Abstract
Leishmania donovani is the etiologic agent of fatal visceral leishmaniasis in man. During their life cycle, Leishmania exist as flagellated promastigotes within the sandfly vector and as nonflagellated amastigotes in the macrophage phagolysosomal compartment of the mammalian host. The transformation from promastigotes to amastigotes is a critical step for the establishment of infection, and the molecular basis for this transformation is poorly understood. To define the molecular basis for amastigote survival in the mammalian host, we previously identified an amastigote stage-specific gene family termed “A2.” In the present study, we have inhibited the expression of A2 mRNA and A2 protein in amastigotes using antisense RNA and show that the resulting A2-deficient amastigotes are severely compromised with respect to virulence in mice. Amastigotes that did survive in the mice had restored A2 protein expression. These data demonstrate that A2 protein is required for L. donovani survival in a mammalian host, and this represents the first identified amastigote-specific virulence factor identified in Leishmania. This study also reveals that it is possible to study gene function in Leishmania through the expression of antisense RNA.
Leishmania donovani protozoa are the causative agents of human visceral leishmaniasis, which is a potentially fatal disseminating infectious disease that effects over 80 countries worldwide (1, 2). To define the molecular basis for amastigote survival in the mammalian host, we previously identified an amastigote stage-specific gene family termed “A2” whose corresponding mRNA and protein are abundant in amastigotes but are largely absent in promastigotes (3–5). There are at least seven members of the A2 gene family that encode a family of proteins ranging from 45 to 100 kDa and that are specific to the amastigote stage (5). A2 proteins are mainly comprised of a repetitive amino acid sequence; each repeat encodes a stretch of 10 amino acids (3, 5) that shares partial identity with the S antigens of the Plasmodium falciparum V1 strain, which is responsible for malaria in man (3). Like A2, the S antigens are stage-specific to the mammlian host and the function has yet to be defined.
To determine whether the A2 proteins are required for the survival of amastigotes in vitro in macrophages and in vivo in a model mammalian host, we undertook to develope A2-deficient L. donovani amastigotes. Although it is possible to block gene expression in Leishmania through creating null mutants by homologues recombination (6–8), we explored the use of antisense RNA to inhibit A2 gene expression and protein synthesis for the following reason. The A2 genes are present as a multigene family alternating with another gene, termed “A2rel,” that is constitutively expressed in promastigotes and amastigotes (4), making it difficult to specifically target the A2 genes without effecting A2rel genes. Although it is possible to make A2 gene knockouts, it would be difficult. Transfecting and selecting cells with an antisense-expressing plasmid construct is technically simpler, and the information gained through this approach may provide justification for subsequently developing the gene knockouts. Finally, if the antisense approach was successful, this would represent a significant advance in studying gene function in Leishmania.
From this study, we present data showing that it is possible to develop A2-deficient amastigotes using the antisense RNA approach. A2 antisense RNA containing amastigotes displayed an absence of both A2 mRNA and corresponding A2 proteins. The A2-deficient amastigotes were viable in culture and proliferated as well as control plasmid-containing amastigotes. A2-deficient amastigotes, however, displayed a reduced ability to multiply in cultured macrophages and were severely compromised with respect to survival in mice. Of interest, the A2-deficient amastigotes that did survive in mice had lost the ability to express A2 antisense RNA and consequently had restored the ability to express A2 protein. These data reveal that A2 can be considered an amastigote-specific virulence factor that is required for L. donovani survival in a mammalian host.
MATERIALS AND METHODS
Plasmid Construction.
The A2 antisense plasmid (pKSneoA2–1R) was constructed by reversing the 2.4-kb BamHI fragment containing a 1.6-kb A2–1 encoding sequence and partial A2 3′ untranslated region (UTR) sequence that was originally derived from the A2 sense plasmid (pKSneoA2–1), which has been described in detail (5).
Cell Culture and Transfection.
The L. donovani 1S/Cl2D cell line was kindly provided by Dennis Dwyer of the National Institutes of Health, Bethesda, MD. These cells can be cycled between promastigotes at 26°C and amastigotes at 37°C in vitro under defined conditions in which both promastigotes and amastigotes remain virulent. The details concerning the formulation of these culture procedures were previously described (9). The medium for promastigotes was 199 plus 10% fetal bovine serum (pH 6.8) and for amastigotes was RPMI 1640 medium plus 20% fetal bovine serum (pH 5.5). The A2 antisense plasmid was electroporated into L. donovani promastigotes, and transfected cells were selected with 20 μg/ml G418 as described (4, 5). After establishment of transfected promastigotes, these transfected cells were routinely cycled between 26°C promastigotes and 37°C amastigotes in the above described media containing 100 μg/ml G418 on a weekly basis.
Northern Blot Analysis.
Total RNAs were isolated from L. donovani promastigotes and in vitro amastigotes with TRIzol reagent (GIBCO). Northern blot analyses were performed as described (3) using 10 μg of total RNA. The hybridizations were carried out for 12 h at 42°C in a solution containing 1% SDS, 1M NaCl, 10% dextran sulfate, and 50 μg/ml salmon sperm DNA. The antisense oligonucleotides [GCGGG(A)CCA(G)ACG(A)GA] specific for A2 mRNA and the sense oligonucleotides [G(C)TCC(T)GTT(C)GGC(T)CCGC] specific for A2 antisense RNA were derived from the repeat region of A2 encoding sequence (3) and labeled with 32p by T4 polynucleotide kinase.
Western Blot Analysis.
Protein samples of L. donovani cells were prepared as described (5) and separated on 10% SDS/PAGE, transferred to nitrocellulose, and analyzed by immunoblotting for A2 proteins with anti-A2 mAb C9 (5). Western blot was performed with an enhanced chemiluminescence kit (Amersham) following the manufacture’s instruction except 10% skim dry milk was used for blocking the membrane and 5% skim dry milk was included in the primary antibody and the second antibody solutions.
Infection of Macrophages in Culture.
Mice bone marrow cell preparation and infection with L. donovani cells were performed as described (10) with some modification. In brief, macrophages were infected in suspension for 24 h at an amastigote-to-cell ratio of 20:1. Noningested amastigotes were removed by centrifugation and three washes in RPMI complete medium, and the infected cells were incubated at 37°C. Amastigote infection level and growth rate in macrophages were evaluated daily by cytospin and Giemsa staining.
Visceral Infection in Mice.
Female BALB/c mice (Charles River Breeding Laboratories) weighing 20–25 g were injected via the tail vein with 1.5 × 108 wild-type amastigotes or A2-deficient amastigotes or amastigotes containing the control vector; 4 weeks after infection, mice were examined for L. donovani parasite burden by counting the number of amastigotes in the Giemsa-stained imprints of the liver. Liver parasite burdens, expressed as Leishman–Donovan Units, were calculated as follows: number of amastigotes per 1000 cell nuclei × liver weight (g) (11). Amastigotes were recovered from the infected mice liver and spleen as described (12), and the recovered amastigotes were cultured in promastigote or amastigote culture medium described above either with or without 20 μg/ml G418. Cells were collected when they reached a density of 3 × 107 cells/ml and subjected to Western blot analysis.
RESULTS
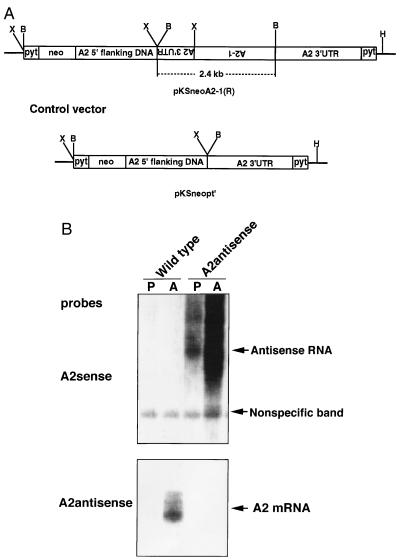
The plasmid construct developed to express A2 antisense RNA and the control plasmid are shown in Fig. 1A. This antisense RNA-expressing construct was designed to express a chimeric A2 antisense RNA (complementary to A2 coding region and part A2 3′ UTR sequence) flanked by A2 5′ UTR and 3′ UTR sequences. We previously have demonstrated that A2 3′ UTR was essential for A2 mRNA accumulation and stabilization in amastigotes (4). Thus, the construct was designed to have the chimeric A2 antisense RNA accumulate at higher levels in Leishmania amastigotes than promastigotes. Plasmids were electroporated into the promastigotes of the L. donovani 1S/CL2D strain, and the recombinant promastigotes were selected with G418 as a pooled population of resistant cells. Northen blot was then performed on RNA isolated from promastigotes and amastigote populations to identify antisense and sense A2 transcripts (Fig. 1B). As predicted, the A2 antisense plasmid-transfected cells contained higher levels of A2 antisense RNA in amastigotes than in promastigotes, and no antisense RNA was present in the wild-type cells (Fig. 1B, Upper). Although A2 mRNA was clearly abundant in the wild-type amastigotes, it was strikingly absent from the amastigotes expressing the A2 antisense transcripts (Fig. 1B, Lower). However, very low amounts of A2 mRNA could be detected when the film was overexposed (data not shown). These data demonstrate that the A2 antisense RNA either inhibited the expression of the A2 genes or impaired the processing and maturation of the A2 transcripts.
Figure 1.
(A) Structures of the A2 antisense RNA expressing vector (pKSneoA2–1 R) construct and the control vector. The reversed 2.4-kb sequence containing 1.6 kb of A2 encoding sequence and part of the 3′ UTR was flanked by a 1.6-kb A2 5′ noncoding upstream sequence and 1.7 kb of A2 3′ UTR. Pyt is a 92-bp synthetic pyrimidine tract that provides the trans-splicing site for the neor gene and insures the correct polyadenylylation of antisense transcripts at the end of A2 3′ UTR. The 2.4-kb BamHI fragment containing A2–1 encoding sequence was removed in the control vector (pKSneopt). Restriction enzyme sites are indicated: X, XbaI; B, BamHI; H, XhoI. (B) Northern blot analysis of RNA isolated from wild-type and pKSneoA2–1 R plasmid-transfected Leishmania cells. Total RNAs (10 μg per lane) isolated from promastigotes (P) or amastigotes (A) from wild-type or transfected cells were subjected to Northern blot analysis with a sense oligonucleotides specific for A2 antisense RNAs (Upper) or an antisense oligonucleotides specific for native A2 mRNAs (Lower). Note: A2 antisense RNAs were detected only in cells transfected with the A2 antisense-expressing plasmid and almost no native A2 mRNA was detected in the amastigotes containing the A2 antisense RNA. Higher levels of A2 antisense RNAs were present in amastigotes than in promastigotes because these transcripts contained the A2 3′ UTR.
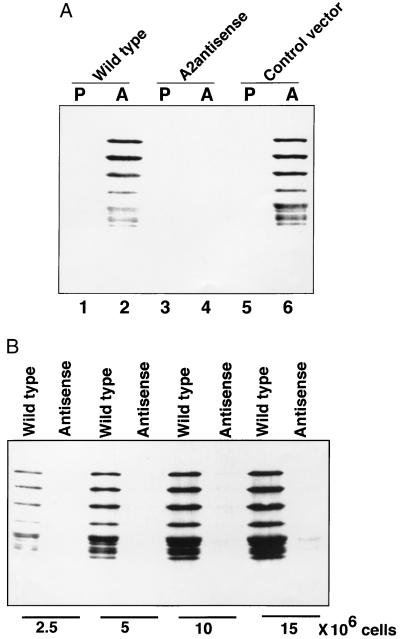
We next examined the level of A2 proteins in the different L. donovani cells by Western blot analysis using previously developed anti-A2 mAbs (5). As expected, the A2 protein family was present in wild-type and control vector transfected amastigotes but not in promastigotes (Fig. 2A, lanes 2 and 6, respectively), and this is consistent with our previous observation that A2 proteins are specific to amastigotes (5). However, the A2 proteins were absent in the amastigotes containing the A2 antisense RNA (Fig. 2A, lane 4), and this is consistent with the data showing that these cells contained dramatically reduced levels of A2 mRNA. To further examine the extent of A2 protein suppression in the A2 antisense RNA containing amastigotes, we compared the level of A2 protein in lysates from increasing amounts of wild-type amastigotes and amastigotes that contained the antisense RNA. As shown in Fig. 2B, in the antisense RNA containing amastigotes, a low level of A2 protein was detectable only in lysates from 15 × 106 cells. Densitometric analyses (NIH image, version 1.57) indicated that the A2 protein level was reduced by more than 94% in amastigotes containing the A2 antisense RNA compared with wild-type amastigotes. Taken together, these data demonstrated that A2 antisense RNA can efficiently block A2 mRNA and protein expression in L. donovani amastigotes.
Figure 2.
(A) Western blot analysis of the A2 amastigote-specific proteins in wild-type and transfected Leishmania cells; P, promastigotes; A, amastigotes. Equal amounts of total protein extracted from 5 × 106 cells were subjected to Western blot analysis with an anti-A2 mAb. Note: There is a dramatic reduction in the amount of A2 protein in the amastigotes containing the A2 antisense RNA (lane 4) whereas the wild-type amastigotes (lane 2) or amastigotes containing the control vector (lane 6) contain relatively high levels of A2. (B) A2 protein levels were compared in total proteins extracted from increasing numbers of wild-type amastigotes and amastigotes expressing the A2 antisense RNA as indicated.
With the development of A2-deficient amastigotes, we initiated studies to define the biological role of A2 protein. A2-deficient cells maintained under promastigote or amastigote culture conditions were indistinguishable from wild-type cells or cells containing the control plasmid with respect to viability and proliferation in culture (data not shown). This demonstrated that A2 protein is not required for proliferation or differentiation of L. donovani grown in culture. This also established that the A2 antisense RNA was not toxic to the L. donovani cells.
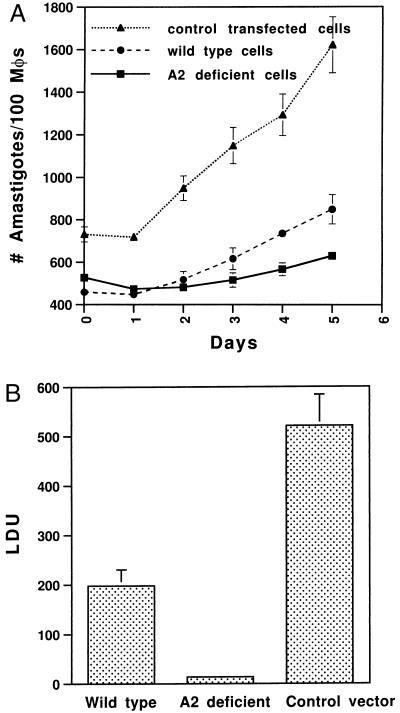
We next determined whether inhibition of A2 expression resulted in altered virulence in vitro in cultured macrophages. Bone marrow-derived macrophages from BALB/C mice were infected for 24 h with A2-deficient, control plasmid-containing, and wild-type amastigotes, and the growth rates of amastigotes within macrophages were subsequently monitored over a 5-day period by cytospin analysis. There was no difference in the ability to invade macrophages during the 24-h infection period. The percentage of infected macrophages for wild-type, A2-deficient, and control plasmid-containing amastigotes was 74%, 80%, and 86%, respectively, and the initial numbers of amastigotes within infected macrophages were similar. However, as shown in Fig. 3A, A2-deficient amastigotes grew slower in macrophages than wild-type cells and much slower than amastigotes containing the control plasmid. This revealed that A2-deficient amastigotes were compromised in their ability to proliferate in cultured macrophages.
Figure 3.
(A) The infection and growth rates of wild-type and transfected Leishmania amastigotes in macrophages in vitro. BALB/c mouse bone marrow-derived macrophages were infected at an amastigote-to-cell ratio of 20:1 for 24 h. Noningested amastigotes were removed by centrifugation. The infected cells were incubated at 37°C. Amastigote infection level and growth rate in macrophages were evaluated daily by cytospin and Giemsa staining. The data are the mean ± SEM (bar) of three independent experiments. (B) Liver parasite burdens of mice infected with wild-type and transfected Leishmania cells as indicated. Female BALB/c mice were injected with amastigotes via the tail vein (1.5 × 108 amastigotes/mouse, three mice per group). Four weeks after infection, mice were examined for hepatic parasite burdens by counting amastigotes from liver impression. Liver parasite burdens, expressed as Leishman–Donovan units (LDU), were calculated as follows: number of amastigotes per 1000 cell nuclei × liver weight (g). The means per group are shown, with the error bars representing SE. These results are from four separate experiments, each which gave very reproducible results. Similar results were obtained when mice were directly infected with wild-type and transfected promastigotes.
The most stringent assay for virulence is the ability of the amastigotes to establish an infection in vivo in an animal model. Therefore, BALB/C mice were infected with A2-deficient, wild-type, and control plasmid-containing amastigotes. Mice were infected via the tail vein (1.5 × 108 amastigotes/mouse, three mice per group), and, 4 weeks after infection, mice were examined for hepatic parasite burdens by counting amastigotes from liver impression. This experiment was repeated four times with consistent results. As shown in Fig. 3B, the parasite burden of mice infected with A2-deficient amastigotes was low compared with the parasite burdens of mice infected with wild-type or control plasmid-containing amastigotes. Similar results were obtained when mice were infected with wild-type or A2-deficient promastigotes (data not shown). It is unclear why amastigotes containing the control plasmid were more virulent than wild-type cells in mice and in cultured macrophages. We do not believe that this was caused by an homologous recombination event resulting in the overexpression of A2 from the plasmid episome because this would have resulted in overexpression of one, but not all, A2 protein species, and there is no evidence of this, as shown in Fig. 2A. The control plasmid-containing cells did, however, undergo a prior selection for the plasmid using G418, which the wild-type cells did not, and this may have somehow selected for a more virulent population. Nevertheless, it is clear that the presence of the plasmid itself did not reduce virulence in these amastigotes. Taken together, these data show that loss of A2 expression was concomitant with reduced virulence in vivo.
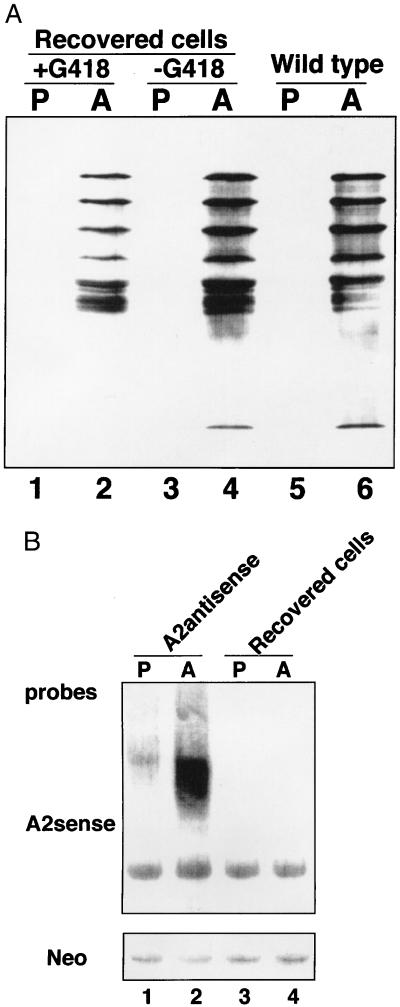
As revealed in Fig. 3B, there was a low but consistent level of infection with the A2-deficient amastigotes. It was therefore of interest to determine the status of the A2 protein in these amastigotes that had survived the 4-week period and had established infection in the liver of these mice. Amastigotes were isolated from the liver and spleen of mice that were originally infected with the A2-deficient amastigotes, and these were placed in promastigote or amastigote culture medium both in the presence and absence of G418. Only amastigotes retaining the transfected plasmid confering Neo resistance would have survived in the G418-containing media. Both promastigotes and amastigotes grew out equally well in the presence or absence of G418. When cells reached a density of 3 × 107 cells/ml, they were harvested, lysed, and subjected to Western blot analysis with the anti-A2 mAb. As shown in Fig. 4A, A2 protein expression was restored in the surviving amastigotes that were originally A2-deficient before infection. This demostrated that during the infection period, there was a selection for A2 protein-containing amastigotes and against A2-deficient amastigotes. Northern blot analysis was performed to determine whether the recovered amastigotes lost the ability to express the antisense RNA. As shown in Fig. 4B, the recovered amastigotes had lost the ability to express the A2 antisense RNA but did retain the ability to express the neo mRNA. Therefore, the surviving amastigotes retained the transfected plasmid but the plasmid selectively lost the ability to express the A2 antisense RNA. Taken together, these data demonstrate that the A2-deficient amastigotes were unable to survive in these mice whereas the amastigotes with restored A2 protein did survive. It is possible that the A2-deficient amastigotes were either unable to establish an infection in the mice or that they were unable to proliferate after infection. In either case, this establishes the A2 protein as an amastigote-specific virulence factor required for survival in the mouse model.
Figure 4.
(A) A2 protein levels are restored in amastigotes isolated from liver and spleen 4 weeks after mice were infected with A2-deficient amastigotes. The amastigotes isolated from infected mice were cultured in vitro in promastigote or amastigote culture medium with or without 20 μg/ml G418. The cells were collected after they reached a density of 3 × 107/ml and were subjected to Western Blot analysis with an anti-A2 mAb. P, promastigotes; A, amastigotes. For comparison, the levels of A2 proteins in wild-type promastigotes and amastigotes are also shown. (B) A2 antisense RNA is lost, but neo RNA is retained in amastigotes isolated from liver and spleen 4 weeks after mice were infected with amastigotes expressing both A2 antisense RNA and neo RNA. Cells were prepared as described above, and Northern blot analysis was performed using probes specific for A2 antisense RNA and neo RNA. P, promastigotes; A, amastigotes.
DISCUSSION
Previous studies (3–5) have shown that the expression of the L. donovani A2 transcripts and protein family are amastigote-specific. In the present study, we examined the role of the A2 protein family in amastigote survival in axenic culture, in infectivity and survival in macrophages cultures in vitro, and in infectivity and survival in BALB/c mice in vivo. The principal observations were that amastigotes deficient in A2 protein were compromised with respect to proliferation in cultured macrophages and were unable to survive in BALB/C mice. Of particular interest, amastigotes that were originally A2-deficient and did survive in BALB/C mice had regained the ability to express the A2 protein. This provides a compelling arguement that A2 protein is required for amastigotes to survive in a mammalian host and thus can be considered an amastigote-specific virulence factor. We have also revealed that antisense RNA can be successfully used to study gene function in Leishmania. Antisense RNA can therefore be considered as an additional genetic tool to study the biology of this importantant parasitic protozoan.
The amastigote-specific expression of A2 is suggestive of a role in the parasite’s survival in macrophages, and this hypothesis is supported by the in vitro and in vivo infection experiments reported within. It has been reported that nitrogen oxides (NO) are the major mediators of macrophage leishmanicidal activity (13). We know that A2-deficient amastigotes and wild-type amastigotes were equally capable of suppressing NO release in infected macrophages (data not shown). This suggests that A2 is not involved in altering host macrophage function, and this is consistent with our previous observation that A2 is located predominantly in the cytoplasm of amastigotes (5). A2 represents a multiprotein family containing repeated subunits, so we are pursuing the possiblility that A2 proteins form a complex, which may have a structural role in the amastigote.
We show that antisense RNA can be used in place of gene targeting to examine gene function in Leishmania. Because of its simplicity and effectiveness, the antisense RNA approach could be widely applied to examine the function of a variety of genes in Leishmania. This could be particularly useful in situations like that of the A2 gene, which is part of a multigene family. Nevertheless, our data also reveal that antisense RNA cannot replace gene knockouts in situations in which a stable phenotype is needed, such as in the development of live, attenuated vaccine strains. For example, it has been shown that gene replacement in the dihydrofolate reductase locus has provided an attenuated strain of L. major, which can induce protection against wild-type L. major in mice (14). Targeting the cysteine protease genes in L. mexicana resulted in parasites retaining the ability to produce s.c. lesions in mice but at a slower rate than the wild-type parasites (15). Based on the data obtained in this study, there is clear justification for targeting the A2 gene in L. donovani, and we are currently undertaking this. With regard to a live, attenuated vaccine strain, it may be advisable to knockout several relavent genes, including the A2 gene, to ensure a stable but viable attenuated phenotype. We also have shown recently that L. donovani can be used to express high levels of recombinant proteins (16); therefore, live attenuated strains can be further engineered to express a variety of protective antigens or immunostimulatory cytokines.
In conclusion, the present study has established the use of antisense RNA to study gene function in Leishmania and has identified the amastigote- specific A2 protein as a virulence factor required for parasite survival in a mammlian host. A2 was not, however, required for parasite survival in axenic culture, and these observations therefore may have implications for the development of a live, attenuated vaccine strain of L. donovani.
Acknowledgments
We thank Dr. D. Dwyer of the National Institutes of Health for providing the L. donovani 1S/Cl2D cell line, E. Ghedin for useful advise on working with this cell line, and S. Buates for the preparation of mouse bone marrow-derived macrophages. This work was supported by the Medical Research Council of Canada. Research at the Institute of Parasitology is partially funded by the Fonds pour la formation de chercheurs et l’aide a la recherche du Quebec. G.M. holds an Medical Research Council of Canada Scientist Award.
ABBREVIATION
- UTR
untranslated region
References
- 1.Molyneux D, Killick-Kendrick R. In: The Leishmaniases in Biology and Medicine. Peters W, Killick-Kendrick R, editors. Vol. 1. London: Academic; 1987. pp. 121–176. [Google Scholar]
- 2.Schlein Y. Parasitol Today. 1993;9:255–258. doi: 10.1016/0169-4758(93)90070-v. [DOI] [PubMed] [Google Scholar]
- 3.Charest H, Matlashewski G. Mol Cell Biol. 1994;14:2975–2984. doi: 10.1128/mcb.14.5.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charest H, Zhang W-W, Matlashewski G. J Biol Chem. 1996;271:17081–17090. doi: 10.1074/jbc.271.29.17081. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W-W, Charest H, Ghedin E, Matlashewski G. Mol Biochem Parasitol. 1996;78:79–90. doi: 10.1016/s0166-6851(96)02612-6. [DOI] [PubMed] [Google Scholar]
- 6.Cruz A, Beverley S. Nature (London) 1990;348:171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- 7.Curotto de Lafaille M, Wirth D. J Biol Chem. 1992;267:23839–23846. [PubMed] [Google Scholar]
- 8.Cruz A, Coburn C M, Beverley S M. Proc Natl Acad Sci USA. 1991;88:7170–7174. doi: 10.1073/pnas.88.16.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle P, Engel J, Pimenta P, Pinto Da Silva P, Dwyer D. Exp Parasitol. 1991;73:326–334. doi: 10.1016/0014-4894(91)90104-5. [DOI] [PubMed] [Google Scholar]
- 10.Moore K J, Labrecque S, Matlashewski G. J Immunol. 1993;150:4457–4465. [PubMed] [Google Scholar]
- 11.Murray H, Oca M, Granger A, Schreiber R. J Clin Invest. 1989;83:1253–1259. doi: 10.1172/JCI114009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiner N. Infect Immunol. 1982;38:1223–1230. doi: 10.1128/iai.38.3.1223-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green S, Nacy C, Meltzer M. J Leuk Biol. 1991;50:93–103. doi: 10.1002/jlb.50.1.93. [DOI] [PubMed] [Google Scholar]
- 14.Titus R, Gueiros-Filho F, De Freitas L A, Beverley S. Proc Natl Acad Sci USA. 1995;92:10267–10271. doi: 10.1073/pnas.92.22.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mottram J, Souza A, Hutchison E, Carter R, Frame M, Coombs G. Proc Natl Acad Sci USA. 1996;93:6008–6013. doi: 10.1073/pnas.93.12.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W W, Charest H, Matlashewski G. Nucleic Acids Res. 1995;23:4073–4080. doi: 10.1093/nar/23.20.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]