Abstract
A cross-maze task that can be acquired through either place or response learning was used to examine the hypothesis that posttraining neurochemical manipulation of the hippocampus or caudate-putamen can bias an animal toward the use of a specific memory system. Male Long-Evans rats received four trials per day for 7 days, a probe trial on day 8, further training on days 9–15, and an additional probe trial on day 16. Training occurred in a cross-maze task in which rats started from a consistent start-box (south), and obtained food from a consistent goal-arm (west). On days 4–6 of training, rats received posttraining intrahippocampal (1 μg/0.5 μl) or intracaudate (2 μg/0.5 μl) injections of either glutamate or saline (0.5 μl). On days 8 and 16, a probe trial was given in which rats were placed in a novel start-box (north). Rats selecting the west goal-arm were designated “place” learners, and those selecting the east goal-arm were designated “response” learners. Saline-treated rats predominantly displayed place learning on day 8 and response learning on day 16, indicating a shift in control of learned behavior with extended training. Rats receiving intrahippocampal injections of glutamate predominantly displayed place learning on days 8 and 16, indicating that manipulation of the hippocampus produced a blockade of the shift to response learning. Rats receiving intracaudate injections of glutamate displayed response learning on days 8 and 16, indicating an accelerated shift to response learning. The findings suggest that posttraining intracerebral glutamate infusions can (i) modulate the distinct memory processes mediated by the hippocampus and caudate-putamen and (ii) bias the brain toward the use of a specific memory system to control learned behavior and thereby influence the timing of the switch from the use of cognitive memory to habit learning to guide behavior.
According to the “multiple memory systems” hypothesis, different forms of memory are organized in independent brain systems. This hypothesis is supported by studies involving several mammalian species, including rats (1–6), monkeys (7–10), and humans (11–14). For example, in rats, findings from double-dissociation experiments using lesion (3–6, 15) and posttraining intracerebral drug injection techniques (16–19) indicate that the hippocampal system and caudate-putamen are parts of independent memory systems.
Characteristics of the psychological operating principles that distinguish multiple memory systems have been proposed by numerous investigators (e.g., refs. 1, 2, 8, 9, 11, 13, 20, and 21), and the design of each of these theories, particularly those derived from animal research, has been influenced to some extent by the historical debate between “cognitive” and “stimulus–response” (S–R) animal learning theorists. The cognitive view, exemplified by the early work of Tolman (22, 23), holds that animals acquire knowledge-based expectations that serve to guide behavior in a purposeful manner, a form of learning in which relationships among multiple stimuli may be acquired. In contrast, the S–R view, exemplified by the early work of Thorndike (24, 25) and Hull (26), holds that animals acquire S–R associations or habits, a form of learning in which reinforcement contingencies influence the ability of stimuli to evoke learned responses, in the absence of cognitive knowledge. The double dissociation observed in learning tasks after manipulations of the hippocampal system and caudate-putamen are consistent with the hypothesis that these brain structures mediate cognitive memory and S–R habit formation, respectively (3–5, 8, 9).
One paradigm in which cognitive and S–R learning may be contrasted involves the use of a cross maze (27, 28). The cross maze is essentially a T maze built such that the two goal arms (e.g., east, west), can be approached from start boxes located on either side of the maze (e.g., north, south). In one version of this task, rats are trained to approach a consistently baited goal-box (e.g., west) from the same start-box location (e.g., south) over several trials. According to the cognitive perspective, rats learn this task by acquiring a “cognitive map” in which knowledge concerning the spatial location of the food reward is represented in memory. The S–R theoretical perspective suggests that rats learn this task by acquiring a response tendency (e.g., a specific directional body turn), a learned S–R habit gradually strengthened by reinforcement, and which does not require spatial knowledge. These learning mechanisms can be contrasted during a probe trial in which trained rats are placed into a novel start-box (e.g., north), and allowed to select a goal-arm. In the example given, rats that have acquired knowledge of the spatial location of the food reward (i.e., “place” learners) would be expected to approach the west maze-arm, whereas rats that had acquired a specific body turn (i.e., “response” learners) would be expected to approach the east maze arm. Numerous studies with the cross maze were conducted to evaluate the merits of cognitive and S–R learning theory; however, the findings were equivocal. Depending in part on experimental conditions, intact rats were found to acquire both place and response learning tendencies (for review, see ref. 28).
Recently, the differential mnemonic roles of the hippocampal system and caudate-putamen were doubly dissociated in a cross-maze task (29). In that study, control rats given a probe trial in the cross maze during an early point in training (day 8) predominantly exhibited place learning, whereas on a second probe trial given after extended training (day 16), rats “shifted” to predominantly displaying response learning. Thus, in a cross-maze task that may be acquired through either a cognitive or S–R learning approach, early learning is controlled by a cognitive mechanism, whereas extended training shifts control over learned behavior to a S–R habit mechanism. Neural inactivation of the hippocampus produced by injection of the local anesthetic lidocaine selectively blocked expression of place learning on the first probe trial and left expression of response learning on the second probe trial intact. In contrast, lidocaine injections into the dorsolateral caudate-putamen left place learning on the initial probe trial intact and on the second probe trial blocked expression of response learning, revealing preserved place learning (29).
The present study was designed to extend these findings by examining the hypothesis that a posttraining memory-enhancing neurochemical manipulation of the hippocampus or caudate-putamen can bias the brain toward the use of a particular memory system in controlling learned behavior and thereby influence the “timing” of the switch from use of cognitive memory to a S–R habit to guide behavior. Accordingly, rats trained in the cross maze received posttraining intracerebral (hippocampus or caudate-putamen) injections of glutamate during early time points in training, and the mnemonic effects of this manipulation were tested on subsequent probe trials. The use of posttraining drug treatments was developed (30, 31) based on early clinical and experimental findings suggesting that immediately after a training experience, memory is in a labile state and over time is “consolidated” into a more permanent state (32–34). Consistent with this notion, the memory modulatory influence of posttraining drug treatments is time-dependent, losing effectiveness as the interval between training and treatment is increased. In addition, the use of posttraining treatments controls for possible drug effects on nonmnemonic factors (e.g., sensory, motor, or motivational) that may influence task performance (31). Glutamate was used in the present study in view of evidence that posttraining intrahippocampal and intracaudate glutamate infusion enhances memory in a task-dependent manner consistent with the hypothesis that these two structures selectively mediate cognitive memory and S–R habit formation, respectively. For example, intrahippocampal injections selectively enhanced memory in a hidden platform water maze task, and intracaudate injection of glutamate selectively enhanced memory in a visible platform water maze task (18).
Materials and Methods
Subjects.
Subjects were 43 male Long-Evans rats (275–300 g; Charles River Breeding Laboratories) individually housed in a temperature-controlled environment on a 12-hour light-dark cycle with the lights on from 7 a.m. to 7 p.m.
Apparatus.
The apparatus was an elevated (75 cm), open wooden cross maze painted flat gray. The maze consisted of four arms (north, south, east, and west) of an eight-arm radial maze (other maze arms were removed). The arms of the cross maze measured 60 × 9 cm. The center platform of the maze connecting the four arms measured 40 cm in diameter. A clear Plexiglas cross-shaped alleyway structure placed on the center platform connected the four arms of the cross maze. The alleyways measured 20 × 9 × 15 cm. A recessed food well was present at the end of the west arm of the maze. The maze was located in a testing room that contained several extramaze cues and was cleaned daily to inhibit intramaze olfactory cues.
Surgery.
Animals were anesthetized with a ketamine (100 mg/kg) and xyline (50 mg/kg) cocktail and implanted with bilateral guide cannula in the dorsal hippocampus (10-mm length) or dorsolateral caudate-putamen (15-mm length) by using standard stereotaxic techniques. The cannulae (23 gauge) were anchored to the skull with jeweler’s screws and dental acrylic. The brain coordinates for the dorsal hippocampal placements were AP = −3.1 mm, ML = ±2.5 mm, and DV = −2.0 mm from bregma. Coordinates for the dorsolateral caudate placements were AP = −0.26 mm, ML = ±4.2 mm, and DV = −4.0 mm from bregma. These coordinates were chosen based on previous research indicating that injection of lidocaine into these hippocampal and caudate sites selectively block expression of place and response learning in a cross maze, respectively (29). Behavioral training began 7–10 days after surgery.
Drugs/Injection Procedures.
l-Glutamic acid (Research Biochemicals, Natick, MA) was dissolved in physiological saline. Injections (0.5 μl) were administered intracerebrally via guide cannula using 30-gauge injection needles connected by polyethylene tubing to 10-μl Hamilton microsyringes. The injections were delivered over 52 sec with an electronically controlled syringe pump (Sage Instruments, Boston), and the injections needles (extending 1 mm from the end of the guide cannula) were left in place an additional 60 sec to allow for diffusion of solution away from the needle tip. Previous findings indicate that an acute posttraining administration of 5 μg of glutamate can enhance memory in an inhibitory avoidance task when injected intracerebroventricularly in mice (35) or directly into the hippocampus in rats (36). In the water maze, posttraining intrahippocampal injection (2 μg) enhanced memory in a hidden platform task, and intracaudate injection (5 μg) enhanced memory in a visible platform task (18). Smaller doses (1 μg, hippocampus; 2 μg, caudate-putamen), which had produced trends toward memory enhancement in the water-maze tasks (18), were used in the present study. Control experiments using injections of cresyl violet into the hippocampus and caudate-putamen at the present stereotaxic coordinates and injection parameters (i.e., 0.5 μl over 52 seconds) result in tissue stain confined largely to the intended target, with some spread observed dorsal to the target site along the outside of the cannula tract. The dye injections suggest a primarily localized effect of the drug treatment. However, in the absence of autoradiographic analyses using glutamate infusion, the possibility of injection spread to adjacent brain structures cannot be completely ruled out.
Histology.
Animals were anesthetized with a 1-ml injection of sodium pentobarbital and perfused with saline followed by a 10% formal-saline solution. The brains were removed and sectioned at 20 μm through the cannula tract region, slide mounted, and stained with cresyl violet. The slides were examined for verification of cannula placement and injection needle tip location using the atlas of Paxinos and Watson (37).
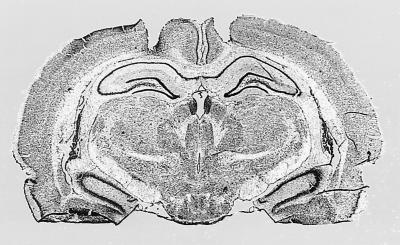
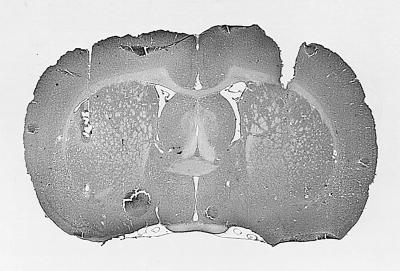
Results of the histological examination for the hippocampal placements indicated that the injection needle tips were located in the dorsal hippocampus, ranging from −3.14 mm to −3.6 mm anterior to posterior from bregma. Caudate placements were located in the dorsolateral caudate, ranging from 0.2 mm to −0.4 mm anterior to posterior from bregma. Photomicrographs of hippocampal and caudate-putamen cannula tracts of two rats that had each received glutamate infusions are provided in Fig. 1. In some animals, a small area of gliosis was observed at the immediate injection site. However, this was observed in a small number of both glutamate- and saline-treated rats, suggesting the damage likely resulted from the repeated intracerebral injections.
Figure 1.
Photomicrogaphs of cannula tracts and placement in dorsal hippocampus (Upper) and dorsolateral caudate-putamen (Lower). Each of these rats received glutamate infusions. The hippocampal rat (Upper) displayed place learning, and the caudate rat (Lower) displayed response learning on both probe trials, respectively.
Behavioral Procedures.
The behavioral procedures were similar to those previously described (29). Before maze training, rats were reduced to 85% of their ad lib feeding weights over 7 days and maintained at this weight throughout the experiment. On two consecutive days, rats were placed into the cross maze in the start box (end of south arm) and allowed to explore the maze for 5 min. No food was present in the maze on either of these two habituation days. Access to the north arm of the cross maze was blocked during habituation and the subsequent food-rewarded training trials with a clear Plexiglas shield. After habituation on both days, rats were allowed to consume 10 45-mg Noyes food pellets in their home cage. Food trials began on day 3. On each food trial, rats were placed into the start box and allowed to traverse the maze and consume a single Noyes food pellet located in the food cup at the end of the goal arm of the maze (west arm). On the initial two food trials only, a trail of four pellets leading to the food cup was placed along the length of the goal arm. Each rat received four food-rewarded trials per day. Entries into the unbaited arm of the maze (east) were scored as incorrect responses during the training trials, and entries into the baited arm (west) were scored as correct responses. A correction procedure was used during training; rats making an incorrect response were allowed to trace back to the baited maze arm and consume the food pellet. If a rat failed to consume the food within 1 min, the trial was terminated. After a trial, the rat was placed into a holding cage located off the maze and behind the start box (i.e., south) for a 30-sec intertrial interval.
On days 4, 5, and 6 of the food-rewarded trials, rats randomly assigned to treatment groups (n = 10–11 per group) received an immediate posttraining intrahippocampal (1.0 μg) or intracaudate (2.0 μg) injection of either glutamate or saline (0.5 μl). On day 8 of training, a single probe trial was given. On the probe trial, rats were placed in the start box opposite that used during training (i.e., end of north arm) and were allowed to make an entry into either the baited west arm or unbaited east maze arm. The entrance to the south arm (i.e., the arm containing the start box used during training) was blocked by a clear Plexiglas shield on the probe trial. Rats entering the west arm on the probe trial were designated place learners (i.e., rats going to the place where food was located during training), and rats entering the east arm on the probe trial were designated response learners (i.e., rats making the same body turn response as during training). The experimenter was blind to the previous posttraining treatments on the probe trials. On day 9 of training, food-rewarded trials (four per day) were reinstated using procedures identical to those of training days 1–7, except that no posttraining drug treatments were administered. On day 16, a second probe trial was given using procedures identical to the probe trial of day 8.
Results
The mean number of errors for the various treatment groups during the preinjection training trials (food days 1–4/trials 1–16), and the postinjection training trials (food days 5–14/trials 17–56) are shown in Table 1. Separate two-way ANOVAs with one repeated measure were computed on the number of incorrect responses made (i.e., selection of the east maze arm) during the pre- and postinjection training trials. The analyses on the preinjection trials revealed no significant group effect (F3,39 = 0.564, not significant), or group × trial interaction (F3,9 = 0.285, not significant). A significant trial effect revealed that all groups improved performance over the preinjection trials (F3,3 = 6.94, P < 0.01). This result indicates that any influence of the subsequent posttraining glutamate injections on the use of place and response learning as assessed on the probe trials is not due to a differential rate of initial task acquisition among groups. Similarly, a two-way one-repeated measures analysis on the postinjection trials revealed no significant group difference (F3,39 = 0.143, not significant), or group × trial interaction (F3,27 = 0.917, not significant). Again, a significant trial effect revealed that all groups improved performance over the postinjection trials (F3,9 = 6.44, P < 0.01). As shown in Table 1, all groups were performing at a mean of approximately one error per four daily trials (range 1.09–1.27), from the last preinjection day (i.e., day 4) through the end of food-rewarded training (day 14). As noted above, statistically significant improvement was seen in all groups over days 5–14, suggesting that some latitude existed for posttraining intracerebral glutamate injections to strengthen memory. However, the high level of performance reached during training days 1–4 may have precluded observation of an enhancing effect of glutamate infusion on memory, at least when the measurement assessed is the number of task errors.
Table 1.
| Group | T 1–4 (day 1) | T 5–8 (day 2) | T 9–12 (day 3) | T 13–16 (day 4) | T 17–56 (days 5–14) |
|---|---|---|---|---|---|
| C-Sal | 1.73 | 1.64 | 1.27 | 1.27 | .636 |
| C-Glu | 1.64 | 1.54 | 1.09 | 1.09 | .673 |
| H-Sal | 1.90 | 1.63 | 1.54 | 1.18 | .655 |
| H-Glu | 1.64 | 1.54 | 1.45 | 1.09 | .591 |
Rats received four daily training trials and were given posttraining injections of glutamate or saline on days 4, 5, and 6. The high level of performance reached by all groups by day 4 may have precluded observation of memory enhancing effects of glutamate when the number of errors is used as a measure of memory. However, an influence of posttraining glutamate infusion on memory was revealed via an influence on the later use of place or response learning on the probe trials (see Fig. 2). T, trial; C, caudate; Sal, saline; H, hippocampus; Glu, glutamate.
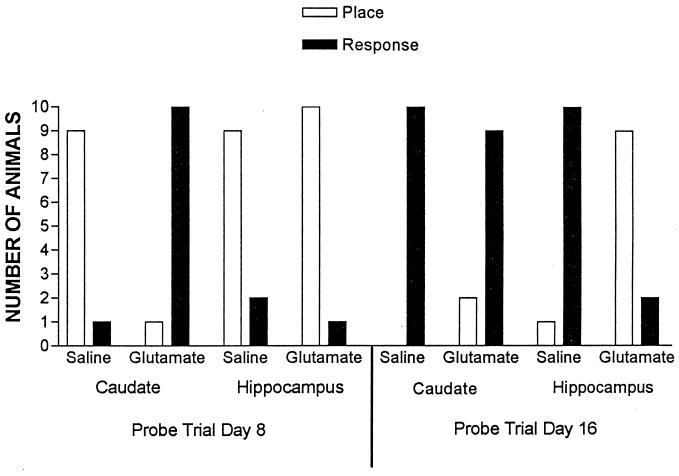
An additional measure of the effects of posttraining intracerebral glutamate infusions on memory is provided by comparing the relative use of place and response learning on the probe trials. Fig. 2 shows the results of the probe trials on days 8 and 16. χ2 analyses (P < 0.05 for all comparisons) were computed to determine whether groups showed a significant tendency to display place or response learning on the probe trials. On the day 8 probe trial, rats previously given posttraining injections of saline into the hippocampus or caudate-putamen were predominantly place learners (i.e., predominantly selected the west arm relative to the training start box, or the place where food was located during training; hippocampus-saline χ2 = 4.45; caudate-putamen saline χ2 = 6.40). Rats given posttraining injections of glutamate into the hippocampus were also predominantly place learners (χ2 = 7.36). In contrast, rats given posttraining injections of glutamate into the caudate-putamen were predominantly response learners on the day 8 probe trial (i.e., predominantly selected the east arm relative to the training start box, making the turning response that was reinforced during training; χ2 = 7.36). These findings indicate that although control and hippocampal-glutamate-treated rats display place learning on this initial probe trial, posttraining intracaudate injections of glutamate bias rats toward a tendency to display response learning.
Figure 2.
Number of rats in each experimental group that exhibited place or response learning on the day 8 and day 16 probe trials.
On the day 16 probe trial, rats previously given posttraining injections of saline into either the hippocampus (χ2 = 7.36) or caudate-putamen (χ2 = 10.00) were predominately response learners. Taken together with the results of the day 8 probe trial, these findings indicate that with extended training, saline-treated rats switched from displaying place learning to response learning. Rats receiving posttraining intracaudate injections of glutamate also predominantly displayed response learning on day 16 (χ2 = 4.45). In contrast, rats receiving posttraining intrahippocampal injections of glutamate predominantly displayed place learning on the day 16 probe trial (χ2 = 4.45). These findings suggest that posttraining intrahippocampal injections of glutamate block the switch to response learning that occurs with extended training in control rats and preserves the use of place learning.
Discussion
Previous findings indicate that both place and response learning can be acquired in a cross-maze task by separate memory systems that include the hippocampus and caudate-putamen, respectively (29, 38, 39). Probe trials used to assess the learning mechanism used by saline-treated rats performing this task indicate that place learning is predominantly in control of learned behavior at an early time point in training (i.e., day 8 probe trial), whereas extended training results in a shift toward the use of response learning (i.e., day 16 probe trial), a phenomenon that has also been observed in intact rats in previous cross-maze studies (e.g., refs. 40 and 41). Thus, in a cross-maze task that can be acquired through either a cognitive or S–R learning approach, early learning is controlled by a cognitive mechanism, whereas extended training shifts control over learned behavior to a S–R habit mechanism. In the present study, rats receiving posttraining intrahippocampal injections of glutamate displayed a predominance for place learning on both the day 8 and day 16 probe trials. Thus, intrahippocampal injections of glutamate appear to selectively enhance cognitive place learning and thereby block the switch to response learning that occurs with extended training. In contrast, rats receiving posttraining intracaudate injections of glutamate displayed a predominance for response learning on both probe trials. Thus, intracaudate injections of glutamate appear to selectively enhance S–R habit formation, producing an earlier shift to response learning. The findings suggest that posttraining neurochemical manipulation of the hippocampus and caudate-putamen can bias the brain toward the use of a specific memory system, and thereby influence the timing of the switch from the use of hippocampal-dependent cognitive memory to use of a caudate-dependent S–R habit to guide learned behavior.
The enhancement of place and response learning produced by intrahippocampal and intracaudate glutamate injections, respectively, adds to a substantial body of evidence supporting the hypothesis that these two brain structures are parts of independent memory systems. In rats, double dissociations of the mnemonic roles of the hippocampal system and caudate-putamen have been observed after reversible (29), and irreversible (3–5) lesions, as well as after posttraining drug treatments affecting dopaminergic (16) and glutamatergic (17, 18) neurotransmitter systems. Studies with human subjects (e.g., refs. 12, 42, and 43) and nonhuman primates (e.g., refs. 10 and 44–47) have also revealed dissociable mnemonic roles of the hippocampal system and caudate-putamen, suggesting an evolutionary conservation of these roles across mammalian species.
Further research is necessary to elucidate the mechanism(s) by which glutamate enhances both hippocampus-dependent and caudate-dependent memory processes. Various sources of excitatory amino acid input to the hippocampal system and caudate-putamen exist, with prominent glutamatergic projections to both structures arising via neocortical pathways. The dorsolateral caudate region targeted in the present study receives a substantial glutamatergic input from the somatosensory cortex (48), and posttraining modulation of this corticostriatal pathway could conceivably enhance caudate-dependent memory processes underlying habit formation in the cross maze, in which a somatic or vestibular cue might influence acquisition of a specific directional body turn. This suggestion is consistent with the proposed role of the caudate-putamen in “egocentric” learning (49, 50). Other evidence indicates that the role of the caudate-putamen in S–R habit formation in tasks involving visual and olfactory cues also is organized on the basis of the cortical sensory input the caudate receives (51). Thus, a functional heterogeneity of S–R learning function that is based on cortical input may be present in lateral aspects of the rodent caudate-putamen (29, 51–53). The neocortical glutamatergic input to the hippocampus originating via the entorhinal cortex/perforant pathway (54) provides the hippocampus with multimodal sensory information. Posttraining modulation of this pathway may enhance hippocampus-dependent processes underlying cognitive memory. A role for glutamate in the various forms of synaptic plasticity proposed to influence memory formation (e.g., long-term potentiation and long-term depression) has also been demonstrated in the rat hippocampus (for review, see ref. 55) and caudate-putamen (56–59), and may in part mediate the mnemonic effects of intracerebral glutamate infusions.
The comparison of place and response learning mechanisms was originally designed to evaluate the relative merits of cognitive and S–R learning theories, and although the cross-maze paradigm was used extensively in this debate, intact rats tested in this task are clearly capable of both place and response learning (for review, see ref. 28). Empirical evidence indicating that these two learning mechanisms are mediated by different memory systems in the brain (e.g., ref. 29, present study) appear to provide, as previously suggested (1, 8, 9), a neurobiological resolution to the historical debate between cognitivist and behaviorist learning theory.
A significant question for future research concerns the nature of the interaction among multiple memory systems. Evidence suggests that in a given learning task, hippocampal and caudate-putamen memory systems can be engaged simultaneously and in parallel (15, 29, present study). The present findings indicate that in a task in which each of these systems provides an adequate learned solution, manipulation of an endogenous neurotransmitter system can bias the brain toward the use of a specific memory system to guide learned behavior. Other findings indicate a role for several experimental parameters that influence the relative use of cognitive or habit learning in the cross maze and which presumably may influence the balance between the use of these two learning mechanisms in other situations as well. For example, the use of correction methods, massed training, and open mazes in visually heterogeneous environments all favor the use of place learning (28). One prediction of the present findings is that manipulation of these types of experimental parameters would also act to bias the brain toward the use of either a hippocampus-based or caudate-based solution in performing various memory tasks. Thus, studies comparing the effects of massed versus spaced training or the use of correction/noncorrection methods of reinforcement may provide information concerning the temporal dynamics underlying the interaction between multiple memory systems.
Finally, it should be noted that in addition to learning tasks in which more than one memory system can provide a competent solution, in other tasks the parallel activation of independent memory systems may interfere with acquisition. For example, lesions of the hippocampal system facilitate the acquisition of caudate-dependent learning in a win–stay radial maze task (3, 5), whereas caudate-putamen lesions have been observed to facilitate learning in a Y maze spatial-discrimination task (60). The presence of functional incompatibility, in which an existing memory system is unable to provide a satisfactory solution when the animal is confronted with novel information or task demands, has been proposed as a driving force in the evolution of multiple memory systems (61) and may underlie the competitive interference among multiple memory systems that is present in some learning tasks. Thus, investigation of the environmental and neurobiological factors that influence the interaction among different memory systems may be productively examined by using tasks in which multiple systems can provide an adequate solution as well as tasks in which competitive interference among systems is present. Such studies will not only increase understanding of the anatomical and neurochemical bases of memory but should also aid in elucidating the psychological operating principles that distinguish multiple memory systems.
Acknowledgments
I thank Jason Schroeder for assistance and James L. McGaugh for helpful comments on an earlier version of the manuscript. This research was supported by National Institutes of Health Grant R29MH56973-01.
Abbreviation
- S–R
stimulus–response
References
- 1.Hirsh R. Behav Biol. 1974;12:421–442. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- 2.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. New York: Oxford Univ. Press; 1978. [Google Scholar]
- 3.Packard M G, Hirsh R, White N M. J Neuorsci. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Packard M G, McGaugh J L. Behav Neurosci. 1992;106:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- 5.McDonald R J, White N M. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Kesner R P, Bolland B L, Dakis M. Exp Brain Res. 1993;93:462–470. doi: 10.1007/BF00229361. [DOI] [PubMed] [Google Scholar]
- 7.Zola-Morgan S, Squire L, Mishkin M. Science. 1982;218:1337–1339. doi: 10.1126/science.6890713. [DOI] [PubMed] [Google Scholar]
- 8.Mishkin M, Petri H L. In: Neurobiology of Learning and Memory. Lynch G, McGaugh J L, Weinberger N M, editors. New York: Guilford; 1984. pp. 65–77. [Google Scholar]
- 9.Petri H L, Mishkin M. Am Sci. 1994;82:30–37. [Google Scholar]
- 10.Zola-Morgan S, Squire L R. J Neurosci. 1984;4:1072–1085. doi: 10.1523/JNEUROSCI.04-04-01072.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen N, Squire L R. Science. 1980;210:207–209. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- 12.Knowlton B J, Mangels J A, Squire L R. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- 13.Graf P, Schacter D L. J Exp Psychol Learn Mem Cognit. 1985;11:501–518. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- 14.Warrington E K, Weiskrantz L. Neuropsychology. 1982;20:233–248. doi: 10.1016/0028-3932(82)90099-9. [DOI] [PubMed] [Google Scholar]
- 15.McDonald R J, White N M. Behav Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- 16.Packard M G, White N M. Behav Neurosci. 1991;105:295–306. doi: 10.1037//0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- 17.Packard M G, Teather L A. Behav Neurosci. 1997;111:543–551. doi: 10.1037//0735-7044.111.3.543. [DOI] [PubMed] [Google Scholar]
- 18.Packard M G, Teather L A. Psychobiology. 1999;27:40–50. [Google Scholar]
- 19.Packard M G, Cahill L, McGaugh J L. Proc Natl Acad Sci USA. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutherland R J, Rudy J W. Psychobiology. 1989;17:129–144. [Google Scholar]
- 21.Cohen N J, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 22.Tolman E C. Purposive Behavior in Animals and Men. New York: Appelton-Century Crofts; 1932. [Google Scholar]
- 23.Tolman E C. Psychol Rev. 1948;56:144–155. doi: 10.1037/h0055304. [DOI] [PubMed] [Google Scholar]
- 24.Thorndike E L. Psychol Rev. 1898;8:28–31. [Google Scholar]
- 25.Thorndike E L. Science. 1933;77:173–175. doi: 10.1126/science.77.1989.173-a. [DOI] [PubMed] [Google Scholar]
- 26.Hull C L. Principles of Behavior. New York: Appleton-Century Crofts; 1943. [Google Scholar]
- 27.Tolman E C, Ritchie B F, Kalish D. J Exp Psychol. 1946;36:221–229. doi: 10.1037/h0060262. [DOI] [PubMed] [Google Scholar]
- 28.Restle F. Psychol Rev. 1957;64:217–228. doi: 10.1037/h0040678. [DOI] [PubMed] [Google Scholar]
- 29.Packard M G, McGaugh J L. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 30.Breen R A, McGaugh J L. J Comp Physiol Psychol. 1961;54:495–501. doi: 10.1037/h0046436. [DOI] [PubMed] [Google Scholar]
- 31.McGaugh J L. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- 32.Muller G E, Pilzecker A. Zeitschrift Psychol. Suppl. 1; 1900. [Google Scholar]
- 33.Duncan C P. J Comp Physiol Psychol. 1949;42:32–44. doi: 10.1037/h0058173. [DOI] [PubMed] [Google Scholar]
- 34.Burnham W H. Am J Psychol. 1904;14:382–396. [Google Scholar]
- 35.Flood J F, Baker M L, Davis J L. Brain Res. 1990;521:197–202. doi: 10.1016/0006-8993(90)91543-p. [DOI] [PubMed] [Google Scholar]
- 36.Izquierdo I, Da Chuna C, Rosat R, Jerusalinsky D, Beatriz M, Ferreira C, Medina J H. Behav Neural Biol. 1992;58:16–26. doi: 10.1016/0163-1047(92)90847-w. [DOI] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 38.DeCastro J M. Behav Biol. 1974;12:373–382. [Google Scholar]
- 39.Thompson W G, Guilford M O, Hicks L H. Physiol Psychol. 1980;8:473–479. [Google Scholar]
- 40.Hicks L H. Psychol Reports. 1964;15:459–462. [Google Scholar]
- 41.Ritchie B F, Aeschliman B, Pierce P. J Comp Physiol Psychol. 1950;43:73–85. doi: 10.1037/h0055224. [DOI] [PubMed] [Google Scholar]
- 42.Martone M, Butters N, Payne M, Becker J, Sax D S. Arch Neurol. 1984;41:965–970. doi: 10.1001/archneur.1984.04050200071020. [DOI] [PubMed] [Google Scholar]
- 43.Heindel W, Butters N, Salmon D. Behav Neurosci. 1988;102:141–150. doi: 10.1037//0735-7044.102.1.141. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Aigner T, Mishkin M. Soc Neurosci Abstr. 1990;16:617. [Google Scholar]
- 45.Divac I, Rosvold H E, Szwarcbart M K. J Comp Physiol Psychol. 1967;63:184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- 46.Buerger A A, Gross C G, Rocha-Miranda C E. J Comp Physiol Psychol. 1974;86:440–446. doi: 10.1037/h0036142. [DOI] [PubMed] [Google Scholar]
- 47.Malamut B L, Suanders R C, Mishkin M. Behav Neurosci. 1984;98:759–769. doi: 10.1037//0735-7044.98.5.759. [DOI] [PubMed] [Google Scholar]
- 48.Fonnum F, Storm-Mathisen J, Divac I. Neuroscience. 1981;6:863–873. doi: 10.1016/0306-4522(81)90168-8. [DOI] [PubMed] [Google Scholar]
- 49.Potegal M. Acta Neurobiol Exp. 1972;32:379–494. [PubMed] [Google Scholar]
- 50.Cook D, Kesner R P. Behav Neural Biol. 1988;49:332–343. doi: 10.1016/s0163-1047(88)90338-x. [DOI] [PubMed] [Google Scholar]
- 51.Viaud M D, White N M. Behav Brain Res. 1989;32:31–42. doi: 10.1016/s0166-4328(89)80069-5. [DOI] [PubMed] [Google Scholar]
- 52.White N M. Life Sci. 1989;43:7–12. doi: 10.1016/0024-3205(88)90230-5. [DOI] [PubMed] [Google Scholar]
- 53.Devan D B, White N M. J Neurosci. 1999;19:2789–2798. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ottersen O P, Storm-Mathisen J. In: The Hippocampus: New Vistas. Chan-Palay V, Kohler C, editors. New York: Liss; 1989. pp. 97–117. [Google Scholar]
- 55.Bliss T V P, Collinridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 56.Calabresi P, Maj R, Pisani A, Mercuri N B, Bernardi G. J Neurosci. 1992;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calabresi P, Pisani A, Mercuri N B, Bernardi G. Eur J Neurosci. 1993;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 58.Charpier S, Deniau J M. Proc Natl Acad Sci USA. 1997;94:7036–7040. doi: 10.1073/pnas.94.13.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Munoz M, Groves P M. NeuroReport. 1992;3:357–360. doi: 10.1097/00001756-199204000-00017. [DOI] [PubMed] [Google Scholar]
- 60.Mitchell J A, Hall G. Q J Exp Psychol B Comp Physiol Psychol. 1988;40:243–258. [PubMed] [Google Scholar]
- 61.Sherry D F, Schacter D L. Psychol Rev. 1987;94:439–454. [Google Scholar]