Abstract
In the visual cortex, as elsewhere, N-methyl-d-aspartate receptors (NMDARs) play a critical role in triggering long-term, experience-dependent synaptic plasticity. Modifications of NMDAR subunit composition alter receptor function, and could have a large impact on the properties of synaptic plasticity. We have used immunoblot analysis to investigate the effects of age and visual experience on the expression of different NMDAR subunits in synaptoneurosomes prepared from rat visual cortices. NMDARs at birth are comprised of NR2B and NR1 subunits, and, over the first 5 postnatal weeks, there is a progressive inclusion of the NR2A subunit. Dark rearing from birth attenuates the developmental increase in NR2A. Levels of NR2A increase rapidly (in <2 hr) when dark-reared animals are exposed to light, and decrease gradually over the course of 3 to 4 days when animals are deprived of light. These data reveal that NMDAR subunit composition in the visual cortex is remarkably dynamic and bidirectionally regulated by sensory experience. We propose that NMDAR subunit regulation is a mechanism for experience-dependent modulation of synaptic plasticity in the visual cortex, and serves to maintain synaptic strength within an optimal dynamic range.
The characteristics of synaptic transmission mediated by N-methyl-d-aspartate receptors (NMDARs) in the visual cortex change over the course of postnatal development. As animals mature, the contribution of NMDARs to visually evoked responses decreases (1, 2), and the duration of NMDAR-mediated synaptic currents shortens (3, 4). The maturation of these properties occurs during the critical period for experience-dependent synaptic plasticity in the visual cortex (5–7). Moreover, the critical period (7–9) and these changes in NMDAR properties are both delayed by rearing animals in complete darkness (2, 3). Because NMDARs play a special role in experience-dependent synaptic plasticity in the visual cortex (10–12), it has been hypothesized that these changes in NMDAR-mediated responses might reflect the mechanisms that bring the critical period to a close (3).
An alternative hypothesis is that these changes in NMDARs are not responsible for ending experience-dependent synaptic plasticity, but instead alter the relationship between NMDAR activation, long-term synaptic potentiation (LTP), and long-term synaptic depression (LTD) during the critical period (13). In the visual cortex, as elsewhere, the long-term consequence of activating glutamate receptors depends on the amount of calcium admitted by postsynaptic NMDARs. Modest elevations in calcium can trigger LTD, whereas large increases trigger LTP (14, 15). According to one theory of synaptic plasticity (16), the LTD-LTP crossover point (called the modification threshold) should “slide,” depending on the history of average cortical activity. The larger (more prolonged) NMDAR currents in the visual cortices of dark-reared animals (3) would be expected to shift the modification threshold to favor LTP (13), and this prediction has now been confirmed experimentally (17).
Research over the past 5 years has suggested a possible molecular mechanism for the observed changes in NMDAR kinetics. NMDARs are heteromeric ion channels composed of NR1 and NR2 subunit proteins. The subtype (A–D) of the NR2 subunit confers distinct functional properties to the receptor (18, 19). In the cerebral cortex at the time of birth, NMDARs contain NR1 and NR2B subunits. Over the course of postnatal development, there is a progressive inclusion of the NR2A subunit (18, 20). Because NR2A-containing receptors have faster kinetics (18, 19), it has been suggested that this molecular change is responsible for the developmental changes in NMDAR properties (21).
In the present study, we monitored changes in NMDAR subunit composition in the visual cortex during postnatal development, and we examined the effects of manipulating visual experience. Our biochemical data provide strong support for the hypothesis that the functional changes in the receptor are accounted for by alterations in the NR2A/B ratio. To see whether the NR2A/B ratio mirrors the potential for synaptic plasticity or the history of cortical activity, we performed manipulations of sensory experience that do not alter the course of the critical period. The results show that NMDAR subunit composition in the visual cortex is bidirectionally modified by experience and deprivation. These data are consistent with the hypothesis that experience-dependent regulation of NMDAR subunit composition is one molecular mechanism for a sliding synaptic modification threshold.
Materials and Methods
Synaptoneurosome Preparation.
Male and female Long Evans rats (Charles River) were anesthetized with methoxyflurane vapor and decapitated after disappearance of corneal reflexes in compliance with the U.S. Department of Health and Human Services and Brown University guidelines. Synaptoneurosomes were prepared by using a procedure adapted from Hollingsworth et al. (22). The primary visual cortex was rapidly dissected in ice-cold dissection buffer (212.7 mM sucrose/2.6 mM KCl/1.23 mM NaH2PO4/26 mM NaHCO3/10 mM dextrose/1.0 mM MgCl2/0.5 mM CaCl2/0.02 mM 6-cyano-7-nitroquinoxaline-2,3-dione/0.1 mM 2-amino-5-phosphonopentanoic acid, and saturated with 95% O2/5% CO2) and immediately homogenized in ice-cold homogenization buffer (10 mM Hepes/1.0 mM EDTA/2.0 mM EGTA/0.5 mM DTT/0.1 mM PMSF/10 mg/liter leupeptin/50 mg/liter soybean trypsin inhibitor/100 nM microcystin). Tissue was homogenized in a glass/glass tissue homogenizer (Kontes, Vineland, NJ), and the homogenate was passed sequentially through two 100-μm-pore nylon mesh filters, followed by a 5-μm-pore filter, and centrifuged at 1000 × g for 10 min. The resulting pellets were resuspended in boiling 1% SDS and stored at −80°C.
Electron Microscopy.
Electron microscopy of our synaptoneurosome preparation was generously performed by Jack Gibbons of the University of Illinois at Chicago. Synaptoneurosomes were primary-fixed with 2.5% glutaraldyhyde/2% paraformaldyhyde in 0.1 M cacodylate, pH 7.4, secondary-fixed with 1% osmium tetroxide in 0.1 M cacodylate buffer, then washed with 25% and 50% ethanol plus 5% uranyl acetate, followed by dehydration in an ascending ethanol series. Pellets were infiltrated with propylene oxide, followed by infiltration with 1:1 propylene oxide to Spurr’s resin. The embedded pellets were polymerized, decapsulated, trimmed, and cut into 70-nm sections. Sections were collected on 200-hex-mesh copper grids, stained with uranyl acetate and lead citrate, dried, and placed into a JEOL 1200EX transmission electron microscope for photomicroscopy.
Immunoblotting.
Equal amounts of synaptoneurosome protein, determined with the bicinchoninic acid assay (BCA; Pierce), were resolved on 12% polyacrylamide gels, transferred to nitrocellulose, and probed with either anti-NR2A or anti-NR2B polyclonal antibodies (1:1000; Upstate Biotechnology) or anti-NR1 monoclonal antibody (1:1000, clone 54.1; PharMingen), followed by the appropriate secondary antibody coupled to horseradish peroxidase (1:3500, Sigma) in Tris-buffered saline, pH 7.3, containing 1% BSA and 0.1% Triton X-100 (Sigma). Visualization of immunoreactive bands was produced by enhanced chemiluminescence (Amersham ECL), and captured on autoradiography film (Amersham Hyper ECL). Digital images, produced by densitometric scans of autoradiographs on a ScanJet IIcx (Hewlett Packard) with DeskScan II software (Hewlett Packard), were quantified with image 1.60 software (National Institutes of Health). The optical density of each band was determined relative to a baseline immediately above and below the band within the same lane, and the values were normalized to the optical density of a protein standard (run on all gels). Group data are represented as the mean ± SEM of the normalized optical density.
Results
Synaptoneurosomes Enrich for Synaptic Proteins.
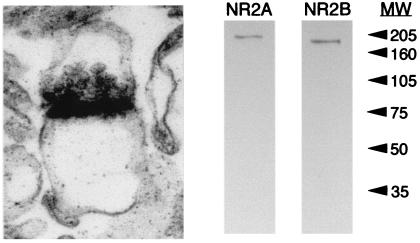
Traditional biochemical methods offer sensitive methods for quantifying small changes in protein levels; however, they offer limited resolution of the locus of these changes. Only a subset of the NMDAR proteins are expressed in the plasma membrane of neurons, and an even smaller fraction is likely to be expressed at synaptic sites (23, 24). Therefore, in an attempt to confine our analyses to synaptic proteins, NMDAR composition was determined in synaptoneurosomes, a subcellular fraction enriched for synaptic proteins (22). Electron micrographs of our synaptoneurosome preparation reveal an enrichment of “snowman-shaped” profiles, resembling native synaptic profiles (Fig. 1). Numerous clear vesicles, diagnostic of glutamatergic presynaptic terminals, opposed the electron-dense postsynaptic density. Immunoblotting equal concentrations of crude homogenate and synaptoneurosome protein prepared from the visual cortex revealed that the synaptoneurosome preparation produced an approximately 3-fold enrichment of synaptic proteins (data not shown). A representative immunoblot of our synaptoneurosomes for the NMDAR subunit proteins NR2A and NR2B is shown in Fig. 1.
Figure 1.
The synaptoneurosome fraction is enriched for synaptic profiles. (Left) Electron micrograph of a synaptoneurosome prepared from the visual cortex of a postnatal day-23 rat. (60,000×.) (Right) Representative immunoblot for the NMDAR proteins NR2A and NR2B. Synaptoneurosomes prepared from a postnatal day-23 visual cortex were resolved on a 12% polyacrylamide gel, transferred to nitrocellulose, and probed with anti-NR2A or anti-NR2B polyclonal antibodies and then with a horseradish peroxidase-coupled, anti-rabbit, secondary antibody. Visualization of immunoreactive bands was produced by enhanced chemiluminescence and captured on autoradiography film. Arrows indicate the positions of molecular weight markers (× 10−3).
NMDAR Subunit Composition Is Regulated by Age and Visual Experience.
To determine the effects of age and sensory experience on visual cortical NMDAR subunit composition, synaptoneurosomes were prepared from primary visual cortices of 1- to 6-week-old Long Evans rats, raised either in a normal light:dark cycle (12:12 hr/day), [light-reared (LR)], or in complete darkness from birth [dark-reared (DR)]. Immunoblotting was performed for each of the NMDAR proteins (NR2A, NR2B, and NR1) present in the visual cortex at these ages.
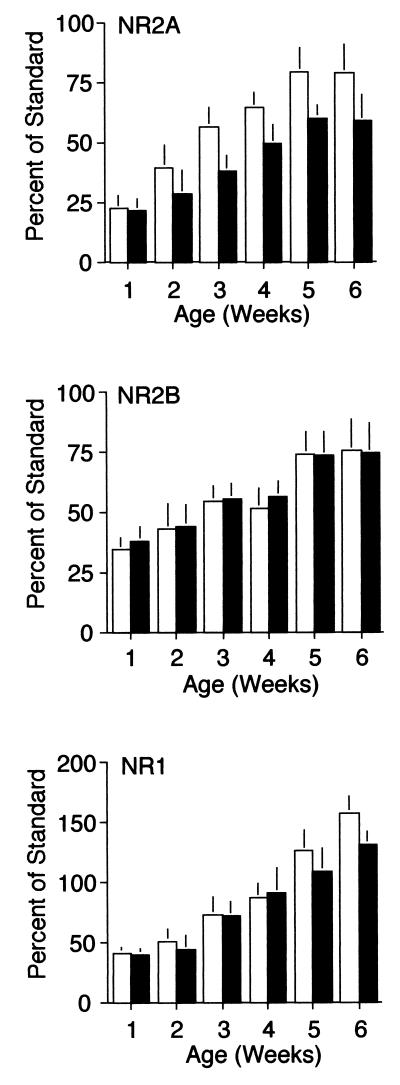
This analysis revealed that in the visual cortices of LR animals, NR2A levels increase approximately 3-fold over development (Fig. 2), reaching a steady state at 5 weeks, which is, according to some estimates (cf. Ref. 6 and 7), the end of the critical period. NR2A levels also increase with age in the visual cortices of DR animals (Fig. 2); however, the level of NR2A protein is reduced in synaptoneurosomes from the visual cortices of DR animals, compared with LR controls. Two-way ANOVA revealed a significant effect of age [F (5, 130) = 9.41, P < 0.01)] and visual experience [F (1, 130) = 6.42, P = 0.01)] on NR2A levels in the visual cortex.
Figure 2.
NMDAR subunit composition is regulated by age and visual experience. NR2A levels (Top) increase over development in both LR (white bars) and DR animals (dark bars), reaching an adult plateau at the age of 5 weeks. The level of NR2A protein is significantly reduced in synaptoneurosomes from the visual cortices of dark-reared animals (two-way ANOVA, P < 0.5, n = 160). NR2B levels (Middle) also increase over development in both LR and DR animals, and reach an adult plateau at the age of 5 weeks. However, dark rearing does not significantly affect the complement of NR2B protein in synaptoneurosomes from the visual cortex (two-way ANOVA, P > 0.1). NR1 levels (Bottom) increase over development in both LR and DR animals, and are not significantly reduced in synaptoneurosomes from the visual cortices of dark-reared animals (two-way ANOVA, P > 0.1).
The levels of NR2B and NR1 protein also increase developmentally in both LR and DR visual cortices. However, dark rearing did not significantly affect the complement of either NR2B or NR1 protein in the synaptoneurosomes at any of the time points examined [two-way ANOVA for NR2B, age F (5, 106) = 8.35, P < 0.01; experience F (1, 106) = 1.53, P > 0.2; ANOVA for NR1, age F (5, 86) = 7.20, P < 0.01; and experience, F (1, 86) = 1.12, P > 0.2]. The effect of dark rearing on NMDAR composition in the visual cortex appears to be a specific consequence of sensory deprivation, because no significant differences in NR2A, NR2B, or NR1 proteins were observed in the hippocampus or frontal cortices of DR vs. LR animals (data not shown).
The NR2A/B Ratio May Account for Changes in NMDAR Current Kinetics.
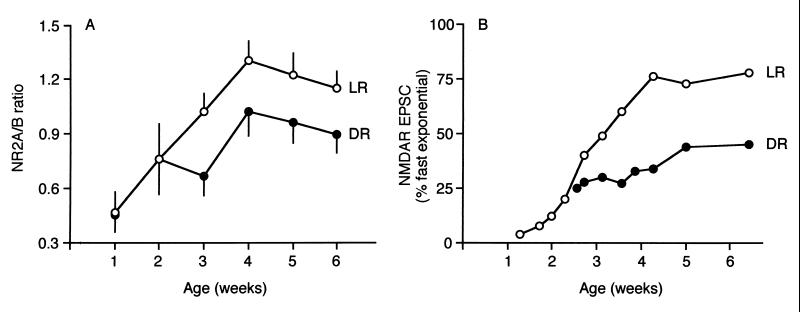
Fig. 3A plots the changes in the NR2A/B ratio during postnatal development in LR and DR animals. The ratio increases linearly over early postnatal development, reaching the adult plateau at 4 weeks. Dark rearing from birth attenuates the developmental increase in NR2A/B; the difference is first manifest between the 2nd and 3rd postnatal weeks, coincident with the onset of functional vision in the rat (6).
Figure 3.
The NR2A/B ratio correlates with the duration of NMDAR-mediated EPSCs. (A) The developmental increase in the NR2A/B ratio is attenuated in DR animals. (B) The developmental decrease in the duration of NMDAR-mediated EPSCs is attenuated in DR animals [data are replotted from Carmignoto and Vicini (3)]. The decay of the NMDAR-mediated EPSC, recorded in layer-IV neurons, was described by a double exponential function with a fast and a slow component. Over the course of development, the duration of NMDAR-mediated EPSCs progressively decreases, evident by the increased contribution of the fast component to the total NMDAR EPSC.
It has been previously established that the duration of NMDAR currents is influenced by the subunit composition; the greater the proportion of NR2A, the faster the decay of the current (18, 19, 21). Our data therefore predict that NMDAR currents will become progressively shorter over development, and that this developmental change will be decreased by dark rearing. Precisely this change in NMDAR kinetics was observed by Carmignoto and Vicini (3). To describe the change in kinetics, they fit a double exponential function (comprised of fast and slow components) to the decay phase of NMDAR-mediated excitatory postsynaptic currents (EPSCs) recorded in visual cortical neurons. Based on the data of Carmignoto and Vicini, we plot in Fig. 3B the developmental changes in the percentage of EPSC decay described by the fast exponential in visual cortical neurons from LR and DR rats. Changes in the ratio of NR2A/NR2B proteins in synaptoneurosomes (Fig. 3A) are highly correlated with changes in the kinetics of NMDAR-mediated currents (Fig. 3B). This analysis suggests that changes in NMDAR subunit levels detected in the synaptoneurosome preparation reflect changes in functional synaptic NMDARs, and that the changes in NMDAR kinetics observed by Carmignoto and Vicini may be largely caused by changes in NMDAR subunit composition.
The NR2A/B Ratio Reflects the History of Cortical Activity.
Previously, we demonstrated a rapid increase in the ratio NR2A/B in the visual cortices of DR animals exposed to light (25). Only 2 hr of visual experience is sufficient to restore the NR2A/B ratio to the value characteristic of age-matched LR animals. We wondered whether this rapid maturation was analogous to flipping a developmental switch, or whether the maintenance of the new NMDAR phenotype required continued exposure to a lighted environment. To address this question, DR animals were exposed to light for 2 hr, and then returned to the dark for varying lengths of time. The light exposure occurred during the fourth postnatal week, a time point when a robust difference is observed in the NMDAR composition across LR and DR animals, yet still within the critical period for synaptic plasticity in the visual cortex (6).
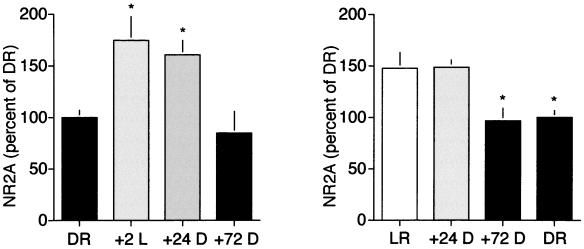
DR animals exposed to light for 2 hr (+2L) showed a robust increase in NR2A levels (174.1 ± 23.2% of DR), that was maintained for 24 hr in the absence of additional visual experience (160.1 ± 13.3% of DR). However, returning +2L animals to the dark for 72 to 96 hr significantly decreased the level of NR2A (84.8 ± 20.8% of DR; see Fig. 4 Left).
Figure 4.
Experience-dependent regulation of NMDAR composition is reversible. (Left) Brief light exposure (2 hr) of DR animals induced a significant increase in NR2A protein that could be reversed by 72 to 96 hr of subsequent visual deprivation [one-way ANOVA, F (3, 18) = 13.14, P = 0.0004. Asterisks (*) denote significant difference vs. dark-reared controls in Student–Newman–Keuls posthoc comparison, P < 0.05)]. (Right) Placing LR animals in the dark for 72 to 96 hr, but not 24 hr, induced a decrease in the level of NR2A protein [one-way ANOVA, F (3, 24) = 6.49, P = 0.0023; asterisks (*) denote significant difference vs. light-reared controls in Student–Newman–Keuls posthoc comparison, P < 0.05)].
To explore further the activity-dependent regulation of NR2A, LR animals were placed in the dark at postnatal day 23 for various times. Light deprivation for 24 hr did not affect the level of NR2A (148.5 ± 6.8% of DR, compared with 147.1 ± 15.8% of DR for LR controls). However, deprivation for 72 to 96 hr did induce a significant decrease in NR2A to levels similar to those in age-matched DR animals (97.0 ± 11.1% of DR; see Fig. 4 Left). Thus, the amount of NR2A protein in the visual cortex synaptoneurosome reflects the history of sensory experience.
Discussion
Visual cortical plasticity is regulated by age and visual experience and depends importantly on activation of NMDARs. Our experiments provide a detailed description of changes in NMDAR subunit proteins during the course of postnatal development in the visual cortices of normal and DR animals. The results show that although all of the NMDAR subunits increase during development, their relative levels are not constant. Of particular interest is the developmental increase in the NR2A/B ratio, which reaches maturity at about the age of 4 weeks in normally reared animals. In addition, our data suggest that the amount of NR2A protein at visual cortical synapses, and consequently the NR2A/B ratio, is also tightly regulated by sensory experience. In the absence of normal visual experience, NR2A levels fall by one-third; when visual experience is restored, they rapidly rise. Thus, NR2A protein at the synapse reflects the history of cortical activation during the critical period.
Changes in NMDAR Proteins Reflect Changes in Function.
Available data strongly suggest that changes in the levels of NMDAR proteins in the synaptoneurosomes reflect changes in the subunit composition of functional synaptic NMDARs. Previous work in heterologous expression systems has established that decreasing the NR2A/B ratio in NMDARs prolongs the time course of deactivation of NMDA responses and increases sensitivity to the noncompetitive, NR2B-selective antagonist ifenprodil (19). Dark rearing, which we show to be accompanied by a reduction in the NR2A/B ratio, is also characterized by slower NMDAR EPSCs (3) and enhanced sensitivity to ifenprodil (25). Similarly, exposing DR animals to light raises the NR2A/B ratio and decreases the ifenprodil sensitivity of NMDAR EPSCs with the same time course. Thus, the biochemical changes we measure appear to have a clear functional impact at visual cortical synapses. We do not know whether specific populations of synapses are more affected than others, but based on previous studies of EPSC kinetics and ifenprodil sensitivity, it is apparent that robust NMDAR changes occur in cortical layers III (25) and IV (3).
Mechanisms for Bidirectional Activity-Dependent Regulation of NR2A.
The observation that visual experience and deprivation can induce bidirectional changes in the composition of NMDARs suggests that a threshold level of activity may be required to maintain a high level of NR2A-containing receptors at cortical synapses. However, up-regulation and down-regulation appear to occur at very different rates. The rapid up-regulation of NR2A-containing receptors is prevented by mRNA translation inhibitors (25), suggesting that the synaptic insertion of receptors requires the synthesis of NR2A or other proteins involved in the trafficking of NMDARs. If new NR2A-containing receptors replace, rather than supplement, existing NR2B-containing receptors, then the turnover rate of cell-surface NMDARs would need to be quite high. On the other hand, the slow loss of NR2A-containing receptors after sensory deprivation would be consistent with a slow turnover rate of synaptic NMDARs. Although the turnover rate for NMDARs in vivo is not known, studies in cultured cerebellar granule cells demonstrate that the metabolic half-life of surface-expressed NR1 protein is approximately 20 hr (24).
It is possible that synaptic activity regulates the rate of NMDAR turnover as well as receptor–subunit composition. Activity-dependent regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor turnover has recently been demonstrated in cultured hippocampal neurons, where increasing synaptic activity leads to a decrease in the rate of turnover of synaptic AMPA receptors (26). Thus, during periods of low activity, such as during sensory deprivation, NR2B-containing NMDARs may turn over rapidly. Increasing the level of synaptic activity may reduce NMDAR turnover, thereby increasing the half-life of the newly synthesized, NR2A-containing, synaptic NMDARs.
A Molecular Mechanism for Sliding Synaptic Modification Threshold?
Do changes in NMDAR subunit composition have an impact on cortical function during development? The developmental increase in the NR2A/B ratio could reflect synaptogenesis (27), onset of experience-dependent plasticity (4), or decline in experience-dependent synaptic plasticity (20). However, based on the bidirectional activity-dependent regulation of NR2A, we propose an alternative hypothesis, that regulation of NMDAR subunit composition by experience is a mechanism for the sliding synaptic modification threshold.
Theoretical investigations suggest that activity-dependent regulation of the LTD-LTP crossover point (the modification threshold) plays a crucial role in cortical development by maintaining the network of modifiable synapses within a useful dynamic range (16). The experience-dependent regulation of the NR2A/B ratio and the concomitant regulation of the duration of synaptic NMDAR currents are likely to have a significant impact on the properties of synaptic plasticity in the developing visual cortex. If we assume that the only consequence of decreasing the NR2A/B ratio is to prolong the EPSC, then down-regulation of NR2A after deprivation would be expected to favor NMDAR-dependent LTP over LTD in response to a given type of stimulation (13). Conversely, activity-dependent increases in NR2A, by reducing the duration of the NMDAR-mediated EPSC, would favor NMDAR-dependent LTD over LTP.
Although studies of synaptic plasticity in LR and DR animals (17) reveal that the modification threshold does correlate with the NR2A/B ratio, a causal relationship remains to be established in the visual cortex. Genetic manipulations in mice may offer exciting opportunities, not only to test this hypothetical mechanism for the sliding modification threshold in the visual cortex, but also to determine its precise contribution to cortical development and plasticity. Indeed, Tang et al. (28) have recently demonstrated that overexpression of the NR2B subunit, which decreases the NR2A/B ratio and prolongs the EPSC, shifts the modification threshold to favor LTP in the CA1 region of the mouse hippocampus.
Acknowledgments
We thank Suzanne Meagher and Erik Sklar for assistance. This work was partially supported by grants from the Human Frontiers Science Program and the National Eye Institute.
Abbreviations
- EPSC
excitatory postsynaptic current
- DR
reared in complete darkness from birth
- LR
normal light/dark-reared
- LTD
long-term synaptic depression
- LTP
long-term synaptic potentiation
- NMDA
N-methyl-d-aspartate
- NMDAR
NMDA receptors
References
- 1.Tsumoto T, Hagihara K, Sato H, Hata Y. Nature (London) 1987;327:513–514. doi: 10.1038/327513a0. [DOI] [PubMed] [Google Scholar]
- 2.Fox K, Sato H, Daw N. J Neurosci. 1989;9:2443–2454. doi: 10.1523/JNEUROSCI.09-07-02443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmignoto G, Vicini S. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- 4.Roberts E B, Ramoa A S. J Neurophysiol. 1999;81:2587–2591. doi: 10.1152/jn.1999.81.5.2587. [DOI] [PubMed] [Google Scholar]
- 5.Daw N W, Fox K, Sato H, Czepita D. J Neurophysiol. 1992;67:197–202. doi: 10.1152/jn.1992.67.1.197. [DOI] [PubMed] [Google Scholar]
- 6.Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 7.Guire E S, Lickey M E, Gordon B. J Neurophysiol. 1999;81:121–128. doi: 10.1152/jn.1999.81.1.121. [DOI] [PubMed] [Google Scholar]
- 8.Cynader M, Mitchell D E. J Neurophysiol. 1980;43:1026–1039. doi: 10.1152/jn.1980.43.4.1026. [DOI] [PubMed] [Google Scholar]
- 9.Mower G D. Dev Brain Res. 1991;58:151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- 10.Bear M F, Kleinschmidt A, Gu Q, Singer W. J Neurosci. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts E B, Meredith M A, Ramoa A S. J Neurophysiol. 1998;80:1021–1032. doi: 10.1152/jn.1998.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 12.Daw N W, Gordon B, Fox K D, Flavin H J, Kirsch J D, Beaver C J, Ji Q, Reid S N, Czepita D. J Neurophysiol. 1999;81:204–215. doi: 10.1152/jn.1999.81.1.204. [DOI] [PubMed] [Google Scholar]
- 13.Gold J I, Bear M F. Proc Natl Acad Sci USA. 1994;91:3941–3945. doi: 10.1073/pnas.91.9.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bear M F, Malenka R C. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 15.Yang S N, Tang Y G, Zucker R S. J Neurophysiol. 1999;81:781–787. doi: 10.1152/jn.1999.81.2.781. [DOI] [PubMed] [Google Scholar]
- 16.Bienenstock E L, Cooper L N, Munro P W. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkwood A, Rioult M G, Bear M F. Nature (London) 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- 18.Monyer H, Burnashev N, Laurie D J, Sakmann B, Seeburg P H. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 19.Vicini S, Wang J F, Li J H, Zhu W J, Wang Y H, Luo J H, Wolfe B B, Grayson D R. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- 20.Sheng M, Cummings J, Roldan L A, Jan Y N, Jan L Y. Nature (London) 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 21.Flint A C, Maisch U S, Weishaupt J H, Kriegstein A R, Monyer H. J Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollingsworth E B. J Neurosci. 1985;5:2240–2253. doi: 10.1523/JNEUROSCI.05-08-02240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall R A, Soderling T R. J Neurosci. 1997;78:361–371. doi: 10.1016/s0306-4522(96)00525-8. [DOI] [PubMed] [Google Scholar]
- 24.Huh K-H, Wenthold R J. J Biol Chem. 1999;274:151–157. doi: 10.1074/jbc.274.1.151. [DOI] [PubMed] [Google Scholar]
- 25.Quinlan E M, Philpot B D, Huganir R L, Bear M F. Nat Neurosci. 1999;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien R J, Kamboj S, Ehlers M D, Rosen K R, Fischbach G D, Huganir R L. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- 27.Tovar K R, Westbrook G L. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Y-P, Shimizu E, Dube G R, Rampon C, Kerchner G A, Zhuo M, Liu G, Tsien J Z. Nature (London) 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]