J, Fienberg AA, Greengard P. D(2) dopamine receptors induce mitogen-activated protein kinase and cAMP response element-binding protein phosphorylation in neurons. Proc Natl Acad Sci U S A. 1999;96:11607–12. doi: 10.1073/pnas.96.20.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabbani N, Hannan MA, Levenson R. Unraveling the dopamine receptor signalplex by DRIPs and DRAPs. Current Proteomics. 2005;2:209–224. [Google Scholar]
- 16.Kabbani N, Levenson R. A proteomic approach to receptor signaling: Molecular mechanisms and therapeutic implications derived from discovery of the dopamine D2 receptor signaling. Eur J Pharmacol. 2007 doi: 10.1016/j.ejphar.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 17.Kabbani N, Negyessy L, Lin R, Goldman-Rakic P, Levenson R. Interaction with neuronal calcium sensor NCS-1 mediates desensitization of the D2 dopamine receptor. J Neurosci. 2002;22:8476–86. doi: 10.1523/JNEUROSCI.22-19-08476.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binda AV, Kabbani N, Levenson R. Regulation of dense core vesicle release from PC12 cells by interaction between the D2 dopamine receptor and calcium-dependent activator protein for secretion (CAPS) Biochem Pharmacol. 2005;69:1451–61. doi: 10.1016/j.bcp.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Ramsey IS, Delling M, Clapham DE. An introduction to trp channels. Annu Rev Physiol. 2006;68:619–47. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 20.Beech DJ. TRPC1: store-operated channel and more. Pflugers Arch. 2005;451:53–60. doi: 10.1007/s00424-005-1441-3. [DOI] [PubMed] [Google Scholar]
- 21.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay B, Cheng KT. TRPC1: The link between functionally distinct store-operated calcium channels. Cell Calcium. doi: 10.1016/j.ceca.2007.01.013. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 22.Beech DJ, Xu SZ, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell Calcium. 2003;33:433–40. doi: 10.1016/s0143-4160(03)00054-x. [DOI] [PubMed] [Google Scholar]
- 23.Lin R, Karpa K, Kabbani N, Goldman-Rakic P, Levenson R. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with filamin A. Proc Natl Acad Sci U S A. 2001;98:5258–63. doi: 10.1073/pnas.011538198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabbani N, Jeromin A, Levenson R. Dynamin-2 associates with the dopamine receptor signalplex and regulates internalization of activated D2 receptors. Cell Signal. 2004;16:497–503. doi: 10.1016/j.cellsig.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Ong HL, Chen J, Chataway T, Brereton H, Zhang L, Downs T, Tsiokas L, Barritt G. Specific detection of the endogenous transient receptor potential (TRP)-1 protein in liver and airway smooth muscle cells using immunoprecipitation and Western-blot analysis. Biochem J. 2002;364:641–8. doi: 10.1042/BJ20020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hannan MA. PhD Dissertation, Vol. PhD. Pennsylvania State University; Hershey, PA: 2007. [Google Scholar]
- 27.Paspalas CD, Goldman-Rakic PS. Microdomains for dopamine volume neurotransmission in primate prefrontal cortex. J Neurosci. 2004;24:5292–300. doi: 10.1523/JNEUROSCI.0195-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paspalas CD, Goldman-Rakic PS. Presynaptic D1 dopamine receptors in primate prefrontal cortex: target-specific expression in the glutamatergic synapse. J Neurosci. 2005;25:1260–7. doi: 10.1523/JNEUROSCI.3436-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paspalas CD, Goldman-Rakic PS. Soc Neuroscience Abstr. 2004 [Google Scholar]
- 30.Karpa KD, Lin R, Kabbani N, Levenson R. The dopamine D3 receptor interacts with itself and the truncated D3 splice variant d3nf: D3-D3nf interaction causes mislocalization of D3 receptors. Mol Pharmacol. 2000;58:677–83. doi: 10.1124/mol.58.4.677. [DOI] [PubMed] [Google Scholar]
- 31.Binda AV, Kabbani N, Lin R, Levenson R. D2 and D3 dopamine receptor cell surface localization mediated by interaction with protein 4.1N. Mol Pharmacol. 2002;62:507–13. doi: 10.1124/mol.62.3.507. [DOI] [PubMed] [Google Scholar]
- 32.Ambudkar IS. Ca2+ signaling microdomains:platforms for the assembly and regulation of TRPC channels. Trends Pharmacol Sci. 2006;27:25–32. doi: 10.1016/j.tips.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Kiselyov K, Kim JY, Zeng W, Muallem S. Protein-protein interaction and functionTRPC channels. Pflugers Arch. 2005;451:116–24. doi: 10.1007/s00424-005-1442-2. [DOI] [PubMed] [Google Scholar]
- 34.Hui H, McHugh D, Hannan M, Zeng F, Xu SZ, Khan SU, Levenson R, Beech DJ, Weiss JL. Calcium-sensing mechanism in TRPC5 channels contributing to retardation of neurite outgrowth. J Physiol. 2006;572:165–72. doi: 10.1113/jphysiol.2005.102889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–89. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 36.Plant TD, Schaefer M. Receptor-operated cation channels formed by TRPC4 and TRPC5. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:266–76. doi: 10.1007/s00210-005-1055-5. [DOI] [PubMed] [Google Scholar]
- 37.Mery L, Strauss B, Dufour JF, Krause KH, Hoth M. The PDZ-interacting domain of TRPC4 controls its localization and surface expression in HEK293 cells. J Cell Sci. 2002;115:3497–508. doi: 10.1242/jcs.115.17.3497. [DOI] [PubMed] [Google Scholar]
- 38.Aman TK, Shen RY, Haj-Dahmane S. D2-like dopamine receptors depolarize dorsal raphe serotonin neurons through the activation of nonselective cationic conductance. Journal of Pharmacology & Experimental Therapeutics. 2007;320:376–85. doi: 10.1124/jpet.106.111690. [DOI] [PubMed] [Google Scholar]
- 39.Mansour A, Meador-Woodruff JH, Bunzow JR, Civelli O, Akil H, Watson SJ. Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. Journal of Neuroscience. 1990;10:2587–600. doi: 10.1523/JNEUROSCI.10-08-02587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki M, Hurd YL, Sokoloff P, Schwartz JC, Sedvall G. D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Research. 1998;779:58–74. doi: 10.1016/s0006-8993(97)01078-0. [DOI] [PubMed] [Google Scholar]
- 41.Sergeeva OA, Korotkova TM, Scherer A, Brown RE, Haas HL. Co-expression of non-selective cation channels of the transient receptor potential canonical family in central aminergic neurones. J Neurochem. 2003;85:1547–52. doi: 10.1046/j.1471-4159.2003.01806.x. [DOI] [PubMed] [Google Scholar]
- 42.Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res Brain Res Rev. 2003;43:70–84. doi: 10.1016/s0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]
- 43.Koh PO, Undie AS, Kabbani N, Levenson R, Goldman-Rakic PS, Lidow MS. Up-regulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients. Proc Natl Acad Sci U S A. 2003;100:313–7. doi: 10.1073/pnas.232693499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kabbani N, Levenson R. Antipsychotic-induced alterations in D2 dopamine receptor interacting proteins within the cortex. Neuroreport. 2006;17:299–301. doi: 10.1097/01.wnr.0000199460.24412.04. [DOI] [PubMed] [Google Scholar]
- 45.Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–45. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, Yuan XB. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–8. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- 47.Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898–904. doi: 10.1038/nature03478. [DOI] [PubMed] [Google Scholar]
- 48.Yoon IS, Li PP, Siu KP, Kennedy JL, Macciardi F, Cooke RG, Parikh SV, Warsh JJ. Altered TRPC7 gene expression in bipolar-I disorder. Biol Psychiatry. 2001;50:620–6. doi: 10.1016/s0006-3223(01)01077-0. [DOI] [PubMed] [Google Scholar]




