Abstract
Although coronary artery disease (CAD) is appreciated to be accelerated in patients with chronic spinal cord injury (SCI), the underlying mechanism of CAD in SCI remains obscure. We have recently shown that platelets from subjects with SCI develop resistance to the inhibitory effect of prostacyclin (PGI2) on the platelet stimulation of thrombin generation. The loss of the inhibitory effect was due to the loss of high-affinity prostanoid receptors, which may contribute to atherogenesis in SCI. Incubation of normal, non-SCI platelets in SCI plasma (n = 12) also resulted in the loss of high-affinity binding of PGI2 (Kd1 = 9.1 ± 2.0 nM; n1 = 170 ± 32 sites per cell vs. Kd1 = 7.2 ± 1.1 nM; n1 = 23 ± 8 sites per cell), with no significant change in the low-affinity receptors (Kd2 = 1.9 ± 0.1 μM; n2 = 1,832 ± 232 sites per cell vs. Kd2 = 1.6 ± 0.1 μM; n2 = 1,740 ± 161 sites per cell) as determined by Scatchard analysis of the binding of [3H]PGE1. The loss of high-affinity PGI2 binding led to the failure of PGI2 to inhibit the platelet-stimulated thrombin generation. The increase of cellular cyclic AMP level, mediated through the binding of PGI2 to low-affinity receptors in platelets, was unaffected in SCI platelets. PAGE and immunoblot of SCI plasma showed the presence of an IgG band, which specifically blocked the binding of [3H]PGE1 to the high-affinity PGI2 receptors of normal platelets. PAGE of the reduced IgG band, the amino acid sequence of the novel band as a heavy chain of IgG that inhibits the binding of [3H]PGE1 to the high-affinity platelet PGI2 receptor, demonstrates that the specific recognition and inhibition of high-affinity PGI2 binding to platelets was due to an anti-prostacyclin receptor antibody present in SCI plasma.
Aggregation of platelets induced by agonists such as ADP, l-epinephrine, collagen, or thrombin, is critically important both in normal blood coagulation and in the pathogenesis of atherosclerosis and thrombosis (1, 2). The aggregation of platelets is counteracted by several autacoids including prostanoids such as prostacyclin [prostaglandin (PG)I2], PGE1, or PGD2, (3, 4) and endothelium-derived relaxing factor/nitric oxide (5). Among the prostanoids, PGI2, an archidonate metabolite of endothelial cells, is the most potent inhibitor of platelet aggregation (1, 3, 5) and is generally believed to be important in the prevention of cardiovascular disease (CAD) through the inhibition of platelet aggregation (6).
The PGI2-induced inhibition of platelet aggregation is mediated by the increase of cellular cyclic AMP level through the activation of membrane adenylate cyclase that has been initiated from the binding of the prostanoid to its receptors on the platelet surface (3–7). There are two classes of PGI2 binding sites reported, one high-affinity–low-capacity and one low-affinity–high-capacity, in the same receptor macromolecules in the membrane bilayers of platelets (8). Furthermore, the binding of PGI2 to the low-affinity binding sites in the prostaglandin receptor results in the inhibition of platelet aggregation due to the increase of the cyclic AMP level (7, 8). The metabolic consequences of binding of PGI2 to high-affinity binding sites is speculative at present.
Cardiovascular disease is currently the leading cause of death in individuals with chronic spinal cord injury (SCI), and it appears prematurely. The mechanism of accelerated CAD in SCI remains obscure, although several risk factors have been associated with the risk of CAD in SCI (9). Because platelet hyperactivity, among other factors, stimulates atheriosclerosis, especially in a permissive milieu, and may lead to CAD, PGI2 as a potent inhibitor of platelet aggregation is believed to have a beneficial role in the inhibition of atherogenesis.
We have recently demonstrated that the platelets from SCI subjects develop resistance to the inhibitory effect of PGI2 in the platelet-induced stimulation of thrombin generation. This failure of the prostanoid in the control of thrombin generation was not related either to the inhibition of platelet aggregation or the synthesis of cyclic AMP but was due to the loss of platelet high-affinity prostanoid receptors (10). Increased platelet-stimulated thrombin generation would be expected to have significant consequences on the pathogenesis of atheriosclerosis. A plasma factor in SCI subjects has been identified that specifically blocks the binding of PGI2 to its high-affinity receptors without effecting the low-affinity binding to platelets.
In this report, the novel occurrence of an anti-prostacyclin receptor antibody specifically capable of recognizing the high-affinity binding site of the receptor on the platelet surface in the circulation of the subjects with chronic SCI is reported. In addition, the antibody-mediated inhibition of PGI2 binding resulted in the failure of the prostanoid to inhibit platelet-stimulated thrombin generation without affecting the prostaglandin-induced synthesis of cyclic AMP. The inhibitory effect of this anti-prostacyclin receptor antibody may be related to increased occurrence of atherosclerosis in SCI.
MATERIALS AND METHODS
Patient Selection.
Patients with chronic SCI (n = 12) between the ages of 35 to 60 years were age matched with 12 able-bodied controls, ranging in age between 30 and 60 years. The protocol was approved by the Institutional Review Board for Clinical Research, Veterans Affairs Medical Center (Bronx, NY) prior to the initiation of the study.
Collection of Blood and Platelet Aggregation.
Blood was drawn from the SCI and non-SCI subjects and anticoagulated by mixing 9 vol of blood with 1 vol of 0.13 M sodium citrate. The subjects were asked to abstain from aspirin and all other medications known to affect platelet aggregation for 2 weeks prior to blood donation. Platelet-rich plasma (PRP) was prepared by centrifuging blood at 200 × g for 15 min at 23°C. Platelet-free plasma was prepared by centrifuging PRP at 10,000 × g for 15 min at 23°C. Aggregation of platelets was studied by using ADP, l-epinephrine, collagen, or thrombin, as described previously (11). The inhibition of platelet aggregation was studied by incubating PRP with different concentrations of prostacyclin for 1 min at 37°C before the addition of aggregating agent to initiate platelet aggregation. The inhibition of platelet aggregation was calculated from the slope of the initial platelet aggregation curve in the presence or absence of prostacyclin.
Determination of Cyclic AMP.
Washed platelets suspended in normal plasma were incubated in the presence or absence of different amounts of plasma protein fractions as described previously (11) for 3 hr at 37°C then treated with 100 nM PGI2 for 1 min at the same temperature. At the end of incubation, the platelet suspension was treated with ice-cold trichloroacetic acid (TCA) (final concentration, 5%) in the presence of 0.1 M HCl to extract cyclic AMP. The TCA extract was centrifuged to remove the cell debris; the clear supernatant was lyophilized to remove trichloroacetic acid. The lyophilized material was reconstituted with 0.1 ml of deionized water, and the cyclic AMP content was determined by the protein kinase binding method (11, 12).
Determination of Platelet-Stimulated Thrombin Generation Time.
Thrombin generation time of PRP was determined according to Hougie (13).
Gel Electrophoresis of Plasma.
Plasma from both control and SCI subjects was analyzed by gel electrophoresis. Samples were electrophoresed on 12% polyacrylamide gel containing 1% SDS as described by Laemmli (14). Molecular weight (Mr) of the proteins was determined with both nonreduced and reduced plasma samples using 0.1 M DTT. Typically, 50 μg of the plasma proteins were reduced by boiling the sample for 2 min in the presence of 2% SDS and 0.1 M DTT (final). In the case of nonreduced proteins, the samples were similarly treated in the absence of the reducing agent. Mr was calculated by using marker proteins of known molecular weights (Bio-Rad). After electrophoresis, the gels were either stained with 0.02% Coomassie brilliant blue or immediately chilled to 0°C. Corresponding protein areas in the unstained gel were cut in slices (1 slice = 0.125 cm). The protein from the slices was eluted in saline and dialyzed overnight in saline solution. In control experiments, portions of the gel without any protein band were similarly treated and dialyzed. The protein band of Mr 47,000 eluted from the gel was checked for homogeneity by PAGE. Identity of the IgG protein band in the gel was ascertained by immunoblotting techniques using anti-human IgG antibody (Bio-Rad), as described previously (15, 16).
Amino Acid Sequence of Reduced Plasma Protein.
Reduced plasma samples were electrophoresed on a prepoured 24-hr-old gel (SDS/PAGE, 12%). The proteins were transferred from the gel to a Millipore PVDF-Immobilon-P (hydrophobic polyvinyldene difluoride) membrane by immunoblotting in a Tris/glycine/SDS/methanol buffer, pH 8.0 (14, 15). The membrane was stained with 1% Coomassie blue CB-R250 for 1 min and then destained in a mixture of 50% methanol and 10% acetic acid. Subsequently, the membrane was rinsed three times in methanol then once with water and air dried. The protein band of interest was excised from the membrane and used for amino acid sequencing. Amino acid sequencing was performed on a Beckman 2090 E gas-phase sequencer (following the manufacturer’s program).
Washed Platelets.
PRP was centrifuged at 3,000 × g for 15 min. The platelet pellet was washed with Tyrode’s buffer, pH 7.4, containing 1.0 mM EDTA, as described previously (11). Next the platelets (≈7 × 108 cells per ml) were suspended in the same buffer, without EDTA, containing 5.0 mM MgCl2 .
Binding of Prostacyclin to Platelet Receptors.
The binding characteristics of prostacyclin to platelets were analyzed by Scatchard plot (17) using [3H]PGE1 as the stable probe, as described previously (8). Because PGI2 and PGE1 bind to the same receptor on the platelet surface and radiolabeled PGI2 as a free-acid form is not commercially available, [3H]PGE1 [(5,6-3H)PGE1; specific activity, 55 Ci/mmol (1 Ci = 37 GBq); New England Nuclear] was used as a stable probe to determine the PGI2 receptor binding in platelets. The platelets (2 × 108) were incubated with 3 nM [3H]PGE1 (30,000 cpm) in a total volume of 200 μl for 20 min to attain equilibrium. The platelet suspension was then filtered over a Whatman glass fiber filter (GF/C), presoaked in Tyrode’s buffer (pH 7.5), containing 5.0 mM MgCl2, under mild vacuum, and washed twice with 5.0 ml of the same buffer. The platelets were adsorbed on the filters, which were then dried, and the radioactivity was determined as described (11). The nonspecific binding was determined by adding excess (15 μM) unlabeled prostanoid to the assay mixture. The specific binding was calculated by subtracting the nonspecific binding from the total binding.
Protein was determined by the method of Lowry et al. (18), and platelet number was determined by using a Coulter counter.
RESULTS
Presence of an IgG-Like Protein in SCI Plasma and Its Effect on Platelet PGI2 Interaction.
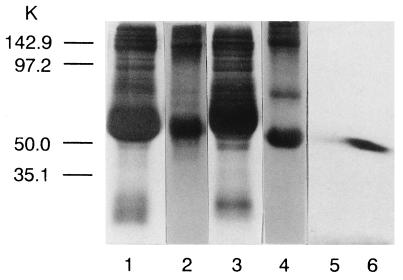
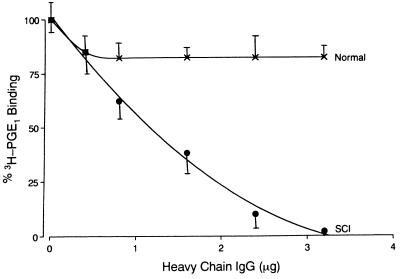
Gel electrophoresis of the SCI plasma under reducing conditions showed the appearance of a novel band of Mr 47,000 when the gels were stained with Coomassie brilliant blue (Fig. 1). This band was determined to be an IgG-like protein by immunoblot technique using anti-human IgG antibody (Fig. 1). In a parallel experiment, on an identical gel that was not stained with the dye to avoid denaturation, the band of Mr 47,000 was cut and the protein from the slices was eluted as described. Normal washed platelets were incubated with increasing concentrations of the eluted protein for 3 hr at 23°C. After incubation the binding of [3H]PGE1 to the platelets was determined. Incubation of normal washed platelets with increasing concentrations of this IgG-like protein eluted from the gel resulted in increased inhibition of [3H]PGE1 binding to the platelet prostacyclin receptors in a dose-dependent manner (Fig. 2). Normal platelets, when incubated with IgG-like protein at a concentration of 3.2 μg protein per ml, resulted in the inhibition of [3H]PGE1 binding that was similar to the SCI platelets. Incubation of normal platelets with eluates from control plasma did not affect prostacyclin binding. Scatchard analysis of the binding of [3H]PGE1 to control non-SCI platelets when incubated with 3.2 μg protein showed the complete loss of high-affinity binding of the probe to the PGI2 receptors on the platelet surface without affecting the low-affinity binding (Table 1).
Figure 1.
SDS/PAGE of reduced and unreduced control and SCI plasma. Reduction and SDS/PAGE of the plasma protein were performed as described in Materials and Methods. Lanes: 1 and 2, reduced and unreduced control (non-SCI) plasma proteins; 3 and 4, reduced and unreduced SCI plasma proteins; 5 and 6, immunoblots of the eluted protein of Mr 47,000 from lanes 1 and 3, respectively, using anti-human IgG antibody. Marker proteins used were: phosphorylase [Mr 142,900 (142.9 K)], BSA [Mr 97,200 (97 K)], ovalbumin [Mr 50,000 (50 K)], carbonic anhydrase [Mr 35,100 (35.1 K)], soybean trypsin inhibitor [Mr 29,700 (29.7 K)], and lysozyme [Mr 21,900 (21.9 K)].
Figure 2.
Inhibition of [3H]PGE1 binding to control platelets by IgG protein identified in SCI plasma. Control, non-SCI, washed platelets (2 × 108 cells per ml) in Tyrode’s buffer, pH 7.4, containing 5 mM MgCl2 were incubated for 3 hr at 37°C with increasing concentrations of the IgG protein. After incubation, the specific binding of [3H]PGE1 was determined (5). X, eluates from control plasma; •, eluates from SCI plasma.
Table 1.
Effect of incubation of control platelets with IgG eluate from SCI plasma on the binding of [3H]PGE1 to prostacyclin receptors compared with prostanoid binding to SCI platelets
| Platelets | Treatment | PGE1/PGI2 receptors
|
|||
|---|---|---|---|---|---|
| High-affinity
|
Low-affinity
|
||||
| Kd1, nM | n1, sites per cell | Kd2, μM | n2, sites per cell | ||
| Control | Buffer | 9.1 ± 2.0 | 170 ± 32 | 1.9 ± 0.1 | 1,832 ± 232 |
| Control | IgG eluate from SCI | 7.2 ± 1.1 | 23 ± 8 | 1.6 ± 0.1 | 1,740 ± 161 |
| Control | IgG eluate from control | 8.9 ± 0.9 | 162 ± 34 | 1.8 ± 0.3 | 1,876 ± 138 |
| SCI | Buffer | 7.5 ± 1.5 | 40 ± 10 | 2.1 ± 0.3 | 1,970 ± 214 |
Platelets were isolated from either control (non-SCI) or SCI subjects and incubated with or without IgG eluates isolated from SCI or control plasma. The binding of [3H]PGE1 to platelets was determined and analyzed by Scatchard plot (10). Results are expressed as means ± SD of six experiments, each performed in triplicate.
Effect of Incubation of Normal Platelets with IgG Eluate on PGI2-Induced Increase of Cellular Cyclic AMP Level.
Incubation of normal platelets with 3.2 μg of IgG-like protein for 3 hr at 23°C did not affect the PGI2-induced increase of cyclic AMP level. Treatment of normal platelets with 100 nM PGI2 for 3 min at 23°C increased the basal cyclic AMP level from 2.1 ± 0.5 to 26.5 ± 5.1 pmol/108 platelets. Under identical conditions, prior incubation of normal platelets with IgG eluate before the addition of PGI2 also increased the basal level of cyclic AMP to similar levels (from 1.91 ± 0.4 to 28.9 ± 4.6 pmol/108 platelets). Treatment of platelets with eluates from control plasma also showed no such effect on the PGI2-induced increase of cyclic AMP level.
Effect of Incubation of Normal Platelets With the IgG Eluates on the Inhibitory Effect of PGI2 on Platelet-Stimulated Thrombin Generation.
The thrombin-generation time of recalcified plasma in the absence of platelets was 210 ± 26 sec, which decreased to 130 ± 15 sec when the platelets were added to the assay mixture (P < 0.001). Treatment of normal platelets with 100 nM PGI2 prior to their addition to the assay mixture increased the thrombin-generation time to 169 ± 12 sec, P < 0.001. In contrast, addition of PGI2 (100 nM) to the assay mixture with platelets previously treated with an IgG-like protein failed to inhibit platelet-stimulated thrombin generation (134 ± 10 sec), P < 0.001. The treatment of platelets with IgG eluate itself had no effect on the stimulation of thrombin generation (172 ± 10 sec), P < 0.001, when compared with control.
Amino Acid Sequence of the Reduced Protein Band Corresponding to the IgG-Like Molecule from SCI Plasma.
To further ascertain the identity of the protein identified by immunoblot to be an IgG, the protein band was sliced from an unstained gel and eluted, and the reduced protein was subjected to SDS/PAGE (12%). The unstained gel was transferred to a Millipore Immobilon-P membrane as described above, and the amino acid sequence of the reduced protein was determined. Amino acid sequence analysis of the reduced protein band of Mr 47,000 was identified with 100% degree of certainty to be an IgG heavy-chain molecule. The partial amino acid sequence of the reduced band from SCI plasma is EVQLVES and it represents the V-III (variable III) region of the heavy chain of IgG. The sequence beginning at position 1 had overlap in seven amino acids (Swiss Protein Data Base), and the N terminus was identified as methionine (Fig. 3).
Figure 3.
Partial amino acid sequence of the reduced IgG from SCI plasma. As described in Materials and Methods, the amino acid sequence of the reduced SCI plasma was determined as illustrated. Amino acid identities of the heavy chain of IgG (V-III region) from SCI plasma (in bold letters) had overlap in seven amino acids when compared with the IgG sequence in the Swiss Protein Data Base.
Inhibition of [3H]PGE1 Binding to Platelets by the Heavy Chain of IgG Isolated from SCI Plasma.
Incubation of normal platelets in plasma containing the heavy-chain IgG eluate obtained by the reduction of IgG isolated from SCI plasma for 3 hr at 23°C resulted in the inhibition of binding of [3H]PGE1 to the prostacyclin receptors on the platelet surface. Scatchard analyses of the binding characteristics showed that, as in the case of IgG-like molecules, the isolated heavy chain of the protein also inhibited the binding of [3H]PGE1 to high-affinity prostacyclin receptors on the platelet without affecting the low-affinity binding (Table 1).
Effect of IgG Heavy Chain from SCI Plasma on the Synthesis of Cyclic AMP in Platelets and the Inhibition of Platelet-Stimulated Thrombin Generation by PGI2.
The effect of IgG eluate isolated from SCI plasma on cyclic AMP formation and platelet-stimulated thrombin generation, as described earlier in this section, could be duplicated by using heavy-chain eluate obtained by the reduction of IgG (reduction of IgG has been previously described in Materials and Methods).
Incubation of normal platelets with 3.2 μg of IgG heavy chain for 3 hr at 23°C followed by treatment of these platelets with 100 nM PGI2 did not affect the cyclic AMP synthesis. The basal level of cyclic AMP in untreated platelets increased in the presence of 100 nM PGI2 to 29.5 ± 5.8 vs. 2.22 ± 1.2 pmol/108 cells. The basal level of cyclic AMP in platelets preincubated with the isolated heavy-chain IgG increased with 100 nM PGI2 to 30.6 ± 6.8 vs. 3.1 ± 1.6 pmol/108 platelets. In contrast, incubation of platelets with the heavy-chain IgG resulted in the failure of PGI2 to inhibit platelet-stimulated thrombin generation (134 ± 8 vs. 131 ± 12 sec).
DISCUSSION
Prostacyclin, through its ability to inhibit platelet aggregation, is believed to have a significant, beneficial role in the prevention of coronary artery disease (16). The prostacyclin effect is mediated through the increase of cellular cyclic AMP level due to the binding of the prostanoid to the low-affinity receptor sites on the platelet surface (7). It then could be expected that the inhibition of PGI2 binding to platelet receptors would lead to the failure of the autacoid to inhibit platelet aggregation. We have shown that incubation of platelets in plasma from SCI subjects resulted in the inhibition of [3H]PGE1 binding to high-affinity binding sites of PGI2 receptors on the platelet surface without affecting the low-affinity binding of the prostanoid. Such treatment of normal platelets simultaneously resulted in the failure of the prostanoid to inhibit platelet-stimulated thrombin generation without any impairment of stimulation of cyclic AMP formation or PGI2-induced inhibition of platelet aggregation. These results indicated the presence of inhibitor(s) in SCI plasma capable of partial impairment of platelet PGI2 interaction, which consequently resulted in partial blockade of the prostacyclin effects in platelets. The inhibitor has been identified herein to be an anti-PGI2 receptor antibody capable of recognizing only high-affinity PGI2-binding sites on the platelet surface. This novel finding was the consequence of the inherent property of the antibody that recognized only the high-affinity PGI2-binding site on platelets. Because both high-affinity and low-affinity binding sites of PGI2 reside on the same receptor macromolecules (8), our results suggest that the antibody in SCI was probably monoclonal in origin and indicate that the occurrence of monoclonal antibody in the system may not always be due to neoplasticity. Neuropathologic factors might be involved in this process. Furthermore, the epitope of the antibody capable of recognizing the high-affinity PGI2-binding sites occurs only in the variable region III of the heavy chain of IgG.
Although the inhibition of PGI2 binding to its high-affinity binding sites on the platelet surface did not result in the impairment of inhibition of platelet aggregation by the prostanoid (10), the inhibition of PGI2 binding to its high-affinity binding sites in the receptor macromolecule by the antibody led to the failure of the autacoid to inhibit platelet-stimulated thrombin generation. It is well established that not only is thrombin important in blood coagulation, but it also has crucially important effects on the enhancement of arteriosclerosis both directly and indirectly (19–24). As such, the inhibition of high-affinity binding of PGI2 on its receptor molecule on the platelet surface may result in the increase of atherogenesis through unregulated, platelet-stimulated thrombin generation rather than in direct thrombogenesis due to uncontrolled platelet aggregation in individuals with SCI per se.
The presence of an anti-high-affinity prostacyclin receptor antibody in the circulation could not be shown in able-bodied subjects with coronary artery disease (unpublished data), in which hyperaggregation of platelets has been previously demonstrated by our group to be a leading factor in thrombogenesis (16). The platelets in these able-bodied patients with coronary artery disease are resistant to the inhibitory effect of PGI2 due to decreased cyclic AMP formation consequent to the decreased binding of PGI2 to low-affinity receptor binding sites on these platelets (11). Results of studies in the SCI and non-SCI populations suggest that the binding of PGI2 to high-affinity and low-affinity binding sites in its receptor molecules on the platelet surface may be related to the prevention of atherogenesis and thrombosis, respectively. The occurrence of anti-high-affinity prostacyclin receptor antibody in the circulation may be postulated to be an immunopathologic risk factor that leads to the development of arteriosclerosis in individuals with SCI.
Acknowledgments
We thank Drs. R. A. Kohanski and Jie Chen for determining the amino acid sequence of the samples.
ABBREVIATIONS
- CAD
coronary artery disease
- SCI
spinal cord injury
- PRP
platelet-rich plasma
References
- 1.Marcus A J, Safier L B. FASEB J. 1993;7:516–522. doi: 10.1096/fasebj.7.6.8472890. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Nature (London) 1993;363:801–809. [Google Scholar]
- 3.Vane J R, Anggard E E, Botting R M. N Eng J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 4.Halbrugge J W, Walter U. In: Protein Kinases in Blood Cell Function. Huang C K, Sha’afi R I, editors. Boca Raton, FL: CRC; 1993. pp. 245–298. [Google Scholar]
- 5.Moncada S, Palmer M J, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 6.Mustard J F, Kinlough-Rathbone R J, Packman M A. In: Hemostasis and Thrombosis. Colman R W, Hirsch J, Marder V J, Salzman E W, editors. Philadephia: Lippincott; 1987. pp. 1073–1088. [Google Scholar]
- 7.Siegle A M, Smith J B, Silver M J, Nicolau K C, Hern D. J Clin Invest. 1979;63:215–220. doi: 10.1172/JCI109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutta-Roy A K, Sinha A K. J Biol Chem. 1987;262:12685–12691. [PubMed] [Google Scholar]
- 9.Samsa G P, Patric C H, Feussner J R. Arch Neurol. 1993;50:909–914. doi: 10.1001/archneur.1993.00540090018005. [DOI] [PubMed] [Google Scholar]
- 10.Kahn N N, Bauman W A, Sinha A K. Proc Natl Acad Sci USA. 1996;93:245–249. doi: 10.1073/pnas.93.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn N N, Muellar H S, Sinha A K. Circ Res. 1990;66:932–940. doi: 10.1161/01.res.66.4.932. [DOI] [PubMed] [Google Scholar]
- 12.Gilman A G. Proc Natl Acad Sci USA. 1970;67:305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hougie C. In: Hematology. William W J, Butler E B, Erslev A J, Rundles R W, editors. New York: McGraw–Hill; 1977. pp. 1642–1645. [Google Scholar]
- 14.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Towbin M, Staehlin Y, Gordon J. Proc Natl Acad Sci USA. 1989;76:43–45. [Google Scholar]
- 16.Kahn N N, Bauman W A, Sinha A K. Am J Physiol. 1995;268:H117–H124. doi: 10.1152/ajpheart.1995.268.1.H117. [DOI] [PubMed] [Google Scholar]
- 17.Scatchard G. Ann NY Acad Sci. 1969;51:660–672. [Google Scholar]
- 18.Lowry O H, Rosebrough N J, Farr L, Randall R J J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Hedin U, Frebelius S, Sanchez J, Dryjski M, Swedenborg J. Arterioscler Thromb. 1994;14:254–260. doi: 10.1161/01.atv.14.2.254. [DOI] [PubMed] [Google Scholar]
- 20.Skultz T C, Mene H E, Abboud H E. Am J Physiol. 1989;257:F366–F374. doi: 10.1152/ajprenal.1989.257.3.F366. [DOI] [PubMed] [Google Scholar]
- 21.Dicorleto P E. Am J Hypertens. 1993;6:3145–3185. [PubMed] [Google Scholar]
- 22.Thompson W A D, Smith E B B, Strirk C M, Kochar A. Blood Coagul Fibrinolysis. 1990;1:489–493. [PubMed] [Google Scholar]
- 23.Naito M, Hayashi T, Kuzuya M, Funaki E, Asai K, Kuzuya F. Atheriosclerosis. 1990;83:9–14. doi: 10.1016/0021-9150(90)90124-2. [DOI] [PubMed] [Google Scholar]
- 24.Dewood M A, Spores J, Notske R, Mouser L T, Burroughs R, Golden M S, Lang H T. N Engl J Med. 1980;303:897–902. doi: 10.1056/NEJM198010163031601. [DOI] [PubMed] [Google Scholar]