Abstract
Experimental autoimmune encephalomyelitis (EAE) is a T cell autoimmune disorder that is a widely used animal model for multiple sclerosis (MS) and, as in MS, clinical signs of EAE are associated with blood–brain barrier (BBB) disruption. SR 57746A, a nonpeptide drug without classical immunosuppressive properties, efficiently protected the BBB and impaired intrathecal IgG synthesis (two conventional markers of MS exacerbation) and consequently suppressed EAE clinical signs. This compound inhibited EAE-induced spinal cord mononuclear cell invasion and normalized tumor necrosis factor α and IFN-γ mRNA expression within the spinal cord. These data suggested that pharmacological intervention aimed at inhibiting proinflammatory cytokine expression within the central nervous system provided protection against BBB disruption, the first clinical sign of EAE and probably the key point of acute MS attacks. This finding could lead to the development of a new class of compounds for oral therapy of MS, as a supplement to immunosuppressive agents.
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS) and the major cause of neurological disability among young adults. The disease evolves through different clinical forms, differing by the presence or absence of more or less complete remission phases. The mechanism by which this autoimmune disease appears and progresses from one given form to another is still unclear. Cytokines are thought to have a role in MS, and a bias in favor of Th2-cell functions has been evoked during remission phases (1, 2), although data accumulated over recent years indicated that immune responses in MS are probably more complex. There is currently no definitive therapy for MS, and conventional immunosuppressive drugs and steroids are not commonly prescribed because of their serious side effects in long-term treatment. Therapeutic strategies targeting early events should be developed, because up to 80% irreversible oligodendrocyte destruction occurs in advanced stages of the disease. Neuropathological changes in MS seem to be induced by damage to the blood–brain barrier (BBB). Indeed, BBB breakdown is temporally correlated with the appearance of clinical symptoms, and the inflammatory cytokines tumor necrosis factor (TNF) α and IFN-γ are believed to be determining factors in this process (3–5).
MS-like immunopathology can be reproduced in Lewis rats. The experimental autoimmune encephalomyelitis (EAE) model clinically simulates the inflammatory phase of an acute attack of the relapsing-remitting form of human MS (6–11). As in MS, EAE is associated with BBB disruption (12) and mononuclear cell invasion, although extensive myelin damages are poorly observed. We describe the effect of SR 57746A (1-[2-(naphth-2-yl)-ethyl]-4-(3-trifluoromethylphenyl)-1,2,5,6-tetrahydropyridine, hydrochloride), a neuroprotective agent (13–16), in the EAE model, with emphasis on its effects on BBB dysfunction.
Materials and Methods
EAE Induction.
Female Lewis rats were used at 8–9 weeks of age. EAE was induced with 100 μg myelin basic protein (MBP) fragment 68–82 (MBP68–82) (Sigma) (17) in suspension in 50 μl of a 0.9% saline solution and 50 μl of incomplete Freund’s adjuvant (IFA) containing 1 mg of Mycobacterium tuberculosis H37RA (Difco). A total of 100 μl of this suspension was injected s.c. into the two hind footpads. Controls were immunized only with IFA supplemented with M. tuberculosis and referred to as CFA. All studies were approved by the Animal Care and Use Committee of Sanofi Recherche.
Treatment and Clinical Grading of EAE.
The SR 57746A-treated groups (n = 10) received doses ranging from 1 to 10 mg/kg by oral route in a volume of 0.5 ml daily, starting from day 0 of EAE induction to the end of the experiment. Control rats (n = 10) received the same volume of SR 57746A vehicle (5% DMSO, 5% Tween 80, and 90% water for injectable preparations). The EAE course was monitored by daily examinations as follows: no neurological sign = grade 0; weak tail = grade 1.5; wobbly walk or limb paresis = grade 2; one leg paralysis = grade 2.5; placed on its back, the animal is unable to return to the normal position = grade 3; hind leg paralysis, nasal bleeding, or incontinence = grade 4; moribund = grade 5; and death = grade 6. Moreover, the animals were weighed daily.
Histological Studies.
Rats were anesthetized and perfused via the left ventricle with 4% paraformaldehyde in PBS as described (18). The lower part of the spinal cord was removed and fixed in 10% formalin. Paraffin-embedded tissues were cut on a microtome and stained with hematoxylin-eosin for histological examination. All detected nucleated cells were counted, and the mean value and SD was determined. In parallel, series of adjacent vibratome sections were incubated sequentially with goat anti-rat TNF-α and IFN-γ antibodies (Genzyme), horseradish peroxidase-conjugated anti-goat antibody (Dako), and diaminobenzidine chromogen.
Biological Measurements.
Cerebrospinal fluid (CSF) samples were obtained by cisternal puncture, and blood samples by retro-orbital puncture. Albumin was determined in plasma or CSF by using a Bio-Rad kit. IgG titers were measured by ELISA using a goat anti-rat IgG unlabeled or labeled with horseradish peroxidase (Southern Biotechnology Associates) for coating and developing antibody. The results are expressed in μg/ml, according to a standard scale performed with a purified rat IgG solution (Southern Biotechnology Associates).
Tissue Sampling.
Mononuclear cells were isolated from spinal cord parenchyma as described (19). Briefly, 25 rats per group were killed, and their spinal cords were removed. An homogeneous cell suspension was obtained after passage through a stainless-steel mesh screen and mixed with a 30% Percoll (Amersham Pharmacia) medium. After 20-min centrifugation at 25,000 × g, mononuclear cells were collected in the pellet.
Relative Quantification of Cytokine mRNA by PCR.
Poly(A)+ mRNA purification and conversion to first-strand cDNA were performed with the PolyATract Series 9600 mRNA Isolation System with cDNA synthesis reagents (Promega) according to the manufacturer’s instructions. Each cDNA sample was amplified by using primer pairs specific to rat TNF-α and IFN-γ (19). Independent PCR amplifications of cytokines and β-2 microglobulin (used as external control) were run in parallel as already described, with quantification performed in the exponential phase of amplification.
MBP Stimulation of Lymph Node Cells for Cytokine Production.
Draining popliteal lymph node cells (PLNC) were removed on day 21 postimmunization. Single cell suspensions were prepared in DMEM (GIBCO) supplemented with 10% FCS (Gibco), 2 × 10−5 M β-2-mercaptoethanol, 50 units/ml penicillin, and 50 μg/ml streptomycin. The cells (5.105 in 100 μl) were cultured in 96-well flat-bottomed plates (Falcon) for 96 h with an optimal concentration of MBP68–82 or Con A (Amersham Pharmacia). After 96 h, the supernatants were harvested and stored at −80°C until testing.
Measurement of Cytokine Production.
TNF-α, IFN-γ, and IL-10 were determined by using ELISA kits from BioSource International (Fleurus, Belgium) and Genzyme. Assays were performed according to the manufacturer’s instructions.
Statistical Analysis.
Statistical significance was determined by the RS/1 multicomparison procedure using a one-way ANOVA and Dunnett’s test for multiple comparisons with a common control group. Differences between means were considered significant when P values were less than 0.05.
Results
Oral Administration of SR 57746A Impaired EAE Development.
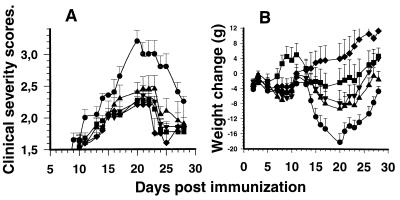
Immunization of adult EAE-susceptible Lewis rats with MBP68–82 resulted in the development of acute EAE in all animals. Paralytic signs indicated ascending EAE, which started on day 12 and peaked around day 20, with a maximum clinical score of 3.0 ± 0.2 (Fig. 1A). At this time, loss of body weight, a characteristic feature of EAE that is correlated with disease severity, reached 18 g for rats weighing 130 g on day 0 (Fig. 1B). By contrast, treatment with daily doses of 10 mg/kg SR 57746A was therapeutically effective in reducing the clinical manifestations of EAE (P < 0.001/controls on day 20) and loss of body weight (P < 0.01/controls on day 20). Furthermore, these two parameters were significantly less severe for these two relevant criteria in rats given 3 mg/kg and 1 mg/kg SR 57746A. The effect observed on the weight parameter, the most objective criteria, is not the result of a direct effect of the compound on weight gain because the molecule by itself does not affect the weight gain of normal animals (data not shown).
Figure 1.
Prevention of MBP68–82-induced EAE by SR 57746A. (A) Effects on clinical severity. (B) Effects on EAE-induced weight loss. Lewis rats were immunized with 100 μg MBP68–82 in CFA on day 0 and given SR 57746A daily by oral route from day 0 until the end of the experiment. Weight loss was documented as the weight relative to that of day 1. Values are means ± SEM for 10 rats. ●, MBP68–82 + vehicle; ■, MBP68–82 + SR 57746A 10 mg/kg; ▴, MBP68–82 + SR 57746A 3 mg/kg; ▾, MBP68–82 + SR 57746A 1 mg/kg; ⧫, CFA + vehicle.
SR 57746A Lowered Inflammatory Infiltration into the CNS.
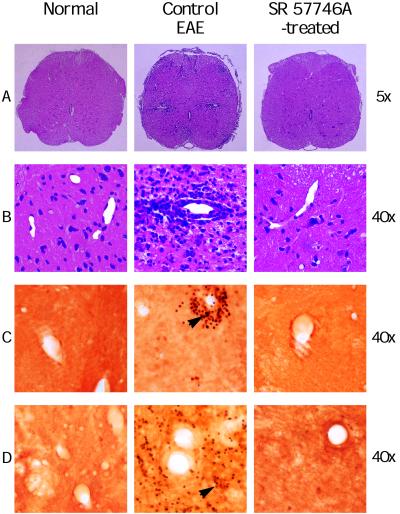
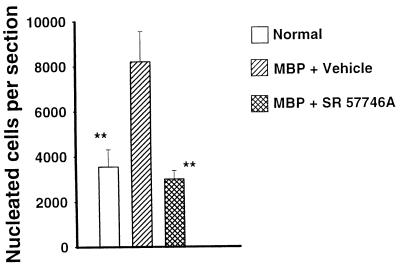
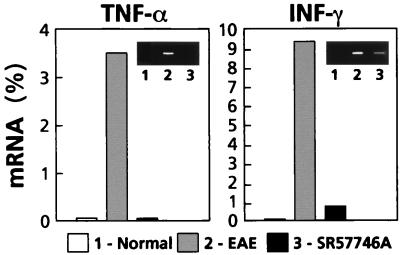
Because the most important pathological changes in rats with EAE are detected in the lumbar part of the spinal cord (20, 21), this section was dissected and carefully processed for histological and immunohistological examinations. All control EAE rats had widespread histological lesions, characterized by heavy perivascular infiltrates forming foci (Fig. 2 A and B), and the mean number of nucleated cells per section was highly enhanced (Fig. 3). Moreover, an immunohistological examination clearly indicated that these perivascular infiltrating cells produce TNF-α and IFN-γ, highlighting that they were in an activated state (Fig. 2 C and D). In EAE animals treated with SR 57746A, only one of six animals developed a visible cellular infiltration, and the mean number of nucleated cells per section was similar to that of normal rats. Moreover, as in normal rats, nucleated cells were class II negative resident cells, with numerous ED1 positive microglial cells, whereas in EAE rats treated with the vehicle, most of the nucleated cells were class II positive infiltrated cells (data not shown). Finally, as in normal rats, no TNF-α- or IFN-γ-producing cells were detected in EAE animals treated with SR 57746A. These results were further confirmed at the mRNA level. TNF-α and INF-γ gene expressions were determined by quantitative reverse transcription–PCR (22, 23) in the early phase of the disease (day 10) in leukocytes extracted from the spinal cord of EAE-induced rats. As expected (19), TNF-α and IFN-γ expressions were highly detected in leukocytes from rats with EAE, contrary to normal rats with barely detectable expression of the two cytokines (Fig. 4). SR 57746A treatment of EAE-induced rats at a 10 mg/kg dose resulted in a significant reduction of these cytokine mRNA expressions. The results of four independent experiments indicated that mRNA expression was inhibited by 98.8% ± 1.2% for TNF-α and 85.2% ± 7.3% for IFN-γ.
Figure 2.
Comparison of the histology of spinal cords in vehicle- and SR 57746A-treated rats. Typical histopathological slices from MBP68–82-immunized rats treated with vehicle, showed several inflammatory infiltrates (A), localized around vessels (B) in the white matter in the lumbar part of spinal cords. In contrast, sections from 10 mg/kg SR 57746A-treated rats immunized with MBP68–82 were similar to those from normal rats. TNF-α (C) and IFN-γ (D) were produced only by perivascular leukocytes (arrows) from rats with EAE.
Figure 3.
SR 57746A impaired inflammatory infiltration in the spinal cords of rats induced for EAE. In hematoxylin/eosin-stained sections, all nucleated cells were counted. For each group, the mean and SD were determined. ∗∗, P < 0.01/MBP + vehicle.
Figure 4.
SR 57746A inhibited the expression of the proinflammatory cytokines TNF-α and IFN-γ. Inflammatory cells were isolated on day 10 postimmunization from the spinal cord of 25 normal rats, 25 rats immunized with MBP68–82, and 25 rats immunized with MBP and treated daily with SR 57746A at a 10 mg/kg dose. Quantification of mRNA was performed by quantitative PCR. The level of mRNA in each sample was normalized for their β-2 microglobulin content and plotted as a percentage of β-2 microglobulin mRNA content. (Insets) Electrophoresed 30-cycle PCR products stained with ethidium bromide.
SR 57746A Impaired BBB Disruption and CNS Production of IgG During EAE.
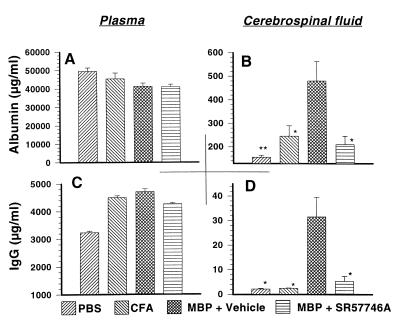
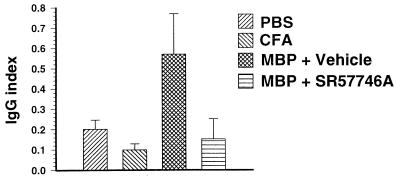
Because of the absence of activated leukocyte in the CNS, we next examined the BBB status. Plasma and CSF were collected for albumin and IgG scoring at the peak of the disease. We observed that plasma-albumin was not significantly affected by inoculation of complete Freund’s adjuvant (CFA) or MBP68–82 in CFA (Fig. 5A), whereas CSF-albumin was markedly increased in rats immunized with MBP68–82 (480 ± 82 μg/ml) compared with control rats (156 ± 8 μg/ml) or rats immunized with CFA alone (246 ± 44 μg/ml) (Fig. 5B). In SR 57746A-treated MBP68–82-immunized rats, CSF-albumin was not significantly increased (210 ± 36 μg/ml). A similar profile was obtained with plasma-IgG (Fig. 5C) or CSF-IgG (Fig. 5D). The IgG index, calculated as (CSF/serum IgG ratio)/(CSF/serum albumin ratio) was increased 5.4-fold in vehicle-treated MBP68–82-immunized rats compared with CFA-immunized rats (Fig. 6), indicating the presence of a humoral immune response within the CNS in EAE rats, as in acute attacks of MS (24). By contrast, the IgG index was not increased in SR 57746A-treated MBP68–82-immunized rats, indicating that there was no humoral immune response within the CNS in SR 57746A-treated rats. Collectively, these data showed that SR 57746A impaired EAE-mediated BBB disruption and CNS production of IgG concomitant with the disease development.
Figure 5.
SR 57746A protected against increased CSF albumin and Ig levels during EAE. Rats were immunized with either PBS, CFA, or MBP68–82, and one group was treated daily with 10 mg/kg SR 57746A. Plasma (A and C) and CSF (B and D) then were collected for albumin (A and B) or Ig (C and D) scoring on day 21. ∗, P < 0.05; ∗∗, P < 0.01/MBP + vehicle.
Figure 6.
SR 57746A impaired the increased IgG index observed during EAE. The IgG index was determined from albumin and IgG measurements in plasma and CSF of the previous experiment.
SR 57746A Did Not Prevent ex Vivo Release of Antigen-Induced Th1 or Th2 Cytokine Secretion by PLNC.
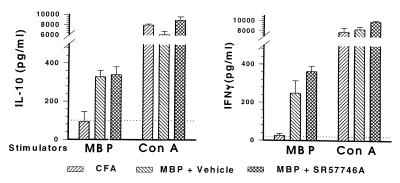
SR 57746A did not impair the generation of an antibody response to MBP68–82 (data not shown). We questioned whether SR 57746A could shift the immune response from a Th1 to a Th2 profile. PLNC draining inflamed immunized footpads, containing a high proportion of MBP-reactive T cells, were used to investigate this issue. PLNC were stimulated with MBP68–82 or with the nonspecific mitogenic agent Con A and assessed for their ability to release IFN-γ as an indicator of Th1 identity or IL-10 as an indicator of Th2 identity. As indicated in Fig. 7, IFN-γ and IL-10 secretions in response to Con A were equivalent in PLNC derived from animals immunized with CFA alone or with MBP68–82 and treated or not with 10 mg/kg SR 57746A. PLNC from rats induced with CFA alone did not respond to MBP68–82. In contrast, PLNC isolated from rats immunized with MBP68–82 strongly reacted by secreting IFN-γ or IL-10, and their responses were similar irrespective of their origin, i.e., control or SR 57746A-treated groups. These data can be expressed in terms of Th1/Th2 cytokine ratios (IFN-γ/IL-10) in vehicle versus SR 57746A-treated groups. The IFN-γ/IL-10 ratio for the vehicle-treated group was thus 0.75 ± 0.27 in MBP-stimulated cells and 1.28 ± 0.21 in Con A-stimulated cells, whereas the same ratios for the SR 57746A-treated group were 1.07 ± 0.21 and 0.91 ± 0.16, respectively. This finding clearly indicates no difference in Th1/Th2 ratios in the two groups of rats.
Figure 7.
SR 57746A did not prevent ex vivo release of Th1 and Th2 cytokines by MBP68–82-specific PLNC. PLNC were harvested 21 days after EAE induction and cultured for 96 h with 111 μg/ml MBP68–82 or 1 μg/ml Con A. Supernatants were evaluated for the presence of IFN-γ and IL-10 by ELISA. The dotted lines indicate IFN-γ and IL-10 levels in the supernatant of PLNC cultured without stimulators.
SR 57746A Down-Regulated TNF-α Production in Lipopolysaccharide (LPS)-Treated Rats.
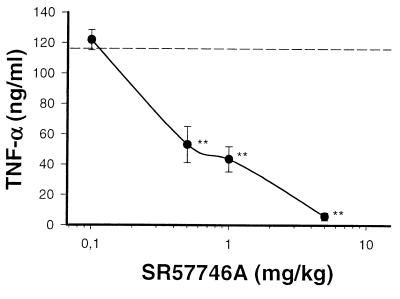
In the CNS, proinflammatory cytokines are produced mainly by astrocytes, microglial cells, and invading activated T cells. On the periphery, they are essentially produced by macrophages and T cells. Here we examined whether SR57746A could modulate cytokine production in a peripheral inflammatory model, induced in Lewis rats by i.p. administration of LPS (25). According to this protocol, SR 577746A treatment did not modify the kinetics of cytokine release (data not shown), but resulted in a strong dose-dependent decrease in TNF-α production (measured at peak time: 1.5 h post-LPS) (Fig. 8). Interestingly, doses required for inhibition of TNF-α synthesis were correlated with those required for EAE protection.
Figure 8.
Protective doses in EAE correlated with those required for the inhibition of TNF-α secretion. Groups of five rats were treated by oral route with SR 57746A at the indicated doses or with the vehicle only (dotted line) 1 h before LPS (1 mg/kg, i.p.). TNF-α was measured in plasma 1.5 h after LPS administration. ∗∗, P < 0.01/vehicle-treated rats.
Discussion
EAE is a widely studied animal model for MS. This model is appropriate for elucidating the role of BBB disruption in the development of this pathology and suitable for evaluation of therapeutic interventions at the BBB level. In the present study, we tested the EAE prevention potential of SR 57746A. This molecule originally was described as having substantial neuroprotective properties in various experimental protocols (intraseptal administration of vincristine, crushing of sciatic nerve, administration of acrylamide, or intraseptal infusion of amyloid-β protein; refs. 13 and 26), and it protects pmn mice against genetic motor neuronopathy (27). In vitro, this molecule potentiates the effect of nerve growth factor (NGF) in PC12 cells and stimulates the synthesis of NGF and brain-derived neurotrophic factor in primary cultures of rat hippocampal astrocytes (13). SR 57746A currently is being evaluated in patients presenting with various neurodegenerative pathologies. In a preliminary worldwide phase I-II clinical trial on 54 patients with amyotrophic lateral sclerosis monitored for 8 months, this agent demonstrated a 40% decrease in disease progression as compared with the placebo group (16). The results reported here indicate that SR 57746A effectively prevents the development of EAE in a dose-dependent manner, as revealed by representative parameters such as clinical grading and change in body weight. Moreover, it prevents peripheral inflammatory activated cells from entering the CNS or being expended at this level, expressing and producing TNF-α and IFN-γ. In addition, the increased CSF/serum ratio for albumin and Ig as well as the increase in the IgG index, conventional markers of BBB disruption and intrathecal Ig synthesis in EAE and MS (24), are prevented by SR 57746A treatment. In contrast, this molecule did not impair the humoral response to MBP68–82 in plasma, the increased secretion of corticosterone (data not shown), and the ex vivo PLNC antigen-specific Th1 and Th2 responses to MBP68–82 (assessed by their respective signature cytokine profiles). SR 57746A therefore did not affect the peripheral immune response from the initiation of EAE, nor the response of the hypothalamic-pituitary-adrenal axis. Together with our histological findings, these data suggest that SR 57746A may mediate its therapeutic effects in EAE, either (i) by altering the transmigration of activated autoreactive T cells into the CNS parenchyma, or (ii) by preventing their expansion at this level. Leukocyte entry into the CNS began by an adhesion step at the BBB level. This process involves endothelial vascular cell adhesion molecule 1 (VCAM-1) expression (28), which is temporally correlated with the onset of clinical signs. TNF-α plays a pivotal role in this VCAM-1 expression on endothelial cells (29) and astrocytes (30). Moreover, it induces intercellular adhesion molecule-1 expression on endothelial cells (31), astrocytes (32), and glial cells (33, 34). TNF-α also favors MHC class II antigen expression on astrocytes and microglial cells (35), which normally express none of these molecules, and thus could act as CNS antigen-presenting cells. The induction of these molecules guides inflammatory leukocytes into and through the CNS, thus contributing to their multiplication and finally to BBB disruption. The mechanism of action of SR57746A at the BBB level is not yet known. However, we noted that orally administered SR 57746A was dose-dependently one of the most potent nonsteroidal drugs inhibiting LPS-induced TNF-α synthesis. These results suggest that the protective effect of SR 57746A against BBB disruption could be mediated through a pharmacological intervention aimed at inhibiting the production of proinflammatory cytokines involved in the development of EAE. The hypothesis of a possible pathogenic role of TNF-α in amyotrophic lateral sclerosis recently has been documented, and even TNF-α-producing cells could differ in amyotrophic lateral sclerosis and EAE (36), which suggests that the protective effect of SR 57746A in these two diseases could be mediated through a common mechanism. The neurotrophin-potentiating activity of SR 57746A previously described (13–16) also may be involved, but additional studies are needed to understand the role of neurotrophins in EAE. Inhibition of pathogenic responses at the BBB level represents a paramount goal of therapeutic approaches to MS. To date, all agents experimentally shown to act on BBB and reported to be efficient in the treatment of MS, i.e., IFN-β (37) and corticosteroids (38), are also potent immunosuppressors, inducing severe side effects and exacerbating risks of infection, thus restricting their use for long-term treatment. SR 57746A, without significant general immunosuppressive properties and active by oral route, could represent a new generation of therapeutic compounds for MS.
Abbreviations
- MBP
myelin basic protein
- EAE
experimental autoimmune encephalomyelitis
- BBB
blood–brain barrier
- CNS
central nervous system
- PLNC
popliteal lymph node cells
- TNF
tumor necrosis factor
- MS
multiple sclerosis
- CSF
cerebrospinal fluid
- CFA
complete Freund’s adjuvant
- LPS
lipopolysaccharide
References
- 1.Correale J, Gilmore W, McMillan M, Li S, McMarthy K, Le T, Weiner L P. J Immunol. 1995;154:2959–2968. [PubMed] [Google Scholar]
- 2.Rieckmann P, Albrecht M, Kitze B, Weber T, Tunami H, Broocks A, Luer W, Poser S. Neurology. 1994;44:1523–1526. doi: 10.1212/wnl.44.8.1523. [DOI] [PubMed] [Google Scholar]
- 3.Selmaj K, Raine C S, Canella B, Brosnan C F. J Clin Invest. 1991;87:949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canella B C, Raine C S. Ann Neurol. 1995;37:424–435. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- 5.Brosnan C F, Selmaj K, Raine C S. J Neuroimmunol. 1988;18:87–94. doi: 10.1016/0165-5728(88)90137-3. [DOI] [PubMed] [Google Scholar]
- 6.Alvord E C, Kies M W, Suckling A J, editors. Prog Clin Biol Res. 1984;146:1–554. [Google Scholar]
- 7.Wekerle H. Curr Opin Neurobiol. 1993;3:779–784. doi: 10.1016/0959-4388(93)90153-p. [DOI] [PubMed] [Google Scholar]
- 8.Ruddle N H, Bergman C M, McGrath K M, Lingenheld E G, Grunnet M L, Padula S J, Clark R B. J Exp Med. 1990;172:1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monastra G, Cross A H, Bruni A, Raine C S. Neurology. 1993;43:153–163. doi: 10.1212/wnl.43.1_part_1.153. [DOI] [PubMed] [Google Scholar]
- 10.Mustafa M, Diener P, Höjeberg B, Van Der Meide P, Olsson T. J Neuroimmunol. 1994;31:165–177. doi: 10.1016/0165-5728(91)90022-y. [DOI] [PubMed] [Google Scholar]
- 11.Issazadeh S, Mustafa M, Ljungdahl A, Höjeberg B, Dagerlind A, Elde R, Olsson T. J Neurosci Res. 1995;40:579–590. doi: 10.1002/jnr.490400503. [DOI] [PubMed] [Google Scholar]
- 12.Villarroya H, Violleau K, Younes-Chennoufi A B, Bauman N. J Neuroimmunol. 1997;64:55–61. doi: 10.1016/0165-5728(95)00151-4. [DOI] [PubMed] [Google Scholar]
- 13.Fournier J, Steinberg R, Gauthier T, Keane P E, Guzzi U, Coude F X, Bougault I, Maffrand J P, Soubrie P, Le Fur G. Neuroscience. 1993;55:629–641. doi: 10.1016/0306-4522(93)90429-j. [DOI] [PubMed] [Google Scholar]
- 14.Pradines A, Magazin M, Schiltz P, Le Fur G, Caput D, Ferrara P. J Neurochem. 1995;64:1954–1964. doi: 10.1046/j.1471-4159.1995.64051954.x. [DOI] [PubMed] [Google Scholar]
- 15.Ruigt G S F, Makkink W K, Konings P M N. Neurosci Lett. 1995;203:9–12. doi: 10.1016/0304-3940(95)12251-6. [DOI] [PubMed] [Google Scholar]
- 16.Fournier J, Keane P E, Ferrara P, Soubrié P. CNS Drug Rev. 1997;3:148–167. [Google Scholar]
- 17.Martin D, Near S L. J Neuroimmunol. 1995;61:241–245. doi: 10.1016/0165-5728(95)00108-e. [DOI] [PubMed] [Google Scholar]
- 18.Jung S, Schluesener H J, Schmidt B, Fontana A, Toyka K V, Hartung H P. Immunology. 1994;83:545–551. [PMC free article] [PubMed] [Google Scholar]
- 19.Tanuma M, Kojima T, Shin T, Aikawa Y, Kohji T, Ishihira A, Matsumoto Y. J Neuroimmunol. 1997;73:197–206. doi: 10.1016/s0165-5728(96)00199-3. [DOI] [PubMed] [Google Scholar]
- 20.Kaltschmidt C, Kaltschmidt B, Lannes-Vieira J, Kreutzberg G W, Wekerle H, Baeuerle P A, Gehrmann J. J Neuroimmunol. 1994;55:99–106. doi: 10.1016/0165-5728(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 21.Zhao W, Tilton R G, Corbett J A, McDaniel M L, Misko T P, Williamson J R, Cross A H, Hickey W F. J Neuroimmunol. 1996;64:123–133. doi: 10.1016/0165-5728(95)00158-1. [DOI] [PubMed] [Google Scholar]
- 22.Marchand J, Bord A, Pénarier G, Lauré F, Carayon P, Casellas P. Cytometry. 1999;35:227–234. doi: 10.1002/(sici)1097-0320(19990301)35:3<227::aid-cyto5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Bouaboula M, Legoux P, Pességué B, Delpech B, Dumont X, Piechaczyk M, Casellas P, Shire D. J Biol Chem. 1992;267:21830–21838. [PubMed] [Google Scholar]
- 24.Anderson M, Alvarez-Cermeno C J, Bernadi G, Cogato I, Fredman P, Frederiksen J, Fredrikson S, Gallo P, Grimaldi L M, Gronning M, et al. J Neurol Neurosurg Psychiatry. 1994;57:897–902. doi: 10.1136/jnnp.57.8.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourrie B, Bouaboula M, Benoit J M, Derocq J M, Esclangon M, Le Fur G, Casellas P. Eur J Immunol. 1995;25:2882–2887. doi: 10.1002/eji.1830251026. [DOI] [PubMed] [Google Scholar]
- 26.Terranova J P, Kan J P, Storme J J, Perrault P, Le Fur G, Soubrié P. Neurosci Lett. 1996;213:79–82. doi: 10.1016/0304-3940(96)12859-7. [DOI] [PubMed] [Google Scholar]
- 27.Duong F, Fournier J, Keane P, Guénet J L, Soubrié P, Warter J M, Borg J, Poindron P. Br J Pharmacol. 1998;124:811–817. doi: 10.1038/sj.bjp.0701885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yednock T A, Cannon C, Fritz L C, Sanchez-Madrid F, Steinman L, Karin N. Nature (London) 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 29.Lechleitner S, Gille J, Johnson D R, Petzelbauer P. J Exp Med. 1998;187:2023–2030. doi: 10.1084/jem.187.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barten D M, Ruddle N H. J Neuroimmunol. 1993;51:123–133. doi: 10.1016/0165-5728(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 31.Majewska E, Paleolog E, Baj Z, Kralisz U, Feldman M, Tchorzewski H. Scand J Immunol. 1997;45:385–392. doi: 10.1046/j.1365-3083.1997.d01-412.x. [DOI] [PubMed] [Google Scholar]
- 32.Ballestas M E, Benveniste E N. Glia. 1995;14:267–278. doi: 10.1002/glia.440140404. [DOI] [PubMed] [Google Scholar]
- 33.Shrikant P, Weber E, Jilling T, Benveniste E N. J Immunol. 1995;155:1489–1501. [PubMed] [Google Scholar]
- 34.Shrikant P, Benveniste E N. J Immunol. 1996;157:1819–1822. [PubMed] [Google Scholar]
- 35.Canella B, Raine C S. J Neuroimmunol. 1989;24:239–248. doi: 10.1016/0165-5728(89)90122-7. [DOI] [PubMed] [Google Scholar]
- 36.Ghezzi P, Bernardini R, Giuffrida R, Bellomo M, Manzoni C, Comoletti D, Di Santo E, Benigni F, Mennini T. Eur Cytokine Network. 1998;9:139–144. [PubMed] [Google Scholar]
- 37.Stone L A, Franj J A, Albert P S, Bash C, Smith M E, Maloni H, McFarland H F. Ann Neurol. 1995;37:611–619. doi: 10.1002/ana.410370511. [DOI] [PubMed] [Google Scholar]
- 38.Barkhof F, Valk J, Hommes O R, Scheltens P. Neurology. 1991;14:1219–1222. doi: 10.1212/wnl.41.8.1219. [DOI] [PubMed] [Google Scholar]