Abstract
Synaptic localization of γ-aminobutyric acid type A (GABAA) receptors is a prerequisite for synaptic inhibitory function, but the mechanism by which different receptor subtypes are localized to postsynaptic sites is poorly understood. The γ2 subunit and the postsynaptic clustering protein gephyrin are required for synaptic localization and function of major GABAA receptor subtypes. We now show that transgenic overexpression of the γ3 subunit in γ2 subunit-deficient mice restores benzodiazepine binding sites, benzodiazepine-modulated whole cell currents, and postsynaptic miniature currents, suggesting the formation of functional, postsynaptic receptors. Moreover, the γ3 subunit can substitute for γ2 in the formation of GABAA receptors that are synaptically clustered and colocalized with gephyrin in vivo. These clusters were formed even in brain regions devoid of endogenous γ3 subunit, indicating that the factors present for clustering of γ2 subunit-containing receptors are sufficient to cluster γ3 subunit-containing receptors. The GABAA receptor and gephyrin-clustering properties of the ectopic γ3 subunit were also observed for the endogenous γ3 subunit, but only in the absence of the γ2 subunit, suggesting that the γ3 subunit is at a competitive disadvantage with the γ2 subunit for clustering of postsynaptic GABAA receptors in wild-type mice.
Nervous system signaling depends on the coordinated function of a variety of cell surface ion channels and receptors that are typically concentrated at specific subcellular domains, such as postsynaptic sites, axon terminals, or nodes of Ranvier (1, 2). Clustering, postsynaptic localization, and synapse-specific targeting of receptors are prerequisites for synaptic function and appear to characterize essential steps in synapse formation during neuronal development. In addition, modulation of synaptic clustering and localization of neurotransmitter receptors provide a potential mechanism for rapid adaptation of synaptic efficacy in mature neurons. This is particularly evident for fast neurotransmission mediated by receptors of the ligand-gated ion channel family.
Inhibitory neurotransmission in the brain is mediated mainly by γ-aminobutyric acid (GABA) acting at heteropentameric GABA type A (GABAA) receptor chloride channels. GABAA receptors comprise an ill-defined number of structurally and pharmacologically distinct receptor subtypes, which emerge by differential expression and assembly of a large number of subunits classified according to their primary structure as α, β, γ, δ, ɛ, π, or ρ subunits (3–5). Most GABAA receptors are composed of variant α and β subunits together with the γ2 subunit and are concentrated at postsynaptic sites. Specific receptor subtypes might be targeted to different types of synapses in distinct cellular domains. For example, in hippocampal pyramidal cells, GABAA receptors containing the α1 subunit are found in all types of GABAergic synapses (on the soma, proximal, and distal dendrites, and the axon initial segment), whereas receptors containing the α2 subunit are selectively localized in the axon initial segment, which is innervated by axo-axonic interneurons (6, 7). Although the γ2 subunit is largely dispensable for assembly and surface expression of GABAA receptors in vivo, it is required for normal channel conductance, postsynaptic clustering, and postsynaptic function of major GABAA receptor subtypes (8–10). Moreover, the γ2 subunit is essential for proper aggregation of the clustering molecule gephyrin at sites apposed to GABAergic terminals (9).
The γ2 and γ3 subunits are 64% identical at the level of their primary structure. They form recombinant receptors with similar unitary channel conductance (11), and both can contribute to the benzodiazepine (BZ) binding site (11, 12). However, the γ3 subunit-containing GABAA receptors are characterized by a very low affinity for the BZ agonist zolpidem (13–15). Because of the low abundance of the γ3 subunit in the brain (11, 13, 14), a functional analysis of the corresponding receptors in vivo has been quite limited. In particular, it has remained elusive whether the γ3 subunit contributes to receptors at postsynaptic sites. To determine whether the γ3 subunit can substitute for the γ2 subunit in the formation of functional postsynaptic GABAA receptors in vivo, a transgenic mouse strain overexpressing the γ3 subunit throughout the brain has been established in a γ2 subunit-deficient background. The results show that ectopically overexpressed γ3 subunit partially restores the formation of BZ-modulated GABAA receptors in vivo and promotes the synaptic localization of gephyrin and GABAA receptors that contribute to miniature postsynaptic currents, similarly to the γ2 subunit.
Methods
Generation of γ3 Transgenic Mice.
The murine γ3 cDNA (16), including the entire γ3 subunit coding region, 23 bp of 5′ flanking sequence, and 115 bp of 3′ flanking sequence, was reconstructed from two partial cDNAs generously provided by N. Walter and J. M. Sikela (University of Colorado, Denver). The cDNA was cloned into pBAP (17) in between 5′ genomic sequences from the human β-actin gene and a 3′ poly(A) signal from simian virus 40. The expression cassette was separated from vector sequences and injected into the pronuclei of fertilized eggs of B6D2F1 hybrid donor mice, and the eggs were transferred into foster mothers as described (18). Transgenic founder mice were identified by PCR analyses of tail biopsies and expanded into lines by crossing with mice that are heterozygous for the GABAA receptor γ2 subunit gene (γ20/+) (8) on a 129/Ola × C57BL/6 background. The offspring were screened for expression of the transgene by Western blotting of brain membranes. One transgenic line was found to express substantial amounts of the γ3 subunit and was subsequently crossed with γ20/+ mice. Offspring that were hemizygous for the γ3 transgene and heterozygous for the null allele of the γ2 subunit (γ3tg/γ20/+) were crossed with γ20/+ mice to obtain γ3tg/γ20/0 homozygous mutants. This breeding scheme maintains hemizygosity at the transgene locus, thus eliminating possible effects due to insertion mutagenesis. The presence of the transgene and the genotype at the γ2 locus were determined by PCR analyses of tail biopsies or embryonic tissue, using the primers 5′-CCGGC CCGGC TTCCT TTGTC C-3′ (γ3tg upper primer) and 5′-TGCCT AATGT TGTTC TTGCT GGTGT CG-3′ (lower primer) and those described (8) for the γ2 gene locus.
Immunoprecipitation.
Affinity-purified α1 subunit-specific Abs were coupled to protein A-agarose beads and incubated overnight at 4°C with deoxycholate membrane extracts prepared from three postnatal day 10–13 (P10–13) brains per genotype as described (13). Following extensive washing with 10 mM Tris⋅HCl (pH 7.5)/150 mM NaCl/1 mM EDTA/0.1 mM PMSF/0.2% Triton X-100, the immunoprecipitates were subjected to Western blot analysis with antibodies against the α1, β2,3, γ2, and γ3 subunits (13).
Autoradiography.
Cryostat brain sections (12 μm) of P14 mice were incubated with 6 nM [3H]flumazenil in the absence or presence of 10 μM zolpidem as described (19). Quantification in the hippocampal region was performed by densitometry in three mice per genotype with an MCID imaging system (Imaging Research, St. Catherine’s, ON, Canada). For each mouse, data were averaged from nine measurements of the CA1 region in three parasagittal sections.
Immunohistochemistry.
Cryostat brain sections (12 μm) of P14 mice were processed for double-immunofluorescence staining as described (7, 9). GABAA receptor subunit expression was investigated with polyclonal antisera raised in guinea pig (α1, α2, α3, γ3) or rabbit (γ3) (13, 20). Gephyrin was detected with the mAb 7a (Connex, Martinsried, Germany) (21). Following overnight incubation with a mixture of two primary Abs, the sections were incubated for 30 min in the corresponding secondary Abs coupled to Cy3 (Jackson ImmunoResearch) or Oregon Green (Molecular Probes), washed, and coverslipped with buffered glycerol.
Data Analysis.
Fluorescent images were captured with a confocal laser scanning microscope (TCS 4D; Leica, Deerfield, IL). For quantification of the number of synaptic receptor clusters, digital images were analyzed with the MCID software using a threshold segmentation algorithm for detection of clusters as described (9). For each mouse (n = 3–4 per genotype), measurements were done in triplicate from three distinct sections. Results are given as mean ± SD. The size of clusters was averaged from individual measurements (n = 500–1300 per genotype).
Electrophysiological Recordings and Neuronal Cultures.
Miniature inhibitory postsynaptic currents (mIPSCs) and currents evoked by pulse application of GABA were recorded with the whole-cell patch-clamp method applied to neocortical neurons from γ2+/+, γ20/0, and γ3tg/γ20/0 embryos cultured for 2–3 wk, as previously described (9).
Results
A transgenic mouse line overexpressing the γ3 subunit under control of the human β-actin promoter was established. In the adult brain, the endogenous γ3 subunit gene is expressed primarily in the olfactory bulb, striatum, and a subset of thalamic nuclei, but is absent, for example, in the cerebellum (11). Hence, to test for ectopic expression of the γ3 subunit in transgenic mice, Western blots were performed using cerebellar and forebrain membrane preparations (Fig. 1A). Whereas no γ3 subunit signal was present in cerebellum of nontransgenic, control mice, ectopic γ3 subunit was readily detected as an intense band in γ3tg cerebellum. The electrophoretic mobility and band pattern of the transgene-encoded γ3 subunit were indistinguishable from those of endogenous γ3 subunit in forebrain, indicating that the protein was properly translated and efficiently processed. The expression level of the γ3 subunit in forebrain was 5–10 times higher in transgenic mice than in controls, indicating that it was expressed throughout the brain (Fig. 1A). The transgene-encoded γ3 subunit was assembled with endogenous α1 and β2,3 subunits, as shown by Western blot analysis of GABAA receptors isolated by immunopurification (Fig. 1B). In the absence of the γ2 subunit, an increased γ3 subunit expression was noted, which was further increased in the presence of the γ3 subunit transgene.
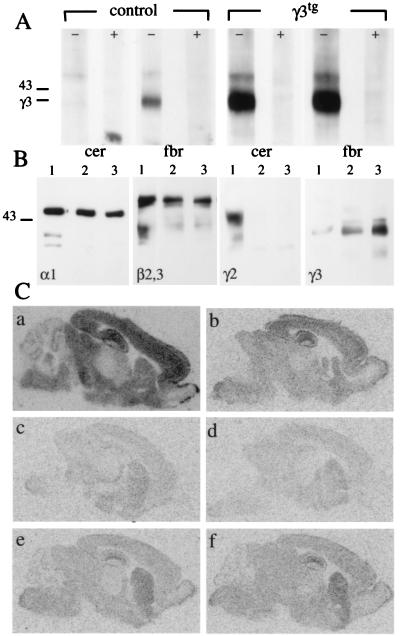
Figure 1.
Expression of γ3 subunit and reconstitution of BZ binding sites in γ3tg/γ20/0 brain. (A) Western blot of γ3 subunit in membranes from cerebellum (cer) and forebrain (fbr) of adult γ2+/+ (control) and γ3tg/γ2+/+ (γ3tg) mice using a γ3 subunit-specific antiserum. Lanes marked with + indicate competition by antigenic peptide (10 μg/ml). Note the low level of γ3 subunit expression in forebrain and the lack of a signal in wt cerebellum. In contrast, the γ3 subunit is abundant in both parts of transgenic brain. The molecular mass (in kDa) of a standard and the position of the γ3 subunit are indicated. (B) Western blot of GABAA receptors immunoprecipitated from brain membranes with an α1 subunit antiserum. Lanes from γ2+/+ (1), γ20/0 (2), and γ3tg/γ20/0 (3) animals were labeled with the Abs indicated. Note the graded up-regulation of the γ3 subunit in γ20/0 and γ3tg/γ20/0 mice. (C) Distribution of total and zolpidem-insensitive BZ binding sites in γ2+/+ (a and b), γ20/0 (c and d), and γ3tg/γ20/0 brain (e and f) as seen by autoradiography with [3H]flumazenil (a, c, and e) or with [3H]flumazenil and 10 μM zolpidem (b, d, and f). Note that nearly all BZ binding sites remaining in γ20/0 mice are zolpidem-insensitive (d), suggesting that they represent GABAA receptors containing the γ3 subunit. In γ3tg/γ20/0 mice, there is a marked increase in BZ binding sites that are likewise zolpidem-insensitive (e and f).
To test whether ectopic γ3 subunit would compensate for loss of function in γ2 subunit-deficient mice, γ3tg mice were crossed with γ20/+ mice (8) and the γ3tg/γ20/+ offspring were subsequently crossed with γ20/+ mice to obtain γ3-transgenic offspring lacking the γ2 subunit (γ3tg/γ20/0). Similar to the γ20/0 phenotype (8), γ3tg/γ20/0 mice displayed perinatal or postnatal lethality with no survivors beyond the third postnatal week, indicating that the γ3 subunit could not fully substitute for the γ2 subunit. The few γ20/0 mice (γ3tg and nontransgenic) that survived to P14 were used for analysis. The γ3tg mice also displayed a phenotype in the γ2+/+ and γ20/+ backgrounds, with a significant number of animals remaining smaller than their nontransgenic littermates [20 of 143 γ3tg/γ20/+ and γ3tg/γ2+/+ mice compared with 3 of 214 γ2+/+ and γ20/+; χ2 (1) = 20.48, P < 0.001]. In addition, fertility was reduced, and spontaneous or handling-induced seizures were sometimes observed in γ3tg/γ20/+ mice, and more of these mice than γ20/+ mice died unexpectedly (18.3% of γ3tg/γ20/+ compared with <1% in γ20/+ mice). Thus, ectopic γ3 subunit expression in both γ20/+ and γ2+/+ mice resulted in a functional deficit indicative of a dominant-negative effect.
Restoration of BZ Binding Sites in γ3-Transgenic Mice.
GABAA receptors containing the γ3 subunit are characterized by zolpidem-insensitive [3H]flumazenil binding sites, similar to receptors containing the α5 subunit in conjunction with the γ2 subunit (11, 22). In γ20/0 and γ3tg/γ20/0 brains, the α5βγ2 receptors are missing, and zolpidem-insensitive flumazenil sites are indicative of γ3 subunit-containing GABAA receptors. To determine whether the γ3 subunit can substitute for the γ2 subunit in vivo, the total number of BZ sites was analyzed by [3H]flumazenil autoradiography of P14 brain sections (Fig. 1C) and quantified in hippocampus. Compared with wild type (wt), the number of [3H]flumazenil binding sites in γ20/0 hippocampus was markedly reduced to 8.4 ± 1.6% (n = 3) in agreement with previous observations (8). The large majority of the γ20/0 [3H]flumazenil sites (73.8%, corresponding to 6.2 ± 1.8% of total wt BZ sites) were insensitive to competition with zolpidem, suggesting that they represented receptors containing the endogenous γ3 subunit (Fig. 1C). In hippocampus of γ3tg/γ20/0 mice, [3H]flumazenil binding was restored to 31.9 ± 2.3% of the wt level, and nearly all of these sites were insensitive to zolpidem (31.5 ± 3.1% of wt level). A 5- to 10-fold increase in γ3 subunit steady-state levels in γ3tg/γ20/0 compared with γ20/0 brain resulted in a corresponding 5-fold increase in hippocampal BZ sites. Thus, the transgene-encoded γ3 subunit assembles with endogenous α and β subunits similarly to endogenous γ3 subunit and restores a substantial proportion of BZ site-containing GABAA receptors. These display the zolpidem-insensitive pharmacology characteristic of γ3 subunit-containing receptors.
A similar analysis was carried out on cerebellum. Loss of the γ2 subunit resulted in complete loss of [3H]flumazenil sites in this location (Fig. 1C) as expected, because the cerebellum is normally devoid of γ1 and γ3 subunits. In contrast, BZ binding sites were clearly detectable in the cerebellum of γ3tg/γ20/0 mice, albeit at a significantly lower level than in wt cerebellum. Thus, the transgene-encoded γ3 subunit can contribute to GABAA receptors in brain regions where it does not naturally occur.
The γ3 Subunit Directs Functional GABAA Receptors to Postsynaptic Sites.
To determine whether the increased number of BZ binding sites in γ3tg/γ20/0 mice was reflected electrophysiologically, we first analyzed the effect of flunitrazepam on GABA-evoked responses in recordings from cultured cortical neurons (14–21 days in vitro). In γ20/0 neurons, the GABA-gated currents were unaffected by flunitrazepam in most cases, or very slightly potentiated, as expected from the loss of most BZ sites (Fig. 2A). In striking contrast, γ3tg/γ20/0 neurons displayed a potentiation by flunitrazepam similar to that observed in wt. Thus, the transgene-encoded γ3 subunit contributes to the formation of BZ-sensitive GABAA receptors in γ2 subunit-deficient neurons.
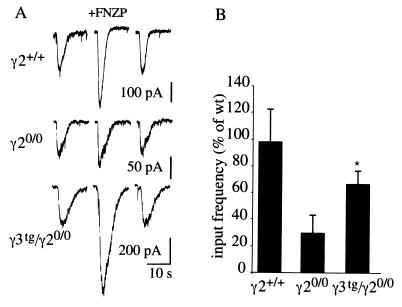
Figure 2.
Restoration of functional BZ-modulated and synaptic GABAA receptors in cultured cortical γ3tg/γ20/0 neurons. (A) Representative recordings illustrating the effect of flunitrazepam (1 μM) on GABA-evoked currents (GABA 1 μM, 2 s) recorded from γ2+/+, γ20/0, and γ3tg/γ20/0 cortical neurons (18 days in vitro). (B) Relative frequency of GABAergic mIPSCs in cortical neurons cultured from γ2+/+, γ20/0, and γ3tg/γ20/0 embryos. GABAergic mIPSCs were identified based on current decay kinetics and pharmacological sensitivity (9). The input frequency was reduced to 33 ± 13.1% of γ2+/+ in γ20/0 neurons and restored to 67 ± 8.9% by overexpression of the γ3 subunit in γ3tg/γ20/0 neurons. Error bars, standard error.
Postsynaptic localization of GABAA receptors in cultured cortical neurons requires the γ2 subunit (9). Consequently, the input frequency of GABAergic mIPSCs in γ20/0 neurons was reduced to 33 ± 13.1% (n = 19) of wt (Fig. 2B), as described (9). In γ3tg/γ20/0 neurons, the input frequency was restored to 67 ± 8.9% (n = 21) of the wt level (Fig. 2B). Thus, in the absence of the γ2 subunit, ectopic γ3 subunit not only assembles with endogenous α and β subunits but also contributes to the formation of a major population of functional postsynaptic GABAA receptors.
Ectopic γ3 Subunit Promotes Synaptic Clustering of α1 and α2 Subunit-Containing GABAA Receptors in Vivo.
A major fraction of hippocampal GABAA receptors is characterized by the presence of the α2 subunit. These receptors are clustered at gephyrin-rich, presumably postsynaptic sites, as indicated by the punctate and colocalized immunoreactivity (IR) for the α2 subunit and gephyrin in the CA1 region (Fig. 3 a and d) (9, 23). In the absence of the γ2 subunit, punctate staining for the α2 subunit and gephyrin was drastically reduced, indicating a major loss of α2 subunit-containing GABAA receptors and gephyrin at postsynaptic sites (Fig. 3 b and e) (9). Little staining was detectable for the endogenous γ3 subunit. Upon overexpression of the γ3 subunit, a substantial recovery of punctate α2 subunit IR was observed in γ3tg/γ20/0 littermates. Moreover, α2 subunit IR colocalized with punctate γ3 subunit staining (Fig. 3c), indicating that the two subunits were located in the same synapse, and perhaps in the same GABAA receptor complex. This pattern was seen in all animals analyzed (n = 4–6 per genotype). Although the γ3 subunit was expressed under control of a ubiquitously active promoter, no γ3 subunit IR was detected in nonneural cells, which is consistent with the notion that the γ3 subunit requires α and β subunits for assembly into stable complexes. The number of α2 subunit clusters in γ3tg/γ20/0 hippocampus (542 ± 18.1 clusters per 104 μm2) was as high as in wt (483.7 ± 22.3), and the average size of α2 subunit clusters [0.198 μm2 ± 0.104 (wt) and 0.194 ± 0.115 (γ3tg/γ20/0)] was also restored. Interestingly, γ3 subunit-containing receptors were colocalized with punctate IR for gephyrin (Fig. 3f), similar to γ2 subunit-containing receptors in wt hippocampus. Thus, in γ3tg/γ20/0 hippocampus, the γ3 subunit substitutes for the γ2 subunit in the formation of clustered, presumably postsynaptic receptors. The two types of receptors are indistinguishable with regard to their colocalization with gephyrin clusters.
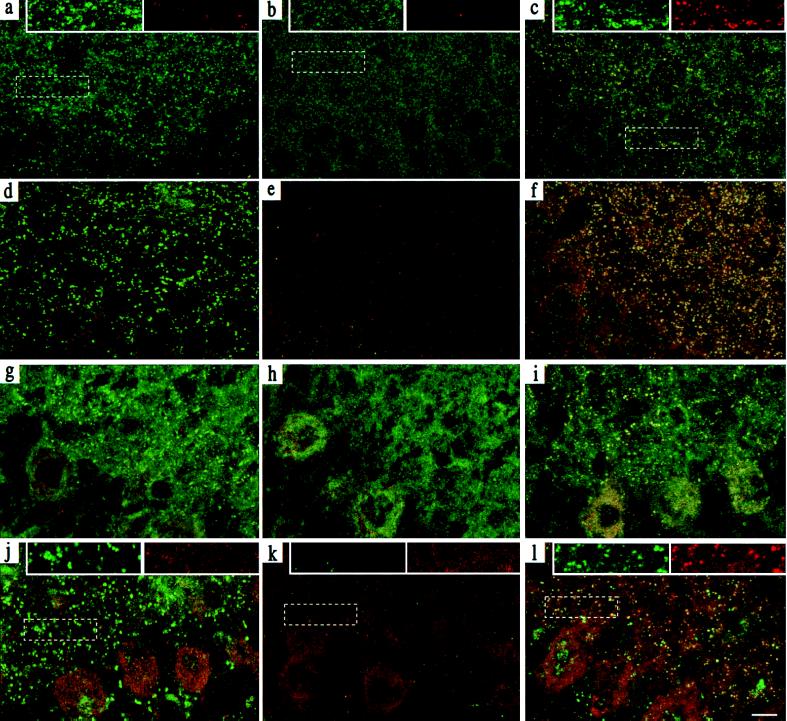
Figure 3.
Clustering of γ3 subunit-containing receptors and gephyrin in γ3tg/γ20/0 hippocampus and cerebellum. Parasagittal sections through the CA1 region of P14 hippocampus (a–f) (n = 4–6 per genotype) were stained with Abs specific for the α2 and γ3 subunits (a–c; α2 green, γ3 red) and for gephyrin and the γ3 subunit (d–f; gephyrin green, γ3 red) and visualized by confocal microscopy. The images were digitally superimposed to illustrate the presence or absence of colocalization between the markers used (yellow puncta). The punctate α2 (a) and gephyrin (d) staining, which is not colocalized with the scarce γ3 subunit IR in wt mice, was completely lost in γ20/0 mice (b and e) and largely restored in γ3tg/γ20/0 mice (c and f). In the latter, it was extensively colocalized with the γ3 subunit, as shown in yellow. Parasagittal sections through the molecular layer of P14 cerebellum (g–l) were stained for the α1 (green) and γ3 subunit (red; g–i) and for gephyrin (green) and the γ3 subunit (red; j–l). The punctate α1 subunit (g) and gephyrin (j) staining in wt brain was completely lost in γ20/0 (h and k) and largely restored in γ3tg/γ20/0 brain (i and l). Whereas specific γ3 subunit IR was absent in wt and γ20/0 cerebellum, it was readily detected and extensively colocalized with the α1 subunit in γ3tg/γ20/0 cerebellum. The data were reproduced with four to six animals per genotype. Insets at the top of panels a–c and j–l show enlargements of the boxed areas to illustrate the colocalization of clustered GABAA receptor and gephyrin IR in color-separated images. (Scale bar, 10 μm.)
A similar analysis was conducted in the molecular layer of the cerebellum, which is largely devoid of α2–6, γ1, γ3, ɛ, and δ subunits, so that virtually all GABAA receptors conform to the α1βγ2 subunit composition (20, 24, 25). Upon double immunofluorescence staining for the α1 and γ3 subunit in wt mice, strong punctate α1 subunit IR was seen in the molecular layer of the cerebellum, probably representing postsynaptic receptors on dendrites of Purkinje, stellate, and basket cells (Fig. 3g). In addition, diffuse staining of the neuropil was evident, indicating extrasynaptic receptors containing the α1 subunit. No γ3 subunit IR was detected in the cerebellum of nontransgenic mice (Fig. 3 g, h, j, and k). In corresponding sections from γ20/0 littermates, the punctate α1 subunit staining was lost entirely, whereas the diffuse α1 subunit staining appeared unaffected, as described (9). Finally, in sections from γ3tg/γ20/0 littermates, the punctate α1 staining was largely reestablished and was extensively colocalized with punctate IR for the γ3 subunit (Fig. 3i). Recovery of GABAA receptor clusters in the cerebellum was again paralleled by a corresponding gain in gephyrin clusters that were colocalized with the γ3 (Fig. 3 j–l) and α1 subunit IR (data not shown). However, the number of α1 subunit clusters in cerebellum was slightly reduced in γ3tg/γ20/0 compared with wt tissue (243.8 ± 97.8 in γ3tg/γ20/0 compared with 274 ± 9.8 clusters per 104 μm2 in γ2+/+), together with a reduction in the mean cluster size [0.28 ± 0.20 μm2 (γ3tg/γ20/0) and 0.35 ± 0.32 μm2 (wt)]. Thus, ectopically expressed γ3 subunit can substitute for the γ2 subunit in the formation of clustered α1 subunit-containing GABAA receptors in the cerebellar molecular layer, but the size and number of clusters do not reach wt levels.
GABAA Receptor Clustering Mediated by the Endogenous γ3 Subunit.
Detailed examination of the [3H]flumazenil autoradiograms revealed that the reticular thalamic nucleus (RTN) and the striatum of γ20/0 P14 brain displayed increased levels of zolpidem-insensitive BZ binding sites compared with the surrounding tissue (Fig. 1B). This indicated that loss of BZ sites was not uniform across the brain, possibly because of higher than average levels of endogenous γ3 subunit in some brain regions. In RTN of P14 γ20/0 brain, staining for the γ3 subunit revealed prominent punctate IR, indicative of synaptically clustered receptors. Moreover, γ3 subunit staining was colocalized with the α3 subunit (Fig. 4a), the most abundant α subunit in the RTN (20), and with gephyrin (Fig. 4b). Thus, in γ20/0 RTN, the endogenous γ3 subunit appears to contribute to postsynaptic GABAA receptors containing the α3 subunit, thereby preventing the loss of GABAA receptor clusters.
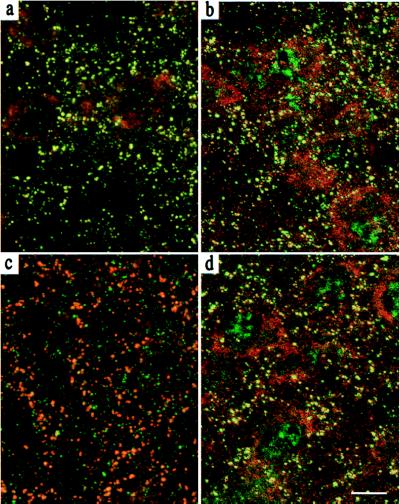
Figure 4.
Clustering of GABAA receptors mediated by the endogenous γ3 subunit. The γ3 subunit in the RTN promotes gephyrin and GABAA receptor α3 subunit clustering in γ20/0 mice. Parasagittal sections through the RTN of P14 mice (n = 4–6 per genotype) were double stained for the α3 and γ3 subunit (a and c; α3 red, γ3 green) or the α3 subunit and gephyrin (b and d; α3 red, gephyrin green) and visualized by confocal microscopy. Yellow puncta in the superimposed red and green images illustrate colocalization of the markers used. In γ20/0 RTN, the α3 subunit IR was colocalized with strong IR for the γ3 subunit (a), as well as for gephyrin (b), whereas in γ2+/+ RTN, there was only a partial colocalization of the α3 subunit IR and the weaker γ3 subunit IR (c), but a prominent colocalization of the gephyrin and α3 subunit IR (d). (Scale bar, 10 μm.)
In the wt RTN, the α3 subunit and gephyrin IR were also colocalized (Fig. 4d). However, the γ3 subunit IR was distinctly weaker than in γ20/0 RTN, forming smaller puncta that were only partially colocalized with the α3 subunit (Fig. 4c). These findings suggest that the γ3 subunit contributes to only a fraction of postsynaptic GABAA receptors in wt RTN, the majority presumably being clustered by the γ2 subunit. Thus, GABAA receptor clustering can be mediated by both the endogenous and the ectopic γ3 subunit, a property best seen in the absence of the γ2 subunit.
Discussion
The γ2 subunit and gephyrin are essential for postsynaptic localization of major γ2 subunit-containing GABAA receptor subtypes. We now report that the γ3 subunit is functionally equivalent to the γ2 subunit in its contribution to postsynaptic localization and function of γ3 subunit-containing GABAA receptors in vivo and for recruitment of gephyrin to GABAergic synapses. In transgenic mice, these receptors were formed and clustered, even in brain regions devoid of endogenous γ3 subunit, indicating that all the factors needed for clustering of γ3 subunit-containing receptors are present and can be recruited in these neurons.
In γ20/0 mice, the γ3 subunit-containing receptor appeared up-regulated (Fig. 1B). A partial compensation for the lack of γ2 subunit was even observed in the RTN, where the endogenous γ3 subunit contributed to postsynaptic clusters containing the α3 subunit and gephyrin. Because γ3 subunit-containing clusters were much fewer in wt RTN, these data suggest that the γ3 subunit is unable to displace the γ2 subunit during formation of synaptically localized receptors. This might be due, in part, to structural differences or to a lower level of expression of the γ3 subunit compared with the γ2 subunit in wt RTN.
Despite the apparent functional similarity between the γ2 and γ3 subunits for the formation of postsynaptic receptors, the overexpressed γ3 subunit did not rescue γ20/0 mice from a lethal phenotype. Crossing of transgenic lines overexpressing the γ2 subunit under control of the same ubiquitous promoter used here for the γ3 transgene allowed full rescue of mice without an overt behavioral phenotype (K.B., C.E., S. Balsiger, M. J. Wick, R. A. Harris, J.M.F., and B.L., unpublished work). This indicates that the level of transgene expression with this promoter can be sufficient to fully restore GABAA receptor function and that ectopic expression of a γ subunit is not in itself toxic. However, the ectopic expression of γ3 subunit appeared to exert a dominant-negative effect, as seen in some γ2+/0 and γ2+/+ transgenic animals.
The phenotype of γ3tg/γ20/0 mice cannot be attributed simply to inefficient assembly of the γ3 subunit with endogenous α and β subunits. In line with previous findings on GABAA receptors immunoprecipitated from brain extracts (13, 14), the γ3 subunit was found to assemble with α and β subunits (Fig. 1B). In the hippocampus, a 5- to 10-fold increase in γ3 subunit expression resulted in a roughly 5-fold increase in BZ binding sites. Furthermore, γ3 subunit-containing receptors show a unitary channel conductance similar to that of γ2 subunit-containing receptors (11). However, synaptic GABAA receptor and gephyrin clusters appeared fully restored in hippocampus but not in cerebellum. Incomplete restoration of synaptic receptors might in part be because of insufficient expression of the γ3 subunit in the cerebellum and possibly other brain regions, possibly contributing to the lack of rescue of γ20/0 mice by the γ3 transgene.
Mechanisms of GABAA Receptor Clustering.
The clustering of glycine receptors at postsynaptic sites requires both receptor activation and interaction with gephyrin (2, 26, 27). It is still unknown, however, whether this is also the case for GABAA receptors (9, 28), or whether protein interactions, involving at least the γ2 or γ3 subunit and gephyrin, are sufficient for clustering at postsynaptic sites. In the absence of the γ2 subunit, GABAA receptors are characterized by a markedly reduced channel conductance (8), which might also account for the lack of clustering. The γ3 subunit can substitute for the γ2 subunit with respect to the conductance of the corresponding GABAA receptors (11). Thus, if receptor activation were required for clustering of GABAA receptors, then it would be the gain in conductance conferred by the presence of the γ3 subunit that would trigger clustering of γ3 subunit-containing GABAA receptors.
Independent of the outcome of further experiments addressing this issue, the γ3 subunit-containing GABAA receptors were colocalized with gephyrin in γ3tg/γ20/0 mice in a manner very similar to the γ2 subunit-containing receptors in wt mice. Therefore, if protein interactions are important for this process, it is very likely that similar domains and factors are involved for both subunits. As the γ2 and γ3 subunits exhibit considerable heterogeneity in their intracellular cytoplasmic loop, it is remarkable that the gephyrin-clustering capability is also present in the γ3 subunit. At glycinergic synapses, gephyrin is thought to mediate clustering of glycine receptors by direct protein–protein interaction with the receptor β subunit. Although gephyrin is also required for postsynaptic localization of GABAA receptors (9), there is so far no evidence for a direct interaction between gephyrin and any GABAA receptor subunit. A candidate protein (GABARAP) that interacts directly with the γ2 subunit and might mediate interaction between the γ2 subunit and gephyrin has recently been identified (29). Interestingly, the portion of the putative γ2 subunit cytoplasmic loop that interacts with GABARAP (residues 394–411) is highly conserved between all γ subunits, indicating that GABARAP might also interact with γ3 subunit-containing receptors (29). These residues might be crucial for protein interactions underlying the clustering of GABAA receptors at postsynaptic sites.
Acknowledgments
We thank S. Balsiger for expert technical assistance, T. Bächi and M. Höchli for generous help with confocal laser microscopy, and H. Mohler for helpful discussions.
Abbreviations
- GABA
γ-aminobutyric acid
- GABAA
GABA type A
- BZ
benzodiazepine
- IR
immunoreactivity
- mIPSCs
miniature inhibitory postsynaptic currents
- Pn
postnatal day n
- RTN
reticular thalamic nucleus
- wt
wild type
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Colledge M, Froehner S C. Proc Natl Acad Sci USA. 1998;95:3341–3343. doi: 10.1073/pnas.95.7.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirsch J, Meyer G, Betz H. Mol Cell Neurosci. 1996;8:93–98. doi: 10.1006/mcne.1996.0048. [DOI] [PubMed] [Google Scholar]
- 3.Mohler H, Fritschy J-M, Lüscher B, Rudolph U, Benson J, Benke D. In: Ion Channels. Narahashi T, editor. Vol. 4. New York: Plenum; 1996. pp. 89–113. [PubMed] [Google Scholar]
- 4.Sieghart W. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 5.Macdonald R L, Olsen R W. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 6.Nusser Z, Sieghart W, Benke D, Fritschy J-M, Somogyi P. Proc Natl Acad Sci USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fritschy J-M, Weinmann O, Wenzel A, Benke D. J Comp Neurol. 1998;390:194–210. [PubMed] [Google Scholar]
- 8.Günther U, Benson J, Benke D, Fritschy J-M, Reyes G, Knoflach F, Crestani F, Aguzzi A, Arigoni M, Lang Y, et al. Proc Natl Acad Sci USA. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Essrich C, Lorez M, Benson J, Fritschy J-M, Lüscher B. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 10.Brickley S G, Cull-Candy S G, Farrant M. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herb A, Wisden W, Lüddens H, Puia G, Vicini S, Seeburg P H. Proc Natl Acad Sci USA. 1992;89:1433–1437. doi: 10.1073/pnas.89.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knoflach F, Rhyner T, Villa M, Kellenberger S, Drescher U, Malherbe P, Sigel E, Mohler H. FEBS Lett. 1991;293:191–194. doi: 10.1016/0014-5793(91)81184-a. [DOI] [PubMed] [Google Scholar]
- 13.Benke D, Honer M, Michel C, Mohler H. Neuropharmacology. 1996;35:1413–1423. doi: 10.1016/s0028-3908(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 14.Tögel M, Mossier B, Fuchs K, Sieghart W. J Biol Chem. 1994;269:12993–12998. [PubMed] [Google Scholar]
- 15.Quirk K, Gillard N P, Ragan I, Whiting P J, McKernan R M. J Biol Chem. 1994;269:16020–16028. [PubMed] [Google Scholar]
- 16.Wilson-Shaw D, Robinson M, Gambarana C, Siegel R E, Sikela J M. FEBS Lett. 1991;284:211–215. doi: 10.1016/0014-5793(91)80687-x. [DOI] [PubMed] [Google Scholar]
- 17.Culiat C T, Stubbs L J, Woychik R P, Russell L B, Johnson D K, Rinchik E M. Nat Genet. 1995;11:344–346. doi: 10.1038/ng1195-344. [DOI] [PubMed] [Google Scholar]
- 18.Hogan B L M, Constantini F, Lacy E. Manipulating the Mouse Embryos: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1986. [Google Scholar]
- 19.Olsen R W, McCabe R T, Wamsley J K. J Chem Neuroanat. 1990;3:59–76. [PubMed] [Google Scholar]
- 20.Fritschy J-M, Mohler H. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 21.Pfeiffer F, Simler R, Grenningloh G, Betz H. Proc Natl Acad Sci USA. 1984;81:7224–7227. doi: 10.1073/pnas.81.22.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lüddens H, Seeburg P H, Korpi E R. Mol Pharmacol. 1994;45:810–814. [PubMed] [Google Scholar]
- 23.Craig A M, Banker G, Chang W, McGrath M E, Serpinskaya A S. J Neurosci. 1996;16:3166–3177. doi: 10.1523/JNEUROSCI.16-10-03166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiting P J, McAllister G, Vassilatis D, Bonnert T P, Heavens R P, Smith D W, Hewson L, O’Donnell R, Rigby M R, Sirinathsinghji D J S, et al. J Neurosci. 1997;17:5027–5037. doi: 10.1523/JNEUROSCI.17-13-05027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laurie D J, Seeburg P H, Wisden W. J Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirsch J, Betz H. Nature (London) 1998;392:717–720. doi: 10.1038/33694. [DOI] [PubMed] [Google Scholar]
- 27.Levi S, Vannier C, Triller A. J Cell Sci. 1998;111:335–345. doi: 10.1242/jcs.111.3.335. [DOI] [PubMed] [Google Scholar]
- 28.Betz H. Nat Neurosci. 1998;1:541–543. doi: 10.1038/2777. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Bedford F K, Brandon N J, Moss S J, Olsen R W. Nature (London) 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]