Abstract
By combining two previously generated null mutations, Ii° and M°, we produced mice lacking the invariant chain and H-2M complexes, both required for normal cell-surface expression of major histocompatibility complex class II molecules loaded with the usual diverse array of peptides. As expected, the maturation and transport of class II molecules, their expression at the cell surface, and their capacity to present antigens were quite similar for cells from Ii°M° double-mutant mice and from animals carrying just the Ii° mutation. More surprising were certain features of the CD4+ T cell repertoire selected in Ii°M° mice: many fewer cells were selected than in Ii+M° animals, and these had been purged of self-reactive specificities, unlike their counterparts in Ii+M° animals. These findings suggest (i) that the peptides carried by class II molecules on stromal cells lacking H-2M complexes may almost all derive from invariant chain and (ii) that H-2M complexes edit the peptide array displayed on thymic stromal cells in the absence of invariant chain, showing that it can edit, in vivo, peptides other than CLIP.
Keywords: peptide loading, antigen presentation, T cell selection, knock-out mice
The maturation and intracellular transport of major histocompatibility complex (MHC) class II molecules appear well adapted to their primary function, the presentation of antigenic peptides derived from extracellular proteins to CD4+ T lymphocytes [for reviews see refs. 1 and 2]. Shortly after their synthesis in the endoplasmic reticulum (ER), class II α and β chains assemble as heterodimers and associate with the invariant chain (Ii). Ii prevents ER-derived peptides from binding to class II molecules by inserting its CLIP segment into the peptide-binding groove (3–7). Ii also facilitates the transport of class II molecules from the ER, through the Golgi, and into endosomal compartments (8–13). There, proteolysis trims Ii to the CLIP segment, which remains encased in the peptide binding groove (6, 14). The replacement of CLIP with peptides encountered in the endocytic pathway (mostly derived from internalized extracellular proteins, but also from endogenous membrane or endosomal proteins travelling the same route) is mediated by the H-2M molecule (15–17). The resulting heterogeneous class II/peptide complexes are then transported to the cell surface, where they can be recognized by CD4+ T cells, triggering their further differentiation and/or proliferation.
Nonetheless, there is ample evidence that class II heterodimers can mature and be loaded with peptides in alternative pathways. For example, class II molecules are found on the surface of cells unable to express Ii. In such cells, class II αβ heterodimers assemble poorly and most remain trapped in the ER or pre-Golgi compartments, largely in the form of high molecular-weight aggregates (10–12, 18–20); however, some do make it to the cell surface, apparently still in association with a heterogenous mix of large polypeptides (7, 18–20). As a consequence, immune functions that are dependent on MHC class II molecules are not completely absent in Ii-deficient cells or mice. As might be expected, presentation of exogenous peptides to T cells is quite efficient (18, 19). More paradoxical perhaps is the fact that a variety of epitopes derived from whole protein antigens can be efficiently processed and loaded onto class II molecules. Some of these originate from endogenous proteins (21–23), but others derive from bona fide external proteins (18, 24–26), suggesting that class II molecules without Ii intersect the endocytic route at some point. In addition, positive selection of CD4+ T cells can take place in thymi from Ii-deficient animals, some specificities being selected quite readily, but others hardly at all (18, 19, 27, 28).
H-2M deficiency also leads to defective functioning of class II molecules, both in mutant lymphoblastoid cells (29–31) and in mice with an engineered null mutation (32–34). Most of the aberrancies characteristic of H-2M-deficient cells and mice can be traced to an inability to replace CLIP with heterogeneous endosomal peptides. Class II Ab molecules are found at normal levels on the cell surface (29–34), but they are predominantly (and sometimes almost exclusively) loaded with CLIP (31–36). Processing and presentation of protein antigens by Ab is abolished (29, 32, 33, 37), and presentation of peptides is strongly affected (32, 33). Ab-mediated positive selection of CD4+ T cells in H-2M-deficient mice is partially reduced, and the selected T cells are strikingly intolerant of MHC molecules from normal mice (32–34).
Thus, producing mice that carry both the Ii° and H-2M° mutations would answer many questions. (i) Would the residual functioning of class II molecules in Ii° mice dependent on H-2M complexes? If so, it would shed light on the compartments in which class II molecules are loaded when devoid of Ii. (ii) Would the aberrations explained by the almost exclusive loading of CLIP in the class II molecules of H-2M° mice disappear in the further absence of Ii? Given current views on class II-peptide loading, as outlined above, it might have been expected that the Ii gene would be epistatic to the H-2M locus, and therefore, that the double mutants would essentially recapitulate the Ii° phenotype. That prediction proved correct in many aspects, but there were some surprises, suggesting that H-2M complexes play a role in certain of the Ii-independent functions of class II molecules.
MATERIALS AND METHODS
Mice.
Ii-, H-2Ma-, and class II-deficient mice have been described (18, 32, 38). The Ii° and H-2M° lines were bred together and intercrossed to obtain the Ii/H-2Ma double-deficient animals, on a mixed C57BL/6(B6) × 129 genetic background. They were maintained in our conventional animal facility (Strasbourg, France).
mAbs and Flow Cytometry.
mAbs reactive against Ab molecules used in this study include Y3P, M5/114, 25.9.17S, Y237, 40B, BP107, Y222, Y248, and Y246 (for review see ref. 39). The following antibodies were used for T cell analysis: biotinylated anti-CD8, phycoerythrin-conjugated anti-CD4 (Caltag, South San Francisco, CA) anti-CD3 (KT3, see ref. 40). Biotinylated antibody was revealed with Cy5 labeled streptavidin and the KT3 antibody with Texas red-labeled anti-rat IgG (Jackson ImmunoResearch). Peanut agglutinin (PNA) receptor was detected using fluorescein-conjugated PNA (Sigma). Immunostainings were performed as described (38).
Biochemical Analyses.
Spleen cells were labeled with [35S]methionine for 0.5 h at 37°C in methionine-deficient culture medium. The newly synthesized molecules were “chased” by addition of cold methionine as indicated in the figure legend, and the cells were lysed in Nonidet P-40 containing lysis buffer. After a preclearing of the lysates, the Ab molecules were immunoprecipitated with the Y3P antibody and analyzed on SDS/PAGE, all essentially as described (32).
Antigen Presentation Assays and Mixed Lymphocyte Reactions.
Antigen presentation assays were performed as previously described (27). T cell hybridomas BO17.10, BO4H91 (41), BEa 20.6 (42), and T49.25 (43) were used to detect the processing and Ab-restricted presentation of ovalbumin 323-339, lyzozyme 74-88, Eα 52-68, and apamin epitopes, respectively. Mixed lymphocyte reactions were performed as described (32). To enrich for CD4+ T cells, lymph nodes cells were incubated with a mixture of mAbs: anti-CD8 (YTS169 and YTS192), anti-B220 (RA3-6B2), and anti-Ab (M5/114) for 30 min at 4°C. Labeled cells were then eliminated by incubation with magnetic beads coated with anti-rat-IgG for 30 min at 4°C, following the manufacturer’s instructions (Dako).
RESULTS
Expression of MHC Class II Molecules by Cells from Ii/H-2Ma-Deficient Mice.
Ii- and H-2Ma-deficient mouse lines (18, 32), both on an H-2b genetic background, were crossed. The resulting double-heterozygotes intercrossed to produce a matched set of littermates: Ii+M+, Ii°M+, Ii+M°, and Ii°M°. The double-mutant Ii°M° animals were generated at frequencies consistent with standard Mendelian transmission, showed no overt developmental defects, and were viable. However, when housed in a conventional animal facility, they often showed runting, and significant mortality ensued after a few weeks of age. Such traits, not seen with either of the parental lines, have been observed in our colony with certain immunocompromised animals, including an MHC class II-deficient line (38).
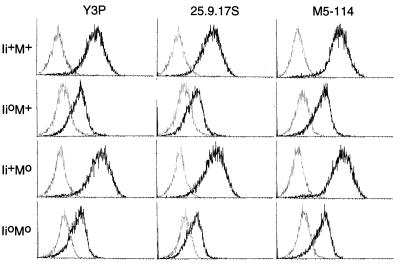
The matched set of littermates was evaluated for cell-surface expression of Ab molecules by staining splenocytes with a panel of anti-Ab reagents. Compiled in Fig. 1 are profiles for gated IgM+ B cells stained with three representative antibodies, recognizing Aβ-specific determinants (M5-114, 25-9-17S), or dependent on the intact AαAβ complex (Y3P). As previously described (32), B cells from Ii°M+ mice showed markedly reduced Ab display (6-fold lower on average) (18–20, 27), while cells from Ii+M° animals exhibited normal levels of expression. In the double-mutants, staining levels were low, matching those for Ii°M+ B cells. Very similar staining profiles were observed with 6 other mAbs. With BP107, a reagent that labels very poorly the Ab molecules from I+M° mice (32), staining remained weak for the double mutants (not shown). Immunofluorescence analyses performed with some of the mAbs on cryostat sections of thymi gave parallel findings: Ab molecules were detected on sections from Ii°M° mice at the same low level as on those from Ii°M+ mutants—both in the cortical epithelium and in medullary dendritic and epithelial cells (not shown).
Figure 1.
Cell surface expression of MHC molecules. Splenocytes from mice of the phenotypes shown were stained using anti-Ab mAbs and analyzed by cytofluorimetry. The darker line represents the Ab specific staining on gated IgM+ B cells. The thin line represents background staining (secondary reagent alone).
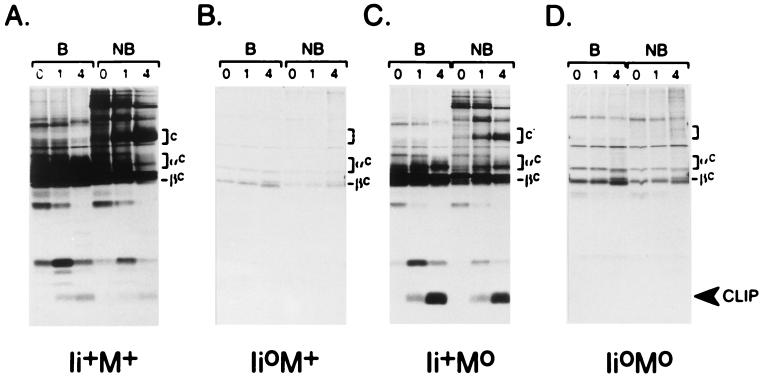
To compare the maturation and transport of MHC class II molecules in cells from these mice, we turned to biochemical analysis, namely immunoprecipitation of proteins from metabolically labeled splenocytes. Full dissociation of the isolated AαAβ complexes was accomplished by boiling the samples in SDS, while their incubation in SDS at room temperature allowed the visualization of stable α/β/peptide complexes (44, 45). As described in refs. 18–20, and illustrated in Fig. 2, the behavior of Ab molecules in splenocytes from Ii°M+ mice was markedly different from wild-type (compare B with A). (i) The absence of Ii led to a reduction in the number of AαAβ complexes assembled, reflected in lower total amounts of immunoprecipitated material. (ii) The transport of complexes out of the ER and their transit through the Golgi were reduced, manifest by the limited number of Aα and Aβ chains that underwent carbohydrate maturation to complex forms, even at 4 h of chase (αc and βc bands). (iii) There was little or no maturation into the usual SDS-stable complexes (c bands), only a small amount of slower mobility material eventually appearing. All of these points were equally true of the samples from Ii°M° mice (Fig. 2D). Also clearly missing in the sample from double-mutant mice were the SDS-stable complexes of slightly reduced mobility characteristic of samples from Ii+M° mice (c′ in Fig. 2C).
Figure 2.
Intracellular transport of MHC class II molecules. Spleen cells were labeled with [35S]methionine for 30 min at 37°C and chased with cold methionine for 0, 1, or 4 h at 37°C. Ab complexes were then immunoprecipitated with the Y3P mAb and analyzed by SDS/PAGE, with (B) or without (NB) prior dissociation at 95°C. Brackets laeled c, c′ are SDS-stable (compact) heterodimers. αc, βc/Aα, and Aβ chains matured to complex glycosylated forms.
Thus, the Ab molecules in Ii°M° mice appear to behave very much like those in Ii°M+ animals. In both cases, there is reduced assembly, maturation, and transport, resulting in lower surface levels and little or no formation of the usual SDS-stable, compact dimers.
Antigen Presentation by Cells from Ii°M° Mice.
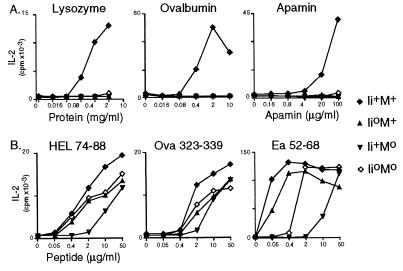
Antigen-presenting cells (APCs) from both Ii- and H-2Ma-deficient mice are known to be severely compromised in their ability to present to T cells: neither can present whole proteins efficiently (save for certain particular epitopes); Ii°M+ APCs can present peptides well, but Ii+M° APCs are poor peptide presenters (18, 19, 32–34). To investigate APC function in the double mutants, we compared the ability of splenocytes from the matched set of littermates to stimulate interleukin 2 production by a panel of Ab-restricted T cell hybridomas recognizing three different antigens. The general findings, depicted in Fig. 3, are clear. Splenocytes from neither the single nor double mutants could present whole protein antigens (Fig. 3A). On the other hand, coexpression of the Ii° mutation largely reversed the reduced ability of Ii+M° APCs to present peptides, such that Ii°M° APCs were almost as active as Ii°M+ or wild-type APCs (Fig. 3B). This was particularly clear for the Ea 52-68 peptide (Fig. 3B Right). We interpret these findings as reflecting that the CLIP prevalently bound to the Ab molecules on Ii+M° APCs hinders the binding of added peptides during the course of the assay; the Ab molecules on Ii°M° APCs, devoid of CLIP, must be complexed with more loosely bound peptides [perhaps the large polypeptides found previously on Ii°M+ cells (7)].
Figure 3.
Antigen presentation. The response of T cell hybridomas to presentation of proteins (Upper) or peptides (Lower) by splenocytes from mice of the indicated phenotypes was evaluated as interleukin 2 production at various doses of antigen.
Selection of CD4+ Cells in Double-Deficient Mice.
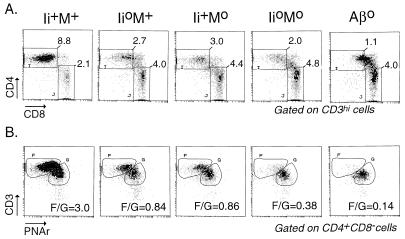
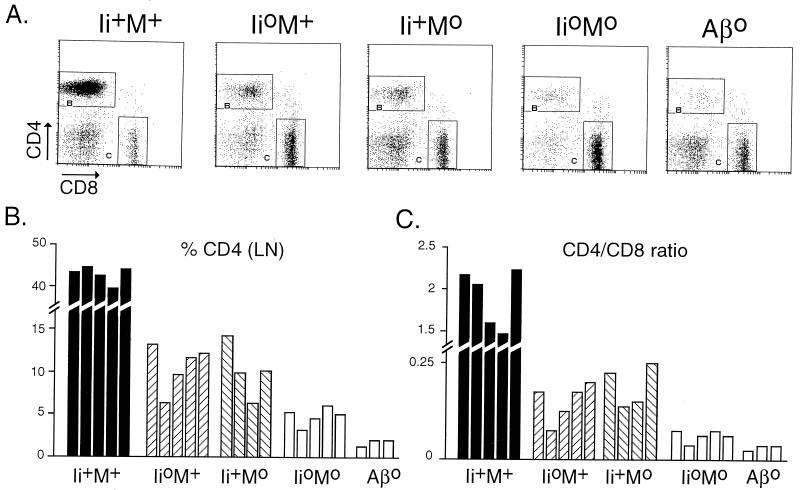
We next compared the CD4+ T cell compartments in the four types of littermates. Typical thymocyte staining profiles, together with reference profiles from a class II-deficient mouse (Aβ°, ref. 38), are presented in Fig. 4. The representative CD4/CD8 plots (Fig. 4A, gated on CD3hi cells to focus on cells actually involved in the positive selection process) illustrate that the CD4+CD8− population in Ii°M° double-mutant animals was reduced to a greater extent than in either of the single mutants. These differences were noted in six independent sets of littermates (Fig. 4, legend). Reduced selection of CD4+CD8− cells in Ii°M+ and Ii+M° mice was accompanied by a shift in the phenotypic distribution of cells, with a higher proportion expressing residual CD8, suggesting that they correspond to early selection intermediates (18, 19, 32–34). This trait was enhanced in Ii°M° mice. A further illustration of the reduced selection of CD4+CD8− cells in the double-mutants compared with their parents could be obtained in the biparametric CD3/PNAr analyses of CD4+CD8− cells (Fig. 4B). The dominant population in wild-type thymi, characterized by the highest level of CD3 staining together with negative PNA staining (gate F), was relatively reduced in Ii°M+ and Ii+M° thymi, in favor of a population with a slightly lower level of CD3 that does stain with PNA (gate G); this shift was further accentuated in Ii°M° thymi, with a further decreased “maturation ratio” (Fig. 4B, legend). According to all of these criteria, positive selection of CD4+8− cells in Ii°M° double-mutant animals was more affected than in either of the single mutants, but it was still slightly superior to the selection in class II-deficient mice (where the few CD4+8− thymocytes consist of MHC class I-restricted selection intermediates and a few bona fide CD4 single-positive cells restricted by nonclassical class I molecules) (46–48).
Figure 4.
Thymocyte phenotypes in mice deficient for Ii and/or M molecules. (A) Dot plots of CD4/CD8 staining of thymocytes expressing high levels of CD3. Values represent the number per thymus (×10−6) of CD3high thymocytes that are CD4+CD8− or CD4−CD8+ in this representative experiment; over six such experiments with mice of various ages, the proportion of CD4+CD8− thymocytes relative to wild-type numbers averaged 33%, 27%, and 17% for Ii+M°, Ii°M+, and Ii°M°, respectively (P < 0.002 and P < 0.05 when comparing Ii+M° and Ii°M+ to Ii°M°, by correlated Student’s t test). (B) CD3/PNAr profiles of gated CD4+CD8− thymocytes, from four-color cytofluorimetry. The values shown are the F/G “maturation ratio”.
The impaired selection of mature CD4+ cells was reflected in the peripheral organs of Ii°M° mice (Fig. 5A). Considering data from several groups of animals (Fig. 5B), we found that, Ii°M° individuals had an average 9-fold reduction, while Ii°M+ and Ii+M° littermates showed only a 4-fold diminution, compared with the numbers in wild-type littermates (P < 0.01). As in the thymus, the few CD4+ cells in Ii°M° lymph nodes were still distinctly, if marginally, more numerous than those in lymph nodes of class II-deficient mice. Like the residual population in II° mice, the CD4+ cells in Ii°M° animals were enriched for those with a memory phenotype—i.e., CD44hi, CD62Llo (data not shown).
Figure 5.
Phenotype of peripheral T cells from mice deficient for Ii and/or M molecules. (A) Dot plots of CD4/CD8 staining of lymph nodes cells. (B) Percentage of CD4+CD8− lymph nodes cells found in several mice of each phenotype. Bars = individual mouse. Average percent CD4: M°, 10.0 ± 3.2; Ii°, 10.6 ± 2.8; M°Ii°, 5.2 ± 0.8; P < 0.01 and P < 0.002 when comparing either of the single mutants to the double mutants, Student’s t test. (C) Ratio of CD4+ T cells to CD8+ T cells.
In short, both in the thymus and periphery, the CD4+ T cell compartments of Ii°M° mice are more severely compromised than their equivalents in single-mutant animals. They appear more similar to those of class II-deficient animals.
Mixed Lymphocyte Reactions.
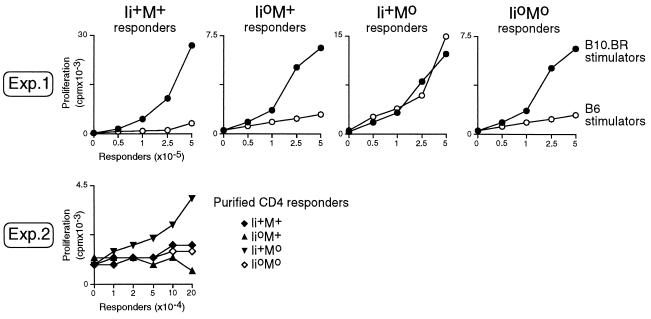
The repertoire of CD4+ T cells selected in Ii+M° mice is quite particular in that these cells exhibit a high reactivity to syngeneic Ab molecules on wild-type APCs (32–34), almost certainly reflecting the fact that T cells that mature in the Ii+M° environment are not tolerized to the panoply of self-peptides normally occupying Ab molecules. No such reactivity is seen in Ii°M+ T cells. The mixed lymphocyte reactions depicted in Fig. 6 were performed to find out whether such a reactivity is also characteristic of the repertoire of CD4+ cells in Ii°M° double-mutants. In the first experiment, responder lymph node cells from the four types of littermates were challenged with syngeneic or allogeneic splenocytes – from B6 or B10.BR mice, respectively. While all four could respond to the control allogeneic stimulators, only the lymph node cells from Ii+M° animals responded to syngeneic B6 stimulators. This observation was confirmed in mixed lymphocyte reactions with highly enriched CD4+ lymph node cells as responders (Exp 2). Thus, Ii°M° mice lack the syngeneic reactivity characteristic of CD4+ T cells from Ii+M° animals.
Figure 6.
Reactivity of T cells from mice of various phenotypes against syngeneic MHC class II molecules of wild type animals. In experiment 1 responder lymph nodes cells from various mice were titrated and stimulated by x-irradiated spleen cells from B6 or B10.BR mice. In experiment 2, CD4+ lymph nodes cells from various animals were purified before stimulation with x-irradiated B6 stimulators. Proliferation was measured as incorporated thymidine after a 3-day culture.
DISCUSSION
These experiments have addressed two complementary issues: whether the residual MHC class II-mediated functions observable in Ii-deficient mice are dependent on H-2M complexes, and whether the functions mediated by class II molecules in H-2M-deficient animals require Ii expression.
In cells and mice lacking Ii, MHC class II molecules reach the surface by a poorly understood alternative pathway(s); here we have addressed whether H-2M complexes influence the behavior of class II molecules as they travel these atypical routes. Cells from single Ii°M+ and double Ii°M° mutant animals exhibited very similar intracellular transport and cell-surface expression of class II molecules, as well as antigen presentation by class II molecules. Yet, in the double mutants, the class II molecules displayed on thymic stromal cells were very inefficient at positively selecting CD4+ T cells: fewer CD4+ cells, with a preponderance of cells with an immature phenotype (Fig. 4). Since the levels and pattern of class II molecule expression in the thymus were similar to those of Ii°M+ mice, and since positive selection is predicated on the peptides presented by class II molecules, the implication is that H-2M complexes can play a role in determining the array of peptides presented by class II molecules in the absence of Ii. In transfectants lacking Ii (and probably H-2M complexes as well), the class II molecules that arrive at the cell surface appear to be complexed mainly with polypeptides of high molecular weight (7). Therefore our observations would suggest that the class II molecules so loaded are poor ligands for positive selection, and that the large polypeptides are edited from class II molecules in Ii°M+ cells by H-2M complexes. H-2M’s editing function in vivo would thus extend beyond its usual CLIP target, in agreement with conclusions from in vitro experiments (15, 49–51).
If H-2M complexes do indeed edit the peptide complement of MHC class II molecules transported in the absence of Ii, one is lead to question where the required interactions take place. The pathways travelled by class II molecules in the absence of Ii have not been clearly delineated, although it is known that they differ from those followed by class II molecules when escorted by Ii (18–20). The Ii-independent routes do end up in compartments where class II molecules can be loaded with peptides, but the precise peptides generated and/or bound are distinct, since the range of epitopes presented from self and exogenous proteins is strongly influenced by the presence or absence of Ii (18, 22–25). It has been proposed that Ii-independent presentation reflects the recycling of class II molecules from the cell surface (52), or that it depends on a transport pathway that parallels the usual one but is mediated by chaperonins other than Ii (e.g., stress proteins) and ends up in different endosomal compartments (53). Our data do not permit a distinction between these two possibilities, but do indicate that the relevant compartment(s) contain functional H-2M complexes, consistent with recent observations that these molecules are broadly distributed throughout the endocytic route (54).
In mice which lack H-2M complexes, the predominant loading of class II molecules with CLIP, in thymic stromal cells in particular, results in the selection of a broad repertoire of CD4+ T cells, although it does not encompass all of the specificities selected in normal mice (32–34) (and our unpublished data). This repertoire is peculiar in that it includes many cells with reactivity against syngeneic class II molecules carrying the normal diverse complement of peptides. In mice lacking H-2M and Ii, and thereby CLIP, this peculiarity is lost. This suggests that the class II molecules in double-mutant animals carry a broad array of self-peptides which, even though ineffectual at positive selection, can tolerize the reactivity to normal syngeneic APCs. By inference, this result also supports our previous contention (32) that the class II molecules of H-2M° mice are almost exclusively loaded with CLIP, and therefore most or all of the CD4+ T cells that emerge in these animals are positively selected on class II/CLIP complexes.
Acknowledgments
We thank C. Waltzinger for help with the flow cytometry; C. Ebel, P. Gerber, and J. Hergueux for assistance; F. Fischer and V. Louerat for maintaining the mice; and P. Eberling for peptides. This work was supported by institute funds from the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, the Centre Hospitalier Universitaire Régional, Bristol-Myers Squibb, by grants to D.M. and C.B. from the Association pour la Recherche sur le Cancer and the Human Frontier Science Program, and by National Institutes of Health Grant 5-ROI-AI34893 (to H.P.). S.T. was supported by fellowships from the Association pour la Recherche sur le Cancer and the Société des Amis des Sciences; T.M. by fellowships from the Institut National de la Santé et de la Recherche Médicale and the Human Frontier Science Program; and P.W. in an Irvington Institute Postdoctoral fellow.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: MHC, major histocompatibility complex; ER, endoplasmic reticulum; Ii, invariant chain; APC, antigen-presenting cell; PNA, peanut agglutinin.
References
- 1.Neefjes J J, Momburg F. Curr Opin Immunol. 1993;5:27–34. doi: 10.1016/0952-7915(93)90077-6. [DOI] [PubMed] [Google Scholar]
- 2.Germain R N. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 3.Roche P A, Cresswell P. Nature (London) 1990;345:615–618. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- 4.Teyton L, O’Sullivan D, Dickson P W, Lotteau V, Sette A, Fink P, Peterson P A. Nature (London) 1990;348:39–44. doi: 10.1038/348039a0. [DOI] [PubMed] [Google Scholar]
- 5.Newcomb J R, Cresswell P. J Immunol. 1993;150:499–507. [PubMed] [Google Scholar]
- 6.Jasanoff A, Park S J, Wiley D C. Proc Natl Acad Sci USA. 1995;92:9900–9904. doi: 10.1073/pnas.92.21.9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busch R, Cloutier I, Sekaly R P, Hammerling G J. EMBO J. 1996;15:418–428. [PMC free article] [PubMed] [Google Scholar]
- 8.Claesson-Welsh L, Peterson P A. J Immunol. 1985;135:3551–3557. [PubMed] [Google Scholar]
- 9.Pieters J, Horstmann H, Bakke O, Griffiths G, Lipp J. J Cell Biol. 1991;115:1213–1223. doi: 10.1083/jcb.115.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaiff W T, Hruska K A, Jr, Bono C, Shuman S, Schwartz B D. J Immunol. 1991;147:603–608. [PubMed] [Google Scholar]
- 11.Lamb C A, Yewdell J W, Bennink J R, Cresswell P. Proc Natl Acad Sci USA. 1991;88:5998–6002. doi: 10.1073/pnas.88.14.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson M S, Miller J. Proc Natl Acad Sci USA. 1992;89:2282–2286. doi: 10.1073/pnas.89.6.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sant A J, Miller J. Curr Opin Immunol. 1994;6:57–63. doi: 10.1016/0952-7915(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 14.Avva R R, Cresswell P. Immunity. 1994;1:763–774. doi: 10.1016/s1074-7613(94)80018-9. [DOI] [PubMed] [Google Scholar]
- 15.Sloan V S, Cameron P, Porter G, Gammon M, Amaya M, Mellins E, Zaller D M. Nature (London) 1995;375:802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- 16.Denzin L K, Cresswell P. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 17.Sherman M A, Weber D A, Jensen P E. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 18.Viville S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, Benoist C, Mathis D. Cell. 1993;72:635–648. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- 19.Bikoff E K, Huang L-Y, Episkopou V, van Meerwijk J, Germain R N, Robertson E J. J Exp Med. 1993;177:1699–1712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott E A, Drake J R, Amigorena S, Elsemore J, Webster P, Mellman I, Flavell R A. J Exp Med. 1994;179:681–694. doi: 10.1084/jem.179.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loss G E, Elias C G, Fields P E, Ribaudo R K, McKisic M, Sant A J. J Exp Med. 1993;178:73–85. doi: 10.1084/jem.178.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodmer H, Viville S, Benoist C, Mathis D. Science. 1994;263:1284–1286. doi: 10.1126/science.7510069. [DOI] [PubMed] [Google Scholar]
- 23.Oxenius A, Bachmann M F, Ashton-Rickardt P G, Tonegawa S, Zinkernagel R M, Hengartner H. Eur J Immunol. 1995;25:3402–3411. doi: 10.1002/eji.1830251230. [DOI] [PubMed] [Google Scholar]
- 24.Nadimi F, Moreno J, Momburg F, Heuser A, Fuchs S, Adorini L, Hämmerling G J. Eur J Immunol. 1991;21:1255–1263. doi: 10.1002/eji.1830210524. [DOI] [PubMed] [Google Scholar]
- 25.Momburg F, Fuchs S, Drexler J, Busch R, Post M, Hammerling G J, Adorini L. J Exp Med. 1993;178:1453–1458. doi: 10.1084/jem.178.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swier K, Miller J. J Immunol. 1995;155:1851–1861. [PubMed] [Google Scholar]
- 27.Tourne S, Nakano N, Viville S, Benoist C, Mathis D. Eur J Immunol. 1995;25:1851–1856. doi: 10.1002/eji.1830250709. [DOI] [PubMed] [Google Scholar]
- 28.Nakano N, Rooke R, Benoist C, Mathis D. Science. 1997;275:678–683. doi: 10.1126/science.275.5300.678. [DOI] [PubMed] [Google Scholar]
- 29.Mellins E, Smith L, Arp B, Cotner T, Celis E, Pious D. Nature (London) 1990;343:71–74. doi: 10.1038/343071a0. [DOI] [PubMed] [Google Scholar]
- 30.Ceman S, Rudersdorf R, Long E O, DeMars R. J Immunol. 1992;149:754–761. [PubMed] [Google Scholar]
- 31.Riberdy J M, Newcomb J R, Surman M J, Barbosa J A, Cresswell P. Nature (London) 1992;360:474–477. doi: 10.1038/360474a0. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki T, Wolf P, Tourne S, Waltzinger C, Dierich A, Barois N, Ploegh H, Benoist C, Mathis D. Cell. 1996;84:531–541. doi: 10.1016/s0092-8674(00)81029-6. [DOI] [PubMed] [Google Scholar]
- 33.Martin W D, Hicks G G, Mendiratta S K, Leva H I, Ruley H E, van Kaer L. Cell. 1996;84:543–550. doi: 10.1016/s0092-8674(00)81030-2. [DOI] [PubMed] [Google Scholar]
- 34.Fung-Leung W P, Surh C D, Liljedahl M, Pang J, Leturcq D, Peterson P A, Webb S R, Karlsson L. Science. 1996;271:1278–1281. doi: 10.1126/science.271.5253.1278. [DOI] [PubMed] [Google Scholar]
- 35.Sette A, Ceman S, Kubo R T, Sakaguchi K, Appella E, Hunt D F, Davis T A, Michel H, Shabanowitz J, Rudersdorf R, Grey H M, DeMars R. Science. 1992;258:1801–1804. doi: 10.1126/science.1465617. [DOI] [PubMed] [Google Scholar]
- 36.Mellins E, Cameron P, Amaya M, Goodman S, Pious D, Smith L, Arp B. J Exp Med. 1994;179:541–549. doi: 10.1084/jem.179.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riberdy J M, Cresswell P. J Immunol. 1992;148:2586–2590. [PubMed] [Google Scholar]
- 38.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 39.Landais D, Beck B N, Buerstedde J-M, Degraw S, Klein D, Koch N, Murphy D, Pierres M, Tada T, Yamamoto K, Benoist C, Mathis D. J Immunol. 1986;137:3002–3005. [PubMed] [Google Scholar]
- 40.Tomonari K. Immunogenetics. 1988;28:455–458. doi: 10.1007/BF00355379. [DOI] [PubMed] [Google Scholar]
- 41.Kobori J A, Hood L, Shastri N. Proc Natl Acad Sci USA. 1992;89:2940–2944. doi: 10.1073/pnas.89.7.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ignatowicz L, Winslow G, Bill J, Kappler J, Marrack P. J Immunol. 1995;154:3852–3862. [PubMed] [Google Scholar]
- 43.Régnier-Vigouroux A, El Ayeb M, Defendini M L, Granier C, Pierres M. J Immunol. 1988;140:1069–1075. [PubMed] [Google Scholar]
- 44.Stern L J, Wiley D C. Cell. 1992;68:465–477. doi: 10.1016/0092-8674(92)90184-e. [DOI] [PubMed] [Google Scholar]
- 45.Germain R N, Hendrix L R. Nature (London) 1991;353:134–139. doi: 10.1038/353134a0. [DOI] [PubMed] [Google Scholar]
- 46.Chan S H, Cosgrove D, Waltzinger C, Benoist C, Mathis D. Cell. 1993;73:225–236. doi: 10.1016/0092-8674(93)90225-f. [DOI] [PubMed] [Google Scholar]
- 47.Lundberg K, Heath X, Kontgen F, Carbone F R, Short-man K. J Exp Med. 1995;181:1643–1651. doi: 10.1084/jem.181.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. J Exp Med. 1995;182:993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kropshofer H, Vogt A B, Moldenhauer G, Hammer J, Blum J S, Hammerling G J. EMBO J. 1996;15:6144–6154. [PMC free article] [PubMed] [Google Scholar]
- 50.Katz J F, Stebbins C, Appella E, Sant A J. J Exp Med. 1996;184:1747–1753. doi: 10.1084/jem.184.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Ham S M, Gruneberg U, Malcherek G, Broker I, Melms A, Trowsdale J. J Exp Med. 1996;184:2019–2024. doi: 10.1084/jem.184.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinet V, Vergelli M, Martin R, Bakke O, Long E O. Nature (London) 1995;375:603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- 53.Romagnoli P, Layet C, Yewdell J, Bakke O, Germain R N. J Exp Med. 1993;177:583–596. doi: 10.1084/jem.177.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pierre P, Denzin L K, Hammond C, Drake J R, Amigorena S, Cresswell P, Mellman I. Immunity. 1996;4:229–239. doi: 10.1016/s1074-7613(00)80431-8. [DOI] [PubMed] [Google Scholar]