Abstract
In cultured oligodendrocytes isolated from perinatal rat optic nerves, we have analyzed the expression of ionotropic glutamate receptor subunits as well as the effect of the activation of these receptors on oligodendrocyte viability. Reverse transcription–PCR, in combination with immunocytochemistry, demonstrated that most oligodendrocytes differentiated in vitro express the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunits GluR3 and GluR4 and the kainate receptor subunits GluR6, GluR7, KA1 and KA2. Acute and chronic exposure to kainate caused extensive oligodendrocyte death in culture. This effect was partially prevented by the AMPA receptor antagonist GYKI 52466 and was completely abolished by the non-N-methyl-d-aspartate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), suggesting that both AMPA and kainate receptors mediate the observed kainate toxicity. Furthermore, chronic application of kainate to optic nerves in vivo resulted in massive oligodendrocyte death which, as in vitro, could be prevented by coinfusion of the toxin with CNQX. These findings suggest that excessive activation of the ionotropic glutamate receptors expressed by oligodendrocytes may act as a negative regulator of the size of this cell population.
Keywords: excitotoxicity, kainate, Ca2+ influx, cell death
The main function of oligodendrocytes is to myelinate axons in the vertebrate central nervous system. This cell type develops mostly soon after the majority of neurons are generated and have extended their axons and, therefore, it is likely that neurons play an important role in regulating oligodendrocyte development. In the rat optic nerve, the first fully differentiated oligodendrocytes appear after birth, and the definitive mature population of oligodendrocytes is reached around 6 weeks later (1, 2). The proliferation and the survival of oligodendrocytes depend, respectively, on the electrical activity of neighboring axons and in the reciprocal contacts they establish (for a recent review, see ref. 3). Axon to oligodendrocyte signaling results in the generation of the precise number of oligodendrocytes necessary to myelinate entirely a given population of axons. Unfortunately, little information is available about the molecules participating in this signaling process.
Oligodendrocytes express neurotransmitter receptors including those activated by glutamate (4). This excitatory amino acid acts at various types of receptors, which can be grouped into two major categories: ionotropic receptors, which gate membrane ion channels permeable to cations; and metabotropic receptors, which are coupled to G proteins. Ionotropic receptors are classified into α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainate, and N-methyl-d-aspartate (NMDA) subtypes, according to their preferred agonist. Molecular cloning has revealed that each receptor subtype is composed of several subunits with high homology within each receptor class (reviewed in ref. 5). Thus, AMPA receptors are formed by GluR1–4, kainate receptors by GluR5–7 and KA1–2, and NMDA receptors by NMDAR1 and NMDAR2A-D subunits (5).
Here, we tested whether glutamate, the most abundant excitatory neurotransmitter in the mammalian brain, acting on its ionotropic receptors, is involved in the shaping of the oligodendrocyte population. To that end, we first analyzed the receptors expressed by this macroglial cell type, and then studied the effects of glutamate agonists on their survival. We show that the main ionotropic glutamate receptors expressed by oligodendrocytes belong to the AMPA and kainate classes and that their activation causes oligodendrocyte death both in vitro and in vivo.
MATERIALS AND METHODS
Oligodendrocyte Cultures.
Oligodendrocyte cultures were established from optic nerves obtained from 7- to 12-day-old Sprague–Dawley rats following essentially the procedure described by Barres et al. (2). Briefly, optic nerves were freed of their meninges and then digested with collagenase (1.25 mg/ml) and 0.125% trypsin/0.02% EDTA in Ca2+- and Mg2+-free Hanks’ buffered salt solution. The resulting cell suspension was gently passed through needles of different gauges (21G, 23G, and 25G), filtered through a nylon mesh (40 μm), and seeded into 24-well plates bearing 12-mm diameter coverslips coated with poly-d-lysine (10 μg/ml) at a density of 1–2 × 104 cells/well. Cells were kept in DMEM (4.5 g/liter glucose/0.11 g/liter sodium pyruvate) supplemented with 2 mM glutamine/1 mg/ml BSA (fraction V)/5 μg/ml bovine pancreatic insulin/100 μg/ml human transferrin/40 ng/ml sodium selenite/60 ng/ml progesterone/16 μg/ml putrescine/30 ng/ml 3,3′,5-triiodo-l-thyronine/40 ng/ml l-thyroxine/10 ng/ml ciliary neurotrophic factor (Boehringer Mannheim)/1 ng/ml neurotrophin-3 (Promega). Cultures were maintained at 37°C and 5% CO2, and fresh medium was added every day. All culture reagents were purchased from Sigma unless otherwise stated.
Oligodendrocytes were identified by their typical mesh-like arborization and by immunofluorescence labeling with a mouse IgG3 anti-galactocerebroside (ref. 6; Boehringer Mannheim; diluted at 3 μg/ml) followed by a rhodamine-conjugated secondary antibody (Sigma).
RNA Isolation and Reverse Transcription–PCR (RT-PCR) Analysis.
Total RNA was extracted from oligodendrocyte cultured for 2–5 days and from adult rat whole brain samples using the guanidinium/phenol/chloroform method (7). Reverse transcription into cDNA was carried out using random hexamer primers and avian myeloblastosis virus reverse transcriptase (United States Biochemical) as described by the supplier. Specific oligonucleotide primers as well as PCR reagent concentrations used for PCR amplification of each AMPA and kainate receptor subunits were as described earlier (8, 9). Primers (20 to 22 mers) specific for NMDA receptor subunits were designed to amplify with equal efficiency all the described splice variants of the NMDAR1 subunit (bases 600 to 1134, clone accession no. L08228), NMDAR2A (bases 3489 to 4017, clone accession no. M91561) and NMDAR2B (bases 3306 to 3786, clone accession no. M91562). The reaction mixture was heated to 95°C for 2 min, cooled to 80°C, and then Taq DNA polymerase (Dynazyme, Finnzymes) was added at this temperature. PCRs were carried out in thin-walled tubes for a total of 40 (50 for NMDA receptor subunits) cycles as follows: 95°C for 50 s, the average of the melting temperature of the corresponding oligonucleotide pair + 5°C for 50 s, 72°C for 50 s, for two cycles; subsequently the annealing temperature was lowered by 1°C every second cycle until it was 9°C lower than the starting annealing temperature. Finally, 22 (32 for NMDA receptor subunits) more cycles were run at the final annealing temperature with an extension time of 1 min. Amplified products were analyzed by electrophoresis in 1.8% agarose gels and viewed with ethidium bromide staining. A φX174 HaeIII digest was used as a size standard.
Glutamate Receptor Immunocytochemistry.
Cells at 3 to 7 days in culture were washed with sodium PBS, fixed in 4% paraformaldehyde in PBS for 20 min, and then processed for immunocytochemistry as described by Puchalski et al. (10). Primary antibodies were applied for 45 min, at concentrations of 2.5 μg/ml for GluR1, GluR2/3, and GluR4 (all from Chemicon), 1 μg/ml for GluR5/6/7 (PharMingen), 6 μg/ml for KA2 (generously provided by R. J. Wenthold, National Institutes of Health, Bethesda, MD), 2 μg/ml for NMDAR1 (Chemicon), and 4 μg/ml for NMDAR2A/B (Chemicon). The immunodetection procedure was carried out using biotinylated secondary antibodies followed by incubation with an avidin-biotin-peroxidase complex (Vector) as indicated by the supplier. Color development was performed using 0.04% 3,3′-diaminobenzidine, 0.06% NiCl2, and 0.02% hydrogen peroxide diluted in 0.1 M Tris⋅HCl, pH 7.4. As a negative control, the primary antibody solution was replaced with rabbit or mouse Igs from nonimmune animals. The whole procedure was carried out at room temperature.
Toxicity Assays.
Cells at 2 to 4 days in culture were exposed to glutamate receptor agonists alone or in conjunction with antagonists for 15 min to 72 hr in feeding medium. Four to 72 hr after drug application, cell viability and cell death were assessed using fluorescein diacetate (60 μg/ml) and propidium iodide (20 μg/ml) according to the method described by Jones and Senft (11). All experiments were carried out in duplicate, and cell counts were taken using a 20× objective from at least 10 randomly selected fields per coverslip. The values provided here are the average of 3–10 independent experiments. Drugs were purchased from Tocris Neuramin (Bristol, U.K.), except GYKI 52466 (Research Biochemicals).
Drug Application in Vivo.
In vivo experiments were performed in adult male New Zealand White rabbits (1.5–2 kg), which were deeply anesthetized with Ketolar (ketamine·HCl, 50 mg/kg body weight, i.m.) and Rompun (tiazine·HCl, 10 mg/kg body weight, i.m.). Surgical procedures were as previously described (12). Vehicle and drugs were continuously delivered through a catheter (24G) implanted under the dura mater enwrapping one optic nerve. The catheter was connected to a tube attached to an osmotic pump (Alzet, Palo Alto, CA) calibrated to infuse 0.5 μl/hr for 7 days. Before surgery, pumps were filled with saline, 0.1–1 mM kainate alone, or in conjunction with 30 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), all diluted in saline. After completion of the treatments, animals were deeply anesthetized and decapitated, and the optic nerves were dissected out and frozen in isopentane chilled at −40°C. Histological analysis of vehicle and drug-treated optic nerves was carried out in cryostat sections (10 μm thick) mounted onto gelatinized slides and counterstained with toluidine blue.
RESULTS
Ionotropic Glutamate Receptor Subunits Expressed by Cultured Oligodendrocytes.
Optic nerve cultures derived from P7 to P12 rats were highly enriched in oligodendrocytes as revealed by immunocytochemistry with antibodies to galactocerebroside (see Fig. 2, GC). After 3 to 5 days in vitro, oligodendrocytes typically constituted at least 95% of the cells present in these cultures. We observed no major changes in the expression of the glutamate receptor subunits analyzed in all the cultures assayed. RT-PCR (40 cycles) of total RNA extracted from these cells resulted in the amplification of some, but not all, AMPA and kainate receptor subunits (Fig. 1). This was in contrast to the results obtained using RNA extracted from the whole rat brain, which showed that all the glutamate receptor subunits assayed were efficiently amplified under the same PCR conditions (Fig. 1).
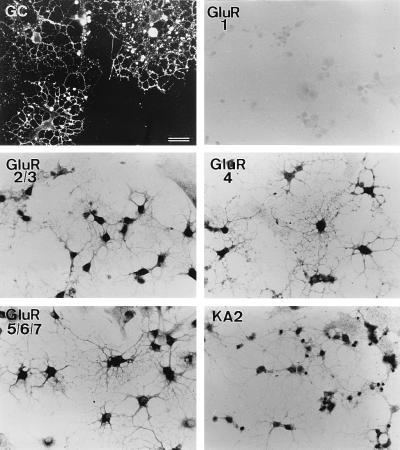
Figure 2.
Expression of ionotropic glutamate receptor subunits in differentiated oligodendrocytes. Cultures were obtained from P7 rat optic nerves and grown for 5 days. The vast majority of the cells in these cultures expressed the oligodendrocyte marker galactocerebroside (GC), and the subunits GluR2–7 and KA2. (Bar = 25 μm.)
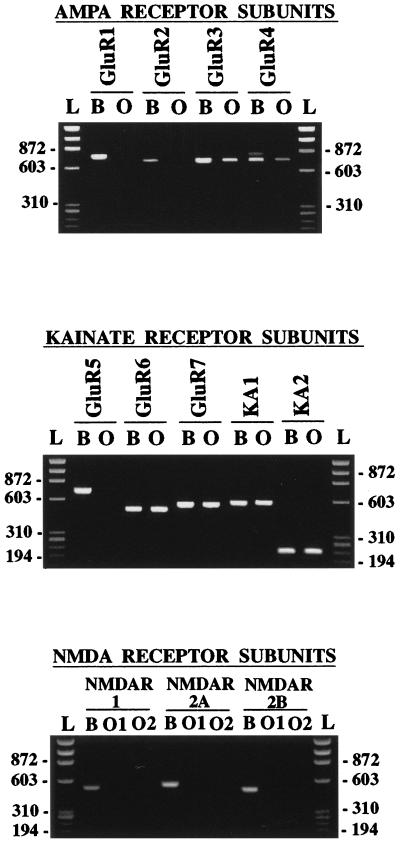
Figure 1.
RT-PCR analyses of total RNAs encoding ionotropic glutamate receptor subunits in oligodendrocytes in vitro (lanes O) derived from the P7 rat optic nerve. All primers efficiently amplified the corresponding subunit from rat brain RNA samples (lanes B). The amplified subunits are indicated above each lane. AMPA and kainate receptor subunits were amplified with 40 PCR cycles (lanes B), while NMDA receptor subunits were amplified with 50 PCR cycles (lanes O). PCR products are of the predicted size in all instances. The expression of subunits was similar in oligodendrocyte cultures derived from optic nerves obtained from P7 and P11 rats, regardless of the time in culture (2–5 days). Molecular standards in base pairs correspond to the φX174 HaeIII digest (lanes L). Numbers on the left indicate size in base pairs. O1 and O2 correspond to samples from two different oligodendrocyte cultures.
Oligodendrocytes expressed only the AMPA receptor subunits GluR3 and GluR4, but not GluR1 and GluR2. The absence of the GluR2 subunit in oligodendrocytes indicates that in these cells the native AMPA receptors are most likely permeable to Ca2+ (5). The primers used to amplify GluR4 also detect the GluR4c variant (13), an isoform that is principally expressed in the cerebellum (14). Indeed, we found that GluR4 along with the GluR4c variant were amplified from our rat whole brain RNA samples, but that the latter was not observed in oligodendrocytes (Fig. 1). Immunocytochemical experiments confirmed that the GluR1 subunit was absent from the oligodendrocyte cultures and showed further that the expressed subunits were present in the vast majority of the cells and that they were located both in the oligodendrocyte somata and in the processes (Fig. 2).
All kainate receptor subunits, except GluR5, were efficiently amplified from RNA extracted from oligodendrocyte cultures (Fig. 1). Consistent with these findings, immunocytochemical analysis with the available antibodies to this glutamate receptor class revealed that these subunits are present in most oligodendrocytes in vitro (Fig. 2). In contrast, no amplification of NMDA receptor subunits was observed after running 40 PCR cycles (not shown), as used for the AMPA and kainate receptors. After 50 PCR cycles, we could observe only very low amounts of amplified fragment for the NMDAR2B, but not for the NMDAR1 and NMDAR2A subunits. Consistently, immunocytochemical results showed no labeling with antibodies to NMDAR1 and near to background stain with antibodies to NMDAR2A/B subunits (not shown). Because native functional NMDA receptors are heterologous in nature and are formed by NMDAR1 and NMDAR2 subunits (15), the significance of the weak expression of NMDAR2B is unclear.
Non-NMDA Ionotropic Glutamate Receptors and Oligodendrocyte Death.
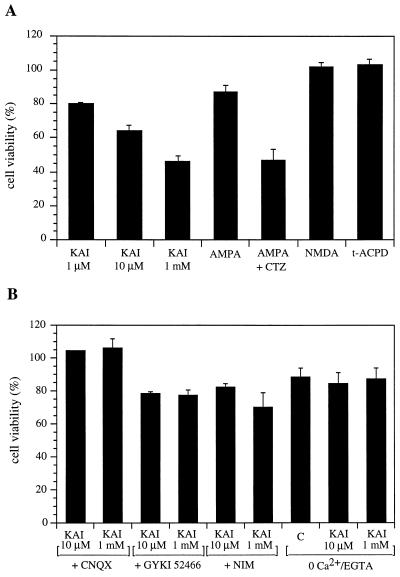
Both acute (15 min) and chronic (24–72 hr) exposure of the cultures to kainate (1–1,000 μM) resulted in a similar oligodendrocyte toxicity (Figs. 3 and 4). Cell death was observed as early as 4 hr after application of glutamate agonists. At 24 hr after the initiation of the treatment with 1 mM kainate, only about 46% of the oligodendrocytes were viable, and the remaining were dead, as measured with the fluorescein diacetate and propidium iodide dyes, respectively. That proportion decreased progressively until most cells were dead 72 hr later. AMPA (100 μM) showed a much weaker oligodendrocyte toxicity (cell viability 87%), while NMDA (100 μM, glycine 300 μM was available in the culture medium), and 1-aminocyclopentane-1,3-dicarboxylic acid (t-ACPD) (100 μM) were ineffective (Fig. 4A). The vulnerability of oligodendrocytes to both kainate and AMPA could be prevented by coapplication of the drugs with CNQX, a nonselective AMPA and kainate receptor antagonist, and also by removing Ca2+ from the culture medium while the receptors were activated by the corresponding agonist (Fig. 4B). The latter indicates that Ca2+ influx into oligodendrocytes mediates the observed toxicity. The partial blockade of oligodendrocyte vulnerability to kainate by nimodipine (10 μM; Fig. 4B), an L-type voltage-activated Ca2+ channel blocker, suggests that Ca2+ influx occurs both through the activated receptors and via Ca2+ channels.
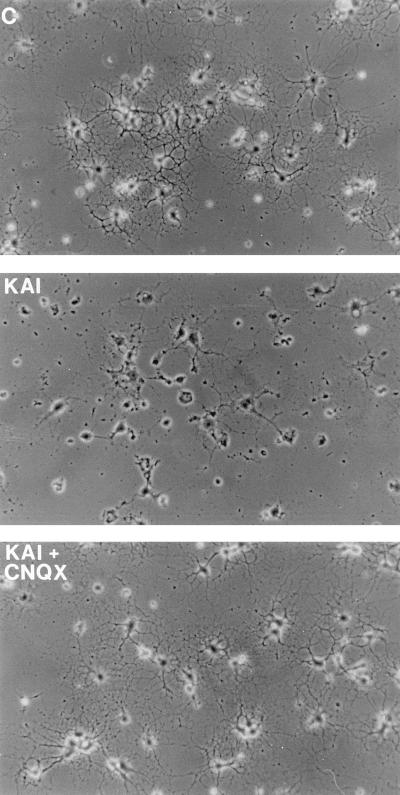
Figure 3.
Kainate toxicity in oligodendrocytes derived from P7 rat optic nerves. Kainate (KAI, 1 mM) alone or together with CNQX (30 μM) was applied to the culture medium for 24 hr and then photographed under phase-contrast microscopy. C, control culture. (Bar = 25 μm.)
Figure 4.
Pharmacological profile of the excitotoxicity mediated by ionotropic glutamate receptors in oligodendrocytes derived from P12 rat optic nerves. (A) Effect of the glutamate receptor agonists kainate (KAI), AMPA (100 μM), AMPA+cyclothiazide (CTZ, both at 100 μM), NMDA and t-ACPD (both 100 μM). (B) Blockade of kainate (KAI, 10 and 1,000 μM) toxicity by CNQX (30 μM), GYKI 52466 (100 μM), nimodipine (10 μM), and with 0 mM Ca2+/0.1 mM EGTA. Values (average ± SEM) are referred to control nontreated cultures (n > 7). Similar values were obtained in cultures from P7 rats. Only glutamate receptor agonists, applied alone at the concentrations indicated, had a significant effect on the viability of oligodendrocytes. C, control (0 Ca2+ + EGTA vs. normal solution).
The low efficacy of AMPA in triggering cell death contrasted with the potency of kainate even at low concentrations. Thus, after application of kainate at 1 μM for 15 min, 20% of the oligodendrocytes were dead 24 hr later (Fig. 4A). However, AMPA (100 μM) toxicity was potentiated with 100 μM cyclothiazide, which blocks desensitization at AMPA-preferring receptors (16), and only 47% of the cells survived after 24 hr of exposure to the agonist. This suggests that the toxic effects may be mediated by both AMPA and kainate receptors. This idea was further strengthened by the fact that GYKI 52466 (100 μM), an AMPA-selective noncompetitive antagonist that has a much lower affinity for kainate receptors (17), prevented only partially the effects of kainate on the viability of oligodendrocytes (Fig. 4B).
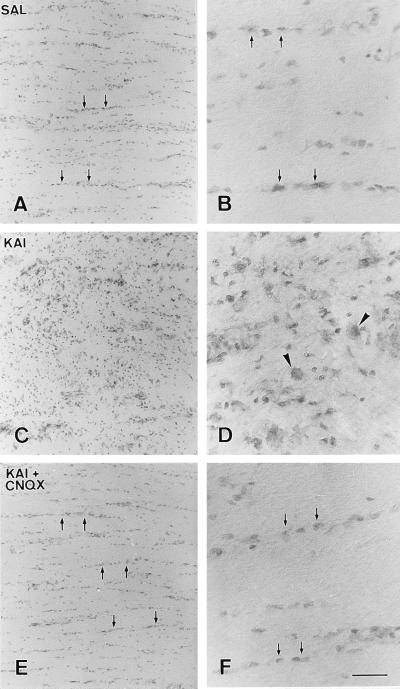
Chronic application of kainate to the surface of rabbit optic nerves resulted in a profound disruption of their histological features. The extent of this disruption was dependent on the concentration of the drug and was not observed when only the vehicle was infused (Fig. 5). Thus, delivery of 100 μM kainate (0.5 μl/hr) over 5–7 days resulted in a great loss of interfascicular oligodendrocytes accompanied by a strong gliotic reaction (Fig. 5 C and D). The area affected by the toxin was located around the site at which the tip of the infusion cannula was located and involved approximately a third of the nerve. Application of 1 mM kainate caused a similar lesion whose extension affected about half of the nerve. Kainate toxicity was prevented by coinfusion of the agonist with the AMPA/kainate receptor blocker CNQX (30 μM; Fig. 5 E and F).
Figure 5.
Kainate histological damage in rabbit optic nerve. Drugs and vehicle were delivered (0.5 μl/hr, 7 days) through a catheter implanted beneath the dura mater. Nerves were infused with saline (SAL), kainate (KAI, 100 μM) or kainate together with CNQX (KAI + CNQX, 1 mM and 30 μM, respectively). Notice that the typical arrangement of interfascicular oligodendrocytes in nerves infused with saline (A and B, arrows) is distorted by kainate (C and D) and that this pathological feature can be prevented when the agonist is applied together with CNQX (E and F, arrows). Damage to oligodendrocytes results in an intense gliotic reaction in which microglial cells (small dark cells) and hypertrophic astrocytes (arrowheads) become evident. [Bar = 120 μm (A, C, and E) or 30 μm (B, D, and F).]
DISCUSSION
The central finding of this work is that activation of the non-NMDA ionotropic glutamate receptors expressed in oligodendrocytes from the optic nerve impairs the viability of these cells both in vitro and in vivo. Furthermore, the receptor-mediated toxicity involves Ca2+ influx through both the receptor channel complexes and voltage-gated channels.
Expression of ionotropic glutamate receptors previously has been observed in cells of the oligodendroglial lineage (for a recent review, see ref. 4). Thus, the oligodendrocyte progenitor cell line CG4 as well as cultured purified cortical O-2A progenitor cells express all known AMPA and kainate receptor subunits, with the exception of the GluR1 and GluR5 subunits (10, 18). These findings are consistent with the observations reported here in differentiated oligodendrocytes derived from the perinatal optic nerve. However, in contrast to the aforementioned studies, our RT-PCR analysis did not reveal the expression of the GluR2 subunit. This feature could be explained by the different cell types assayed (CG4 or O-2A cells versus oligodendrocytes) and the various areas from which the cultured cells were obtained (cerebral cortex versus optic nerve). The lack of the GluR2 subunit in the native glutamate receptors expressed by oligodendrocytes is relevant to the functioning of these receptors because the presence of its edited version, the most common in the brain, renders the AMPA receptors impermeable to Ca2+ (e.g., see ref. 5). Indeed, the toxicity mediated by the AMPA receptors expressed in oligodendrocytes appears to be mediated, at least in part, by Ca2+ influx through the ligand-gated channels and thus, strongly suggests that GluR2 does not contribute substantially to the native receptors present in oligodendrocytes.
Our RT-PCR analysis indicated that very few transcripts encoding NMDA receptor subunits are present in differentiated oligodendrocytes in vitro. This finding correlates well with the fact that in the current study we did not observe NMDA toxicity in oligodendrocytes and also by the absence of electrophysiological responses to NMDA in oligodendrocytes in vitro reported before (e.g., ref. 18). In addition, these observations parallel results obtained in situ, indicating that very few transcripts encoding functional NMDA receptors are found in glial cells of the white matter (19, 20).
Several lines of evidence suggest that kainate toxicity appears to be mediated by both AMPA and kainate-selective receptors. First, AMPA lethality is potentiated by cyclothiazide, an agent that fully blocks desensitization of AMPA-preferring receptors (16). Second, oligodendrocyte vulnerability can be partially prevented by GYKI 52466, which at the concentration used in this study is an AMPA receptor-specific antagonist (17). And lastly, kainate induces oligodendrocyte death at concentrations that mainly activate kainate-selective receptors (18, 21). In addition to toxicity, previous findings indicated that kainate actions, such as the induction of early genes, in cells of the oligodendroglial lineage can be mediated by both AMPA and kainate receptors (22). Thus, it appears that activation of these two receptor classes may contribute to the same overall biological effects in oligodendroglial cells.
Oligodendrocyte vulnerability to AMPA and kainate receptor activation can be fully prevented by removal of Ca2+ from the culture medium, indicating that it is triggered by the influx of this cation through the plasma membrane and not by its release from internal stores. The depolarization caused by activated AMPA and kainate receptors may open Ca2+ channels located in the vicinity of these receptors. Because of that, we examined whether blocking of Ca2+ channels with nimodipine, at concentrations known to block all voltage-gated Ca2+ channels in at least oligodendrocyte progenitors (22), had a protective effect on the viability of these cells. Indeed, we found that this blocker only partially reversed the toxicity induced by kainate application. Therefore, it appears that Ca2+ influx through both voltage-gated channels and non-NMDA receptors is responsible for the observed oligodendrocyte damage.
AMPA receptors lacking the GluR2 subunit are permeable to Ca2+ (5). Accordingly, the lack of GluR2 expression in the oligodendrocyte cultures derived from the optic nerve correlates well with the notion that Ca2+ influx through AMPA receptors expressed by these cells contributes to the overall damage observed upon activation of this receptor class. Similarly, the fact that the inhibition by Ca2+ channel blockers of the toxicity mediated by low concentrations of kainate is only partial indicates that kainate receptors in cultured oligodendrocytes may be permeable to Ca2+. Consistent with this, the predominant isoform of the GluR6 subunit present in oligodendrocytes was the unedited version of the Q/R site (not shown), an isoform that may render the kainate receptor-channel complex permeable to Ca2+ (23). The results we obtained in oligodendrocytes are apparently in contrast to those observed in hippocampal neurons in vitro, in which the expressed kainate receptors have a very low Ca2+ permeability (21). Yet, kainate receptors in hippocampal neurons are formed principally by the unedited variant Q of the GluR6 subunit (24). It is thus conceivable that cell type specific dependent mechanisms different from editing may account for the putative differences in the Ca2+ permeability of kainate receptors expressed by oligodendrocytes and neurons. That is a possibility that remains to be investigated.
Our results in cultured oligodendrocytes parallel in at least two regards those observed in situ and therefore may be relevant to the functioning of oligodendroglial glutamate receptors present in vivo. First, cells of the oligodendroglial lineage in vitro and in situ express Ca2+ permeable AMPA receptors (4, 25) and kainate receptors with an editing profile compatible with that of Ca2+ permeable receptors have been found in glial cells of the white matter (9). And second, moderate concentrations of kainate applied to oligodendrocytes cultured from the optic nerve or to the nerve itself triggered massive oligodendrocyte death, which could be prevented with the non-NMDA antagonist CNQX. It thus appears that the ionotropic glutamate receptors present in oligodendrocytes may negatively regulate the survival of these cells and consequently participate in shaping the oligodendrocyte population during development and in the mature brain.
Glutamate receptors in oligodendrocytes can be activated by the enhanced release of glutamate from the nerve, which occurs after the propagation of action potentials (26). This possibility raises an important question as to whether demyelinating diseases, in which abnormal oligodendrocyte death is a common feature, may arise from an excessive activity of the AMPA and kainate receptors located in oligodendrocytes. In addition, and perhaps more importantly, the glutamate receptor-mediated toxicity that we describe may be used as an experimental paradigm to design strategies aiming at developing clinical agents, which improve oligodendrocyte survival. In summary, the results reported here provide evidence that oligodendrocytes, both in vitro and in vivo, are vulnerable to non-NMDA glutamate receptor agonists and that the excitotoxicity is mediated by Ca2+ influx.
Acknowledgments
We thank Dr. R. J. Wenthold for providing antibodies to the KA2 subunit, Dr. F. Pérez-Cerdá for help with the histology, and D. J. Fogarty for NMDA receptor primer design and for critically reading the manuscript. We also are indebted to Drs. E. Cavalheiro and J. Lerma for their suggestions and for reviewing the manuscript. This work was supported by grants from the Ministerio de Educación y Ciencia (PB94-1377), the Gobierno Vasco (PI94-106), and the National Institute of Neurological Disorders and Stroke (R.M. NS23284). M.V.S.-G. held a fellowship from the Gobierno Vasco.
ABBREVIATIONS
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- NMDA
N-methyl-d-aspartate
- RT-PCR
reverse transcription–PCR
References
- 1.Miller R, David S, Patel R, Abney E, Raff M. Dev Biol. 1985;111:35–41. doi: 10.1016/0012-1606(85)90432-4. [DOI] [PubMed] [Google Scholar]
- 2.Barres B A, Hart I K, Coles H S R, Burne J F, Voyvodic J T, Richardson W D, Raff M C. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- 3.Barres B A, Raff M. In: Glial Development: Basic Principles and Clinical Relevance. Jessen K R, Richardson W D, editors. Oxford: Bios Scientific; 1996. pp. 71–83. [Google Scholar]
- 4.Steinhäuser C, Gallo V. Trends Neurosci. 1996;19:339–345. doi: 10.1016/0166-2236(96)10043-6. [DOI] [PubMed] [Google Scholar]
- 5.Hollmann M, Heinemann S. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 6.Ranscht B, Clapshaw P A, Price J, Noble M, Seifer W. Proc Natl Acad Sci USA. 1982;79:2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 8.Jensen A M, Chiu S Y. J Neurosci. 1993;13:1664–1675. doi: 10.1523/JNEUROSCI.13-04-01664.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Barcina J M, Matute C. Eur J Neurosci. 1996;8:2379–2387. doi: 10.1111/j.1460-9568.1996.tb01201.x. [DOI] [PubMed] [Google Scholar]
- 10.Puchalski R B, Louis J C, Brose N, Traynelis S F, Egebjerg J, Kukekov V, Wenthold R J, Rogers S W, Lin F, Moran T, Morrison J H, Heinemann S F. Neuron. 1994;13:131–147. doi: 10.1016/0896-6273(94)90464-2. [DOI] [PubMed] [Google Scholar]
- 11.Jones K H, Senft J A. J Histochem Cytochem. 1985;33:77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- 12.Hodges-Savola C, Rogers S D, Ghilardi J R, Timm D R, Mantyh P W. Glia. 1996;17:52–62. doi: 10.1002/(SICI)1098-1136(199605)17:1<52::AID-GLIA5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Gutiérrez-Igarza K, Fogarty D J, Pérez-Cerdá F, Doñate-Oliver F, Albus K, Matute C. Visual Neurosci. 1996;13:61–72. doi: 10.1017/s0952523800007136. [DOI] [PubMed] [Google Scholar]
- 14.Gallo V, Upso L M, Hayes W P, Vyklicky L, Winters C A, Buonanno A. J Neurosci. 1992;12:1010–1023. doi: 10.1523/JNEUROSCI.12-03-01010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg P H. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 16.Patneau D K, Vickliky L, Jr, Mayer M L. J Neurosci. 1993;13:3496–3509. doi: 10.1523/JNEUROSCI.13-08-03496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paternain A V, Morales M, Lerma J. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- 18.Patneau D K, Wright P W, Winters C, Mayer M L, Gallo V. Neuron. 1994;12:357–371. doi: 10.1016/0896-6273(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 19.Matute C, Miledi R. Proc Natl Acad Sci USA. 1993;90:3270–3274. doi: 10.1073/pnas.90.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matute C, Miledi R. Fourth IBRO Congress Neurosci Abstr. 1995;1:103. [Google Scholar]
- 21.Lerma J, Paternain A V, Naranjo J R, Mellström B. Proc Natl Acad Sci USA. 1993;90:11688–11692. doi: 10.1073/pnas.90.24.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pende M, Holtzclaw L A, Curtis J L, Russell J T, Gallo V. Proc Natl Acad Sci USA. 1994;91:3215–3219. doi: 10.1073/pnas.91.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burnashev N. Curr Opin Neurobiol. 1996;6:311–317. doi: 10.1016/s0959-4388(96)80113-9. [DOI] [PubMed] [Google Scholar]
- 24.Ruano D, Lambolez B, Rossier J, Paternain A V, Lerma J. Neuron. 1995;14:1009–1017. doi: 10.1016/0896-6273(95)90339-9. [DOI] [PubMed] [Google Scholar]
- 25.Fulton B P, Burne J F, Raff M C. J Neurosci. 1992;12:4816–4833. doi: 10.1523/JNEUROSCI.12-12-04816.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinrich D, Hammerschlag R. Brain Res. 1975;84:137–142. doi: 10.1016/0006-8993(75)90807-0. [DOI] [PubMed] [Google Scholar]