Abstract
The MEK1 (MAP kinase/ERK kinase)/ERK (extracellular-signal-responsive kinase) pathway has been implicated in cell growth and differentiation [Seger, R. & Krebs, E. G. (1995) FASEB J. 9, 726–735]. Here we show that the MEK/ERK pathway is activated during focal cerebral ischemia and may play a role in inducing damage. Treatment of mice 30 min before ischemia with the MEK1-specific inhibitor PD98059 [Alessi, D. R., Cuenda, A., Cohen, P., Dudley, D. T. & Saltiel, A. R. (1995) J. Biol. Chem. 270, 27489–27494] reduces focal infarct volume at 22 hr after ischemia by 55% after transient occlusion of the middle cerebral artery. This is accompanied by a reduction in phospho-ERK1/2 immunohistochemical staining. MEK1 inhibition also results in reduced brain damage 72 hr after ischemia, with focal infarct volume reduced by 36%. This study indicates that the MEK1/ERK pathway contributes to brain injury during focal cerebral ischemia and that PD98059, a MEK1-specific antagonist, is a potent neuroprotective agent.
MEK1 is a dual-specificity kinase that phosphorylates extracellular-signal-responsive kinase (ERK)1/2 on threonine and tyrosine residues and activates these mitogen-activated protein (MAP) kinases (1). The ERK MAP kinases are phosphorylated in the hippocampus in response to global brain ischemia (2, 3). The use of general tyrosine kinase inhibitors, such as genistein, decreases ERK2 phosphorylation in this model and is associated with protection against neuronal cell damage (2). These data, however, do not establish a direct relationship between ERK2 phosphorylation and cell injury. Tyrosine kinase inhibitors, such as genistein, also inhibit protein kinase-independent systems, such as fatty acid synthesis, mitochondrial oxidative phosphorylation, and lactate transport (4). It has been shown that inhibition of MEK1 protects hippocampal neurons in a cell-culture model of seizure (5). In this study, we show that inhibition of the MEK1/ERK pathway leads to neuroprotection from brain injury resulting from occlusion of the middle cerebral artery (MCA).
Methods
Ischemia Model.
Adult male SV-129 mice (18–22 g, Taconic Farms, Germantown, NY) were housed under diurnal lighting conditions and allowed food and water ad libitum. Animals were anesthetized with 1.5% halothane and maintained in 1.0% halothane in 70% N2O and 30% O2 by using a Fluotec 3 vaporizer (Colonial Medical, Amherst, NH). Ischemia was induced with a 8.0 nylon monofilament coated with silicone resin/hardener mixture (Xantopren and Elastomer Activator, Bayer Dental, Osaka, Japan) as described previously (6).
Two microliters of PD98059, SB203580, or DMSO was injected intracerebroventricularly (bregma −0.9 mm lateral, −0.1 mm posterior, −3.1 mm deep) 30 min before ischemia by using a Hamilton injection syringe. The concentrations used are stated in Results.
Immunohistochemistry.
Mice were deeply anesthetized with an overdose of sodium pentobarbital (100 mg/kg i.p.) and then transcardially perfused with 0.9% saline solution, followed by 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), pH 7.4. The brains were quickly removed and stored in the same fresh buffer containing 20% sucrose. Brains were cut with a freezing microtome into coronal sections 40 μm thick. The sections were processed by the free-floating method as described previously (7).
Western Blot Analysis.
Postischemic and nonischemic brains were Dounce homogenized in 1 ml of potassium phosphate buffer [10 mM potassium phosphate, pH 7.05/1 mM EDTA/5 mM EGTA/10 mM magnesium chloride/50 mM β-glycerophosphate/1 mM sodium vanadate/1 mM dithiothreitol (DTT)/0.5% Nonidet P-40/0.1% Brij-35]. Lysates were clarified by centrifugation at 14,000 × g for 10 min. The protein concentration in the supernatant was determined by the Bradford assay (Bio-Rad). To test for phosphorylation of ERK1/2, 40 μg of total cell lysate was subjected to electrophoresis on an SDS/10% polyacrylamide gel and transferred onto Immobilon-P membrane (Millipore). Western blotting was performed with phospho-specific p44/42 MAP kinase antibodies (1:1000) (New England Biolabs). Proteins were detected by using enhanced chemiluminescence (ECL; Amersham). To analyze the protein levels of p42/44 within each lane, the blot was stripped and reprobed, as described by Amersham, using C-14 anti-ERK2 antibody (1:1000) (Santa Cruz Biotechnology).
Analysis of Brain Infarction.
After 22 hr of postischemic reperfusion, brains were removed and sliced into five coronal sections 2 mm thick by using a mouse brain matrix (RBM-2000C; Activational System, Warren, MI). Brain slices were treated with 2% 2,3,5-triphenyltetrazolium chloride (Sigma), followed by 10% formalin overnight as described previously (8). The infarcted areas, outlined in white, were measured on the posterior surface of each section by an image analysis system (MCID version 3, Imaging Research, St. Catherine’s, ON, Canada) and infarction volume was calculated by summing the infarction volume of sequential 2-mm-thick sections. For evaluation at 72 hr after reperfusion the mice were deeply anesthetized with an overdose of sodium pentobarbital and fixed with 30 ml of 10% formalin in 0.1 M PBS administered by intracardiac infusion. The brains were quickly removed and saturated with 20% sucrose. Fifty-micrometer-thick coronal sections were cut on a freezing microtome, and every 20th section from the frontal pole was mounted on a glass slide and stained with 0.05% thionin. A coverslip was applied with Permount (Fisher). The infarction areas were measured as described above and quantitated by summing the infarction areas of 6 sequential sections. The data were analyzed by Student’s t test or ANOVA.
Physiology.
In randomly selected animals (n = 5, 200 μM PD98059; n = 4, 0.4% DMSO), regional cerebral blood flow (rCBF) was measured with a laser-Doppler flow meter (PF2B, Perimed, Stockholm, Sweden) using a flexible 0.5-mm fiberoptic extension to the master probe. The tip of the probe was secured 2 mm posterior and 6 mm lateral to bregma on the ipsilateral hemisphere in the animals. Steady-state baseline values were recorded before MCA occlusion, and rCBF during and after occlusion was expressed as percentage of the baseline values. rCBF and arterial blood pressure were monitored by using a MacLab/8 data acquisition system (AD Instruments, Milford, MA) equipped with an ETH 400 transducer amplifier (AD Instruments, Milford, MA) by means of a femoral artery catheterized with PE-10 polyethylene tubing. Arterial blood samples (50 μl) were analyzed for pH, oxygen (pO2), and carbon dioxide (pCO2) by using a blood gas/pH analyzer (Corning 248, Ciba-Corning Diagnostics, Medford, MA). Core temperature was maintained at approximately 36.5°C during MCA occlusion with a thermoregulator (FHC; Brunswick, ME) and a heating pad (Watlow, St. Louis). Mice were kept in a warming chamber (Thermocare, Incline Village, NV) for 3 hr after reperfusion.
Results
Increase in phospho-ERK after focal cerebral ischemia.
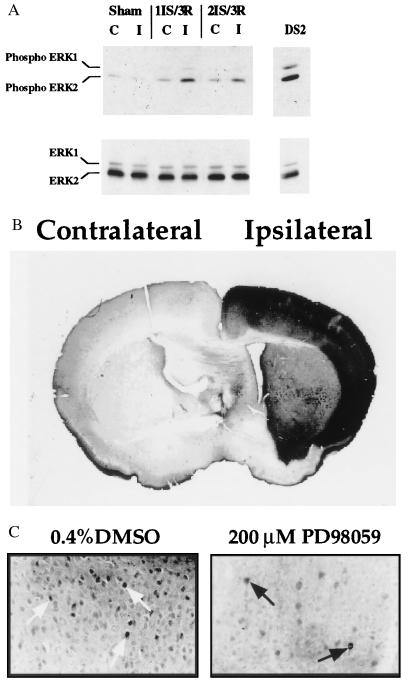
There is an increase in ERK1/2 phosphorylation after 1 or 2 hr of MCA occlusion followed by 3 min of reperfusion (Fig. 1A). After 1 hr of ischemia and 3 min of reperfusion an increase in phosphorylated ERK was detected in the nuclei of cortical cells within the MCA territory by immunohistochemistry using the phospho-specific ERK1/2 antibody (New England Biolabs) (Fig. 1B).
Figure 1.
(A) Increase in ERK1/2 phosphorylation after ischemia and reperfusion. Western blot analysis was performed on lysates (40 μg per lane) from the contralateral (C) and ipsilateral/ischemic (I) side of the brain after 1 hr of ischemia (1IS) followed by 3 min of reperfusion (3R) or 2 hr of ischemia (2IS) followed by 3 min of reperfusion (3R). Western analysis was performed with phospho-specific ERK1/2 antibodies (New England Biolabs) (1:1000 dilution) (Upper). The blot was stripped and reprobed with C-14 ERK2-specific antibodies (Santa Cruz Biotechnology) (1:1000 dilution) (Lower). DS2 total cell lysate (40 μg) was used as a control for ERK1/2 phosphorylation. DS2 is a clonal cell line that exhibits constitutively active ERK1/2 (23). (B) Increased ERK1/2 phosphorylation in the cortical region of the brain after 1 hr of focal cerebral ischemia and 3 min of reperfusion. Immunohistochemistry of brain sections (40 μm thick) was performed with phospho-specific ERK1/2 antibodies. Contralateral (nonischemic) and ipsilateral (ischemic) sides of the brain are indicated. (×100.) (C) Pretreatment of mice with PD98059 leads to decreased ERK1/2 phosphorylation in the cortical region of the brain after 2 hr of focal cerebral ischemia and 3 min of reperfusion. Immunohistochemistry of brain sections (40 μm thick) was performed with the phospho-specific ERK1/2 antibodies. Nuclear staining is indicated by the arrows. (×400.) Dunnett’s posthoc tests. (C) PD98059 neuroprotection is maintained 3 days after ischemia. Brain sections were analyzed as described in the text. Numbers reflect the values from seven mice per treatment; ∗, P < 0.05. The data were analyzed by Student’s t test. Note: The number above each bar reflects the mean infarct volume as a percentage of the contralateral hemisphere to correct for edema, and was calculated using the following formula: (contralateral volume − ipsilateral undamaged volume) × 100/contralateral volume (8). (D) Pretreatment with SB203580, an inhibitor of p38 MAP kinase, is not neuroprotective. Mice were pretreated with 2 μl of 100 μM SB203580 or 0.2% DMSO 30 min before 2 hr of focal cerebral ischemia followed by 22 hr of reperfusion. The numbers reflect the values from four mice per treatment.
PD98059, the MEK1/2 inhibitor, is neuroprotective, resulting in a decrease in infarct volume.
To evaluate whether ERK1/2 activation plays a direct role in brain injury, we used a specific inhibitor of MEK1 (9), PD98059. Two microliters of 200 μM PD98059 was injected into the lateral ventricle 30 min before the induction of ischemia. Pretreatment with PD98059 reduced phospho-ERK1/2 immunostaining in the cortex within the MCA territory after 2 hr of ischemia and 3 min of reperfusion (Fig. 1C).
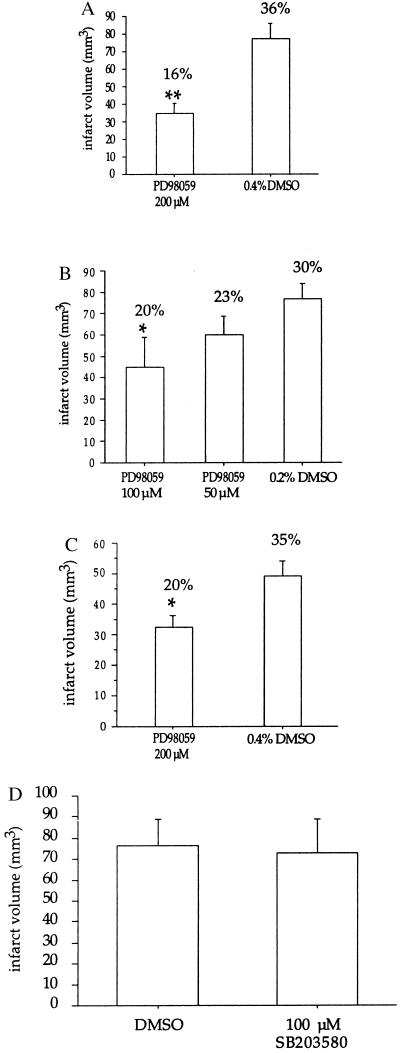
PD98059 attenuated infarct size by 55% (P < 0.01) in mice that were pretreated with 200 μM PD98059 as assessed by 2,3,5-triphenyltetrazolium chloride (TTC) staining (8) 22 hr after reperfusion (Fig. 2A). A decrease in infarct size was seen in both the cortical and subcortical regions, with a 59% reduction in damage (P < 0.004) within the cortex and 47% (P < 0.020) within the subcortex.
Figure 2.
Pretreatment with PD98059 is neuroprotective. (A) Mice were pretreated with 200 μM PD98059 or 0.4% DMSO 30 min before 2 hr of focal cerebral ischemia followed by 22 hr of reperfusion. Brains were removed and sliced into five coronal sections (2 mm thick). The sections were treated with 2% 2,3,5-triphenyltetrazolium chloride (Sigma), followed by 10% formalin overnight. The infarcted areas were measured by an image analysis system (MCID version 3) on the posterior surface of each section, and infarction volume was calculated by summing the infarction volume of sequential 2-mm-thick sections. Numbers reflect the values obtained from seven mice per treatment (±SEM); ∗∗, P < 0.01. The data were analyzed by Student’s t test. (B) Dose-dependent neuroprotection with PD98059. Mice were pretreated with 50 or 100 μM PD98059 or 0.2% DMSO 30 min before 2 hr of focal cerebral ischemia followed by 22 hr of reperfusion. Infarcted volume values were obtained as described for A. The numbers reflect the values from seven mice per treatment; ∗, P < 0.05. The data were analyzed by ANOVA followed by
Neuroprotection by PD98059 is dose dependent.
Neuroprotection was dose dependent, with a 42% (P < 0.05) decrease in infarct volume when 100 μM PD98059 was injected (Fig. 2B). The degree of protection is similar to that obtained previously when the immunosuppressive drug FK506 (10), the noncompetitive N-methyl-d-aspartate (NMDA) receptor agonist MK801 (11), or the caspase inhibitor ZVAD-FMK (12) was used . We measured the mean grain number per 250 μm × 250 μm square of the immunohistochemical staining in brain section layers 2–3 and subtracted from each value the background (i.e., staining with secondary antibody alone). We obtained the following values (±SEM): 100 ± 11 for DMSO (vehicle), 66 ± 10 for 100 μM, and 26 ± 14 for 200 μM drug (linear regression analysis of the dose response yielded r = 0.75, P = 0.0004). Thus, the immunostaining quantitation reveals a reduction in phospho-ERK staining that correlates with reduced infarct volume.
Neuroprotection was sustained for 3 days, accompanied by an attenuation of neurological deficits.
The neuroprotective effect was sustained for at least 3 days; in animals treated with 200 μM PD98059 there was a 36% decrease in infarct volume at 3 days after ischemia (P < 0.05) (Fig. 2C), accompanied by significantly attenuated neurological deficits (P < 0.05) (Table 1). Neurological deficits caused by ischemia were scored according to Huang et al. (6) (see Table 1). Neurological grading 3 days after 2 hr of MCA occlusion and reperfusion was 0.71 ± 0.29 (±SEM) and 2.2 ± 0.3 (P < 0.05) in PD98059-treated and vehicle-treated mice, respectively (Table 1). These data indicate that the inhibition of the MEK1/ERK pathway has long-term neuroprotective significance.
Table 1.
Neurological deficit score
| Treatment | No. with score
|
n | Mean score | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |||
| DMSO (0.4%) | 0 | 1 | 3 | 2 | 0 | 6 | 2.2 ± 0.3 |
| PD98059 (200 μM) | 3 | 3 | 1 | 0 | 0 | 7 | 0.7 ± 0.3* |
Neurological scoring was determined after 2 h of MCA occlusion and 72 h of reperfusion in mice treated with 200 μM PD98059 (n = 7) or vehicle (n = 6). The numeral abbreviations are as follows: 0, normal motor function; 1, flexion of contralateral torso and forelimb upon lifting of the animal by the tail; 2, circling to the contralateral side but normal posture at rest; 3, leaning to contralateral side at rest; and 4, no spontaneous motor activity. Mean scores are given ± SEM; *, P < 0.05. The data were analyzed by Mann–Whitney U test.
PD98059 does not alter respiratory and cardiovascular parameters, and body and head temperatures.
PD98059 does not affect respiratory and cardiovascular parameters, nor produce hypothermia. Ipsilateral rCBF during 2 hr of MCA occlusion was 15.9% ± 1.7% and 25.2% ± 5.7% of baseline levels for PD98059-treated and vehicle-treated animals, respectively. After 30 min of reperfusion, the rCBF values were 94.9% ± 8.9% and 102.5% ± 6.8% of baseline values for PD98059-treated and vehicle-treated animals, respectively (Table 2). Therefore, the decrease in ERK1/2 phosphorylation or protection against injury in the presence of PD98059 was not due to higher blood flow. Mean arterial pressure, partial oxygen (pO2) and carbon dioxide (pCO2) tensions, blood pH, and body temperature were unaffected by the presence or absence of 200 μM PD98059 (Table 2). Body temperature was unaltered up to 22 hr after reperfusion in the PD98059-treated animals compared with the vehicle controls, with values of 34.6 ± 0.8°C and 35.9 ± 0.4°C, respectively. In addition, temporal muscle temperature, reflective of head temperature, was unaltered up to 3 hr after reperfusion in the PD98059-treated animals, 35.5 ± 0.1°C versus 35.2 ± 0.3°C in vehicle-treated animals.
Table 2.
Respiratory and cardiovascular parameters
| Treatment | Ischemia | Temp., °C | MABP, mmHg | rCBF, % | pCO2, mmHg | pO2, mmHg | pH |
|---|---|---|---|---|---|---|---|
| DMSO (0.4%) | Before | 36.2 ± 0.1 | 80.7 ± 3.8 | 100 | ND | ND | ND |
| (n = 4) | During | 36.1 ± 0.1 | 82.3 ± 1.0 | 25.2 ± 5.7 | 48.5 ± 2.5 | 155.2 ± 6.9 | 7.32 ± 0.03 |
| 30 min after | 36.1 ± 0.1 | 82.8 ± 6.8 | 102.5 ± 6.8 | ND | ND | ND | |
| PD98059 (200 μM) | Before | 36.2 ± 0.1 | 86.4 ± 4.9 | 100 | ND | ND | ND |
| (n = 5) | During | 36.2 ± 0.1 | 81.8 ± 1.9 | 15.9 ± 1.7 | 49.9 ± 1.6 | 139.1 ± 9.7 | 7.32 ± 0.03 |
| 30 min after | 36.2 ± 0.2 | 86.0 ± 5.5 | 94.9 ± 8.9 | ND | ND | ND |
Results are mean ± SEM. MABP, mean arterial blood pressure; ND, not determined.
p38 and c-Jun (Ser-63) phosphorylation are not increased early in focal cerebral ischemia; SB203580 is not neuroprotective.
No increase in phosphorylated p38 MAP kinase or phosphorylated (at Ser-63) c-Jun, a substrate for SAPKs/JNKs (13), was observed by Western blot analysis and immunohistochemistry using phospho-specific p38 and phospho-specific c-Jun antibodies (New England Biolabs) (data not shown). There was no protection against brain injury afforded by SB203580, an inhibitor of p38 MAP kinase (1–2 μM), and SAPKs/JNKs (>10 μM) (13) (Fig. 2D). Two microliters of 100 μM SB203580 was injected into the lateral ventricle 30 min before ischemia.
Discussion
It has been demonstrated in some systems that the MEK/ERK pathway may have anti-apoptotic effects that oppose the pro-apoptotic effects associated with activation of the JNK and p38 MAP kinases (14). In this study we have shown that the activation of the MEK1/ERK pathway is important for ischemic brain injury. Phillis et al. (15) showed that inhibition of tyrosine phosphorylation by genistein during cerebral ischemia in rats attenuates the release of amino acid neurotransmitters, including aspartate, glutamate, and glycine. Excessive amounts of glutamate are excitotoxic, resulting in neuronal cell death (16). Since ERK1/2 are phosphorylated on tyrosine as well as on threonine, it was postulated that the release of excitatory amino acids was likely the result of ERK1/2 phosphorylation followed by activation of cytosolic phospholipase A2 (cPLA2) (15), a substrate for ERK1/2 (17). Consistent with this hypothesis, a reduction in the infarct volume after focal cerebral ischemia has been observed in cPLA2-null mice (18). ERK has also been shown to phosphorylate synapsin I, a major phosphoprotein found in nerve terminals (19, 20). Synapsin I allows for the maintenance of synaptic vesicle contact with actin filaments. Phosphorylation of this protein leads to the dissociation of the vesicles, resulting in neurotransmitter release. Therefore, it is possible that inhibition of the MEK/ERK pathway blocks synapsin I phosphorylation and prevents the release of excitotoxic amino acids, such as glutamate, thus explaining the protection from ischemia.
Inhibition of the ERK1/2 pathway after focal cerebral ischemia may also lead to transcriptional and/or translational stability of gene products such as c-fos, resulting in protection against damage. In our studies, immunohistochemical analysis revealed an enhanced c-fos signal in the cortex of PD98509treated animals compared with vehicle-treated control animals (data not shown). Enhanced neuronal c-fos expression has been associated with cell survival in brain ischemia models (7, 11). Fasting of mice before an ischemic insult results in a reduction in the infarct volume that correlates with more intense c-fos mRNA expression in the ischemic cortex (21). Treatment of embryonic rat hippocampal neurons with neuroprotective concentrations of cycloheximide, a protein synthesis inhibitor, leads to an increase in c-fos and bcl-2 mRNA and protein (22).
In conclusion, the inhibition of the MEK1/ERK pathway results in protection of the brain from ischemic injury, and it reveals a role for this pathway in the initial stages of stroke pathophysiology. MEK1 represents a previously unrecognized target for development of approaches for the treatment and/or prevention of brain injury resulting from stroke and/or perioperative ischemia during neurosurgical procedures.
Acknowledgments
We thank Drs. Thomas Force, Adam Sapirstein, and Raymond Erikson for their helpful suggestions regarding the manuscript. This work was supported in part by grants from the National Kidney Foundation of Rhode Island and Massachusetts and the American Heart Association, Massachusetts Affiliate, to A.A., Special Coordination Funds for Promoting Science and Technology, Japan, to S.N., MERIT R37–DK39773, to J.V.B., and National Institutes of Health Massachusetts General Hospital Interdepartmental Stroke Project Grant 2P50NS10828 to M.A.M. and J.V.B.
Abbreviations
- MAP
mitogen-activated protein
- ERK
extracellular-signal-responsive kinase
- MEK
MAP kinase/ERK kinase
- MCA
middle cerebral artery
- rCBF
regional cerebral blood flow
- SAPK/JNK
stress-activated protein kinase/c-Jun N-terminal kinase
References
- 1.Seger R, Krebs E G. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 2.Kindy M S. J Cereb Blood Flow Metab. 1993;13:372–377. doi: 10.1038/jcbfm.1993.50. [DOI] [PubMed] [Google Scholar]
- 3.Campos-Gonzalez R, Glenney J R., Jr Cell Regul. 1991;2:663–673. doi: 10.1091/mbc.2.8.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young S W, Poole R C, Hudson A T, Halestrap A P, Denton R M, Tavare J M. FEBS Lett. 1993;316:278–282. doi: 10.1016/0014-5793(93)81308-m. [DOI] [PubMed] [Google Scholar]
- 5.Murray B, Alessandrini A, Cole A J, Yee A G, Furshpan E J. Proc Natl Acad Sci USA. 1998;95:11975–11980. doi: 10.1073/pnas.95.20.11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Z, Huang P L, Panahian N, Dalkara T, Fishman M C, Moskowitz M A. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 7.Uemura Y, Kowall N W, Moskowitz M A. Brain Res. 1991;552:99–105. doi: 10.1016/0006-8993(91)90665-i. [DOI] [PubMed] [Google Scholar]
- 8.Bederson J B, Pitts L H, Germano S M, Nishimura M C, Davis R L, Bartkowski H M. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 9.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 10.Sharkey J, Butcher S P. Nature (London) 1994;371:336–339. doi: 10.1038/371336a0. [DOI] [PubMed] [Google Scholar]
- 11.Uemura Y, Kowall N W, Beal M F. Brain Res. 1991;542:343–347. doi: 10.1016/0006-8993(91)91589-s. [DOI] [PubMed] [Google Scholar]
- 12.Hara H, Friedlander R M, Gagliardini V, Ayata C, Fink K, Huang Z, Shimizu S M, Yuan J, Moskowitz M A. Proc Natl Acad Sci USA. 1997;94:2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen P. Trends Cell Biol. 1997;7:353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- 14.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 15.Phillis J W, Song D, O’Regan M H. Neurosci Lett. 1996;207:151–154. doi: 10.1016/0304-3940(96)12521-0. [DOI] [PubMed] [Google Scholar]
- 16.Mattson M P. Adv Exp Med Biol. 1990;268:211–220. doi: 10.1007/978-1-4684-5769-8_24. [DOI] [PubMed] [Google Scholar]
- 17.Nemenoff R A, Winitz S, Qian N X, Van P V, Johnson G L, Heasley L E. J Biol Chem. 1993;268:1960–1964. [PubMed] [Google Scholar]
- 18.Bonventre J V, Huang Z, Taheri M R, O’Leary E, Li E, Moskowitz M A, Sapirstein A. Nature (London) 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 19.Matsubara M, Kusubata M, Ishiguro K, Uchida T, Titani K, Taniguchi H. J Biol Chem. 1996;271:21108–21113. doi: 10.1074/jbc.271.35.21108. [DOI] [PubMed] [Google Scholar]
- 20.Jovanovic J N, Benfenati F, Siow Y L, Sihra T S, Sanghera J S, Pelech S L, Greengard P, Czernik A J. Proc Natl Acad Sci USA. 1996;93:3679–3683. doi: 10.1073/pnas.93.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin L-L, Wartmann M, Lin A Y, Knopf J L, Seth A, Davis R J. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 22.Furukawa K, Estus S, Fu W, Mark R J, Mattson M P. J Cell Biol. 1997;136:1137–1149. doi: 10.1083/jcb.136.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alessandrini A, Greulich H, Huang W, Erikson R L. J Biol Chem. 1996;271:31612–31618. doi: 10.1074/jbc.271.49.31612. [DOI] [PubMed] [Google Scholar]