Abstract
Severe acute respiratory syndrome (SARS) is a disease characterized by diffuse alveolar damage. We isolated human alveolar type II cells and maintained them in a highly differentiated state. Type II cell cultures supported SARS-CoV replication as evidenced by RT-PCR detection of viral subgenomic RNA and an increase in virus titer. Virus titers were maximal by 24 h and peaked at approximately 105 pfu/mL. Two cell types within the cultures were infected. One cell type was type II cells, which were positive for SP-A, SP-C, cytokeratin, a type II cell-specific monoclonal antibody, and Ep-CAM. The other cell type was composed of spindle-shaped cells that were positive for vimentin and collagen III and likely fibroblasts. Viral replication was not detected in type I-like cells or macrophages. Hence, differentiated adult human alveolar type II cells were infectible but alveolar type I-like cells and alveolar macrophages did not support productive infection.
Keywords: SARS, Lung, Alveolar macrophage, ACE2
Background
SARS coronavirus (SARS-CoV) emerged in 2002–2003 and presented primarily as a fulminant atypical pneumonia with a case fatality rate of about 10%. Since its disappearance at the end of the 2003 pandemic, the virus and the associated syndrome have been the focus of intense study. Pathologic studies on the lungs of fatal cases often report the presence of marked diffuse alveolar damage and uniformly report SARS-CoV antigen and/or RNA in the alveolar epithelium and alveolar macrophages (CD68+ cells) (Ding et al., 2004, Franks et al., 2003, Gu et al., 2005, He et al., 2006, Hwang et al., 2005, Nicholls et al., 2006, Shieh et al., 2005, To et al., 2004, Ye et al., 2007). These studies indicate that alveolar epithelial cells are important cells involved with SARS infection and subsequent diffuse alveolar damage and respiratory failure. When the alveolar epithelium is further differentiated into type I and type II cells, the various studies report infection of one, the other, or both cell types. The primary receptor of SARS-CoV was identified as angiotensin-converting enzyme 2 (ACE2) (Li et al., 2003). ACE2 is distributed among numerous tissues, including abundant expression on the pulmonary and intestinal epithelia accounting for the pulmonary and GI symptoms observed with infection (Hamming et al., 2004, He et al., 2006).
The alveolar epithelium consists of two main cell types. Type I cells are thin, flat cells that cover over 90% of the alveolar surface and comprise 8% of total lung cells (Stone et al., 1992a). The primary function of the type I cells is to facilitate gas exchange between the lumen of the alveoli and the blood. Alveolar type II cells are cuboidal epithelial cells that comprise 15% of total lung cells and maintain the alveolar microenvironment through production and secretion of pulmonary surfactant, transepithelial sodium transport and alveolar fluid homeostasis, proliferation to maintain the epithelium, and ultimate transdifferentiation into type I cells (Mason, 2006). The alveolar wall and air space also contains alveolar macrophages and other inflammatory cells, endothelial cells, and fibroblasts as well as cells within the microvasculature. All of the cells could be theoretic targets for SARS-CoV infections, but ACE2 has been reported to be heavily expressed on alveolar type II cells in the human lung (Ding et al., 2004, Hamming et al., 2004, He et al., 2006).
Many in vitro models have been explored for their utility in studying SARS-CoV infection of the pulmonary epithelium. A549 cells, a lung carcinoma cell line that has been used as a model of alveolar epithelial cells although it does not express the surfactant proteins, has been refractory to SARS-CoV infection unless provided with exogenous ACE2 despite expressing some endogenous ACE2 (Gillim-Ross et al., 2004, Hamming et al., 2004, Lieber et al., 1976, Mossel et al., 2005). Calu-3 cells, a bronchial epithelial adenocarcinoma cell line, are permissive to SARS-infection and are a useful model for infection (Ren et al., 2006, Tseng et al., 2005). Two groups have reported that differentiated airway ciliated cells can be infected with SARS-CoV and that the infection correlated with ACE2 expression (Jia et al., 2005, Sims et al., 2005). However, infection of primary cultures of alveolar epithelial cells has not been reported.
Using a multi-step isolation and purification technique, we produced cultures of highly differentiated type II and type I-like alveolar epithelial cells (Wang et al., 2007). Here we examine the ability of SARS-CoV to infect these cell types. An understanding of the cells of the alveoli permissive to SARS-CoV infection is an important step in understanding SARS pathogenesis. We found that differentiated alveolar type II cells could be infected with SARS-CoV. An unexpected finding in these studies was the detection of SARS-CoV antigen in pulmonary fibroblasts in our cultures. This is the first report of SARS-CoV infection in differentiated adult human alveolar type II cells in vitro.
Results
Characterization of primary human type II and type I-like pneumocytes
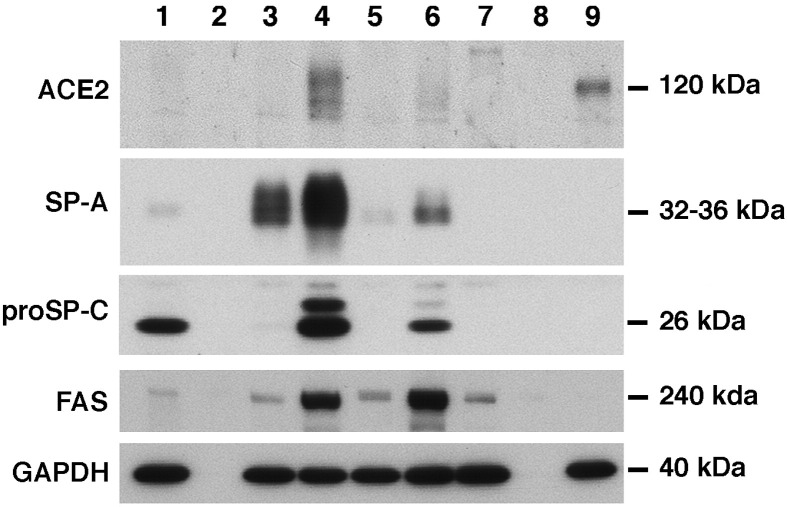
The type II cell and type I-like cell cultures have been fully characterized previously (Wang et al., 2007). The type II cell cultures expressed the surfactant proteins SP-A, SP-B, and proSP-C at the mRNA and protein level and had characteristic lamellar inclusions and apical microvilli (Wang et al., 2007). As shown in Fig. 1 , these cells express SP-A and proSP-C as well as fatty acid synthase (FAS) and ACE2 when cultured with keratinocyte growth factor (KGF), isobutylmethylxanthine (IBMX), 8-Br-cAMP (A), and dexamethasone (Dex, D) collectively referred to as KAID, in the presence of 1% charcoal-stripped fetal bovine serum (CS-FBS). By both Western and RNA analyses, ACE2 expression was highest in the presence of KAID in 1% CS-FBS (Fig. 1 and data not shown). The type I-like cells expressed caveolin-1, receptor for advanced glycated end products (RAGE), and cytokeratin, but expression of ACE2, SP-A, SP-B, and proSP-C was below the limit of detection (Fig. 1 and data not shown) (Wang et al., 2007).
Fig. 1.
Type II cell cultures express ACE2 as well as the surfactant proteins. Alveolar type II cells or Vero cells were cultured as described in the Methods section, and extracts were prepared for immunoblotting. Type II cells were cultured with specific additives on a Matrigel-rat tail collagen gel for 6 days and type I-like cells were cultured on rat-tail collagen-coated wells. Lane 1 contains the extract of freshly isolated type II cells; Lane 2 is a blank lane; Lane 3 contains extract of cells with 1% CS-FBS alone; Lane 4 1% CS-FBS + KAID; Lane 5 5% FBS; Lane 6 5% FBS + KAID; Lane 7 5% FBS with cells on a collagen-coated well (type I phenotype); Lane 8 blank lane; and Lane 9 Vero cells. These results are representative of four separate experiments.
SARS-CoV replicates in type II but not type I-like alveolar cells in vitro
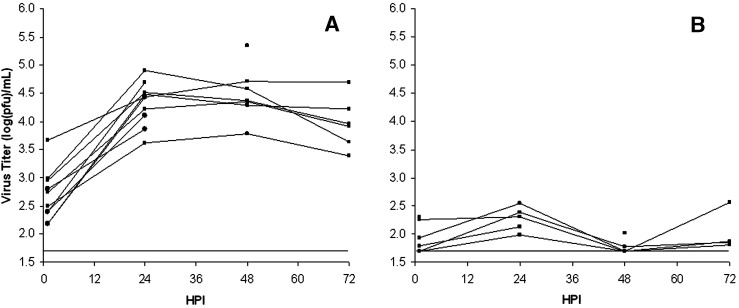
Type I-like and type II cell cultures were infected with SARS CoV at MOI = 2–3, and supernatant titers were determined by plaque assay at the indicated time points to determine their ability to support SARS-CoV replication (Figs. 2A, B). Type II cells cultured on the Millicell inserts proved difficult to wash clean of residual inoculum resulting in relatively high titers detected at 1 hpi. However, in each of the 11 different experiments on cells isolated from nine different lungs, detectable virus in the supernatant increased 10–100× at 24–48 hpi. Maximal titers were generally reached by 24 hpi and were maintained or dropped slightly through 72 hpi. Cytopathic effect (CPE) was not detectable in the type II cell cultures. Type I-like cells did not appear to support SARS-CoV replication. After < 0.5 log increase in detectable extracellular virus 24 hpi, virus was undetectable in most cultures through 48 and 72 hpi (n = 6 different lungs for type I-like cell cultures). In addition, alveolar macrophages did not support productive infection (data not shown). For the type II cell donors, the age range was 21–74 years (44 ± 20 years, mean ± S.D.) with nearly equal numbers of men and women and smokers and nonsmokers. There was no obvious effect of sex or smoking status on the results in this small cohort. The type I-like cell cultures that did not support viral production were all from donors whose type II cells supported viral production.
Fig. 2.
Virus replication in primary human alveolar type II cell and type I-like cell cultures. Cell monolayers were infected at MOI = 2–3 after 7–8 days in culture. At each time point, an aliquot of supernatant was removed and frozen for plaque assay. The results for type II cells are shown in Panel A and type I-like cells in Panel B. Each curve represents virus growth in cells derived from different donor lungs and measured in three wells. The solid horizontal line indicates the assay limit of detection.
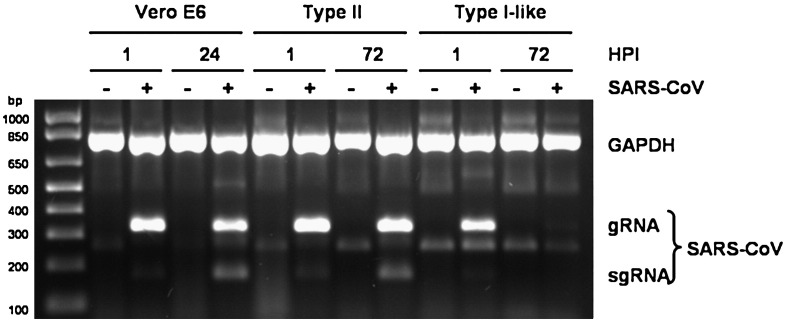
These observations were confirmed by application of the multiplex RT-PCR method previously described by Gillim-Ross et al. (2004). This method allows for simultaneous amplification of genomic and sub-genomic SARS-CoV RNA as well as a GAPDH internal control. Vero E6, type II, and type I-like cell cultures were infected at MOI = 2–3. At the indicated time points, RNA was extracted from the monolayers and amplified. 1 hpi, SARS-CoV genomic RNA was present in all three infected cultures (Fig. 3 ), indicative of incomplete removal of the inoculum. SARS-CoV genomic and subgenomic RNA is present 24 hpi in Vero E6 cells and 72 hpi in type II cell cultures, further evidence of SARS-CoV replication in these cells. However, at 72 hpi, SARS-CoV genomic and subgenomic RNA was below the limit of detection in type I-like cells.
Fig. 3.
SARS-CoV genomic and subgenomic RNA in infected Vero E6, type II, and type I-like cell cultures. Cell monolayers were infected at MOI = 2–3. Total RNA was extracted from the monolayers 24 hpi (Vero E6) or 72 hpi (type II and type I-like cells). SARS-CoV genomic and subgenomic RNA and cellular GAPDH RNA were simultaneously amplified by multiplex RT-PCR as described.
Immunofluorescent staining of SARS-CoVinfected type II and type I-like cell cultures
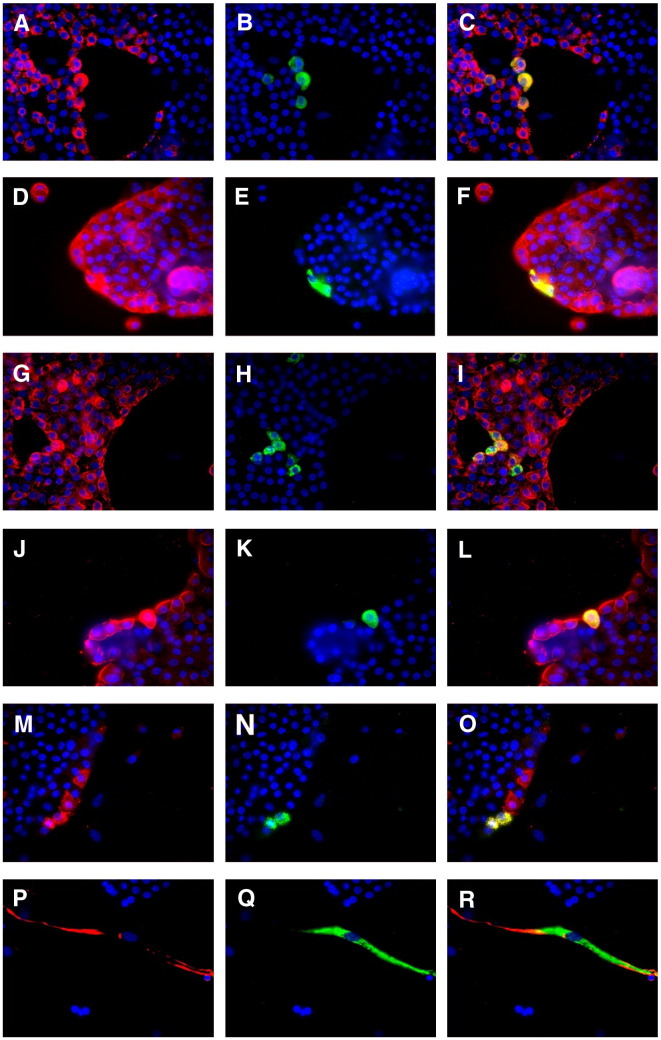
The 1–2 log increase in virus titer in the supernatants of type II cell cultures, while reproducible, was unexpectedly low. Monolayers infected as above were immunostained with anti-SARS-N to determine the extent of SARS-CoV infection and a variety of cell markers to identify the cells infected. Within the type II cell cultures, very few cells (estimated at < 1 per 250) stained positive for SARS-N (Figs. 4B, E, H, K, N, Q). Further, the SARS-N positive cells had two distinct morphologies. One was small, cuboidal cells typically appearing in large, cobbled islands, which were type II cells, and the other was a larger flat or spindle-shaped cell. The small, cuboidal cells stained positive for SP-A, cytokeratin, EP-CAM, and a type II cell-specific antibody obtained from Dr. Leland Dobbs, University of California, San Francisco (Fang et al., 2006) (Figs. 4A–L), and proSP-C (data not shown) confirming their type II phenotype. These cells were also negative for CD-31, CD-45, CD-68, collagen III and vimentin (data not shown). The larger, spindle-shaped cells that stained positive for SARS-N were vimentin (Figs. 4P–R) and collagen III positive and were SP-A, cytokeratin, EP-CAM, CD-45, CD-68 and CD-31 negative (data not shown), indicating that these infected cells are fibroblasts. Within the type II cell cultures, a rare CD-31 positive cell could be identified as also being SARS-N positive, indicating that endothelial cells may also be occasionally infected with SARS-CoV.
Fig. 4.
SARS-CoV infection in primary human alveolar type II cell cultures. Type II cell cultures were stained with antibodies to SARS-CoV nucleocapsid (SARS-N) protein (green), and a cellular marker (red). Each marker is shown in three frames: marker/DAPI, SARS-N/DAPI, and marker/SARS-N/DAPI. The small cuboidal cells were positive for SP-A (A–C), cytokeratin (D–F), EP-CAM (G–I), and a type II cell monoclonal antibody (J–L). Cells positive for SARS-N were also positive for the SARS-CoV receptor ACE2 (M–O). The larger spindle-shaped cells were vimentin positive (P–R).
As expected, the type II cells that were infected with SARS-CoV were also positive for the SARS-CoV receptor, ACE2 (Figs. 4M–O). However, also evident were cells that were positive for ACE2 but negative for SARS-CoV. None of the cells in these cultures stained with the Oct-4 antibody (SC-9081, Santa Cruz Biotechnology, Santa Cruz, CA, USA), which identifies a putative lung stem cell that can be infected with SARS (Ling et al., 2006) (data not shown). Despite the low level of infection in these cultures, we observed multinucleated cells of both type II and the spindle-shaped morphology (Fig. 5 ). Immunofluorescent staining of type I-like cell cultures indicated less vimentin-positive cell contamination. Further, type I-like monolayers were completely devoid of SARS-N staining (data not shown).
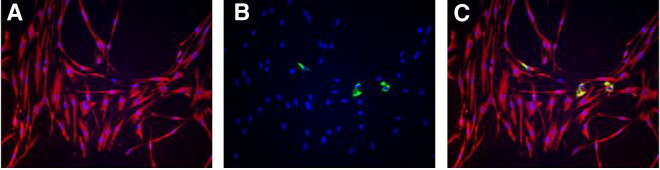
Fig. 5.
Multinucleated cells expressing SARS-N protein. Type II cell cultures were examined for the expression of SARS-CoV nucleocapsid protein (green). Nuclei were stained with DAPI. (A) A tri-nucleated cell of cuboidal morphology, likely of type II cell origin. (B) A bi-nucleated spindle shaped cell. Cells on this filter were additionally stained for SP-A. The morphology of this cell and the lack of SP-A staining suggest that this cell was derived from fibroblasts in the culture.
SARS-CoV infects rare primary human lung fibroblasts cultured in KAID-containing medium
Because we cannot further purify type II cells from the contaminating cell types, we examined the replication of SARS-CoV in primary human lung fibroblasts. Fibroblasts from five donors were cultured on Millicell inserts under the same conditions as the type II cell cultures. Fibroblasts from only one of these donors appeared to support a limited replication of SARS-CoV and these results were confirmed by immunohistochemistry (Fig. 6 ). However, a later passage of this fibroblast isolate that was initially positive for SARS was negative. Hence, we were not able to confirm this initial finding.
Fig. 6.
SARS-CoV infection of fibroblasts. Five different lung fibroblast isolates were evaluated and only one was positive. This picture represents the positive experiment. Panel A is vimentin staining; panel B is SARS-N protein; and panel C is the composite.
Discussion
Models currently used in pathologic studies of SARS and SARS-CoV in human cells are limited by two factors. First, most cell culture models are not adequately representative of primary cells or of cells in the context of tissue. Notable exceptions to this are the bronchial epithelial cell models of Sims et al. (2005) and Jia et al. (2005). Second, pathological specimens have the advantage of examining primary cells in their natural context, but by necessity have focused on the more severe or fatal cases, in which there is excessive tissue injury and cell recognition is difficult. It is not known if the diffuse alveolar damage is due primarily to alveolar epithelial infection, a cytokine storm, or a combination of these and other host factors, and if any special cell–cell interactions or protease exposure is required for alveolar epithelial infections. In addition, as reviewed by Subbarao and Roberts, animal models of SARS have significant limitations and do not fully replicate the severe disease in humans (Subbarao and Roberts, 2006).
In this report, we describe a model for the study of SARS-CoV infection of primary human alveolar epithelial cells. In our culture system, type II alveolar epithelial cells from all nine donors supported SARS-CoV replication. Immunofluorescent analysis suggests that viral replication occurs in a limited number of cells within our cultures. Replication of SARS-CoV did not, however, occur in the type I-like cells from any of the six donors examined. However, it should be noted that while these type I-like cells express many of the markers of type I cells in vivo, they were derived from type II cells in vitro and may not express all of the surface proteins of type I cells in the lung. We also observed the occasional infection of pulmonary fibroblasts, which was variable in different cultures. The number of contaminating fibroblasts varied with the donor and cell isolate. Because infected type II cells and fibroblasts were observed within the same cultures, it is not clear whether one or both of the infections is productive. Cultured monocyte derived macrophages are not susceptible to SARS-CoV infection as determined by virus production (Cheung et al., 2005). We extended this observation to human alveolar macrophages in primary culture. These results contrast with the several pathological studies that describe widespread detection of SARS-CoV RNA and/or antigen in the alveolar epithelium, both in type II and type I cells, though not always both cell types in every study (Ding et al., 2004, Franks et al., 2003, Gu et al., 2005, He et al., 2006, Hwang et al., 2005, Lang et al., 2003, Nicholls et al., 2006, Shieh et al., 2005, To et al., 2004, Ye et al., 2007). In addition, in these pathologic studies, alveolar macrophages were shown to be positive for SARS-CoV antigen and/or RNA. Giant multi-nucleated cells or syncytia within the lungs are characteristic of fatal SARS-CoV infection, though these do not always represent infected cells (Ding et al., 2003, Gu et al., 2005, Hwang et al., 2005, Nicholls et al., 2006, To et al., 2004). The SARS-CoV positive multinucleated giant cells can be derived from epithelial cells (cytokeratin positive) or macrophages (CD-68 positive) (Franks et al., 2003, Nicholls et al., 2003).
ACE2 has been previously reported to be expressed on type II cells (Hamming et al., 2004, Wiener et al., 2007). In murine lung, Weiner et al. recently reported ACE2 in type II cells, Clara cells, and smooth muscle cells from small and medium vessels, but not in type I cells or in capillaries in the alveolar wall (Wiener et al., 2007). However, with our antibodies and immunofluorescent techniques the level of expression of ACE2 is below the limit of detection in most cells in our cultures. ACE2 expression in type II cell cultures was increased by the addition of IL-13 to the basal medium but not to KAID medium in 1%CS-FBS, and the addition of IL-13 did not result in increased infection efficiency (data not shown). Additionally, as Figs. 4M–O demonstrates, numerous cells can be identified that express ACE2 but do not appear to be infected with SARS-CoV.
It is possible that other factors contributed to the inefficiency of SARS-CoV infection. Surfactant protein D (SP-D) might aggregate the virus and inhibit infection. SP-A and SP-D are secreted by type II cells and have a variety of immunoregulatory functions. Recently SP-D has been reported to bind to the spike glycoprotein on SARS-CoV (Leth-Larsen et al., 2007), and this could possibly limit infection. Type II cells also produce numerous cytokines that may have an effect on permissiveness to SARS-CoV infection. Beta-defensin-2 is a protein produced by type II cells shown in some cases to have direct anti-viral activity (Ganz, 2002, Sun et al., 2005). Efforts were made to remove surfactant proteins and other soluble factors from the cultures at the time of infection, but none resulted in increased infection efficiency. There also may be co-stimulating molecules for receptor binding or cathepsins needed for maximal viral infection that are missing from these primary cultures (Huang et al., 2006). Therefore, ACE2 expression alone may not account for the scarcity of infected cells. Another difference between the in vitro situation and the in vivo situation is the low volume of apical fluid such that a very high MOI likely exits in vivo. The alveolar fluid volume in an adult human is thought to be only 35 mL spread over a surface area of about 100 m2 (Stone et al., 1992b).
Another possible explanation for the low level of infection is the choice of subjects. Pathological reports focus on clinical, most often severe or fatal, cases of SARS. The cells used in this study are acquired from random members of the populace. If individuals progressing to severe disease represent a small proportion of those exposed, then it is possible that by utilizing cells from the general population, we are biasing our studies toward mimicking mild or subclinical-type infections. Despite these possibilities, the most likely explanation for the low level of infection is that we have not discovered the means of stimulating a high level of ACE2 expression throughout our cultures.
The infection of fibroblasts was unexpected. Recently, more detailed evaluation of pathologic material has indicated that fibroblasts can be infected in vivo (Ye et al., 2007). In addition, Lang et al. reported SARS RNA in interstitial cells as well as type II cells and bronchiolar epithelial cells (Lang et al., 2003). However, previous data indicated that fibroblast cell lines (MRC-5, HEL) were not permissive to SARS-CoV infection (Gillim-Ross et al., 2004, Kaye, 2006, Mossel et al., 2005). The infection of fibroblasts may have been related to particular characteristics of our cultures. The infection only occurred in one of five low passage cultures of primary lung fibroblasts. The infection that occurred in the one permissive sample was much more apparent in 1% CS-FBS plus KAID than in media with 5% FBS. The cells in our primary cultures of alveolar epithelial cells were considered fibroblasts because they expressed vimentin and collagen III but not CD31 or CD45. It is possible that these particular fibroblasts were derived from epithelial cells by epithelial mesenchymal transition (EMT), but we believe this possibility is unlikely. EMT in vitro requires exogenous transforming growth factor beta (TGF-β), which was not added to these cultures (Willis et al., 2005, Yao et al., 2004).
In conclusion, we have developed an in vitro culture system to evaluate SARS-CoV in highly differentiated adult human type II cells. Type II cells could be infected with SARS-CoV but we were unable to demonstrate productive infections with alveolar macrophages or alveolar type I-like cells in vitro.
Methods
Cells and virus
Vero E6 cells (American Type culture Collection (ATCC), Manassas, VA, USA) were propagated in MEM (Invitrogen, Carlsbad, CA, USA) supplemented with 5% FBS. SARS-CoV strain Urbani, obtained as a seed stock from Centers for Disease Control and Prevention, Atlanta, GA, USA was propagated in Vero E6 cells. All techniques using replication competent SARS-CoV were performed under BSL-3 containment. SARS-CoV titrations were performed on Vero E6 cells as described previously (Sainz et al., 2004) with the modification that SeaKem LE agarose (Cambrex, East Rutherford, NJ, USA) was used in the overlays.
Isolation and culture of human alveolar type II and type I-like cells
Type II cells were isolated from deidentified human lungs that were not suitable for transplantation and donated for medical research. The lungs were obtained through The National Disease Research Interchange (Philadelphia, PA, USA) and the International Institute for the Advancement of Medicine (Edison, NJ, USA). The Committee for the Protection of Human Subjects at National Jewish Medical and Research Center approved this research. The isolation method has been published previously (Wang et al., 2007). Briefly, the middle lobe was perfused, lavaged, and then instilled with elastase (12.9 units/mL, Roche Diagnostics, Indianapolis, IN, USA) and incubated for 50 min at 37 °C. The lung was minced, and the cells were isolated by filtration and partially purified by centrifugation on a discontinuous density gradient made of Optiprep (Accurate Chemical Scientific Corp., Westbury, NY, USA) with densities of 1.080 and 1.040 and by negative selection with CD-14-coated magnetic beads (Dynal Biotech ASA, Oslo, Norway) and binding to IgG-coated Petri dishes (Sigma Chemicals, Inc., St. Louis, MO, USA).
The isolated cells were resuspended in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% FBS, 2 mM glutamine, 2.5 μg/mL amphotericin B, 100 μg/mL streptomycin, 100 units/mL penicillin G, and 10 μg/mL gentamicin (Gibco BRL, Rockville, MD, USA). The cells were plated on 4.2 cm2 Millicell inserts (Millipore Corp., Bedford, MA, USA) at a density of 8 × 105/cm2 in DMEM with 10% FBS. After 24–48 h, the medium was changed to 1% CS-FBS, 10 ng/mL (KGF, K) (R&D Systems, Inc., Minneapolis MN, USA), 0.1 mM (IBMX, I), 0.1 mM 8-Br-cAMP (A), and 10 nM dexamethasone (Dex, D) (Sigma) in addition to glutamine, amphotericin B, streptomycin, penicillin, and gentamicin as above. Medium was replaced every 48 h. Some experiments utilized cells that were frozen down after isolation, stored in liquid nitrogen, thawed and processed the same as the fresh cells except that the plating density was 1 × 106 cells/cm2.
To transdifferentiate type II cells into type I-like cells, type II cells were plated on rat tail collagen-coated dishes or collagen-coated glass coverslips at a density of 0.5–1 × 105/cm2 in DMEM with 10% FBS (Wang et al., 2007). After 24–48 h for adherence, medium was changed to DMEM with 5% FBS without KAID additives above and cultured for an additional 6 days (Wang et al., 2007).
Isolation and culture of primary human lung fibroblasts
Fibroblasts were isolated from normal lung by a technique reported previously (Frankel et al., 2006). In brief, lung tissue was minced into 1 mm3 sections and cultured with 10% heat inactivated FBS on scored tissue culture dishes. After 10–14 days, the fibroblasts derived from the explants were trypsinized and maintained in culture. Cells at low passage (3–5) were used in these experiments. Some of the experiments were done on cells that had been frozen down. All the cells were vimentin positive.
SARS-CoV infection of type II and type I-like cells and primary fibroblasts
Medium was aspirated from both sides of the Millicell inserts (type II cells) or from the wells (type I cells). Monolayers were washed briefly with 2 mL MEM supplemented with 2% FBS (MEM/2%). A second wash was added and left to stand 15 min at room temperature (RT). The second wash was aspirated and to the interior chamber of each insert (type II) or well (type I) was added 250 μL SARS-CoV stock (MOI = 2–3) or 250 μL MEM/2% for mock-infections. Cells were placed at 37 °C in 5% CO2 atmosphere for 1 h. After adsorption, inoculum was aspirated and cell monolayers were washed four times with MEM/2%. After the final wash of type II cell cultures, 0.5 mL media was added to the interior of the Millicell insert and 1.5 mL media was added to the exterior. After the final wash of type I-like cells, 2 mL type I cell medium was added to each well. Cultures were incubated at 37 °C in 10% CO2 atmosphere. At indicated time points 100 μL aliquots of medium were removed and stored at − 80 °C until analysis and replaced with equal volumes of cell type appropriate fresh medium.
Western blotting
Protein expression was measured by Western blotting according to protocols described previously (Wang et al., 2007). Polyacrylamide gradient gels (8–16%) from Invitrogen Corp., Carlsbad, CA, USA, were run in Tris glycine buffer to separate proteins. Proteins were run in their reduced state except for SP-B, which was run unreduced. For Western blotting, protein loading was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Multiplex RT-PCR
After removal of the culture medium, 250 μL TRIzol LS reagent (Invitrogen) was added to the cell monolayers for 2–4 min at room temperature. RNA was extracted from the solution per the manufacturer's protocol. Multiplex RT-PCR for the simultaneous detection of SARS-CoV genomic and subgenomic RNA as well as cellular GAPDH was performed as described by Gillim-Ross et al. (2004).
Immunocytochemistry
Type II cells were fixed and stained on intact inserts. Type I-like cells were fixed and stained on rat tail collagen-coated coverslips within 6-well tissue culture plates. Cells were fixed 10–15 min in 100% methanol at − 20 °C or overnight in 4% paraformaldehyde at 4 °C. Coverslips and inserts were stored in PBS at 4 °C up to 2 weeks prior to staining. Immunostaining was done as previously reported (Wang et al., 2007). Paraformaldehyde fixed filters were permeabilized with 0.2% triton X-100. Methanol-fixed cells were not treated with triton. After blocking with normal serum, the cells were incubated with primary antibodies for 1 h. The primary antibodies were: SARS nucleocapsid (IMG-548, Imgenex, San Diego, CA, USA), SP-A (PE-10, a gift from Dr. Yoshio Kuroki, Sapporo, Japan), cytokeratin (CAM5.2, Becton Dickinson, San Jose, CA, USA), Ep-CAM (Chemicon International, Temecula, CA, USA), an antibody specific for human type II cells (generously provided by Leland Dobbs, UCSF (Fang et al., 2006), vimentin (V9, Santa Cruz Biotechnology, Santa Cruz, CA, USA), CD-31 (JC70A, Dako, Carpinteria, CA, USA), CD-45 (BD Biosciences, San Jose, CA, USA) and collagen III (SC-28888, Santa Cruz Biotechnology). The secondary antibodies, Alexa 488 anti-rabbit IgG and/or Alexa 594 anti-mouse IgG (Invitrogen) were incubated with the cells for 1 h. Cells were mounted with Vectashield containing DAPI (Vector Laboratories, Burlingame, CA, USA) to visualize nuclei.
Acknowledgments
This study was supported by the National Institutes of Health grant PO1 AI59576, HL-29891, and by the Exxon Mobil Foundation. The authors would like to thank Dr. Leland Dobbs, University of California, San Francisco for the generous gift of the type II cell-specific antibody. Special thanks to Xueni Chen for helping with the type II cell isolations and to Dr. Kevin Brown for his help with lung procurement. Finally, we wish to thank Teneke M. Warren and Caroline Cook for manuscript preparation.
References
- Cheung C.Y., Poon L.L., Ng I.H., Luk W., Sia S.F., Wu M.H., Chan K.H., Yuen K.Y., Gordon S., Guan Y., Peiris J.S. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 2005;79(12):7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., Cai J., Li X., Kang W., Weng D., Lu Y., Wu D., He L., Yao K. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X., Song Y., Hirsch J., Galietta L.J., Pedemonte N., Zemans R.L., Dolganov G., Verkman A.S., Matthay M.A. Contribution of CFTR to apical–basolateral fluid transport in cultured human alveolar epithelial type II cells. Am. J. Physiol., Lung Cell. Mol. Physiol. 2006;290(2):L242–L249. doi: 10.1152/ajplung.00178.2005. [DOI] [PubMed] [Google Scholar]
- Frankel S.K., Cosgrove G.P., Cha S.I., Cool C.D., Wynes M.W., Edelman B.L., Brown K.K., Riches D.W. TNF-alpha sensitizes normal and fibrotic human lung fibroblasts to Fas-induced apoptosis. Am. J. Respir. Cell Mol. Biol. 2006;34(3):293–304. doi: 10.1165/rcmb.2005-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks T.J., Chong P.Y., Chui P., Galvin J.R., Lourens R.M., Reid A.H., Selbs E., McEvoy C.P., Hayden C.D., Fukuoka J., Taubenberger J.K., Travis W.D. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Human Pathol. 2003;34(8):743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Antimicrobial polypeptides in host defense of the respiratory tract. J. Clin. Invest. 2002;109(6):693–697. doi: 10.1172/JCI15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillim-Ross L., Taylor J., Scholl D.R., Ridenour J., Masters P.S., Wentworth D.E. Discovery of novel human and animal cells infected by the severe acute respiratory syndrome coronavirus by replication-specific multiplex reverse transcription-PCR. J. Clin. Microbiol. 2004;42(7):3196–3206. doi: 10.1128/JCM.42.7.3196-3206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z., Zhuang H., Wu B., Zhong H., Shao H., Fang W., Gao D., Pei F., Li X., He Z., Xu D., Shi X., Anderson V.M., Leong A.S. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Ding Y., Zhang Q., Che X., He Y., Shen H., Wang H., Li Z., Zhao L., Geng J., Deng Y., Yang L., Li J., Cai J., Qiu L., Wen K., Xu X., Jiang S. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006;210(3):288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I.C., Bosch B.J., Li F., Li W., Lee K.H., Ghiran S., Vasilieva N., Dermody T.S., Harrison S.C., Dormitzer P.R., Farzan M., Rottier P.J., Choe H. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J. Biol. Chem. 2006;281(6):3198–3203. doi: 10.1074/jbc.M508381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D.M., Chamberlain D.W., Poutanen S.M., Low D.E., Asa S.L., Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod. Path. 2005;18(1):1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J., Farzan M., Wohlford-Lenane C., Perlman S., McCray P.B., Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005;79(23):14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye M. SARS-associated coronavirus replication in cell lines. Emerg. Infect. Dis. 2006;12(1):128–133. doi: 10.3201/eid1201.050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang Z.W., Zhang L.J., Zhang S.J., Meng X., Li J.Q., Song C.Z., Sun L., Zhou Y.S., Dwyer D.E. A clinicopathological study of three cases of severe acute respiratory syndrome (SARS) Pathology. 2003;35(6):526–531. doi: 10.1080/00313020310001619118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leth-Larsen R., Zhong F., Chow V.T., Holmskov U., Lu J. The SARS coronavirus spike glycoprotein is selectively recognized by lung surfactant protein D and activates macrophages. Immunobiology. 2007;212(3):201–211. doi: 10.1016/j.imbio.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M., Smith B., Szakal A., Nelson-Rees W., Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int. J. Cancer. 1976;17(1):62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- Ling T.Y., Kuo M.D., Li C.L., Yu A.L., Huang Y.H., Wu T.J., Lin Y.C., Chen S.H., Yu J. Identification of pulmonary Oct-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro. Proc. Natl. Acad. Sci. U. S. A. 2006;103(25):9530–9535. doi: 10.1073/pnas.0510232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R.J. Biology of alveolar type II cells. Respir. Suppl. 2006;11:S12–S15. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- Mossel E.C., Huang C., Narayanan K., Makino S., Tesh R.B., Peters C.J. Exogenous ACE2 expression allows refractory cell lines to support severe acute respiratory syndrome coronavirus replication. J. Virol. 2005;79(6):3846–3850. doi: 10.1128/JVI.79.6.3846-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J.M., Poon L.L., Lee K.C., Ng W.F., Lai S.T., Leung C.Y., Chu C.M., Hui P.K., Mak K.L., Lim W., Yan K.W., Chan K.H., Tsang N.C., Guan Y., Yuen K.Y., Peiris J.S. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361(9371):1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J.M., Butany J., Poon L.L., Chan K.H., Beh S.L., Poutanen S., Peiris J.S., Wong M. Time course and cellular localization of SARS-CoV nucleoprotein and RNA in lungs from fatal cases of SARS. PLoS Med. 2006;3(2):e27. doi: 10.1371/journal.pmed.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Glende J., Al-Falah M., de Vries V., Schwegmann-Wessels C., Qu X., Tan L., Tschernig T., Deng H., Naim H.Y., Herrler G. Analysis of ACE2 in polarized epithelial cells: surface expression and function as receptor for severe acute respiratory syndrome-associated coronavirus. J. Gen. Virol. 2006;87(Pt 6):1691–1695. doi: 10.1099/vir.0.81749-0. [DOI] [PubMed] [Google Scholar]
- Sainz B., Jr., Mossel E.C., Peters C.J., Garry R.F. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) Virology. 2004;329(1):11–17. doi: 10.1016/j.virol.2004.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh W.J., Hsiao C.H., Paddock C.D., Guarner J., Goldsmith C.S., Tatti K., Packard M., Mueller L., Wu M.Z., Rollin P., Su I.J., Zaki S.R. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Human Pathol. 2005;36(3):303–309. doi: 10.1016/j.humpath.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims A.C., Baric R.S., Yount B., Burkett S.E., Collins P.L., Pickles R.J. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J. Virol. 2005;79(24):15511–15524. doi: 10.1128/JVI.79.24.15511-15524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K.C., Mercer R.R., Freeman B.A., Chang L.Y., Crapo J.D. Distribution of lung cell numbers and volumes between alveolar and nonalveolar tissue. Am. Rev. Respir. Dis. 1992;146(2):454–456. doi: 10.1164/ajrccm/146.2.454. [DOI] [PubMed] [Google Scholar]
- Stone K.C., Mercer R.R., Gehr P., Stockstill B., Crapo J.D. Allometric relationships of cell numbers and size in the mammalian lung. Am. J. Respir. Cell Mol. Biol. 1992;6(2):235–243. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- Subbarao K., Roberts A. Is there an ideal animal model for SARS? Trends Microbiol. 2006;14(7):299–303. doi: 10.1016/j.tim.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Finnegan C.M., Kish-Catalone T., Blumenthal R., Garzino-Demo P., La Terra Maggiore G.M., Berrone S., Kleinman C., Wu Z., Abdelwahab S., Lu W., Garzino-Demo A. Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J. Virol. 2005;79(22):14318–14329. doi: 10.1128/JVI.79.22.14318-14329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.F., Tong J.H., Chan P.K., Au F.W., Chim S.S., Chan K.C., Cheung J.L., Liu E.Y., Tse G.M., Lo A.W., Lo Y.M., Ng H.K. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases. J. Pathol. 2004;202(2):157–163. doi: 10.1002/path.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.T., Tseng J., Perrone L., Worthy M., Popov V., Peters C.J. Apical entry and release of severe acute respiratory syndrome-associated coronavirus in polarized Calu-3 lung epithelial cells. J. Virol. 2005;79(15):9470–9479. doi: 10.1128/JVI.79.15.9470-9479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Edeen K., Manzer R., Chang Y., Wang S., Chen X., Funk C.J., Cosgrove G.P., Fang X., Mason R.J. Differentiated Human Alveolar Epithelial Cells and Reversibility of Their Phenotype in vitro. Am. J. Respir. Cell Mol. Biol. 2007;36(6):661–668. doi: 10.1165/rcmb.2006-0410OC. (Jun) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener R.S., Cao Y.X., Hinds A., Ramirez M.I., Williams M.C. Angiotensin converting enzyme 2 is primarily epithelial and is developmentally regulated in the mouse lung. J. Cell. Biochem. 2007;101(5):1278–1291. doi: 10.1002/jcb.21248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis B.C., Liebler J.M., Luby-Phelps K., Nicholson A.G., Crandall E.D., du Bois R.M., Borok Z. Induction of epithelial–mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am. J. Pathol. 2005;166(5):1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H.W., Xie Q.M., Chen J.Q., Deng Y.M., Tang H.F. TGF-beta1 induces alveolar epithelial to mesenchymal transition in vitro. Life Sci. 2004;76(1):29–37. doi: 10.1016/j.lfs.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Ye J., Zhang B., Xu J., Chang Q., McNutt M.A., Korteweg C., Gong E., Gu J. Molecular pathology in the lungs of severe acute respiratory syndrome patients. Am. J. Pathol. 2007;170(2):538–545. doi: 10.2353/ajpath.2007.060469. [DOI] [PMC free article] [PubMed] [Google Scholar]