Abstract
In pre-B lymphocytes, productive rearrangement of Ig light chain genes allows assembly of the B cell receptor (BCR), which selectively promotes further developmental maturation through poorly defined transmembrane signaling events. Using a novel in vitro system to study immune tolerance during development, we find that BCR reactivity to auto-antigen blocks this positive selection, preventing down-regulation of light chain gene recombination and promoting secondary light chain gene rearrangements that often alter BCR specificity, a process called receptor editing. Under these experimental conditions, self-antigen induces secondary light chain gene rearrangements in at least two-thirds of autoreactive immature B cells, but fails to accelerate cell death at this stage. These data suggest that in these cells the mechanism of immune tolerance is receptor selection rather than clonal selection.
The developmental transition of pre-B cells to the immature IgM+ B cell phenotype is the earliest stage at which cells bear a complete receptor capable of binding to self-antigen, and such cells are in fact exquisitely sensitive to tolerance (1–5). This transition is marked by V(D)J recombinase-dependent Ig light chain gene assembly, surface expression of BCR, and suppression of further Ig gene rearrangements (i.e., allelic exclusion) (6, 7). Mutational analysis has demonstrated that BCR expression and signaling function is required to complete this transition, which is sometimes referred to as positive selection (8). Immune tolerance in these immature cells can occur either by apoptosis (2, 5, 9) or by an induced reprogramming of the specificity of the BCR through nested light chain gene rearrangements (receptor editing) (3, 10–12). While roles for clonal deletion and receptor editing are seen in different experimental contexts, the developmental stage specificity of these forms of regulation and the extent to which each mechanism contributes to B cell tolerance are unclear.
Normal homeostasis and aberrant receptor gene assembly also may lead to developmental arrest and massive cell loss (8, 13), making it difficult to determine the extent to which immune tolerance contributes to cell death in normal immune systems. Although in vivo analyses of auto-Ab transgenic (Tg) mice demonstrated that expression of antigen in the bone marrow (BM) depletes reactive Tg B cells from the peripheral lymphoid organs (14–18), self-reactive cells with a low density of BCR are retained in the BM (17, 19–21). Moreover, in some of these Tg models, deletion did not cause complete B cell loss, and the remaining B cells in the peripheral lymphoid organs were shown to have lost self-reactive specificity through secondary rearrangements at the endogenous light chain gene loci, or to a lesser extent, at the heavy chain gene loci (4, 10, 14, 16, 17, 22). The interpretation of these Tg experiments is hindered, however, because of uncertainty about the extent to which this in vivo escape from tolerance-mediated elimination represents induced receptor editing in the BM or peripheral selection of rare variant B cells. In vitro tolerance studies using Tg and normal, non-Tg B cells have come to somewhat conflicting conclusions regarding the mechanisms of tolerance. In some studies, immature B cells cultured in the presence of antigen or anti-BCR antibodies failed to manifest decreased survival, although apoptosis was not directly measured (19, 23). In these studies, antigen or anti-BCR treatment prevented immature B cells from acquiring cell surface maturation markers such as sIgD, CD23, CD21, and L-selectin (19), and led to the up-regulation or maintenance of recombinase activator gene (RAG) expression and light chain gene rearrangement activity (23). In other studies, in which non-Tg immature B cells (IgM+, IgD−) were highly purified prior to culture, treatment with anti-IgM antibodies induced apoptosis in many B cells (2, 5, 9), but a substantial fraction of B cells appeared to be apoptosis-resistant.
In this study, we modified a well characterized interleukin 7 (IL-7)-driven BM culture system (24) to analyze the induction of self-tolerance and positive selection during B cell development. Normal and 3–83Tg B cell precursors were grown either in the absence of antigen, or in its continuous presence, a situation that is more likely to occur in the BM in vivo than is the acute introduction of antigen. Using this experimental system, we were able to ask questions about the precise stage specificity and mechanisms of central B cell tolerance and to simultaneously quantify tolerance-induced apoptosis and receptor editing. The results indicate that at this early stage of B cell development receptor editing plays a dominant role in immune tolerance.
EXPERIMENTAL PROCEDURES
Animals.
Mice were 3–5 week old B10.D2nSn/J that expressed the 3–83Tg (25) or non-Tg littermates. In some experiments 3–83Tg and non-Tg B10.D2nSn/J mice bearing one inactivated κ allele (κ+/−) (26) were used.
Cell Lines and Culture Conditions.
3–83Tg and non-Tg cultures of B cell precursors were grown as described (27). Primary cultures: Briefly, BM cells (depleted of red blood cells) were cultured for 5 days in the presence of 50–100 units/ml of recombinant IL-7. BM cells from non-Tg mice were depleted of sIgM+ cells by panning prior to culture. In some experiments, BM cells were cultured in the presence of anti-idiotypic (id) antibody (Ab) 54.1 (14) or with rat anti-mouse κ mAb 187 (American Type Culture Collection). In these experiments, B cells were collected after 5 days for analysis or washed and recultured without IL-7 for another period of 24–48 hr prior to analysis. Secondary cultures: To maintain and further expand the B cell precursors, cells were collected, washed, and recultured with rIL-7 (50–100 units/ml) as described (28–30) on monolayers of 2,000 rad irradiated antigen-negative stromal cell line S17 (a gift from K. Dorshkind, ref. 28), or on irradiated antigen-bearing stromal cell line op42 (a gift from P. Kincade, ref. 31). Stroma cell lines were tested for expression of the class I molecule Kb or Kk (the antigens recognized by the 3–83 BCR) by staining using the high affinity mAb, Y3 (4). Differentiation was induced by removal of IL-7 (27–30).
Flow Cytometry.
Expression of cell surface molecules was detected using the following antibodies: Goat anti-mouse-λ fluorescein isothiocyanate (Caltag, South San Francisco, CA); goat anti-mouse IgM phycoerythrin (Caltag); goat anti-mouse κ-biotin (Southrn Biotechnology Associates); and anti 3–83 BCR clonotype, 54.1-biotin (14). All biotinylated Abs were visualized with streptavidin-TriColor (Caltag) for three-color analysis. Expression of IgMa, B220, L-selectin, IgDa CD23, and CD21 was detected as described (27). Three-color fluorescence-activated cell sorter analysis was carried out on a FACScan instrument (Becton Dickinson).
Terminal Deonucleotidyltransferase-Mediated UTP End Labeling (TUNEL) Assay.
Apoptosis was determined by a TUNEL assay (Boehringer Mannheim) according to the protocol supplied by the manufacturer. Cells were analyzed by FACScan in a single color analysis with forward and side scatter gates adjusted to include all cells and to exclude debris. For each sample a control TUNEL mix without the enzyme terminal deoxynucleotidyltransferase was included.
Nucleic Acids Analysis.
PCR amplification from genomic DNA templates was performed using cell lysates prepared as described (27). In some experiments genomic DNA was isolated from cells as above and purified using the phenol-chloroform method. Total RNA purification and reverse transcription to cDNA were as described (10, 27).
Conditions and primer sequences for DNA PCR reactions for α-actin and Vκ-Jκ1 and for RT-PCR for RAG-2 and CD19 were as described in ref. 27, and for VHJ558L-JH2, VHQ52-JH2, VH7183-JH2, and VHGAM3–8-JH2 DNA rearrangements as described in ref. 32. PCR cycle conditions Vκ-Jκ5 were 40 sec at 94°C, 40 sec at 58°C, and 1.5 min at 72°C, for 22 cycles. The sequences are as follows: Vκ-Jκ5, 5′ Vκ(FW3) (27) and 3′Jκ5 intervening sequence, 5′-TGCCACGTCAACTGATAATGAGCCCTCTC-3′.
PCR products were run on a gel transferred to membrane and hybridized with specific probes as described (27). Blots were then washed and exposed to x-ray film or were scanned with a PhosphorImager (Molecular Dynamics) to quantify signal intensity. To obtain a semiquantitative estimate of gene expression, signal intensity of the Vκ-Jκ1 and Vκ-Jκ5 PCR products were normalized to the α-actin signal and those of the RAG-2 were normalized to the CD19 signal.
Genomic Southern blot for the detection of κ rearrangements was performed as follows. Ten micrograms of genomic DNA were digested with HindIII restriction enzyme for 6 hr at 37°C, electrophoresed on 1% agarose gel, vacuum transferred to Zeta probe membrane, hybridized overnight with 32P-labeled Jκ1–5 DNA probe, and exposed to x-ray film.
RESULTS
Self-Antigen Arrests the Development of Autoreactive B Cells and Blocks Positive Selection.
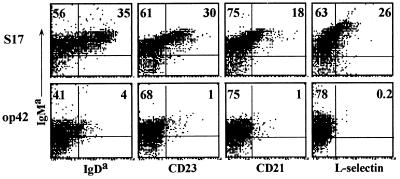
The 3–83Tg encodes a BCR with known antigen specificity that supports growth and developmental progression in IL-7 cultures (27). We used this system to probe the effects of self-tolerance on B cell development. 3–83Tg B cells grown in primary BM cultures were recultured in the presence of IL-7 for a further 5 days on irradiated stromal cell line monolayers that bear the 3–83 cognate antigen (H-2Kk,b) (op42) or on control, non-reactive stroma (S17). After secondary culture, cells were harvested and directly analyzed, or recultured for 1–2 days without IL-7, a protocol that promotes differentiation. Fig. 1 shows that after secondary IL-7 culture Tg cells expanded without antigen expressed high levels of sIgM on >90% of the cells, whereas those grown with antigen expressed a much lower level of sIgM. Significantly, in the absence of antigen, B cells progressed in development, up-regulating the maturation markers IgDa, CD23, CD21, and L-selectin (35%, 30%, 18%, and 26%, respectively), whereas B cells growing on antigen-bearing stroma did not express these markers. Similar results were observed in secondary cultures in which IL-7 was removed (not shown). No significant differences were found between the two different stroma layers in terms of supporting B cell growth, as equivalent numbers of B cells were observed in both cultures, indicating that the inability of the antigen-bearing op42 cells to foster 3–83Tg B cell maturation was due to their expression of 3–83 cognate antigen. This was confirmed in an analysis of primary cultures (grown in the absence of exogenous stroma), which indicated that developmental progression, but not growth, was specifically prevented when anti-3–83 id mAb 54.1 was included (not shown). Thus, under the culture conditions described, self-reactivity blocked B cell maturation (positive selection; ref. 8).
Figure 1.
Self-antigen arrests the in vitro maturation of 3–83Tg B cells as revealed by three-color flow cytometry analysis of cell surface markers. B cell precursors from 3–83Tg mice were grown for 5 days on antigen-free (S17) or antigen-bearing (op42) stromal cell lines in the presence of IL-7 and stained for the expression of surface IgM, the pan B cell marker B220, and the indicated cell surface markers. Results shown were gated on the B220+ population to exclude stromal cell contamination.
Antigen Fails to Accelerate B Cell Apoptosis in IL-7-Driven Cultures.
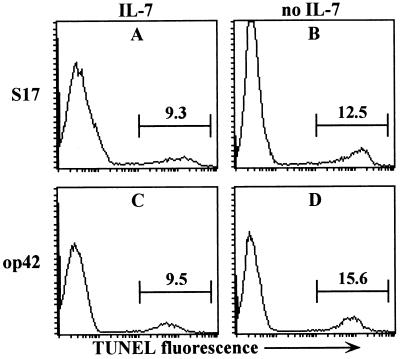
Several studies have suggested that antigen-induced apoptosis is a major mechanism of central tolerance (2, 5, 9), but in IL-7-driven cultures of 3–83Tg B cells antigen challenge failed to increase apoptosis (Fig. 2, compare A and C). Upon IL-7 withdrawal, an approximate 50% increase in apoptosis could be detected, but the presence of antigen did not significantly enhance this level (compare B and D). Similarly, in cultures lacking exogenous stroma that were challenged with mAb 54.1, no increase in apoptosis was observed before or after IL-7 withdrawn (not shown). Because these cultures generated B cells at high purity (96–99%) it is unlikely that our measurements of apoptotic cells were substantially underestimated because of rapid engulfment of apoptotic lymphocytes by small numbers of contaminating phagocytes. Taken together, these data show that although auto-antigen blocks positive selection and the development of B cells, it does not accelerate their apoptosis.
Figure 2.
Self-antigen does not accelerate apoptosis in 3–83Tg B cell cultures. 3–83Tg B cell precursors expanded for 5 days on S17 or op42 stroma were recultured for 24 hr on the same stroma in the presence or absence of IL-7 and apoptotic cells were detected by TUNEL assay. In control cultures treated with 5 μM Beauvericin (Sigma) 80–90% of the cells were apoptotic.
Developmentally Arrested 3–83Tg B Cells Undergo Secondary Rearrangements.
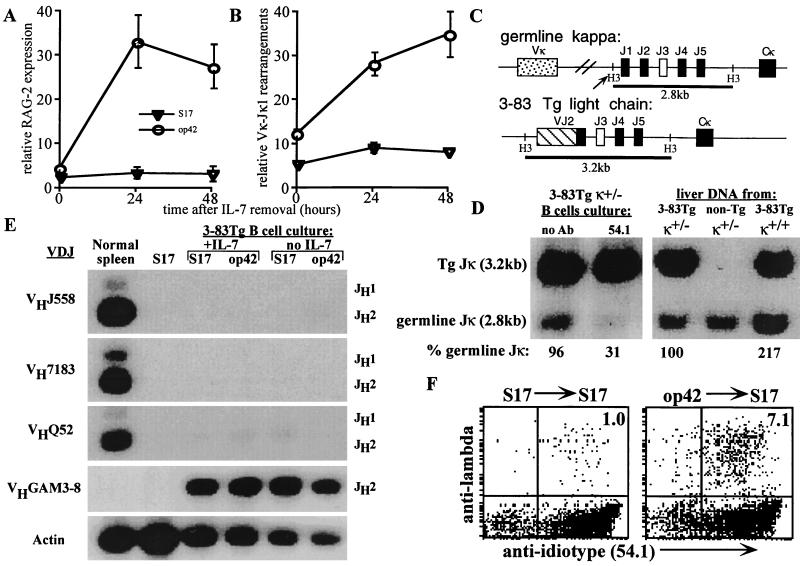
We measured antigen-induced rearrangements of endogenous Ig light chain genes in 3–83Tg B cell cultures as an indicator of receptor editing. When IL-7 was continuously present, cultures containing cognate 3–83 antigen had 2–3 fold elevated RAG-2 mRNA levels compared with control cultures (Fig. 3A). A highly significant, 9–10-fold antigen-induced increase in RAG mRNA expression was revealed when cells were deprived of IL-7 during the final 1–2 days of culture (Fig. 3A). To detect endogenous DNA rearrangements at the κ light chain locus, a PCR was designed to amplify Vκ-to-Jκ1 rearrangements using a pan-reactive Vκ framework 3 primer and a Jκ1-specific oligonucleotide. 3–83Tg B cells grown in the continual presence of IL-7 had a 2-fold increase in endogenous Vκ-Jκ1 DNA rearrangements on antigen-bearing stroma compared with antigen-free cultures (Fig. 3B), and, as in the case of RAG expression, withdrawal of IL-7 in the antigen-containing cultures resulted in a further significant increase in Vκ-Jκ1 rearrangements that peaked after 48 hr, while no significant changes were detected in B cells in the absence of antigen (Fig. 3B). To further quantitate rearrangement at the endogenous κ-locus, the loss of germline Jκ DNA was analyzed by genomic Southern blot in cultures lacking exogenous stroma (to minimize the presence of non-B cell contaminating DNA) (Fig. 3D). In cultured 3–83Tg cells that were heterozygously deficient for the natural Jκ-Cκ locus (3–83Tg κ ±) (which ensures that band intensity is proportional to the fraction of cells lacking rearrangements) anti-id treatment specifically induced κ-gene rearrangements in about two-thirds of the cells (as only 31% of germline Jκ band was detected), whereas little rearrangement was detected in the nonantigen-treated cultures (96% of the germline Jκ band was retained).
Figure 3.
Antigen induces secondary light chain gene rearrangements in 3–83Tg B cell cultures. 3–83Tg cultures were generated as described in Fig. 2 and analyzed at 0, 24, or 48 hr after IL-7 withdrawal. (A) RT-PCR analysis of mRNA expression of RAG-2 normalized to that of CD19. Results are shown as mean ± SEM of 8 separate experiments. (B) PCR analysis of VκJκ1 rearrangements in cell lysates normalized to that of α-actin gene. Results are shown as mean ± SEM of eight separate experiments. (C and D) Restriction analysis of κ rearrangements from primary 3–83Tg cultures grown in the presence or absence of anti-id mAb 54.1. IL-7 was withdrawn after the fifth day of a 7-day culture. The strategy to detect rearrangement is depicted in C, which shows HindIII restriction sites and the expected sizes of fragments detected by Jκ probe. Southern blot (D) shows the loss of the germline Jκ fragment upon exposure to 54.1 mAb (second lane). Control liver DNAs with one (±) or two (+/+) copies of the germline Jκ gene verified the quantitation of the germline Jκ band intensity. Germline Jκ band was quantitated and normalized to that of the Tg Jκ and is expressed as percent of control 3–83Tg κ ± liver DNA (which is 100%). (E) PCR analysis of heavy chain gene rearrangements. Cells were analyzed with PCRs detecting α-actin control gene or DNA rearrangements involving the indicated VH gene families, as described in Experimental Procedures. (F) antigen induces expression of endogenous λ-chain on 3–83Tg B cells. 3–83Tg BM cells stimulated with IL-7 on antigen-free S17 stroma (Left) or antigen-bearing op42 stroma (Right) were recultured on S17 for 48 hr in the absence of IL-7 and analyzed by three-color flow cytometry. Histograms depicts surface expression of 3–83 id and λ chain among the gated, B220+ cells.
DNA samples from the same cultures used to analyze κ-rearrangements were tested for endogenous rearrangements at the heavy chain gene loci by a PCR using an oligonucleotide that primed 3′ to JH2 in conjunction with V-region primers homologous either to sequences shared by members of the J558L, VQ52, V7183, or to the VGAM3–8 VH families (the latter primer also detects the heavy chain Tg). No elevation in endogenous heavy chain gene rearrangements was detected in the presence of antigen (Fig. 3E).
Despite undergoing extensive endogenous light chain gene rearrangements in the presence of antigen, most 3–83Tg B cell precursors were developmentally arrested and unable to mature, suggesting that they continued to express the autoreactive receptor and therefore were not positively selected. To confirm this prediction, IL-7 and antigen were withdrawn 48 hr prior to staining to prevent continued growth and to allow receptor re-expression. Flow cytometry analysis revealed substantial retention of the Tg-encoded specificity despite significant endogenous κ and λ-chain gene rearrangement. Cells challenged with antigen during culture largely retained the Tg-encoded clonotype determinant, but many coexpressed new light chains, as revealed in part with an anti-λ Ab, which labeled ≈7% of the cells (Fig. 3F, Right) (and up to 15% in cultures of 3–83Tg κ−/− B cells, not shown). This light chain coexpression is possible because the insertion site of the 3–83Tg is not in the normal κ-locus context and, unlike the naturally assembled κ genes, the κ-Tg is rarely extinguished by nested rearrangements. Collectively, these results further indicate that although the Tg-encoded BCR mediates substantial feedback suppression of V(D)J recombination, stimulation with antigen can specifically overcome light chain, but not heavy chain, allelic exclusion.
In Normal B Cell Cultures Treated with Anti-κ Ab, κ-Expressing Cells Are Developmentally Arrested but Not Killed.
To extend these findings to normal, non-Tg B cells, anti-κ mAb (187) was used as surrogate antigen in primary IL-7 cultures of normal B cells. Cells grown in the presence or absence of 187 Ab, were stained for expression of κ and λ chains and analyzed for the occurrence of apoptosis by staining for DNA fragmentation using the TUNEL assay. In control cultures, 30–40% of the cells expressed sIgM (not shown). As shown in Fig. 4B Left, 38% of the cells expressed κ chains and the κ/λ ratio was comparable to that found in vivo (15:1). In the 187-treated cultures, a similar number of B cells was observed, but only 15–20% of them expressed detectable sIgM (not shown). Relative to control cultures, treatment with 187 Ab suppressed the developmental progression of κ-expressing cells to a CD23+ phenotype (Fig. 4A) and stimulated a two-fold increase in the frequency and absolute number of λ+ cells (Fig. 4B). Unlike the κ-expressing B cells, λ+ cells in the 187-treated cultures were able to advance in maturity as measured by their acquisition of CD23 (Fig. 4A) and other markers such as IgD and CD21 (not shown). Moreover, as found in the 3–83Tg system (Fig. 2) the presence of 187 Ab did not induce cell death as only 11% apoptotic cells were detected compared with 14% in the untreated control (data not shown). Thus, in the presence of anti-κ, B cells expressing κ were developmentally arrested, but viable, whereas λ-cells were more numerous and could progress in development. The observed increase in λ-expressing cells in the presence of 187 Ab probably indicates that many cells underwent secondary light chain rearrangements, but only a subset of these encoded functional λ-chains.
Figure 4.
Anti-κ chain treatment induces λ-chain expression, but not apoptosis in cultures of normal, non-Tg B cells. B10.D2 BM depleted of IgM+ cells was cultured in the presence or absence of anti-κ mAb 187 for 6 days and analyzed for expression of CD23 (A) and surface light chain (B). In all cultures, IL-7 was withdrawn 24 hr before analysis. Representative results from three experiments are shown. For CD23 expression, gates were set to include all CD23+ cells that were then analyzed for expression of λ light chain. Cells positive for CD23 but negative for λ were considered as κ.
Secondary Rearrangements in Normal B Cells Developing in the Presence of Anti-κ Ab.
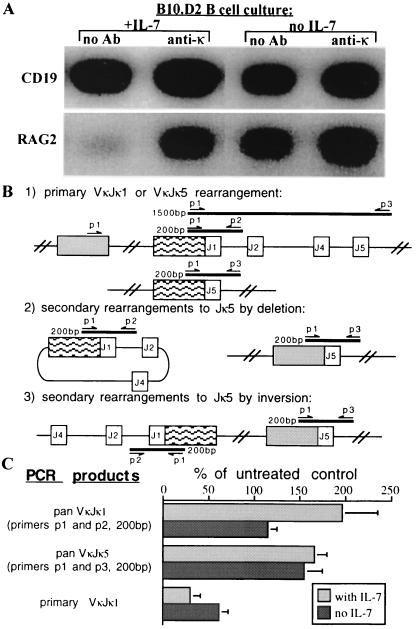
To further test the prediction that anti-κ treatment promotes receptor editing in IL-7-driven cultures of normal B cells, RNA and DNA were isolated from treated or control cultures and monitored for evidence of recombinase activity. As shown in Fig. 5A, anti-κ Ab induced a significant increase in RAG-2 expression over controls both in cultures in which IL-7 was present throughout and, to a lesser extent, in cultures in which IL-7 was subsequently withdrawn (Fig. 5A). To determine if this increased RAG expression correlated with light chain gene rearrangements, DNA rearrangements in the κ-locus were assessed using the PCR strategy described in Fig. 5B. The 5′ consensus VκFW3 primer detects most Vκs (p1) and was used with two different 3′ primers, p2 and p3, which hybridize 3′ of Jκ1 and Jκ5, respectively. While PCR with p1 and p2 detects all Vκ-to-Jκ1 rearrangements, PCR with p1 and p3 amplifies only primary Vκ-to-Jκ1 rearrangements (Fig. 5B Top), and fails to detect Vκ-to-Jκ1 rearrangements if subsequent deletional or inversional rearrangements occur on the same allele (Fig. 5B Middle and Bottom, respectively). The use of the p1 and p3 combination does, however, amplify all Vκ-to-Jκ5 rearrangements (Fig. 5B). Fig. 5C summarizes 187-induced κ rearrangements relative to control rearrangements (which is 100%). Anti-κ treatment stimulated total VκJκ1 and VκJκ5 rearrangements (Fig. 5C Top and Middle, respectively), while reducing primary VκJκ1 rearrangements by ≈50% because of increased secondary rearrangements to downstream Js (Fig. 5C Bottom). Taken together, these data show that normal B cell precursors grown in the presence of anti-κ Ab undergo secondary rearrangements to downstream Jκs and to λ, indicating that extensive receptor editing can occur in normal B cells.
Figure 5.
Normal non-Tg B cells undergo secondary rearrangements when grown in the presence of anti-κ mAb. (A) B10.D2 BM cultured as described in Fig. 5 were analyzed by reverse transcriptase-PCR for RAG-2 and CD19 mRNA expression as described in Fig. 3A. (B) Strategy for the PCR analysis of primary and secondary κ gene rearrangements showing the locations of the different primers and the approximate sizes of the expected products. (C) Quantitation of κ gene rearrangements induced by anti-κ mAb treatment. Rearrangements (normalized to α-actin) are shown for cells at various times after IL-7 withdrawal and are expressed as percent of untreated controls [calculated as (treated/untreated) × 100%, thus, the control amount is always 100%].
DISCUSSION
Using a novel in vitro system to study immature B cell tolerance, we have shown here that tolerance to self cell surface antigen is mediated by a block in B cell maturation, which promotes receptor editing, but does not appreciably accelerate apoptosis over the short term. Under these experimental conditions, self antigen induced secondary light chain gene rearrangements in at least two-thirds of the autoreactive immature B cells, but failed to accelerate cell death at this stage, suggesting that receptor editing plays a major role in rescuing formerly self-reactive B cells. If not relieved by receptor editing, this tolerance-induced developmental block will presumably lead to eventual B cell death, but as a result of the limited cellular carrying capacity of the tissue microenvironment, rather than by B cell autonomous suicide. This central tolerance mechanism that operates during B cell development is rather different than that of peripheral B cell tolerance, in which self-antigen specifically accelerates B cell death and lowers the steady state numbers of autoreactive B cells in peripheral lymphoid tissues (4, 15, 25, 33, 34). On the contrary, in the immature B cell compartment, central B cell tolerance results in the specific accumulation of cells with autoreactive specificities, which presumably await escape through receptor editing (19, 20). In normal, non-Tg B cells this escape through receptor editing is efficient, as it is facilitated by the organization of the Ig κ-locus, with its unique ability to undergo nested V-J rearrangements that inactivate and replace light chains contributing to autoreactive receptors (10, 12, 35). In this study we have demonstrated that central tolerance maintains light chain gene rearrangement for a considerable period of time in the absence of cell death, thus confirming the prediction that receptor editing provides an important salvage function.
The experimental system described here has many advantages for the study of central B cell tolerance. B cells are generated at high purity and are challenged with auto-antigen immediately upon BCR expression, a situation that should mimic tolerance to natural auto-antigen in the BM. This allowed us to simultaneously monitor the contributions of receptor editing and apoptosis to tolerance in 3–83Tg B cells, which bear an autoreactive receptor of predefined specificity. Furthermore, we were able to generate cultures of developing B cells from normal, non-Tg BM in which receptor editing and apoptosis could be similarly studied in response to anti-κ Ab. These experiments confirmed that receptor editing is a major mechanism of central tolerance in normal, non-Tg B cells. This experimental system should also facilitate further biochemical studies of tolerance mechanisms.
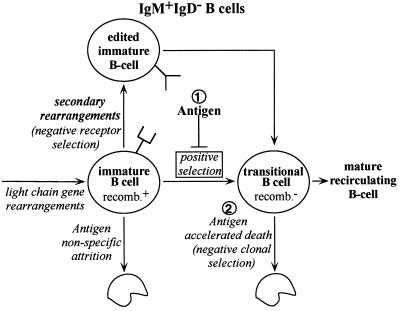
Other studies have indicated that cell death in immature B cells encountering self-antigen is surprisingly modest (19, 23, 33), but these prior studies did not directly measure apoptosis and have been challenged by other work (18), including studies that analyzed central tolerance in highly purified immature B cells (9), or in putative tumor models of central tolerance (36). In this study, we have carefully monitored apoptosis, cell recoveries, differentiation markers, and receptor editing in a highly homogeneous system of nontransformed B cells and find that at the immature B cell stage apoptosis is not accelerated by challenge with auto-antigen. In the case of the 3–83Tg cells, this lack of death was not merely due to the removal of the self-reactive receptors by receptor editing because editing was relatively inefficient in these cells, probably because of the chromosomal location of the Tg-encoded light chain gene outside of its normal context. On the other hand, receptor editing in normal, non-Tg B cells could be readily observed in anti-κ-treated cultures, and was reflected in an increase in both light chain rearrangement activity and the absolute number of λ+/κ− B cells generated. One way that the disparate results in the literature regarding the apoptosis sensitivity of immature B cells can be accommodated is by postulating that immature cells become exquisitely sensitive to apoptosis-mediated negative selection shortly after positive selection (Fig. 6, pathway marked “2”). Prior to this positive selection step, self-reactivity blocks developmental progression, prevents down-regulation of light chain gene rearrangements, and therefore promotes receptor editing (Fig. 6, pathway “1”). This might also explain the presence of an apoptosis-resistant population among IgM+IgD− immature cells treated with anti-μ Ab (9).
Figure 6.
A proposed model for the induction of receptor editing and cell death during B cell development. Productive light chain rearrangements allow progression of pre-B cells to the immature, IgM+/recombination+ (recomb.+) phenotype that, in the absence of antigen, matures to a transitional, IgMbright/recombination− stage (positive selection). In the presence of antigen (pathway marked “1”), however, this transition does not occur, allowing continued expression of VJ recombination, new light chain gene expression, and receptor editing. If receptor editing extinguishes self-specificity, the IgM+/recombination+ B cell can resume its maturation. However, if an appropriate receptor cannot be made, the cell eventually dies, but no more quickly than a cell that is unable to assemble in-frame rearrangements during the small pre-B II stage. In this sense, antigen reactivity in the IgM+/recombination+ B cell does not directly accelerate B cell death, but blocks differentiation. In contrast, transitional, IgMbright/recombination− B cells encountering antigen (pathway marked “2”) are reported to be highly sensitive to rapid antigen-induced death (18).
In the absence of auto-antigen, the 3–83Tg BCR can drive B cell developmental progression both in vitro and in vivo, as defined by the acquisition of cell surface molecules characteristic of peripheral B cells, such as sIgD, CD23, CD21, and L-selectin, and by the down-regulation of VJ recombination (21, 27). This study shows that auto-antigen can block progression in an entire cohort of cells developing in vitro. It is perhaps significant that the ability to promote positive selection is not shared by all Tg-encoded BCRs, possibly because of their intrinsic autoreactivity (21, 37). The nature of B cell positive selection is not understood in detail, but clearly requires BCR surface expression and signal transduction (8, 38–40). The data presented in this paper therefore show that in immature B cells, as in immature T cells (41), the same antigen receptor can signal both positive and negative selection, depending upon the quality or quantity of extracellular ligand. The developmentally arrested, autoreactive, immature IgM+ B cell has in fact several striking similarities to the immature TCR+ thymocyte that fails to receive a positive selection signal from major histocompatibility complex antigens of the thymic epithelium. Both of these cells continue to express V(D)J recombinase and to undergo receptor gene rearrangements despite surface antigen receptor expression (this study and refs. 4, 10, 42) and these receptor gene rearrangements frequently occur through nested V-J recombination (this study and refs. 12, 35, 43). Furthermore, both cells fail to advance in development until they produce an appropriate receptor, which is perceived by the cells as one that provides the proper degree of signaling (8, 44). Thus, in both B cell central tolerance and in T-cell “neglect” the developmental arrest is reversible, and the cells can be efficiently rescued and allowed to mature through secondary rearrangements that provide the appropriate specificity.
Acknowledgments
This work was supported by National Institutes of Health (Grants RO1 AI33608, K04 AI01161, R01 GM44809), the Arthritis Foundation, and the Human Frontier Foundation.
ABBREVIATIONS
- BCR
B cell receptor
- Tg
transgenic
- RAG
recombinase activator gene
- BM
bone marrow
- IL-7
interleukin 7
- idiotype
id
- TUNEL
terminal deonucleotidyltransferase-mediated UTP end labeling
- Ab
antibody
References
- 1.Klinman N R. Immunity. 1996;5:189–195. doi: 10.1016/s1074-7613(00)80314-3. [DOI] [PubMed] [Google Scholar]
- 2.Monroe J G. J Immunol. 1996;156:2657–2660. [PubMed] [Google Scholar]
- 3.Radic M Z, Zouali M. Immunity. 1996;5:505–511. doi: 10.1016/s1074-7613(00)80266-6. [DOI] [PubMed] [Google Scholar]
- 4.Lang J, Jackson M, Teyton L, Brunmark A, Kane K, Nemazee D. J Exp Med. 1996;184:1685–1697. doi: 10.1084/jem.184.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nossal G J. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- 6.Hardy R R, Carmack C E, Shinton S A, Kemp J D, Hayakawa K. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storb U, Roth P, Kurtz B K. Immunol Res. 1994;13:291–298. doi: 10.1007/BF02935620. [DOI] [PubMed] [Google Scholar]
- 8.Rajewsky K. Nature (London) 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 9.Norvell A, Mandik L, Monroe J G. J Immunol. 1995;154:4404–4413. [PubMed] [Google Scholar]
- 10.Tiegs S L, Russell D M, Nemazee D. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radic M Z, Erikson J, Litwin S, Weigert M. J Exp Med. 1993;177:1165–1173. doi: 10.1084/jem.177.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prak E L, Weigert M. J Exp Med. 1995;182:541–548. doi: 10.1084/jem.182.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osmond D G, Rico-Vargas S, Valenzona H, Fauteux L, Liu L, Janani R, Lu L, Jacobsen K. Immunol Rev. 1994;142:209–230. doi: 10.1111/j.1600-065x.1994.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 14.Nemazee D A, Burki K. Nature (London) 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 15.Murakami M, Tsubata T, Okamoto M, Shimizu A, Kumagai S, Imura H, Honjo T. Nature (London) 1992;357:77–80. doi: 10.1038/357077a0. [DOI] [PubMed] [Google Scholar]
- 16.Hartley S B, Crosbie J, Brink R, Kantor A B, Basten A, Goodnow C C. Nature (London) 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Nagy Z, Radic M Z, Hardy R R, Huszar D, Camper S A, Weigert M. Nature (London) 1995;373:252–5. doi: 10.1038/373252a0. [DOI] [PubMed] [Google Scholar]
- 18.Carsetti R, Kohler G, Lamers M C. J Exp Med. 1995;181:2129–2140. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartley S B, Cooke M P, Fulcher D A, Harris A W, Cory S, Basten A, Goodnow C C. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 20.Nemazee D, Buerki K. Proc Natl Acad Sci USA. 1989;86:8039–8043. doi: 10.1073/pnas.86.20.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spanopoulou E, Roman C A, Corcoran L M, Schlissel M S, Silver D P, Nemazee D, Nussenzweig M C, Shinton S A, Hardy R R, Baltimore D. Genes Dev. 1994;8:1030–1042. doi: 10.1101/gad.8.9.1030. [DOI] [PubMed] [Google Scholar]
- 22.Erikson J, Radic M Z, Camper S A, Hardy R R, Carmack C, Weigert M. Nature (London) 1991;349:331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 23.Hertz M, Nemazee D. Immunity. 1997;6:429–436. doi: 10.1016/s1074-7613(00)80286-1. [DOI] [PubMed] [Google Scholar]
- 24.Melchers F, Rolink A, Grawunder U, Winkler T H, Karasuyama H, Ghia P, Andersson J. Curr Opin Immunol. 1995;7:214–227. doi: 10.1016/0952-7915(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 25.Russell D M, Dembic Z, Morahan G, Miller J F, Burki K, Nemazee D. Nature (London) 1991;354:308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Trounstine M, Kurahara C, Young F, Kuo C C, Xu Y, Loung J F, Alt F W, Huszar D. EMBO. 1993;12:821–830. doi: 10.1002/j.1460-2075.1993.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melamed D, Kench J A, Grabstein K, Rolink A, Nemazee D. J Immunol. 1997;159:1233–1239. [PubMed] [Google Scholar]
- 28.Cumano A, Dorshkind K, Gillis S, Paige C J. Eur J Immunol. 1990;20:2183–2189. doi: 10.1002/eji.1830201006. [DOI] [PubMed] [Google Scholar]
- 29.Hardy R R, Kishimoto T, Hayakawa K. Eur J Immunol. 1987;17:1769–1774. doi: 10.1002/eji.1830171214. [DOI] [PubMed] [Google Scholar]
- 30.Rolink A, Haasner D, Nishikawa S I, Melchers F. Blood. 1993;81:2290–2300. [PubMed] [Google Scholar]
- 31.Smithson G, Medina K, Ponting I, Kincade P W. J Immunol. 1995;155:3409–3417. [PubMed] [Google Scholar]
- 32.Costa T E F, Suh H, Nussenzweig M C. Proc Natl Acad Sci USA. 1992;89:2205–2208. doi: 10.1073/pnas.89.6.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fulcher D A, Basten A. J Exp Med. 1994;179:125–134. doi: 10.1084/jem.179.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cyster J G, Hartley S B, Goodnow C C. Nature (London) 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 35.Chen C, Prak E L, Weigert M. Immunity. 1997;6:97–105. doi: 10.1016/s1074-7613(00)80673-1. [DOI] [PubMed] [Google Scholar]
- 36.Scott D W. Adv Immunol. 1993;54:393–425. doi: 10.1016/s0065-2776(08)60539-8. [DOI] [PubMed] [Google Scholar]
- 37.Andersson J, Melchers F, Rolink A. Scand J Immunol. 1995;42:21–33. doi: 10.1111/j.1365-3083.1995.tb03621.x. [DOI] [PubMed] [Google Scholar]
- 38.Kitamura D, Rajewsky K. Nature (London) 1992;356:154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- 39.Torres R M, Flaswinkel H, Reth M, Rajewsky K. Science. 1996;272:1804–1808. doi: 10.1126/science.272.5269.1804. [DOI] [PubMed] [Google Scholar]
- 40.Papavasiliou F, Misulovin Z, Suh H, Nussenzweig M C. Science. 1995;268:408–411. doi: 10.1126/science.7716544. [DOI] [PubMed] [Google Scholar]
- 41.Jameson S C, Hogquist K A, Bevan M J. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 42.Borgulya P, Kishi H, Uematsu Y, Von Boehmer H. Cell. 1992;69:529–537. doi: 10.1016/0092-8674(92)90453-j. [DOI] [PubMed] [Google Scholar]
- 43.Harada K, Yamagishi H. J Exp Med. 1991;173:409–415. doi: 10.1084/jem.173.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saint-Ruf C, Ungewiss K, Groettrup M, Bruno L, Fehling H J, Von Boehmer H. Science. 1994;266:1208–1212. [PubMed] [Google Scholar]