Abstract
Selective inhibition of T cell costimulation using the B7-specific fusion protein CTLA4-Ig has been shown to induce long-term allograft survival in rodents. Antibodies preventing the interaction between CD40 and its T cell-based ligand CD154 (CD40L) have been shown in rodents to act synergistically with CTLA4-Ig. It has thus been hypothesized that these agents might be capable of inducing long-term acceptance of allografted tissues in primates. To test this hypothesis in a relevant preclinical model, CTLA4-Ig and the CD40L-specific monoclonal antibody 5C8 were tested in rhesus monkeys. Both agents effectively inhibited rhesus mixed lymphocyte reactions, but the combination was 100 times more effective than either drug alone. Renal allografts were transplanted into nephectomized rhesus monkeys shown to be disparate at major histocompatibility complex class I and class II loci. Control animals rejected in 5–8 days. Brief induction doses of CTLA4-Ig or 5C8 alone significantly prolonged rejection-free survival (20–98 days). Two of four animals treated with both agents experienced extended (>150 days) rejection-free allograft survival. Two animals treated with 5C8 alone and one animal treated with both 5C8 and CTLA4-Ig experienced late, biopsy-proven rejection, but a repeat course of their induction regimen successfully restored normal graft function. Neither drug affected peripheral T cell or B cell counts. There were no clinically evident side effects or rejections during treatment. We conclude that CTLA4-Ig and 5C8 can both prevent and reverse acute allograft rejection, significantly prolonging the survival of major histocompatibility complex-mismatched renal allografts in primates without the need for chronic immunosuppression.
Unmodified organ transplantation between genetically nonidentical individuals invariably results in immunological rejection of the organ through T cell-dependent mechanisms. Successful transplantation of allogeneic organs has therefore required the administration of drugs directed at suppressing recipient T cell function. Both calcineurin phosphatase inhibitors and glucocorticosteroids are used clinically, and both prevent the T cell-mediated release of activating cytokines, particularly IL-2. Therapy with these agents is imperfect, however. Both act by impairing T cell antigen receptor (TCR) signal transduction, the sole mediator of T cell antigen recognition, thus minimizing the potential for specific immune interaction between the recipient and donor. They also act on all T cells indiscriminately. In addition, the effect of these drugs is not lasting, such that cessation of immunosuppression has generally resulted in graft loss even after prolonged rejection-free survival. Thus, transplant patients are required to suffer the consequences of nonspecific immunosuppression to avoid rejection. These consequences include an increased risk to the patient of infection and malignancy as well as significant drug related expense and toxicity.
Data establishing that T cell activation requires both TCR-mediated signals and simultaneously delivered costimulatory signals have accumulated over the past 20 years (1). These important costimulatory signals are provided at least in part by the T cell-based CD28 molecule when bound to its counter receptors CD80 (B7–1) or CD86 (B7–2) on antigen-presenting cells (APCs) and perhaps parenchymal cells (1–3). The interaction of CD40 and its T cell-based ligand, CD40L (CD154), also plays an important role in T cell activation at least in part by up-regulating CD80/86 (B7) (4, 5). In addition, CD40 and CD40L play a fundamental role in establishing T cell-dependent B cell activity (6, 7). Further studies have shown that the T cell molecule CTLA4 (CD152) appears to down-regulate costimulation and TCR-mediated activation, probably by competing with CD28 for B7 and by delivering a unique negative signal to the TCR signal transduction complex (8).
Several groups have shown in rodents that T cell activation can be blocked and allograft survival prolonged by treatment with the B7 specific fusion protein CTLA4-Ig (9–11). Others have demonstrated that B7 up-regulation can be prevented by the CD40L-specific monoclonal antibody MR1 (4). Because both agents appear to be dependent on TCR engagement for their effectiveness, the specificity of the T cell response theoretically can be exploited rather than depending on pan-T cell suppression. In addition to in vitro efficacy, in rodents these agents have shown dramatic in vivo effects, allowing for the acceptance of fully mismatched skin grafts, a result not obtainable with currently available immunosuppression (12). It has thus been hypothesized that CTLA4-Ig and the human homologue to MR1, the CD40L-specific monoclonal antibody 5C8, may be capable of inducing long-term survival or perhaps even tolerance to allografted tissues in humans. To test this hypothesis in a relevant preclinical model, CTLA4-Ig and 5C8 were tested alone and in combination on rhesus peripheral blood leukocytes in vitro and in rhesus monkeys transplanted with primarily vascularized renal allografts.
MATERIALS AND METHODS
Reagents.
Human CTLA4-Ig and a control fusion protein, IgG1, were prepared as previously described (2) and shipped in solution by Genetics Institute (Cambridge, MA). The anti-CD40 ligand antibody 5C8 was prepared as previously described (6, 7) and shipped in solution by Biogen. The hamster anti-mouse CD28 monoclonal antibody PV-1 (IgG1, clone G62) was purified from hybridoma culture supernatants and used as an isotype control monoclonal antibody.
MHC Typing and Donor-Recipient Selection.
The experiments described in this study were conducted according to the principles set forth in the Guide for the Care and Use of Laboratory Animals (13). Donor-recipient combinations and animals chosen for third-party cells were selected based on genetic nonidentity at both major histocompatibility complex (MHC) class I and class II. Class I disparity was established by one-dimensional isoelectric focusing as previously described (14). Class II disparity was established based on the results of unidirectional mixed lymphocyte reactions (MLRs). In addition, the animal’s DRB loci were verified to be disparate by denaturing gradient gel electrophoresis and direct sequencing of the second exon of DRB as previously described (15). Vigorous in vitro T cell responsiveness of the recipient toward the donor was confirmed in vitro for all donor–recipient pairs.
In Vitro Cellular Analysis.
Unidirectional MLRs were performed on all animals prior to transplantation and on rejection-free survivors after 100 and 150 days. Each animal was tested against all potential donors to establish the highest responder pairs for transplantation. Responder cells (3 × 105) were incubated with irradiated stimulator cells (1 × 105) at 37°C for 5 days. Cells were pulse-labeled with [3H]thymidine, and proliferation was monitored by [3H]thymidine incorporation. Polyclonal stimulation with concanavilin A (25 μg/ml) served as a positive control. A stimulation index was calculated by normalization to self-reactivity, which in all cases was near background incorporation. For in vitro dose-response studies, CTLA4-Ig or 5C8 was added to the MLR on day 1 at concentrations ranging from 100 μg/ml to 0.01 μg/ml. Combined treatments were performed by varying the CTLA4-Ig concentration and holding the 5C8 concentration steady at 50 μg/ml.
Peripheral blood lymphocyte phenotype analysis was performed prior to transplantation and periodically during and after drug therapy. Assays evaluated 0.2 ml of heparinized whole blood diluted with phosphate-buffered saline and 1% fetal calf serum. Fluorescein isothiocyanate-labeled T11, B1 (Coulter), and FN18 (the generous gift of David M. Neville, Jr., National Institutes of Health, Bethesda, MD) monoclonal antibodies were used to assess the percentage of CD2 (T cell/natural killer cell), CD20 (B cell), and CD3 (T cell) positive cells respectively. Red blood cells were removed from the preparation by lysis buffer (0.15 M NH4Cl/1.0 mM KHCO3/0.1 mM Na2EDTA, pH 7.3) treatment following staining. Cells were subjected to flow cytometry immediately or following fixation in 1% paraformaldehyde. Flow cytometry was performed using a Becton Dickinson FACScan.
Renal Allografts.
Renal allotransplantation was performed as previously described (14). Briefly, outbred juvenile (1 to 3 years of age) rhesus monkeys, seronegative for simian immunodeficiency virus, simian retrovirus, and herpes B virus, were obtained from the Primate Center (University of Wisconsin, Madison) or Laboratory Animal Breeding Services (Yemassee, SC). Procedures were performed under general anesthesia using ketamine (1 mg/kg, i.m.), xylazine (1 mg/kg, i.m.), and halothane (1%, inhaled). Transplantation was performed between genetically distinct donor–recipient pairs as determined by the MHC analysis described above. The animals were heparinized during organ harvest and implantation (100 units/kg). The allograft was implanted using standard microvascular techniques to create an end-to-side anastamosis between the donor renal artery and recipient distal aorta as well as the donor renal vein and recipient vena cava. A primary ureteroneocystostomy was then created. Bilateral native nephrectomy was completed prior to closure.
Animals were treated with intravenous fluid for approximately 36 hr until oral intake was adequate. Trimethaprim-sulfa was administered for 3 days for surgical antibiotic prophylaxis. Each animal received 81 mg of aspirin on the day of surgery. The need for analgesia was assessed frequently, and analgesia was maintained with intramuscular butorphanol. Animals were weighed weekly. Skin sutures were removed after 7–10 days. CTLA4-Ig and/or 5C8 was given intravenously at doses and dosing schedules that varied based on accumulating experience with the agents. No other immunopharmaceuticals were administered. Serum creatinine and whole-blood electrolytes (Na+, K+, Ca2+) and hemoglobin were determined every other day until stable and then weekly.
Pathological Analysis.
Biopsies were performed on animals suspected of having rejection using a 20-gauge needle-core device (Biopty-Cut, Bard, Covington, GA). Standard staining with hematoxylin and eosin was performed on frozen or formalin-fixed tissue to confirm the diagnosis of rejection. Animals were euthanized at the time of anuria or if a weight loss of 15% of pretransplant body weight occurred in accordance with American Association for the Accreditation of Laboratory Animal Care standards. All animals underwent complete gross and histopathological evaluation at the time of death.
RESULTS
CTLA4-Ig and 5C8 Synergistically Prevent T Cell Proliferation in Vitro.
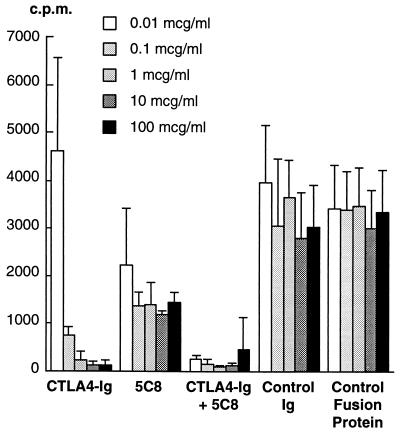
Both CTLA4-Ig and 5C8 inhibited rhesus MLRs in a dose-dependent fashion (Fig. 1). CTLA4-Ig was, however, more effective than 5C8 as a single agent in preventing T cell proliferation. Substantial reduction in thymidine incorporation was seen at a CTLA4-Ig concentration of 0.1 μg/ml, and further inhibition was achieved at higher concentrations. Modest reduction in proliferation was achieved with 5C8 concentrations of 0.01 μg/ml, but inhibition was not substantially improved by increasing concentrations. Both agents acted synergistically, with the combination inhibiting proliferation approximately 100 times more effectively than either agent alone. Dose-response studies were repeated for three separate naive animals with identical results.
Figure 1.
The effect of CTLA4-Ig and 5C8 alone and in combination on unidirectional rhesus monkey mixed lymphocyte reactions. Increasing concentrations of CTLA4-Ig result in progressive suppression, whereas the effects of 5C8 are more modest. The combination is more effective than either drug alone at 100-fold or greater concentrations. Results shown were reproduced in three independent experiments. c.p.m., counts per minute from incorporated [3H]thymidine.
CTLA4-Ig and 5C8 Synergistically Prevent Allograft Rejection in Vivo.
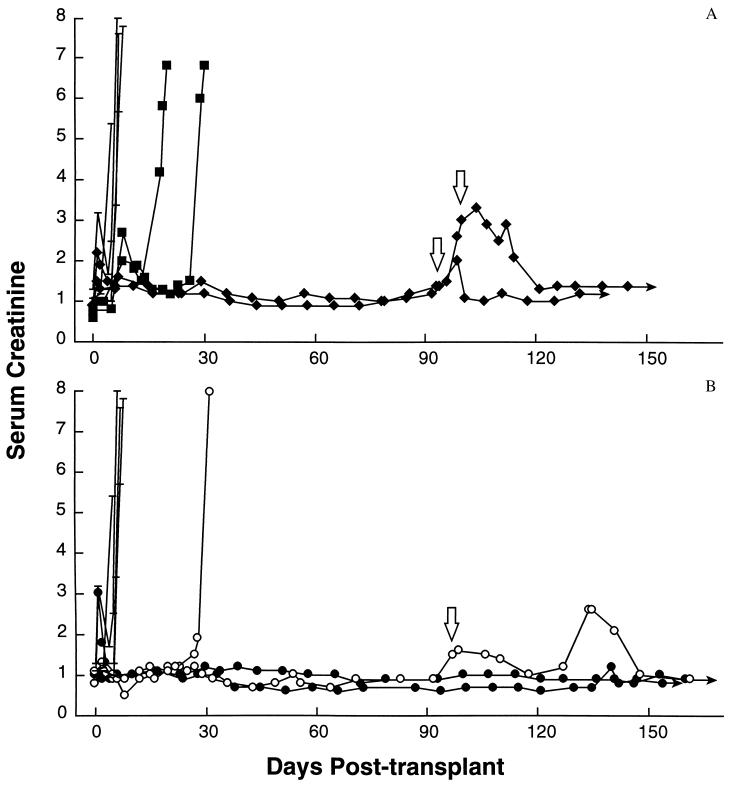
Twelve renal allotransplants were performed (Fig. 2). Four animals received transplants without any immunological intervention. These animals rejected in 5, 7, 7, and 8 days. Histological examination of their kidneys showed acute cellular rejection characterized by diffuse interstitial and tubular lymphocytic infiltration with edema and cellular necrosis (Fig. 3A). One animal was given a 5-day course of CTLA4-Ig (10 mg/kg per day) beginning at the time of transplantation and had graft survival prolonged to 20 days (Fig. 2A). Graft loss was due to cellular rejection indistinguishable from that seen in the control animals. One animal was treated with 20 mg/kg CTLA4-Ig on the day of transplantation followed by a 12-day course of 10 mg/kg every other day and had graft survival prolonged to 30 days (Fig. 2A). Again, graft loss was due to acute cellular rejection. Extrapolating from previously published work in the rat heterotopic cardiac allograft model of Turka and coworkers (9), a donor-specific transfusion of lymph node-derived lymphocytes (108) was given at the time of transplantation to these two animals.
Figure 2.
(A) Survival and renal function as determined by serum creatinine following unmodified allogeneic renal transplantation (–) or transplantation following induction with CTLA4-Ig alone (▪) or 5C8 alone (⧫). Open arrows indicate re-treatment during biopsy-proven rejection. Solid arrows indicate continued survival. (B) Survival and renal function as determined by serum creatinine following unmodified allogeneic renal transplantation (–) or transplantation following induction with both CTLA4-Ig and 5C8. ○ indicate treatment on days 0, 2, 4, 6, 8, 10, and 12 posttransplant. • indicate treatment on days 0, 2, 4, 6, 8, 12, 16, and 28 posttransplant. Open arrows indicate re-treatment during biopsy-proven rejection for the animal depicted in ○. Solid arrows indicate continued survival free of rejection since transplantation.
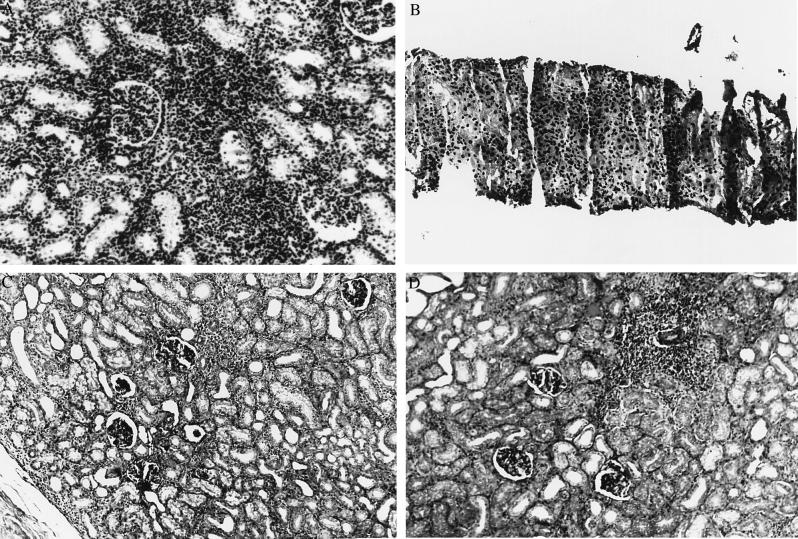
Figure 3.
(A) Renal allograft histology showing acute cellular rejection following unmodified renal allotransplantation in rhesus monkeys. (B) Renal allograft histology showing acute cellular rejection prior to reversal with 5C8. (C) Normal renal allograft histology from an animal with normal renal function 163 days after transplantation and induction with CTLA4-Ig and 5C8. (D) A perivascular lymphoid aggregate within the allograft shown in C. These nests of lymphocytes exist in the allograft despite normal function and the absence of immunosuppression. All micrographs are ×250.
Two animals were treated with 5C8 alone (Fig. 2A). Both animals received 20 mg/kg every other day beginning on the day of surgery and continuing for 14 postoperative days (8 doses, total). No donor-specific transfusion was performed. Both animals experienced extended rejection-free survival, although transient creatinine elevations were recorded during the second and fourth postoperative weeks. Both animals rejected between 95 and 100 days posttransplant. Biopsy was performed on each animal to confirm the diagnosis (Fig. 3B). Both animals were then re-treated with 7 doses of 5C8 (20 mg/kg; one animal every other day and one animal daily), and both returned to normal graft function with no demonstrable adverse effects. They remain alive and well more than 150 days after transplantation at the time of this writing.
Two animals were given 20 mg/kg each of CTLA4-Ig and 5C8 following transplantation (Fig. 2B). Again, each drug was given every other day beginning on the day of surgery and continuing for 14 postoperative days without donor-specific transfusion. One animal rejected 32 days after surgery. The other remained free of rejection for 100 days, but like those animals treated with 5C8 alone, it rejected at that time. Similarly, a biopsy showed acute cellular rejection. The initial regimen of CTLA4-Ig and 5C8 was repeated, and the creatinine returned to baseline (1.0). MLR analysis following this treatment showed a donor-specific loss of reactivity. Third-party responsiveness was maintained (data not shown). At 165 days posttransplant, the animal was sacrificed as required by protocol due to weight loss. Graft function at that time was normal. At autopsy, the animal was found to have shigella and camphylobacter enterocolitis, a common infection in rhesus monkeys. This illness had infected multiple animals in the original primate colony, including several untreated animals. No other pathological abnormality was found; specifically, there was no evidence of lymphoproliferative disease or opportunistic infection. Histologically, the graft had isolated nests of lymphocytes in the interstitium but no evidence of tubular infiltration, glomerular damage, or parenchymal necrosis (Fig. 3C).
Like the animals treated with 5C8 alone, both of these animals had transient increases in their creatinine combined with an increase in graft size during the fourth postoperative week. It was hypothesized that this graft swelling reflected a second wave of infiltrating lymphocytes and therefore led to a modified dosage schedule, such that both reagents were given on the day of surgery and on postoperative days 2, 4, 6, 8, 12, 16, and 28 without donor-specific transfusion.
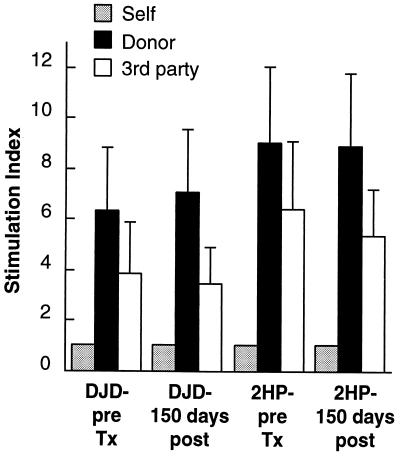
Two animals were treated with this modified regimen (Fig. 2B). Both have experienced rejection-free survival and have been free of illness or alterations in renal function for more than 150 days. Both remain alive and well at the time of this writing. After 100 days of rejection-free survival, MLRs were repeated against donor cells and third-party cells. No changes in in vitro reactivity were observed (data not shown). These studies were repeated after 150 days of rejection-free survival with identical results (Fig. 4). Both animals maintain vigorous in vitro responses toward donor and third-party cells but fail to reject their allografts.
Figure 4.
Mixed lymphocyte responses against donor lymphocytes and third-party lymphocytes for two rhesus monkeys 150 days after allotransplantation with rejection-free survival and normal renal function and without any chronic therapy. Both donor and third-party responsiveness are maintained.
No animal has demonstrated toxicity from any of the therapies employed. Specifically, there has been no fever, anorexia, or hemodynamic abnormalities, and no opportunistic infections have occurred. Animals have been housed in standard conditions and have been allowed contact with the other animals in the colony. They have maintained normal weight gain. Laboratory chemistries and hematological parameters such as hemoglobin and white blood cell counts have remained normal. The percentages of cells expressing CD2, CD3, and CD20 were unaffected by any treatment regimen (data not shown). Specifically, no reductions in T cell counts were observed during or after treatment in any animal.
DISCUSSION
This report documents initial experience in primates with a new class of reagents directed at modifying T cell costimulation rather than focused on T cell suppression or elimination. Herein, strategies designed to interfere with the interaction of B7 and its ligand or with the up-regulation of B7 are shown to have dramatic effects on T cell responsiveness in vitro and on allograft survival in vivo—including prevention of rejection and the reversal of established, biopsy-proven rejection. In addition, these data demonstrate that anti-rejection activity can persist long after drug administration has stopped. Although these studies are preliminary, they are nonetheless striking. Such success in outbred rhesus monkeys clearly demonstrates that prolonged, rejection-free allograft survival can be induced with these agents, and it suggests that allograft tolerance might be an achievable goal in humans using this or a similar therapeutic approach. Longer follow-up and additional study will be required to fully assess the potential of this new therapy.
It is encouraging that this regimen was remarkably simple, involving two agents alone or in combination administered through a standard peripheral intravenous catheter, and that it was tolerated so well by the recipients. This is in stark contrast to other regimens used to achieve lasting graft acceptance in primates that require ionizing radiation, administration of donor-derived bone marrow, and significant perioperative immunosuppression (16, 17). The animals treated in this study displayed no evidence of T cell activation or cytokine release typically observed following treatment with antibodies directed at CD3, and prolonged survival has not carried with it a demonstrable cost in terms of opportunistic infection. In addition, no alterations in peripheral blood hematological parameters were noted during these studies. Long-term survival was achieved without apparent clearing or global reductions in T or B cells and without loss of in vitro T cell responsiveness. It is therefore unlikely that the observed effect is attributable to T cell destruction following antibody or fusion-protein opsonization.
The mechanism and relative contribution of each agent remains a matter of speculation at this juncture. The successes of CD40L blockade alone suggest that any basal costimulation signaling is less important in maintaining the rejection response than B7 up-regulation. Indeed, 5C8 resulted in impressive rejection-free survival when used alone, whereas CTLA4-Ig’s effects were more transient. This is in keeping with the findings of Sayegh et al. (10) that costimulation blockade with CTLA4-Ig has increased effectiveness in rodents when therapy is delayed for 2 days after transplantation to allow for up-regulation of costimulation molecules. The temporal expression of CD40, B7, and their ligands clearly deserves critical attention in future studies. Furthermore, non-T cell events could be critical in establishing reactivity against the allograft, particularly given the recent discovery that CD40L is expressed on nonmyeloid cells such as vascular endothelium and smooth muscle (18) and that B7–1 can be induced on fibroblasts (3) and hepatocytes (19). Denying the immune system access to critical parenchymal adhesion and costimulatory signals at the time of transplantation thus might provide significant protection. The distribution of these molecules therefore must be evaluated in addition to the timing of their expression in clinically relevant models.
It is interesting that the MLR results have not demonstrated donor-specific hyporesponsiveness despite a clear lack of rejection in vivo. The differences in activation induced by donor parenchyma compared with activation induced by lymphoid cells could explain the preservation of in vitro reactivity to donor lymphocytes despite normal graft function. This could also explain the general poor correlation between MLR reactivity and graft outcome seen in human transplantation. The observed results alternatively could be interpreted as a critical indicator that the recipient has retained the ability to recognize the graft in some way. Recognition is certainly required for the identification of friend or foe. However, despite the apparent differences in in vitro and in vivo activity, the effects of CTLA4-Ig and 5C8 were shown to be synergistic in both systems. Perhaps CTLA4-Ig provides insurance against B7 expression that escapes the effects of 5C8. In that instance, considerable time seems to be required to mount an effective acute rejection with the few cells that escape initial blockade.
Because this strategy was successful in reversing established, biopsy-proven acute rejection, it would appear that the rejection process must be maintained by continuous costimulation rather than by a process that, once set into motion, proceeds unless the effector cells are eliminated or rendered incapable of TCR signaling. Teleologically, the body is best served by inflammation that is easily controlled. Thus, in the absence of direction to attack, retreat may be the tacit order. This suggests that exploitation of the immune system’s natural propensity to down-regulate may be more advantageous than pan-suppression.
The rhesus monkey model used in this study has been shown repeatedly to be a rigorous test of immune manipulation—one that is exquisitely sensitive to even minor changes in allograft function or adverse effects on recipient wound healing and immune function (14, 16, 20). In addition, it has obvious biological similarity to human renal transplantation. Specifically, genes that encode MHC proteins are well conserved between rhesus monkeys and humans (21–23), and their rejection of vascularized organs closely parallels that seen clinically (14, 16, 20). Nevertheless, issues of optimal dosing and treatment time course remain to be resolved. Although rodent models have been successful with a single dose of CTLA4-Ig given on postoperative day 2 in combination with donor-specific transfusion (9), it is clear that a more aggressive approach is required in primates. Nonetheless, a transient, well tolerated treatment that exploits the specificity of the immune system and gives lasting, rejection-free survival would appear to be nearing clinical applicability.
Acknowledgments
This work was supported by Naval Medical Research and Development Command Grant EW.0095.003.1412, Office of Naval Research Grant N00014–96-1–1282, and the University of Wisconsin Medical Foundation. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Navy, the Department of Defense, nor the United States Government.
ABBREVIATIONS
- TCR
T cell antigen receptor
- APC
antigen-presenting cell
- MLR
mixed lymphocyte reaction
- MHC
major histocompatibility complex
References
- 1.June C H, Bluestone J A, Nadler L M, Thompson C B. Immunol Today. 1994;15:321–325. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 2.Gimmi C D, Freeman J G, Gribben G, Gray G, Nadler L M. Proc Natl Acad Sci USA. 1993;90:6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pechhold K, Patterson N B, Craighead N, Lee K P, June C H, Harlan D M. J Immunol. 1997;158:4921–4929. [PubMed] [Google Scholar]
- 4.Yang Y, Wilson J M. Science. 1996;273:1862–1864. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 5.Grewal I S, Foellmer H G, Grewal K D, Xu J, Hardardottir F, Baron J L, Janeway C A, Flavell R A. Science. 1996;273:1864–1867. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 6.Lederman S, Yellin M J, Krichevsky A, Belko J, Lee J J, Chess L. J Exp Med. 1992;175:1091–1101. doi: 10.1084/jem.175.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lederman S, Yellin M J, Inghirami G, Lee J J, Knowles D M, Chess L. J Immunol. 1992;149:3817–3826. [PubMed] [Google Scholar]
- 8.Marengere L E M, Waterhouse P, Duncan G S, Mittrucker H-W, Feng G-S, Mak T W. Science. 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 9.Lin H, Bolling S F, Linsley P S, Wei R-Q, Gordon D, Thompson C B, Turka L A. J Exp Med. 1993;178:1801–1806. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sayegh M H, Akalin E, Hancock W W, Russell M E, Carpenter C B, Linsley P S, Turka L A. J Exp Med. 1995;181:1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolling S F, Lin H, Wei R Q, Linsley P, Turka L A. J Surg Res. 1994;57:60–64. doi: 10.1006/jsre.1994.1110. [DOI] [PubMed] [Google Scholar]
- 12.Larsen C P, Elwood E T, Alexander D Z, Ritchie S C, Hendrix R, Tucker-Burden C, Cho H R, Aruffo A, Hollenbaugh D, Linsley P S, Winn K J, Pearson T C. Nature (London) 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 13.Committee on Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. Bethesda: Natl. Inst. Health; 1985. , DHHS. Publ. No. (NIH) 86–23. [Google Scholar]
- 14.Knechtle S J, Vargo D, Fechner J, Zhai Y, Wang J, Hanaway M J, Scharff J, Hu H, Knapp L, Watkins D, Neville D M. Transplantation. 1997;63:1–6. doi: 10.1097/00007890-199701150-00002. [DOI] [PubMed] [Google Scholar]
- 15.Knapp L A, Cadavid L F, Eberle M E, Knechtle S J, Bontrop R E, Watkins D I. Immunogenetics. 1997;45:171–179. doi: 10.1007/s002510050186. [DOI] [PubMed] [Google Scholar]
- 16.Thomas J, Carver M, Cunningham P, Park K, Gonder J, Thomas F. Transplantation. 1987;43:332–338. doi: 10.1097/00007890-198703000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Kawai T, Cosimi A B, Colvin R B, Powelson J, Eason J, Kozlowski T, Sykes M, Monroy R, Tanaka M, Sachs D H. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 18.Mach F, Schonbeck U, Sukhova G K, Bourcier T, Bonnefoy J-Y, Pober J S, Libby P. Proc Natl Acad Sci USA. 1997;94:1931–1936. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mochizuki K, Hayashi N, Katayama K, Hiramatsu N, Kanto T, Mita E, Tatsumi T, Kuzushita N, Kasahara A, Fusamoto H, Yokochi T, Kamada T. Hepatology. 1997;25:713–718. doi: 10.1002/hep.510250337. [DOI] [PubMed] [Google Scholar]
- 20.Thomas J M, Carver F M, Kaston-Jolly J, Haisch C E, Rebellato L M, Gross U, Vore S J, Thomas F T. Transplantation. 1994;57:101–115. doi: 10.1097/00007890-199401000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Gyllensten U B, Sundvall M, Erlich H A. Proc Natl Acad Sci USA. 1991;20:233–235. doi: 10.1073/pnas.88.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slierendregt B L, Otting N, Jonker M, Bontrop R E. Hum Immunol. 1991;30:11–17. doi: 10.1016/0198-8859(91)90064-g. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z W, McAdam S N, Hughes A L, Dogon A L, Letvin N L, Watkins D I. J Immunol. 1992;148:2547–2554. [PubMed] [Google Scholar]