Abstract
Glial-cell-line-derived neurotrophic factor (GDNF) is a potent neurotrophic factor for adult nigral dopamine neurons in vivo. GDNF has both protective and restorative effects on the nigro-striatal dopaminergic (DA) system in animal models of Parkinson disease. Appropriate administration of this factor is essential for the success of its clinical application. Since it cannot cross the blood–brain barrier, a gene transfer method may be appropriate for delivery of the trophic factor to DA cells. We have constructed a recombinant adenovirus (Ad) encoding GDNF and injected it into rat striatum to make use of its ability to infect neurons and to be retrogradely transported by DA neurons. Ad-GDNF was found to drive production of large amounts of GDNF, as quantified by ELISA. The GDNF produced after gene transfer was biologically active: it increased the survival and differentiation of DA neurons in vitro. To test the efficacy of the Ad-mediated GDNF gene transfer in vivo, we used a progressive lesion model of Parkinson disease. Rats received injections unilaterally into their striatum first of Ad and then 6 days later of 6-hydroxydopamine. We found that mesencephalic nigral dopamine neurons of animals treated with the Ad-GDNF were protected, whereas those of animals treated with the Ad-β-galactosidase were not. This protection was associated with a difference in motor function: amphetamine-induced turning was much lower in animals that received the Ad-GDNF than in the animals that received Ad-β-galactosidase. This finding may have implications for the development of a treatment for Parkinson disease based on the use of neurotrophic factors.
Keywords: recombinant adenovirus
Glial-cell-line-derived neurotrophic factor (GDNF) was isolated recently and is structurally related to members of the transforming growth factor β superfamily. In vitro, GDNF promotes survival, high-affinity dopamine uptake, and neurite outgrowth of embryonic dopaminergic (DA) neurons (1). GDNF also stimulates recovery of developing DA neurons after damage by 1-methyl-4-pyridinium (2). Intracerebral administration of GDNF over the substantia nigra (SN) completely prevents nigral cell death and atrophy in a rat model of Parkinson disease (3). Axotomy results in loss of tyrosine hydroxylase (TH)-containing neurons in the SN but this is largely prevented by repeated injections of GDNF adjacent to the SN (4). In mice, GDNF injected over the SN or into the striatum before 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine potently protects the DA system, and with both modes of administration, motor activity is increased above normal levels (5). Recently, intracerebral injection of the factor into rhesus monkeys showed its ability to protect the nigrostriatal DA pathway in a species closely related to humans (6). For all these reasons, this trophic factor is a potential candidate for the treatment of Parkinson disease.
An appropriate mode of administration of this factor is essential for its clinical efficacy. Intracerebral injection is necessary because this type of trophic factor cannot cross the blood–brain barrier. However, the direct delivery of purified proteins into the brain would necessitate repeated injections. A gene transfer method may, therefore, be a good alternative to obtain continuous expression after a single injection. In addition, as GDNF is a disulfide-bonded homodimer protein that is heterogeneously glycosylated (7), its constant delivery in its biologically correct form may best be obtained through exogenous expression after gene transfer. Intracerebral gene transfer to neural cells has been demonstrated with adenovirus (Ad) expressing β-galactosidase (βGal). We have recently shown that recombinant Ad is a vector of choice for in vivo gene therapy of diseases affecting the central nervous system (8, 9). Direct intracerebral injection of Ad vector results in efficient gene transfer to all cell types of the central nervous system. In neurons, the recombinant Ad can be transported retrogradely via axonal flow (10). This vector is also currently being improved for clinical applications.
We constructed a recombinant Ad encoding GDNF (Ad-GDNF). The Ad-GDNF directed production of large amounts of GDNF protein that was biologically active: it increased the survival rate of DA neurons in vitro. We injected the Ad-GDNF into rat striatum to allow expression in both DA axon terminals and DA cell bodies via retrograde transport. The animals were then subjected to a unilateral progressive lesion of nigral DA neurons by using the model of Parkinson disease developed by Sauer and Oertel (11). Mesencephalic nigral dopamine neurons of animals that received the Ad-GDNF were protected, whereas those of animals that received the Ad-βGal were not. Lesioned rats responded to amphetamine by a turning behavior. The better protection of DA cells in animals that received Ad-GDNF was associated with significantly less amphetamine-induced turning, indicating functional recovery.
MATERIALS AND METHODS
Construction of the GDNF Recombinant Ad.
The Ad-βGal, a gift from M. Perricaudet, has been described previously (12). To generate the Ad-GDNF, a cDNA containing the entire coding sequence of GDNF was isolated by reverse transcription of cultivated rat embryonic astrocyte mRNA followed by polymerase chain reaction (PCR). The astrocytes were cultivated as described by Lundberg et al. (13). The 5′ and 3′ PCR primers contain restriction enzyme sites to facilitate subsequent cloning, a Kozak consensus sequence to optimize initiation of translation (14), and GDNF-hybridizing sequences to isolate the rat cDNA (5′, 5′-CCGTCGACCTAGGCCACCATGAAGTTATGGGATGTCG-3′; 3′, 5′-CCGTCGACATGCATGAGCTCAGATACATCCACACC-3′). The PCR product was digested with SalI and ligated into the SalI site of a Bluescript plasmid (Stratagene) containing the polyadenylylation signal of the simian virus 40 large tumor gene. The rat GDNF cDNA was sequenced on both strands and found to be identical to that published by Lin et al. (1). The GDNF sequence and downstream polyadenylylation signal were isolated as a ClaI–KpnI fragment. This fragment was then inserted into pAd (a gift from M. Perricaudet), between the ClaI and EcoRV sites, downstream from the long terminal repeat of the Rous sarcoma virus promoter. The GDNF recombinant Ad was generated as previously described (9) in a BL3 controlled environment in accordance with French legislation. A high titer of Ad-GDNF virus [1011 plaque-forming units (pfu)/ml] was obtained.
In Vitro Adenoviral Gene Transfer.
Embryonic day 14 rat neuronal mesencephalic cells were isolated and cultivated in serum-free N-2 supplemented Dulbecco’s modified Eagle’s medium (DMEM). Rat primary astroglial cells prepared from embryonic day 15 striatum (13) and the HeLa cell line were cultivated in DMEM containing 10% fetal calf serum. All cells were plated on 48-well plates at an initial density of 100,000 cells per cm2. Quantities of GDNF were determined by using an ELISA kit from Promega (with a detection limit of 30 pg of GDNF). Infections were performed by incubation of the cells with Ad-GDNF or Ad-βGal, at a multiplicity of infection (MOI) of 25–200 pfu per cell, for 1 h at 37°C. The medium was replaced with fresh medium 24 h before collecting the conditioned medium 3 days after infection.
To assay the biological activity of GDNF, the DA-cell survival rate was quantified. Embryonic day 14 mesencephalic cells (100,000 cells per cm2) were plated on a layer of primary astroglial cells (100,000 cells per cm2) previously infected with Ad-GDNF or Ad-βGal at a MOI of 50 or 100. Six days later, the cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) and were processed for TH immunocytochemistry. The number of TH+ cells present in each well was counted under the microscope.
In Vivo Ad Gene Transfer in a Rat Model of Parkinson Disease.
Young adult female Sprague–Dawley rats (Charles River Breeding Laboratories) were used in all experiments. All surgery was performed under equithesin (3–4 ml/kg, i.p.) anesthesia and followed by subcutaneous injection of 5 ml of 5% glucose to prevent dehydration. The injection of Ad-GDNF was given in a BL3 controlled environment in accordance with French legislation. A total of 1.5 × 108 pfu of Ad-GDNF diluted in 9 μl of PBS was injected into nine sites (1 μl per site) of the striatum as described by Horellou et al. (9). Control animals received either 1.5 × 108 pfu of Ad-βGal virus diluted in 9 μl of PBS or were naive animals that did not receive treatment before 6-hydroxydopamine (6-OHDA). We also tested the effect of sham operation on amphetamine-induced turning after 6-OHDA by comparing the effect of intrastriatal injections of the vehicle (PBS) with naive animals that did not receive treatment before 6-OHDA.
To generate partial retrograde lesions, we used the rat model of Parkinson disease described by Sauer and Oertel (11). We adapted this lesion model to the virus injection procedure by injecting 6-OHDA in the center of the virus injection tracts in three deposits to obtain optimal protective effect of the virus. Six days after injecting the virus, the rats were anesthetized with equithesin (2–3 ml/kg, i.p.) and received a stereotaxic injection of 6-OHDA into their left striatum. Appropriate preparation of 6-OHDA is essential for the reproducibility of the lesion. 6-OHDA is unstable and its characteristics vary between batches. We therefore first divided one batch of 50 mg of 6-OHDA-HCl (Sigma) into 4- to 5-mg aliquots and kept them at −20°C before use. To dissolve the toxin, a stock solution of ascorbate saline (0.2 mg/ml, pH 4.30) was prepared on the day of the experiment and kept at 4°C. Each aliquot of 6-OHDA was dissolved immediately before use in ice-cold ascorbate saline (6-OHDA⋅HCl, 4 μg/μl). The preparation was kept on ice and protected from light during the experiment. A total of 5 μl of 6-OHDA was infused at a speed of 1 μl/min and was equally distributed between three sites (the cannula was left in place another 4 min before being withdrawn) at the following coordinates: AP +1.2 mm from bregma; L +2.5 mm lateral to midline; V −5 mm, 4.6 mm, and 4.2 mm ventral to dural surface (toothbar set at the level of the interaural line).
Histological Analysis.
After intrastriatal Ad delivery and 3 weeks after 6-OHDA injection, animals were perfused and their brains were processed for TH immunohistochemistry as described (9). The number of TH+ cell bodies present in the SN (ventral tegmental area excluded) was determined in every sixth serial coronal section (14 μm thickness) between the coordinates AP −4.3 and −6.4 mm from bregma. A Zeiss microscope at high magnification (objective, ×20) was used with the observer blind to the experimental group. DA survival was calculated as percentage of TH+ cells counted in the contralateral nonlesioned SN. The degree of TH innervation in the striatum was microscopically estimated by comparison with the density of TH+ fibers observed on the contralateral nonlesioned side. Ten to 12 brain sections per animal (distributed between the coordinates AP +1.7 and +0.2 mm from bregma) were processed for TH immunostaining. To assess general toxicity to the tissue of the various treatments, the size of the striatum was semiquantitatively determined on the same TH-stained sections. The maximal lateral extension of the striatum was measured with an ocular microscope equipped with a grid (Zeiss) and compared with the contralateral nonlesioned striatum to calculate the percentage of atrophy.
To visualize in vivo βGal-transgene expression, 14-μm-thick coronal sections through the caudate putamen and the SN were processed for immunohistochemistry using specific polyclonal antibodies (15).
Behavioral Analysis.
The injected animals were tested for amphetamine-induced turning 1, 2, and 3 weeks after intracerebral injection. Motor asymmetry was monitored in automated rotometer bowls [Imetronic, Bordeaux, France (16)] for 90 min after an injection of d-amphetamine sulfate (Sigma, 5 mg/kg, i.p.). At the end of the session, the animals received a subcutaneous injection of 5 ml of 5% glucose. A net rotation asymmetry score for each test was calculated by subtracting turns contralateral to the 6-OHDA lesion from turns ipsilateral to the lesion.
Statistical Analysis.
All values are expressed as the mean ± SEM. Differences among means were analyzed by using one-factor analysis of variance (ANOVA). When ANOVA showed significant differences, pair-wise comparisons between means were tested by the Scheffé post-hoc test. Correlations were performed by calculating the correlation coefficient, and subsequent simple linear regression was performed. In all analyses, the null hypothesis was rejected at the 0.05 level.
RESULTS
In Vitro Protective Effect of Ad-GDNF.
The Ad-GDNF was constructed by inserting the coding sequence of the rat GDNF precursor protein (1) under the control of the Rous sarcoma virus long terminal repeat promoter into a human type 5 E1E3-defective Ad. Mesencephalic and astrocytic cultures and HeLa cells were infected with various amounts of Ad-GDNF or Ad-βGal (MOI, 25–200). The ability of Ad-GDNF to induce GDNF expression was verified by reverse transcription-coupled PCR (data not shown) and by ELISA assay of GDNF protein. Maximal GDNF production was obtained at a MOI of 100 with all three cell types. The concentration of endogenous GDNF, measured in noninfected sister cultures, was close to the detection limit of the assay (35 pg/ml) in mesencephalic culture. In astrocytes it was about 5-fold higher (148 ± 10 pg/ml), in agreement with the capacity of this cell type to produce GDNF (17). Three days after infection by Ad-GDNF, the secretion of the trophic factor was increased by 54-fold in mesencephalic cells (1,980 ± 110 pg/ml) and by 12-fold in astrocytes (1,780 ± 90 pg/ml). Infection by the control Ad-βGal did not modify endogenous GDNF secretion levels in any of the cultures at any viral dose (data not shown), demonstrating that GDNF produced after infection by Ad-GDNF was a consequence of the gene transfer rather than a nonspecific induction of the endogenous GDNF gene by the infection. HeLa cells produced the factor after infection, although to a lower extent than neural cells (550 ± 20 pg/ml).
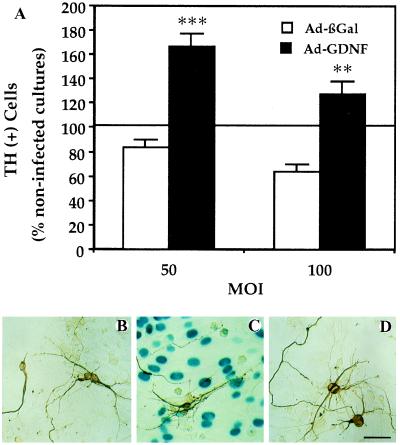
To demonstrate the neurotrophic activity of the secreted GDNF, we tested the ability of the Ad-GDNF to increase the survival rate of embryonic DA neurons after cocultivating these cells on a layer of infected astrocytes. The survival rate of DA neurons cultivated on layers of astrocytes infected with Ad-GDNF was 2-fold (at a MOI of 50 and 100) higher than that on layers of Ad-βGal-infected astrocytes (Fig. 1). The morphology of the DA cells cultivated on Ad-GDNF-infected astrocytes was clearly more differentiated than that of DA cells on control astrocytes, whether or not infected with Ad-βGal. Microscopic analysis revealed larger cell bodies, stronger TH immunoreactivity, and longer and more branched processes (Fig. 1).
Figure 1.
Increased survival of DA neurons in cultures infected with Ad-GDNF. (A) DA-cell counts by TH immunocytochemistry. Embryonic mesencephalic cells (100,000 cells per cm2) were plated on a layer of primary astroglial cells (100,000 cells per cm2) previously infected with Ad-GDNF or Ad-βGal. DA cells were identified with TH immunocytochemistry and counted. The values represent the percentages of total DA cells counted in noninfected cultures (means ± SEM of three determinations). In the control noninfected cultures, there were 982 ± 47 DA cells per well. ∗∗, P < 0.01; ∗∗∗, P < 0.001 versus Ad-βGal. (B–D) Differentiation of DA neurons visualized by TH immunocytochemistry. Photomicrographs illustrating the morphology of DA cells plated on a layer of primary astroglial cells previously or not infected. (B) Noninfected astrocytes, (C) astrocytes infected with Ad-ßGal (50 pfu per cell), (D) with Ad-GDNF (50 pfu per cell). βGal expression is visualized enzymatically with 5-bromo-4-chloro-3-indolyl β-d-galactoside as a substrate giving rise to a blue color. Note that the differentiation of DA neurons is much more advanced in cultures producing the transgenic GDNF than in control or Ad-βGal-infected cultures. (Bar = 100 μm.)
In Vivo Protective Effect of Ad-GDNF: Correlation Between DA-Cell Survival in the SN and Turning Behavior.
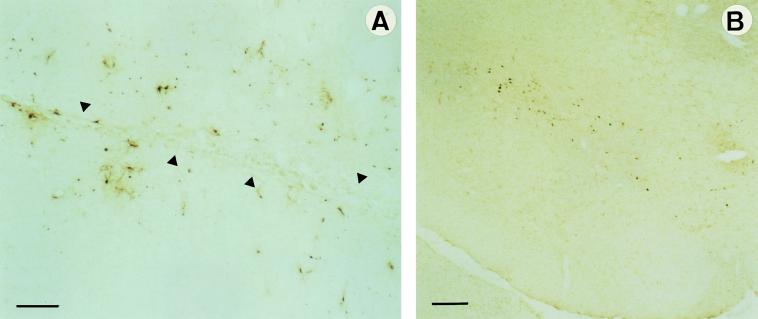
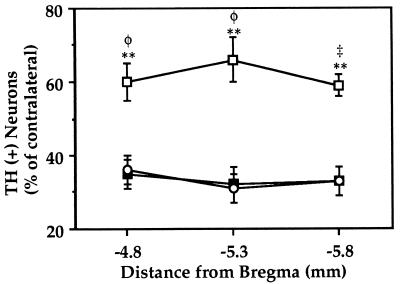
The efficacy of Ad-mediated GDNF gene transfer in vivo was tested in the rat model of Parkinson disease of Sauer and Oertel (11), which allows progressive degeneration of DA cells. GDNF was delivered at both DA terminals and DA cell bodies by injecting the virus unilaterally into the striatum to obtain expression at the site of the injection as well as in the SN via retrograde transport of the virus. After 6 days, the rats received 6-OHDA in their previously injected striatum. This toxin injected into the striatum causes ipsilateral nigral DA neuron loss (11). Three weeks after the unilateral 6-OHDA lesion, the animals were sacrificed. Immunohistochemical analysis using specific anti-Escherichia coli βGal antibodies showed numerous infected cells in the injected striatum and in the ipsilateral SN (Fig. 2). Substantial transgenic βGal expression was detected for at least 4 weeks after Ad delivery. This suggests that Ad-GDNF drove a high level of production of transgenic GDNF. The survival of DA neurons was analyzed throughout the SN between the coordinates AP −4.3 and −6.4 mm from bregma (Fig. 3 and Table 1). The animals treated with 6-OHDA alone or with Ad-βGal 6 days before the lesion showed a similar degree of DA-neuron degeneration. The survival of DA cells was only about 30% throughout the SN. That for the Ad-GDNF group was 60–62%, showing a significantly better protection than in the 6-OHDA alone group (P = 0.0003) or than in the Ad-βGal group (P = 0.0009): twice as many DA neurons survived in animals that received the Ad-GDNF than in those that did not or received Ad-βGal.
Figure 2.
Analysis of adenoviral transgene expression using βGal immunohistochemistry. Pictures of 14-μm-thick coronal sections through the caudate putamen (A) and SN (B) showing βGal-expressing cells 4 weeks after intrastriatal injection of Ad-βGal. Anti-E. coli βGal antibodies were used to distinguish the transgenic from the endogenous βGal activity. In the striatum, βGal+ cells are found along the needle tract (indicated by arrows in A) and up to 2 mm from the site of injection. Numerous infected cells can be observed in the SN compacta (B) after retrograde transport of viral particles delivered in the caudate putamen. (Bar = 200 μm.)
Figure 3.
Survival of DA neurons in the SN of 6-OHDA lesioned rats. The animals received intrastriatal Ad injections followed by 6-OHDA 6 days later. Three weeks after 6-OHDA injection, animals were sacrificed for TH immunohistochemistry. The number of TH+ cell bodies present in the SN at the coordinates AP −4.8, −5.3, and −5.8 mm from bregma (three or four sections per region of each animal) was determined. The values reported are means ± SEM for 6–11 rats per group and are expressed as percentages of TH+ cell counts in the contralateral nonlesioned SN. The survival of DA neurons is significantly higher in animals injected with Ad-GDNF (□) than with Ad-βGal (▪) or than in animals that received 6-OHDA alone (○). ∗∗, P < 0.01 versus 6-OHDA alone; φ, P < 0.01 and ‡, P < 0.001 versus Ad-βGal.
Table 1.
Survival of DA neurons and degree of TH innervation in 6-OHDA-lesioned rats subjected to different treatments
| Group | DA cellsa | TH SNb | Striatal sizec | TH striatumd |
|---|---|---|---|---|
| 6-OHDA (n = 10) | 31 ± 4 | + | −2.1 ± 0.7 | + |
| Ad-βGal (n = 8) | 31 ± 3† | + | −13.6 ± 1.8* | + |
| Ad-GDNF (n = 7) | 62 ± 5*‡ | +++ | −13.1 ± 2.3* | +++ |
Animals were injected with 6-OHDA 6 days after treatment (n, number rats per group). Coronal sections of SN and striatum were processed for TH immunohistochemistry. Values for DA cells and TH SN correspond to the analysis of five or six brain sections for each animal, where TH+ cell bodies were counted only in the SN and restricted to the coordinate AP − 5.3 mm from bregma. Values for striatal size and TH striatum correspond to the analysis of 10–12 brain sections per animal (between the coordinates AP +1.7 and +0.2 mm from bregma). DA cell bodies and DA neurites were more protected from 6-OHDA toxicity by Ad-GDNF than by Ad-βGal.
TH+ cells in SN (percent contralateral; mean ± SEM).
Estimation of TH-neurite density in SN: ++++, 100%; +++, 75%; ++, 50%; +, 25% contralateral.
Decrease in striatum size (percent contralateral; mean ± SEM).
Estimation of TH neurite density in the striatum (scale as above).
P < 0.001 versus 6-OHDA alone.
Not significant versus 6-OHDA alone.
P < 0.001 versus Ad-βGal.
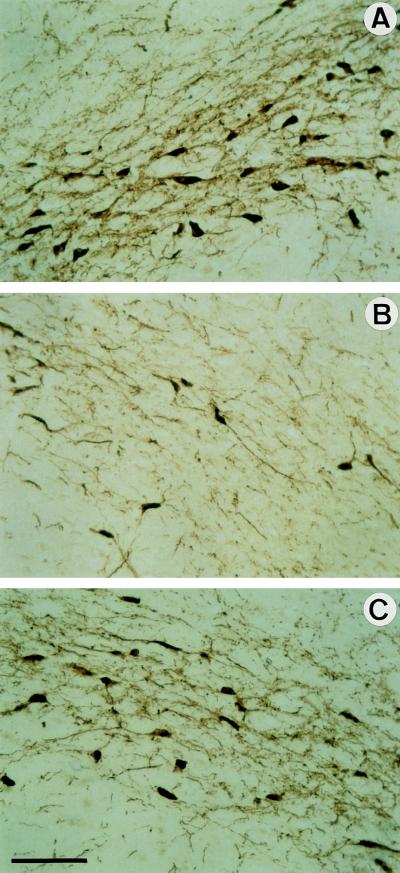
The Ad vector injected into the brain induces inflammation. We therefore investigated the toxicity of the virus by histological analysis. The injected striatum of animals treated with Ad vectors was more inflammatory and atrophied than striatum treated with 6-OHDA alone (about 13% versus 2%, Table 1). The inflammation and atrophy induced by the Ad-βGal or the Ad-GDNF were not significantly different (Table 1). To evaluate the toxicity of the virus injection, we measured the number of DA cells in animals that received Ad-βGal alone without injection of 6-OHDA. There was a reduction of 37 ± 6% (n = 4) in the number of DA cells 3 weeks after intrastriatal injection (data not shown). Interestingly, Ad toxicity was not additive with 6-OHDA toxicity (Table 1). Ad-GDNF may compensate for not only the toxicity induced by 6-OHDA but also that induced by the first-generation Ad used in this study. The overall protective action of Ad-GDNF was not only apparent as an increased survival of DA neurons but also as more TH innervation in the striatum and SN after 6-OHDA administration than in Ad-βGal-treated animals (Table 1 and Fig. 4). Therefore, it appears that the Ad-GDNF injection in the striatum protected DA cell bodies as well as DA terminals in the striatum from the toxicity of 6-OHDA.
Figure 4.
Histological analysis of SN DA neurons of treated rats. Representative pictures of 14-μm-thick coronal sections through the SN processed for TH immunohistochemistry are shown. (A) Section contralateral to the lesion. (B and C) Sections ipsilateral to the lesion of animals injected with Ad-βGal (B) or Ad-GDNF (C). The aspect of the ipsilateral SN from rats that received only 6-OHDA is comparable to those of rats that received 6-OHDA and Ad-βGal (see B). (Bar = 100 μm.) The number of TH+ cell bodies and density of TH-stained fibers were both higher in rats that received Ad-GDNF than Ad-βGal (C versus B).
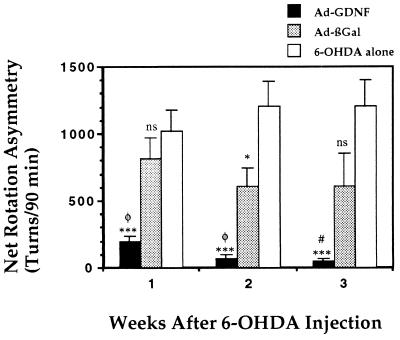
To evaluate the behavioral consequence of the DA-neuron degeneration, we monitored amphetamine-induced turning 1, 2, and 3 weeks after the lesion (Fig. 5). Control animals that received 6-OHDA had a mean rotation score of 1,020 ± 160 net ipsilateral turns over a 90-min period 1 week after the lesion. This turning behavior was stable for at least 3 weeks after the lesion. Injection of Ad-βGal 6 days prior to the lesion slightly decreased the rotation score compared with 6-OHDA alone to 810 ± 150 at 1 week after lesion. The score decreased but not significantly, thereafter. The rotation score of the animals that received Ad-βGal was not significantly different from that of the animals that received 6-OHDA alone 1 week after lesion (P = 0.38). A statistical difference was observed 2 weeks after lesion (P = 0.03) but did not persist to the third week after lesion (P = 0.06) (Fig. 5). Injection of Ad-GDNF 6 days prior to the lesion reduced the rotation score to 200 ± 30 at 1 week after lesion. The rotation score decreased further to 70 ± 25 after 2 weeks and 47 ± 17 after 3 weeks. The difference in rotation score between animals injected with Ad-GDNF and animals that received Ad-βGal (P = 0.004, 0.002, and 0.03 at 1, 2, and 3 weeks, respectively) or 6-OHDA alone (P = 0.0008, 0.0002, and 0.0001) was highly significant (Fig. 5).
Figure 5.
Effect of Ad-GDNF on amphetamine-induced rotational behavior in 6-OHDA-lesioned rats. Ad-GDNF (n = 7) or Ad-βGal (n = 8) were delivered into the left striatum of animals by stereotaxic injection. Six days thereafter, 20 μg of 6-OHDA⋅HCl was injected into the left striatum of all two groups of animal. A third group of animal received no preinjection before 6-OHDA lesion (6-OHDA only, n = 10). The ability of the different treatments to counteract the neurotoxin action was assessed by following asymmetric rotational behavior induced by amphetamine administration 1, 2, and 3 weeks after 6-OHDA injection. The values reported are the means ± SEM (bars) of net ipsilateral turns over 90 min (turns contralateral to the lesion subtracted). ∗, P < 0.05; ∗∗∗, P < 0.001; and ns, not significant versus 6-OHDA alone. #, P < 0.05 and φ, P < 0.01 versus Ad-βGal.
We also tested the effect of sham operation on amphetamine-induced turning after 6-OHDA. A group of animals were subjected to intrastriatal injections of the vehicle (PBS). Six days later, this group of animals and a group of naive animals received intrastriatal 6-OHDA and were tested for amphetamine-induced turning. The rates of rotation of these two groups were not significantly different (data not shown). Thus neither the Ad-βGal injection nor the sham operation induced a protective effect, demonstrating the functional effect of the Ad-GDNF in the model of Parkinson disease used.
The correlation between the extent of DA-neuron survival in the SN and the rate of amphetamine-induced rotation 3 weeks after 6-OHDA injection was analyzed by plotting the two variables against each other. A significant correlation was found between amphetamine rotation (Y) and the percentage of surviving DA cells (X): (Y = 1,652 − 23.8X; r2 = 0.447; P = 0.0003; n = 25). Because the groups of rats that received 6-OHDA alone or Ad-βGal before 6-OHDA had similar DA-cell survival rates (Fig. 3) and similar amphetamine-induced rotation rates (Fig. 5), they were pooled for this regression analysis (Y = 1,811 − 27.5X; r2 = 0.259; P = 0.03; n = 18). Interestingly, the animals that received Ad-GDNF 6-days before 6-OHDA gave a regression curve with a much smaller slope (Y = 187 − 2.2X; r2 = 0.597; P = 0.04; n = 7) than the other groups. This difference in the linear regression curves illustrates the fact that animals that received the Ad-GDNF had a better motor functional score (lower amphetamine-induced rotation response) than predicted by their higher DA-cell survival rate in comparison to animals receiving 6-OHDA alone or 6-OHDA and Ad-βGal. Histological analysis showed a higher protection and/or sprouting of axonal terminal in the striatum and of dendrites in the SN of animals that received Ad-GDNF (Table 1 and Fig. 4). These observations suggest that not only the improved DA-cell survival but also the protection of DA neurites in the striatum and/or in SN contribute to reduce turning behavior. It can be concluded that Ad-GDNF protected DA cells in the SN and protected or stimulated DA-neurite arborization resulting in better motor function.
DISCUSSION
We evaluated the capacity of Ad-mediated GDNF gene transfer to protect DA neurons from the degeneration associated with Parkinson disease. We used a rat model of the disease obtained by intrastriatal injection of 6-OHDA that induces a progressive ipsilateral nigrostriatal degeneration (11). Unlike lesions by intranigral injection of 6-OHDA, DA-nigral cells do not lose their DA phenotype but mostly undergo apoptosis (11, 18). DA cells start degenerating 1 week after 6-OHDA injection, but extensive death of nigral neurons is observed only 4 weeks after lesion (3). In this paradigm, administration of recombinant GDNF protein to the SN, starting on the day of lesion, completely prevents DA cell death and atrophy (3). Immunostaining using antibodies specific for E. coli βGal protein shows the efficiency of the gene transfer using Ad vectors both in the striatum and in SN. In most animals, a large number of cells expressing the transgene was detected within the denervated striatum and in the SN. The labeled cells were dispersed throughout the entire caudate putamen with a pattern similar to that previously observed (9). The substantial βGal-transgene expression for at least 4 weeks after Ad delivery suggests that the protective effect observed after Ad-GDNF injection is probably due to the production of exogenous GDNF.
The sites of 6-OHDA injection were slightly less lateral and more dispersed (three deposits along the needle track) than those described by Sauer and Oertel (11). This led to a sustained amphetamine-induced rotation as early as 1 week after the lesion and for at least 3 weeks. Recently, Winkler et al. (19) reported that two deposits of 6-OHDA caused amphetamine-induced rotation. An important consequence of the simplicity of the behavioral test is that it facilitates the analysis of recovery after treatment. Amphetamine-induced rotation has been used previously as a marker of the DA depletion for partial lesions studies (20, 21). The dose we used (5 mg/kg) is a “standard rotational” (22) that has been widely used (23).
Amphetamine-induced rotation correlates significantly with DA depletion in the lesioned striatum (23). In our study, the number of DA-cell bodies present in the SN of the lesioned animals was determined 3 weeks after 6-OHDA injection. We show a significant correlation between amphetamine-induced rotation and the extent of DA-cell loss in the lesioned SN after intrastriatal 6-OHDA lesion. The comparison between animals receiving GDNF recombinant Ad and animals receiving the control Ad appears to be the most appropriate for determining the value of Ad as a tool to express GDNF. The inflammatory response, the destruction of neuronal tissue, and the gliosis induced in the striatum at the sites of injection were relatively limited and similar in both the Ad-GDNF and the Ad-βGal groups. We found a statistically significant difference in the rate of amphetamine-induced rotation and in the degree of DA survival between the Ad-GDNF and the Ad-βGal groups. The protective effect of Ad-GDNF can be explained by expression of the transgene in the striatum and in the SN via retrograde transport of the Ad vector. Indeed, GDNF can be retrogradely transported by DA neurons of the nigrostriatal pathway via a specific receptor-mediated uptake mechanism operating in the adult (24). In our study, the availability of the neurotrophic factor to both the DA-cell bodies and to the DA-nerve terminals prevented not only DA-cell death but also striatal denervation. This most probably allowed the functional recovery that was observed after Ad-GDNF administration. Interestingly, in a similar lesion model, Winkler et al. (19) injected GDNF protein above the SN and reported no reduction of rotational behavior, although DA-cell survival improved. Recently, Choi-Lundberg et al. (25) reported a similar study using the same lesion model as the one we used with injection of GDNF-recombinant Ad near the SN. However, they show a protection effect of the Ad-GDNF only on DA survival, and they report no behavioral data. The recent study of Winkler et al. (19) where GDNF protein was injected near the SN and yielded high rates of DA-cells survival without any behavioral effect. Therefore, the value of Ad-GDNF injection near the SN for therapy appears small. Our study suggests that GDNF expression in both striatum and SN not only allows protection of striatal DA innervating fibers and of DA-cell bodies but also limits motor impairment, suggesting possible therapeutic value of this method.
The Ad appears to have toxic effects: the size of the striatum was slightly reduced in both the Ad-GDNF- and Ad-βGal-injected animals; and 37% of DA neurons were destroyed after Ad-βGal delivery (omitting toxin injection). The mechanism of this toxicity is not clearly understood. It may be caused by envelope virion particles, by expression of viral proteins, and/or by antigen-mediated cytotoxicity (26). It is thus likely that the Ad-GDNF compensates not only for the toxicity induced by 6-OHDA but also for that induced by the Ad. In this case, the protective activity of the Ad-GDNF may be higher than that observed. Work is underway to increase the therapeutic action of the Ad-GDNF by using third-generation Ad, a less toxic version recently developed (27) and by association with antiinflammatory drugs to diminish the Ad-induced toxicity.
Our study demonstrates that a recombinant Ad encoding GDNF significantly improved DA-cell survival in the SN and DA-neurite arborization in SN and in striatum ipsilateral to the injection site. This effect is associated with reduction in turning behavior 1, 2, and 3 weeks after 6-OHDA lesion. These results suggest that GDNF gene transfer mediated by an Ad vector might be of therapeutic value for Parkinson disease.
Acknowledgments
We thank Prof. A. Björklund for his advice; Drs. M.-H. Buc-Caron and M. Nosten-Bertrand for critical reading of the manuscript; and N. Dufour, M.-L. Samolyk, and M.-C. Geoffroy for excellent technical assistance; and Drs. M. Perricaudet, G. Hasse and A. Kahn for giving access to their BL3 facilities. This study was supported by grants from the Centre National de la Recherche Scientifique, Human Science Frontier Program, the European Science Foundation ENP program, the European Community BIOMED 2 Program (BMH 4.CT96-1012), Association Française contre les Myopathies, and Rhône-Poulenc Rorer. A. B.-B. was supported by Swiss National funds, the Ciba Geigy–Jubilaeums Stiftung and the Freiwillige Akademische Gesellschaft–Basel.
ABBREVIATIONS
- 6-OHDA
6-hydroxydopamine
- Ad
adenovirus
- βGal
β-galactosidase
- DA
dopaminergic
- GDNF
glial-cell-line-derived neurotrophic factor
- MOI
multiplicity of infection
- pfu
plaque-forming unit
- SN
substantia nigra
- TH
tyrosine hydroxylase
References
- 1.Lin L F, Doherty D H, Lile J D, Bektesh S, Collins F. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 2.Hou J G, Lin L F, Mytilineou C. J Neurochem. 1996;66:74–82. doi: 10.1046/j.1471-4159.1996.66010074.x. [DOI] [PubMed] [Google Scholar]
- 3.Sauer H, Rosenblad C, Björklund A. Proc Natl Acad Sci USA. 1995;92:8935–8939. doi: 10.1073/pnas.92.19.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck K D, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen R A, Rosenthal A, Hefti F. Nature (London) 1995;373:339–341. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- 5.Tomac A, Widenfalk J, Lin L-F, Kohno T, Ebendal T, Hoffer B J, Olson L. Proc Natl Acad Sci USA. 1995;92:8274–8278. doi: 10.1073/pnas.92.18.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gash D M, Zhang Z, Ovadia A, Cass W A, Yi A, Simmerman L, Russell D, Martin D, Lapchak P A, Collins F, Hoffer B J, Gerhardt G A. Nature (London) 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 7.Lin L F, Zhang T J, Collins F, Armes L G. J Neurochem. 1994;63:758–768. doi: 10.1046/j.1471-4159.1994.63020758.x. [DOI] [PubMed] [Google Scholar]
- 8.Le Gal La Salle G, Robert J J, Berrard S, Ridoux V, Stratford-Perricaudet L D, Perricaudet M, Mallet J. Science. 1993;259:988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- 9.Horellou P, Vigne E, Castel M N, Barnéoud P, Colin P, Perricaudet M, Delaère P, Mallet J. NeuroReport. 1994;6:49–53. doi: 10.1097/00001756-199412300-00014. [DOI] [PubMed] [Google Scholar]
- 10.Ridoux V, Robert J J, Zhang X, Perricaudet M, Mallet J, Le Gal La Salle G. Brain Res. 1994;648:171–175. doi: 10.1016/0006-8993(94)91919-4. [DOI] [PubMed] [Google Scholar]
- 11.Sauer H, Oertel W H. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- 12.Stratford-Perricaudet L D, Makeh I, Perricaudet M, Briand P. J Clin Invest. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundberg C, Horellou P, Mallet J, Björklund A. Exp Neurol. 1996;139:39–53. doi: 10.1006/exnr.1996.0079. [DOI] [PubMed] [Google Scholar]
- 14.Kozak M. Nucleic Acids Res. 1985;12:857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabaté O, Horellou P, Vigne E, Colin P, Perricaudet M, Buc-Caron M H, Mallet J. Nat Genet. 1995;9:256–260. doi: 10.1038/ng0395-256. [DOI] [PubMed] [Google Scholar]
- 16.Ungerstedt U, Arbuthnott G W. Brain Res. 1970;24:485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- 17.Schaar D G, Sieber B A, Dreyfus C F, Black I B. Exp Neurol. 1993;124:368–371. doi: 10.1006/exnr.1993.1207. [DOI] [PubMed] [Google Scholar]
- 18.Bowenkamp K E, David D, Lapchak P L, Henry M A, Granholm A C, Hoffer B J, Mahalik T J. Exp Brain Res. 1996;111:1–7. doi: 10.1007/BF00229549. [DOI] [PubMed] [Google Scholar]
- 19.Winkler C, Sauer H, Lee C S, Björklund A. J Neurosci. 1996;16:7206–7215. doi: 10.1523/JNEUROSCI.16-22-07206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hefti F, Melamed E, Wurtman R J. Brain Res. 1980;195:123–137. doi: 10.1016/0006-8993(80)90871-9. [DOI] [PubMed] [Google Scholar]
- 21.Heikkila R E, Shapiro B S, Duvoisin R C. Brain Res. 1981;195:123–137. doi: 10.1016/0006-8993(81)90614-4. [DOI] [PubMed] [Google Scholar]
- 22.Björklund A, Stenevi U. Brain Res. 1979;177:555–560. doi: 10.1016/0006-8993(79)90472-4. [DOI] [PubMed] [Google Scholar]
- 23.Hudson J L, van Horne C G, Stromberg I, Brock S, Clayton J, Masserano J, Hoffer B J, Gerhardt G A. Brain Res. 1993;626:167–174. doi: 10.1016/0006-8993(93)90576-9. [DOI] [PubMed] [Google Scholar]
- 24.Tomac A, Lindqvist E, Lin L-F, Ogren S O, Young D, Hoffer B J, Olson L. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 25.Choi-Lundberg D L, Lin Q, Mohajeri H, Chang Y N, Chiang Y L, Hay C M, Davidson B L, Bohn M C. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- 26.Byrnes A P, MacLaren R E, Charlton H M. J Neurosci. 1996;16:3045–3055. doi: 10.1523/JNEUROSCI.16-09-03045.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh P, Dedieu J F, Orsini C, Vigne E, Denèfle P, Perricaudet M. J Virol. 1996;70:559–565. doi: 10.1128/jvi.70.1.559-565.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]