Abstract
Metabotropic glutamate receptors (mGluRs) are G-protein-coupled receptors and are densely expressed in the forebrain of adult rats. Accumulative evidence suggests a critical role of mGluRs in the regulation of normal physiological activity of neurons and pathogenesis of mental illnesses such as schizophrenia, depression, and substance addiction. In this study, we investigated alterations in mGluR8 subtype mRNA expression in the rat forebrain in response to repeated intraperitoneal administration of amphetamine (twice daily for 12 days, 5 mg/kg per injection) using quantitative in situ hybridization. We found that mGluR8 mRNA levels were profoundly increased in the dorsal (caudate putamen) and ventral (nucleus accumbens) striatum 1 day after the discontinuation of amphetamine treatments. Such increases were sustained up to 21 days of withdrawal. Increases in mGluR8 mRNAs were also found in the cerebral cortex, including the cingulate and sensory cortex but not the piriform cortex, at 1 and 21 days. These data demonstrate a positive response of mGluR8 in mRNA abundance in most forebrain regions to repeated stimulant exposure.
Keywords: hybridization, dopamine, cocaine, cortex, striatum, caudate, nucleus accumbens
Metabotropic glutamate receptors (mGluRs) are G-protein-coupled receptors that are densely expressed in brain cells. Eight subtypes of mGluRs so far cloned from the rat brain are classified into three functional groups. Group I mGluRs (mGluR1/5) are positively coupled to phosphoinositol hydrolysis, and activation of them increases phosphoinositol hydrolysis followed by intracellular Ca2+ release and protein kinase C activation [4]. Both group II (mGluR2/3) and group III (mGluR4/6/7/8) receptors are negatively coupled to adenylate cyclase. Activation of these receptors leads to decreased protein kinase A (PKA) activity and cAMP production [4]. A large number of anatomical studies using in situ hybridization and immunohistochemistry have demonstrated expression of most mGluR subtypes in the striatum at variable levels [27,29]. The enriched mGluR distribution in this region indicates putative involvements of mGluRs in the regulation of striatal function.
Behavioral studies have shown the important role of striatal mGluRs in the regulation of psychomotor activity. Mutant mice lacking mGluR5 showed a deficit of cocaine-stimulated locomotion [3]. The mGluR2/3 agonist blocked locomotor sensitization to amphetamine (AMPH) [11]. Similarly, the group III mGluR agonists suppressed acute behavioral responses to dopamine stimulants [14,26]. At a molecular level, mGluRs are linked to inducible gene expression in the striatum in response to different forms of stimulant exposure [29]. An acute AMPH injection stimulated c-fos and neuropeptide (prodynorphin and proenkephalin) mRNA expression in the striatum, which was attenuated by an mGluR selective antagonist (+)-α-methyl-4-carboxyphenyl-glycine (MCPG), indicating a mediating role of mGluRs in AMPH-stimulated gene expression [16,30]. Furthermore, acute AMPH-induced increases in c-fos and proenkephalin mRNA expression were attenuated by an mGluR5 selective antagonist 2-methyl-6- (phenylethynyl) pyridine hydrochloride (MPEP) [19,20]. As to group II/III mGluRs, their negative coupling to the PKA/cAMP pathway may render them the ability to downregulate striatal gene expression [29].
The subtype of mGluR8 is expressed in the forebrain [17] and is thought to play a significant role in the regulation of normal mental function and in the development of various forms of psychiatric illnesses [18,22]. However, no attempt has been made so far, to our knowledge, to examine the effect of psychostimulants on expression of mGluR8 mRNAs in the rat brain. In this study, we investigated the effect of chronic AMPH administration on basal levels of mGluR8 mRNA transcripts in striatal and cortical areas. A regimen requiring twice daily injection of AMPH (5 mg/kg, i.p.) for twelve days was utilized in adult rats. Quantitative in situ hybridization was conducted to analyze alterations in mRNA levels in the target areas 1 and 21 days after the cessation of AMPH administration.
Adult male Wistar rats weighing 200–225 g (Charles River, New York, NY) were individually housed in clear plastic cages. An at least 5-day accommodation period was allowed prior to the commencement of the experiment. Animals were maintained on a 12/12 h light dark cycle; lights were turned on at 7:00 am. The housing environment was maintained at 23°C and humidity at 50 ± 10% with food and water available ad libitum. Animals were brought to a quiet procedure room at least 1–2 h prior to injection. All procedures performed were approved by the Institutional Animal Care and Use Committee and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Rats were randomly divided into 4 groups (n = 5 per group) and received intraperitoneal (i.p.) injection of saline or D-amphetamine sulphate (Sigma, St. Louis, MO). AMPH was calculated as its salt and freshly prepared each day. AMPH was injected at 5 mg/kg twice daily for 12 days; the first daily injection was made between 8:30 and 9:30 am and the second injection between 3:30 and 4:30 pm. Rats were sacrificed 1 or 21 days after the last injection. The selection of an early (1 day) versus a late (21 days) withdrawal period was based on numerous studies of this kind showing time-dependent changes in gene expression in response to psychostimulant exposure [15].
In situ hybridization histochemistry was conducted according to procedures standardized in this laboratory [13,19,20]. Briefly, rats were deeply anesthetized with Equithesin (5 ml/kg, i.p.). Brains were removed and immediately frozen in isopentane at −40°C. The brains were sectioned (12 μm) in a cryostat, and 3 coronal sections from one brain were mounted per slide. The sections were fixed with 4% paraformaldehyde and defatted with chloroform. This was followed by hybridization with oligonucleotide probes (45 bases in length) complimentary to 214–228 amino acids of mGluR8 [23]. The oligonucleotide probes were labeled with α-35S-ATP and terminal deoxynucleotidyl transferase at the 3′ end to a specific activity of 7–12 × 105 cpm/μl. The slides were incubated with 1 × 106 cpm/25 μl hybridization buffer/section overnight at 37°C, washed, and apposed to Kodak X-OMAT AR film along with a 14C standard (American Radiolabeled Chemicals). The films were developed after 7–10 days of exposure.
Quantification of mRNA hybridization signals from the films was done by running Scion Image (Windows version of the public domain NIH image program) on a Dell computer as described previously [19,20]. Briefly, uneven illumination was corrected by measuring the background and saving it as “blank field”. The calibration curve was plotted by measuring the 14C standards and plotting them against known dpm/mg values, followed by conversion to 35S equivalents. The density slice option was used, the lower value was set to the bottom of the LUT scale and the upper value was set to exclude background signals. The density slice values were not changed throughout the measurement of films. Measurements of the hybridization signals in a specific region were made using a drawing that outlined the target area (Fig. 1). Measurements were expressed as 1) area, which represents the number of label pixels per area, 2) mean density of tissue in dpm/mg, and 3) integrated density which is the product of area times mean density [19,20]. Values from 3 adjacent sections were averaged as the value for each rat and taken as n = 1.
Fig. 1.
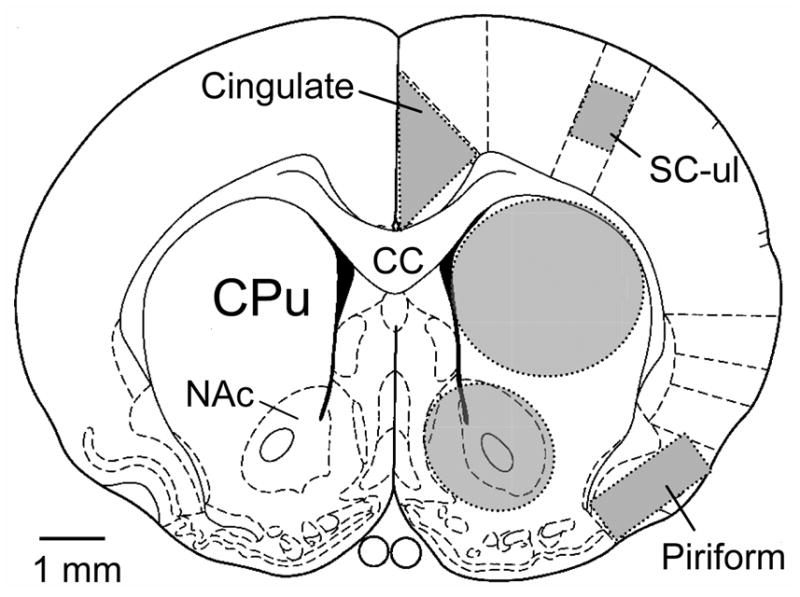
Schematic diagram of a cross-section of the rat forebrain depicting the forebrain areas analyzed for quantification of mRNA hybridization signals. Shadow areas represent the analyzed regions, which include the cingulate cortex, the upper limb area of sensory cortex, the piriform cortex, the dorsal striatum, and the ventral striatum. Abbreviations, cc, corpus callosum; CPu, caudate putamen; NAc, nucleus accumbens; SC-ul, upper limb area of sensory cortex.
The results are presented as mean ± SEM. Area, mean density, and integrated density measurements were evaluated using a two-way ANOVA followed by a Bonferroni’s (Dunn) comparison of groups using least squares-adjusted means. Results were considered statistically significant at probability levels less than 0.05.
The effect of chronic saline or AMPH treatments on mGluR8 expression in the forebrain was examined at the two withdrawal time points: 1 and 21 days after the final AMPH injection. In saline-treated rats, levels of constitutive mRNA hybridization signals were quite low in the entire striatum (Fig. 2A). As compared to this low level of signals in the striatum, a moderate level of mGluR8 mRNAs was revealed in the inner and middle layers (IV-VI layers) of cortical regions (Fig. 2A). A high level of hybridization signals was displayed in the piriform cortex (Fig. 2A). In AMPH-treated rats, visual analysis of the autoradiogram in the striatum showed that 1 day following repeated AMPH treatments slightly more intense mGluR8 hybridization signals were seen in both the dorsal/caudate putamen (CPu) and ventral/nucleus accumbens (NAc) striatum of AMPH-treated rats as compared to that of saline-treated animals (Fig. 2B vs. 2A). At 21 days, AMPH sustained its ability to elevate mGluR8 signals in the striatum as compared to saline-treated rats at the same time point (Fig. 2D vs. 2C). In fact, a more robust increase was induced in the dorsal striatum at 21 days as compared to that seen at 1 day. Moreover, in the dorsal striatum, the elevated mGluR8 signal had a heterogeneous distribution in which patches (100–300 μm wide) of heightened labeling were scattered in the dorsal region (Fig. 2D). In the cerebral cortex, changes in mGluR8 hybridization signals occurred in most areas. In the cingulate cortex, increases in mGluR8 signals were evident 1 and 21 days after the discontinuation of AMPH treatments (Fig. 2). Marked increases in the three inner layers (IV, V, and VI) of sensory/motor cortex were seen in AMPH-treated rats at the two time points. However, the mGluR8 hybridization signals in the piriform cortex seemed to be stable as their levels of expression were not significantly altered by AMPH at either withdrawal period (Fig. 2). In addition to these striatal and cortical areas, expression of mGluR8 hybridization signals in the corpus callosum was increased at 21 days (Fig. 2D).
Fig. 2.
Photomicrographs illustrating effects of repeated administration of AMPH on mGluR8 mRNA expression in the rat striatum at different time points. Saline or AMPH (5 mg/kg, i.p.) was injected twice a day for 12 days. Rats were sacrificed 1 (A and B) or 21 (C and D) days after the final injection of saline (A and C) or AMPH (B and D) for in situ hybridization measurements of mRNA expression. Note that mGluR8 hybridization signals were increased in the cortical and striatal regions 1 day after repeated AMPH injections. At 21 days, the increases in mGluR8 hybridization signals in the cortex and the dorsal striatum were even greater as compared to the results seen at 1-day time point.
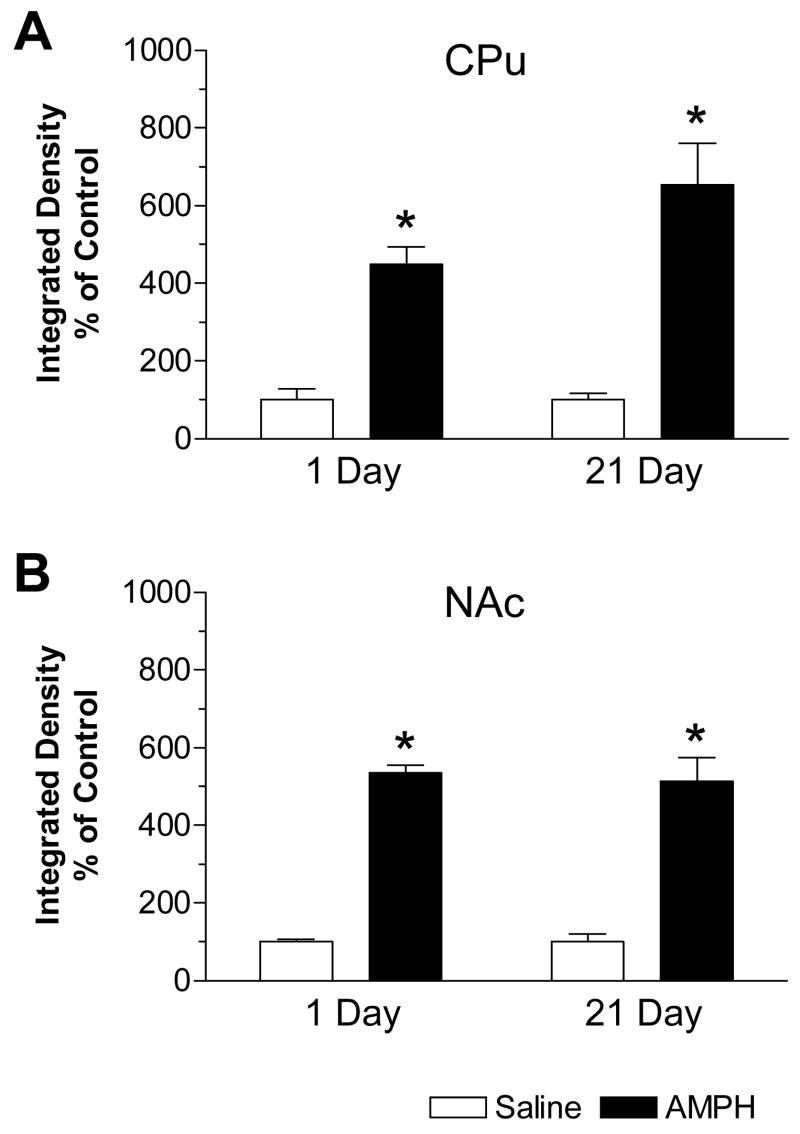
Quantification of mRNA signals of mGluR8 in the striatum was made and data are expressed in terms of integrated density (Fig. 3). The drug-induced alterations in integrated density primarily reflect a change in the number of labeled particles per area rather than the mean density of hybridization signals (data not shown). As expected based on the above image impression, at the first time point (1 day after final chronic injection), mGluR8 mRNA levels in the CPu of AMPH-treated rats were increased by 349% over control (Fig. 3A). At 21 day, a greater increase in mGluR8 mRNAs (553% over control) was induced in the same region (Fig. 3A). Similarly, in the NAc, mGluR8 mRNA levels were increased by 435% and 413% over control at 1 and 21 days, respectively, after drug injection (Fig. 3B).
Fig. 3.
Quantitative results of changes in mGluR8 hybridization signals in the dorsal and ventral striatum in response to repeated AMPH administration. In both the caudate putamen (CPu, A) and nucleus accumbens (NAc, B), mGluR8 mRNAs showed sustained increases from 1 to 21 days after repeated AMPH injections. Values are expressed in terms of integrated density and expressed as mean ± SEM (n = 5 per group). *: p < 0.05 versus saline-treated rats at 1-day time point.
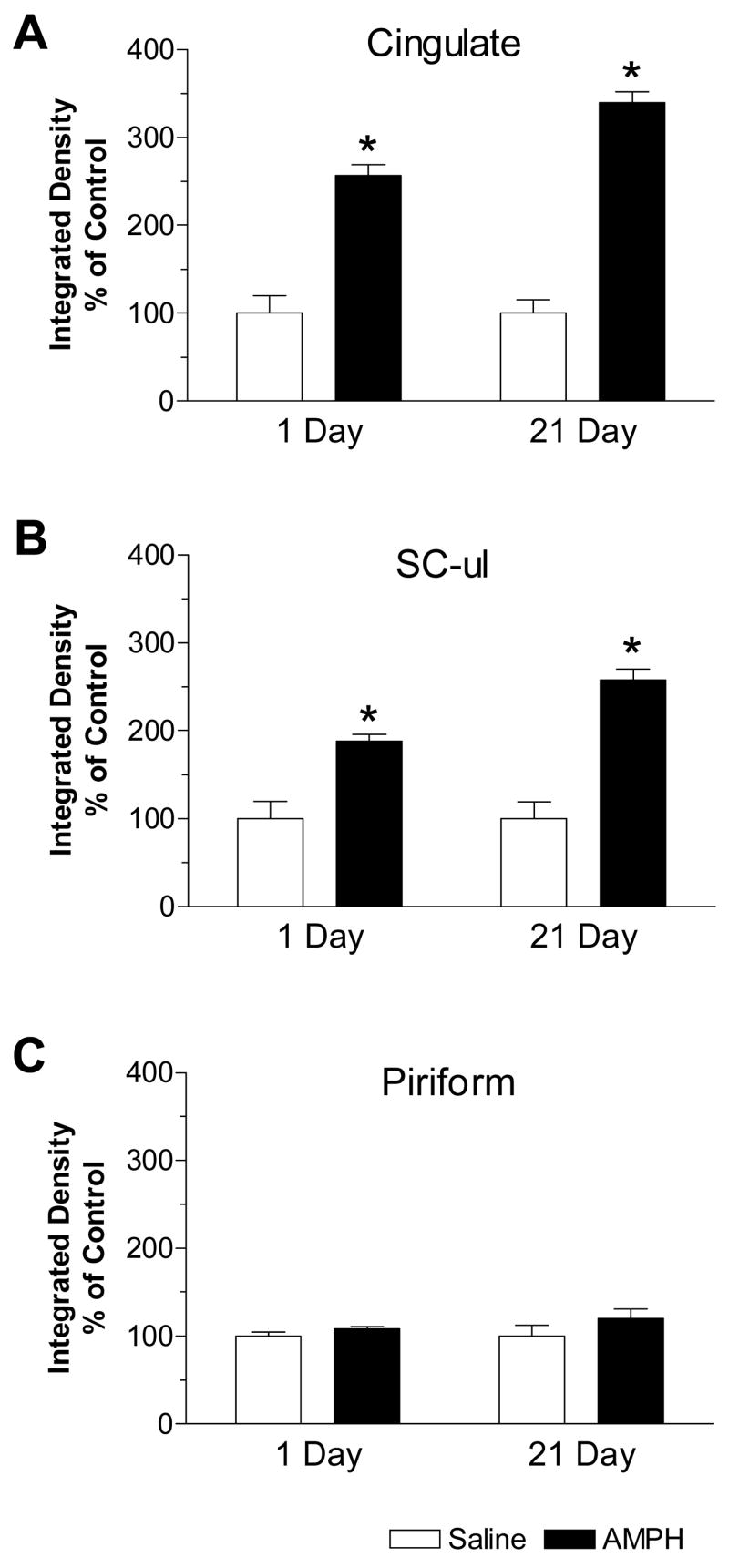
Quantitative analysis of alterations in mGluR8 mRNA levels was also made in the three cortical regions. In the cingulate cortex, basal levels of mGluR8 mRNAs were increased by 157% and 240% over control at 1 and 21 days, respectively (Fig. 4A). Similar results were observed in the upper limb area of sensory cortex (SC-ul) as 88% and 157% increases over control were seen at the respective early and late time points (Fig. 4B). In contrast, in the piriform cortex, no significant change in mGluR8 mRNA levels was seen at both the time points following AMPH administration (Fig. 4C).
Fig. 4.
Quantitative results of changes in mGluR8 hybridization signals in multiple cortical regions in response to repeated AMPH administration. In both the cingulate cortex (A) and upper limb area of sensory cortex (SC-ul, B), mGluR8 mRNAs showed sustained increases from 1 to 21 days after repeated AMPH injections. No significant change was seen in the piriform cortex (C). Values are expressed in terms of integrated density and expressed as mean ± SEM (n = 5 per group). *: p < 0.05 versus saline-treated rats at 1-day time point.
This study investigated the effect of chronic AMPH administration on the constitutive mRNA expression of the mGluR8 subtype in the rat forebrain in vivo using quantitative in situ hybridization. The results indicate that mGluR8 is a sensitive target for the regulation of mRNA expression by AMPH. Repeated AMPH administration induced a profound and long-lasting upregulation of mGluR8 in mRNA abundance in the both dorsal and ventral striatum. Similarly, AMPH elevated mGluR8 mRNA levels in the cingulate and sensory cortex. In contrast, AMPH did not alter mGluR8 mRNA expression in the piriform cortex. These data suggest that mGluR8 in addition to group I mGluRs (mGluR1 and 5) [15] is a subtype that is sensitive in its expression to chronic stimulant administration. The altered mGluR8 gene expression may implicate this subtype of mGluRs in long-term changes in neuroplasticity related to addictive properties of psychostimulants.
Plastic changes in the limbic circuit derived from repeated drug administration are characterized by a remarkably long-lived nature. An altered genomic response, if it lasts long enough, could be a critical molecular mechanism for the nearly permanent neural and behavioral modification [10]. The upregulated mGluR8 gene expression discovered from this study showed such a long-lasting nature as an obvious increase in mGluR8 mRNA levels was exhibited even 21 days after the discontinuation of drug treatments. The long-term nature of mGluR8 gene responses certainly indicates this change as an important link in the molecular mechanisms responsible for addictive action of drugs. Another important characteristic of altered mGluR8 gene expression is its profound changes in the cerebral cortex in addition to the striatum. The cerebral cortex is known to project massively and topographically upon the striatum, while it is also the major recipient structure of the basal ganglia [2,7]. Since mGluR8, like other group III subtypes, is predominantly presynaptic [25,28], this subtype of group III mGluRs is believed to reside upon the corticostriatal terminals although this needs to be proven experimentally. Thus, the upregulated mGluR8 mRNA expression detected in cell bodies of neurons in the cortex may translate to a parallel increase in receptor proteins in presynaptic terminals of the corticostriatal projection within the striatum. Moreover, positive neurons with upregulated mGluR8 mRNA signals were particularly numerous in cortical areas mainly occupying layers IV–VI. Thus, a subset of corticostriatal projections may host the upregulation of mGluR8 gene expression after stimulant exposure.
While the mGluR8-specific role is unclear at present due to the lack of data, group III mGluRs in the forebrain [9] are found to play a significant role in the regulation of transmitter release and behavioral plasticity in the limbic system, according to a number of pharmacological studies with the group III selective agents. As a group of Gi/o-protein-coupled receptors, group III mGluRs upon activation by the selective group III agonist such as L-2-amino-4-phosphonobutyrate (L-AP4) reduced extracellular basal levels of dopamine in the striatum [8,12], indicating a tonic inhibitory tone of group III mGluRs on basal dopamine release. L-AP4 also depressed the excitatory postsynaptic potentials at rat corticostriatal synapses evoked by cortical stimulation [1,21] and the 4-aminopyridine-evoked glutamate release in striatal synaptosomal preparations [5]. Since L-AP4 did not alter the postsynaptic response to focal application of glutamate, the L-AP4 effect was believed to be mediated via a presynaptic mechanism, i.e., an existence of the group III autoreceptor-mediated presynaptic inhibition of excitatory transmission at corticostriatal synapses. In adult rat striatal or hippocampal slices, L-AP4 showed the ability to suppress forskolin-stimulated cAMP formation [6,24]. Behaviorally, local injections of L-AP4 into the dorsal striatum blocked hyperlocomotion induced by acute injection of cocaine or amphetamine, and the effect of L-AP4 was reversed by a group III antagonist α-methyl-4-phosphonophenylglycine (MPPG) [14]. These data indicate that the enhanced group III mGluR glutamatergic transmission by an exogenous ligand is capable of suppressing behavioral responses to acute exposure of dopamine stimulants. Together, regardless the accumulative data signifying the role of group III mGluRs, the specific contribution of mGluR8 to the group III mGluR-mediated functions remains elusive. At present, it can only be assumed that the upregulated mGluR8 gene expression, if it can translate to enhanced inhibitory tone of mGluR8 over dopaminergic or glutamatergic transmission following repeated stimulant exposure, may represent a compensatory response to repeated drug administration. It counteracts the stimulation of the dopamine/glutamate system in the limbic system to homeostatically normalize the cellular and neural responses to drugs. Further studies are necessary to elucidate the precise role of mGluR8 in regulating long-term effects of stimulants.
Acknowledgments
This work was supported by grants from the NIH (DA010355 and MH061469).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calabresi P, Pisani A, Mercuri NB, Bernardi G. Heterogeneity of metabotropic glutamate receptors in the striatum: electrophysiological evidence. Eur J Neurosci. 1993;5:1370–1377. doi: 10.1111/j.1460-9568.1993.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 2.Calabresi P, Pisani A, Mercuri NB, Bernardi G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci. 1996;19:279–280. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- 3.Chiamulera C, Epping-Jordan M, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nature Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 4.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 5.East SJ, Hill MP, Brotchie JM. Metabotropic glutamate receptor agonists inhibit endogenous glutamate release from rat striatal synaptosomes. Eur J Pharmacol. 1995;277:117–121. doi: 10.1016/0014-2999(95)00119-6. [DOI] [PubMed] [Google Scholar]
- 6.Genazzani AA, Casabona G, L’Episcopo MR, Condorelli DF, Dell’Albani P, Shinozaki H, Nicoletti F. Characterization of metabotropic glutamate receptors negatively linked to adenylyl cyclase in brain slices. Brain Res. 1993;622:132–138. doi: 10.1016/0006-8993(93)90811-z. [DOI] [PubMed] [Google Scholar]
- 7.Gerfen CR. Synaptic organization of the striatum. J Electron Microsc Tech. 1988;10:265–281. doi: 10.1002/jemt.1060100305. [DOI] [PubMed] [Google Scholar]
- 8.Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther. 1999;289:412–416. [PubMed] [Google Scholar]
- 9.Hudtloff C, Thomsen C. Autoradiographic visualization of group III metabotropic glutamate receptors using [3H]-L-2-amino-4-phosphonobutyrate. Br J Pharmacol. 1998;124:971–977. doi: 10.1038/sj.bjp.0701910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Vezina P. The mGlu2/3 receptor agonist LY379268 blocks the expression of locomotor sensitization by amphetamine. Pharmacol Biochem Behav. 2002;73:333–337. doi: 10.1016/s0091-3057(02)00827-4. [DOI] [PubMed] [Google Scholar]
- 12.Mao L, Lau YS, Wang JQ. Activation of group III metabotropic glutamate receptors inhibits basal and amphetamine-stimulated dopamine release in rat dorsal striatum: an in vivo microdialysis study. Eur J Pharmacol. 2000;404:289–297. doi: 10.1016/s0014-2999(00)00633-6. [DOI] [PubMed] [Google Scholar]
- 13.Mao L, Conquet F, Wang JQ. Augmented motor activity and reduced striatal preprodynorphin mRNA induction in response to acute amphetamine administration in metabotropic glutamate receptor 1 knockout mice. Neuroscience. 2001;106:303–312. doi: 10.1016/s0306-4522(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 14.Mao L, Wang JQ. Distinct inhibition of acute cocaine-stimulated motor activity following microinjection of a group III metabotropic glutamate receptor agonist into the dorsal striatum of rats. Pharmacol Biochem Behav. 2000;67:93–101. doi: 10.1016/s0091-3057(00)00307-5. [DOI] [PubMed] [Google Scholar]
- 15.Mao L, Wang JQ. Differentially altered mGluR1 and mGluR5 mRNA expression in rat caudate nucleus and nucleus accumbens in the development and expression of behavioral sensitization to repeated amphetamine administration. Synapse. 2001;41:230–240. doi: 10.1002/syn.1080. [DOI] [PubMed] [Google Scholar]
- 16.Mao L, Wang JQ. Activation of metabotropic glutamate receptor mediates upregulation of transcription factor mRNA expression in rat striatum induced by acute administration of amphetamine. Brain Res. 2002;924:167–175. doi: 10.1016/s0006-8993(01)03230-9. [DOI] [PubMed] [Google Scholar]
- 17.Messenger MJ, Dawson LG, Duty S. Changes in metabotropic glutamate receptor 1–8 gene expression in the rodent basal ganglia motor loop following lesion of the nigrostriatal tract. Neuropharmacology. 2002;43:261–271. doi: 10.1016/s0028-3908(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 18.Ossowska K, Pietraszek M, Wardas J, Wolfarth S. Potential antipsychotic and extrapyramidal effects of (R,S)-3,4-dicarboxyphenylglycine [(R,S)-3,4-DCPG] a mixed AMPA anagonist/mGluR8 agonist. Pol J Pharmacol. 2004;56:295–304. [PubMed] [Google Scholar]
- 19.Parelkar NK, Wang JQ. Preproenkephalin mRNA expression in rat dorsal striatum induced by selective activation of metabotropic glutamate receptor subtype-5. Synapse. 2003;47:255–261. doi: 10.1002/syn.10174. [DOI] [PubMed] [Google Scholar]
- 20.Parelkar NK, Wang JQ. MGluR5-dependent increases in immediate early gene expression in the rat striatum following acute administration of amphetamine. Mol Brain Res. 2004;122:151–157. doi: 10.1016/j.molbrainres.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Pisani A, Calabresi P, Centonze D, Bernardi G. Activation of group III metabotropic glutamate receptors depresses glutamatergic transmission at corticostriatal synapse. Neuropharmacology. 1997;36:845–851. doi: 10.1016/s0028-3908(96)00177-3. [DOI] [PubMed] [Google Scholar]
- 22.Robbins MJ, Starr KR, Honey A, Soffin EM, Rourke C, Jones GA, Kelly FM, Strum J, Melarange RA, Harris AJ, Rocheville M, Rupniak T, Murdock PR, Jones DN, Kew JN, Maycox PR. Evaluation of the mGluR8 receptor as a putative therapeutic target in schizophrenia. Brain Res. 2007;1152:215–227. doi: 10.1016/j.brainres.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 23.Saugstad JA, Kinzie JM, Shinohara MM, Segerson TP, Westbrook GL. Cloning and expression of rat metabotropic glutamate receptor 8 reveals a distinct pharmacological profile. Mol Pharmacol. 1997;51:119–125. doi: 10.1124/mol.51.1.119. [DOI] [PubMed] [Google Scholar]
- 24.Schaffhauser H, Cartmell J, Jakob-Rotne R, Mutel V. Pharmacological characterization of metabotropic glutamate receptors linked to the inhibition of adenylate cyclase activity in rat striatal slices. Neuropharmacology. 1997;36:933–940. doi: 10.1016/s0028-3908(97)00079-8. [DOI] [PubMed] [Google Scholar]
- 25.Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swanson CJ, Kalivas PW. Regulation of locomotor activity by metabotropic glutamate receptors in the nucleus accumbens and ventral tegmental area. J Pharmacol Exp Ther. 2000;292:406–414. [PubMed] [Google Scholar]
- 27.Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wada E, Shigemoto R, Kinoshita A, Ohishi H, Mizuno N. Metabotropic glutamate receptor subtypes in axon terminals of projection fibers from the main and accessory olfactory bulbs: a light and electron microscopic immunohistochemical study in the rat. J Comp Neurol. 1998;393:493–504. [PubMed] [Google Scholar]
- 29.Wang JQ, Mao L, Parelkar NK, Tang Q, Liu Z, Sarwar S, Choes ES. Glutamate-regulated behavior, transmitter release, gene expression and addictive plasticity in the striatum: roles of metabotropic glutamate receptors. Current Neuropharmacol. 2003;1:1–20. [Google Scholar]
- 30.Wang JQ, McGinty JF. Intrastriatal injection of the metabotropic glutamate receptor antagonist MCPG attenuates acute amphetamine-stimulated neuropeptide mRNA expression in rat striatum. Neurosci Lett. 1996;218:13–16. doi: 10.1016/0304-3940(96)13107-4. [DOI] [PubMed] [Google Scholar]