Abstract
Experimental autoimmune encephalomyelitis (EAE) induced with myelin proteolipid protein (PLP) residues 139–151 (HSLGKWLGHPDKF) can be prevented by treatment with a T cell receptor (TCR) antagonist peptide (L144/R147) generated by substituting at the two principal TCR contact residues in the encephalitogenic peptide. The TCR antagonist peptide blocks activation of encephalitogenic Th1 helper cells in vitro, but the mechanisms by which the antagonist peptide blocks EAE in vivo are not clear. Immunization with L144/R147 did not inhibit generation of PLP-(139–151)-specific T cells in vivo. Furthermore, preimmunization with L144/R147 protected mice from EAE induced with the encephalitogenic peptides PLP-(178–191) and myelin oligodendrocyte protein (MOG) residues 92–106 and with mouse myelin basic protein (MBP). These data suggest that the L144/R147 peptide does not act as an antagonist in vivo but mediates bystander suppression, probably by the generation of regulatory T cells. To confirm this we generated T cell lines and clones from animals immunized with PLP-(139–151) plus L144/R147. T cells specific for L144/R147 peptide were crossreactive with the native PLP-(139–151) peptide, produced Th2/Th0 cytokines, and suppressed EAE upon adoptive transfer. These studies demonstrate that TCR antagonist peptides may have multiple biological effects in vivo. One of the principal mechanisms by which these peptides inhibit autoimmunity is by the induction of regulatory T cells, leading to bystander suppression of EAE. These results have important implications for the treatment of autoimmune diseases where there are autopathogenic responses to multiple antigens in the target organ.
Keywords: altered peptides, immune deviation, regulatory T cells
T cell epitopes derived from protein antigens may induce immunity or suppression (1). An immunogenic epitope can be converted to a suppressor epitope by changing a single amino acid. A clear example of this was described in H-2s mice which develop helper T cell responses to the copolymer of Glu and Ala (GA) and tolerogenic responses to the copolymer of Glu, Ala, and Tyr (GAT) (2, 3). Therefore substitution of tyrosine in the GA polymer induced suppressor T cells that were crossreactive with the immunogenic GA molecule and converted immunogenic GA into an immunosuppressive molecule. This result was attributed to distinct binding of immunogenic and tolerogenic epitopes to different H-2-linked genes (Ir versus Is genes). However, the failure to identify distinct Is genes has led researchers in the field to reexamine this phenomenon. We have been studying the mechanisms by which autoimmune disease can be regulated with a view that the nature and role of regulatory cells can be tested in vivo in an autoimmune disease setting.
Analogs of encephalitogenic peptides have been known to protect animals from the induction of experimental autoimmune encephalomyelitis (EAE) for a number of years (4, 5). Originally it was postulated that the principal mechanism by which peptide analogs mediate protection was major histocompatibility complex (MHC) blockade, and this was shown to be the case for peptides with high affinity for MHC class II molecules (6, 7). However, a number of observations raised the possibility that MHC blockade was not the only mechanism responsible for protection (8, 9). The description of altered peptide ligands generated by single amino acid substitution of the antigenic peptide that could act as T cell receptor (TCR) antagonists or partial agonists of various T cell clones provided a framework to understand the actions of many peptide analogs (10–13). We have shown that an analog of the encephalitogenic myelin proteolipid protein (PLP)-(139–151) peptide (14–16), L144/R147 (with substitutions at the two main TCR contact residues), is a powerful TCR antagonist for encephalitogenic PLP-(139–151)-specific T cell clones in vitro and is able to protect animals from the induction of EAE (17). This effect was in contrast with that of a second, weaker, TCR antagonist analog L144, which had little or no effect on the development of clinical disease. While the in vivo protective effects of L144/R147 could have been due to antagonism of PLP-(139–151)-specific T cells, the observation that mice coimmunized with L144/R147 plus the encephalitogenic peptide PLP-(139–151) and without signs of clinical disease developed inflammatory foci within the central nervous system suggested that L144/R147 was not simply ablating EAE by inhibiting the development of a PLP-(139–151)-specific T cell response.
To investigate the mechanism by which the TCR antagonist peptide L144/R147 mediated its protective effects in vivo, we tested the effects of L144/R147 on the induction of EAE with other unrelated encephalitogenic peptides and proteins derived from central nervous system myelin. We found that, as well as protecting from EAE induced with the encephalitogenic peptide PLP-(139–151), the L144/R147 peptide can protect animals from EAE induced by another encephalitogenic PLP peptide and by the unrelated myelin antigens myelin basic protein (MBP) (18) and myelin oligodendrocyte glycoprotein (MOG) (19), excluding the possibility that protection was simply due to TCR antagonism in vivo. Our data further suggest that the L144/R147 altered peptide induces bystander suppression by generating regulatory T cells that crossreact with the native encephalitogenic peptide PLP-(139–151) and that transfer of these crossreactive regulatory T cell lines can protect against the development of EAE.
MATERIALS AND METHODS
Animals.
Four- to 6-week-old female SJL mice were purchased from The Jackson Laboratory and housed under virus-free conditions. They were maintained in accordance with the guidelines of the Committee on Animals of Children’s Hospital and Harvard Medical School.
Antigens.
Peptide antigens were synthesized by Richard Laursen on a Milligen model 9050 synthesizer using Fmoc chemistry. Milligen PAL amide resins were used to produce peptides with C-terminal amides. Most peptides were >90% pure, as determined by HPLC. The peptides used in these experiments were PLP-(139–151) (HSLGKWLGHPDKF), L144 (HSLGKLLGHPDKF), Q144 (HSLGKQLGHPDKF), L144/R147 (HSLGKLLGRPDKF), PLP-(178–191) (NTWTTCQSIAFPSK), MOG-(92–106) (DEGGYTCFFRDHSYQ), and neuraminidase (Nase)-(101–120) (EALVRQGLAKVAYVYKPNNT). MOG-(92–106) and mouse MBP were the kind gift of A. Al-Sabbagh (Autoimmune Inc., Lexington, MA).
Preimmunization, Induction, and Assessment of EAE.
Mice were preimmunized by injecting s.c. at two sites with the relevant peptide (100 or 200 μg per mouse) emulsified in complete Freund’s adjuvant (CFA; Difco) supplemented with Mycobacterium tuberculosis H37 RA (500 μg per mouse; Difco). Three to 6 weeks later mice were immunized with the disease-inducing peptide or protein (25–200 μg) emulsified in CFA and supplemented with M. tuberculosis H37 RA (500 μg per mouse). On this day and on day 3 after immunization each mouse was also injected i.v. with 109 heat-killed Bordetella pertussis bacilli (pertussis vaccine lot no. 285, Massachusetts Public Health Biological Laboratories, Boston). Mice were examined daily, beginning on day 9, for disease, which was assessed clinically according to the following criteria: 0 = no disease, 1 = limp tail, 2 = hindlimb weakness, 3 = hindlimb paralysis, 4 = hindlimb plus forelimb paralysis, and 5 = moribund or dead.
In Vitro Proliferation and Cytokine Assays.
Mice were injected s.c. at five sites with antigen emulsified in CFA containing a total of 250 μg of M. tuberculosis H37 RA. Mice immunized with a single peptide received a total of 100 μg of antigen; mice immunized with a mixture of PLP-(139–151) and an analog peptide received 100 μg of PLP-(139–151) and either 100 μg or 300 μg of second peptide (i.e., a total of 200 or 400 μg of antigen per mouse). On day 12 lymph nodes were removed and lymph node cells (LNC) were prepared from them. LNC (4 × 105 per well) were cultured in triplicate in 96-well round-bottom plates (Falcon, Becton Dickinson), in the presence of antigen, for 48 hr, then [3H]thymidine [1 μCi (37 kBq) per well] was added for the last 16 hr before harvesting the cells. [3H]Thymidine incorporation was determined in triplicate wells in a Beckman scintillation counter (model LS 5000). The data were expressed as a stimulation index, which was calculated by dividing the proliferation (cpm incorporated) measured in the presence of antigen by the proliferation measured with medium alone.
To measure the concentration of cytokines, supernatants were collected from activated T cells (5 × 104 T cells plus 5 × 105 syngeneic irradiated spleen cells per well in the presence or absence of antigen), 40 hr after activation and diluted with an equal volume of fresh culture medium. Interferon (IFN)-γ and interleukins IL-2, IL-4, and IL-10 were measured by quantitative capture ELISA according to the supplier’s guidelines as previously described (20).
Derivation and Adoptive Transfer of T Cell Lines and Clones.
T cell lines were generated from LNC from mice immunized with PLP-(139–151) plus L144/R147 and cultured in syngeneic serum with the appropriate antigen (20 μg/ml) for 5 days. T cell blasts were purified over a Ficoll/Hypaque gradient and expanded. Long-term culture and cloning were carried out as described (20).
T cell lines for adoptive transfer were prepared from mice immunized by using the same protocol used for the in vitro proliferation assays. Lymph nodes were removed on day 10 and LNC were resuspended at a concentration of 6–10 × 106 cells per ml in culture medium containing 0.5% syngeneic serum in place of fetal bovine serum. Cells were cultured in the presence of various antigens (20 μg/ml) for 4 days, then harvested and purified over a Ficoll/Hypaque gradient. Cells were resuspended in PBS at 25 × 106 cells per ml and injected i.v. into recipient animals (0.2 ml, 5 × 106 cells per mouse), then recipient mice were immunized with the peptide PLP-(178–191) (10–100 μg per mouse) as described above to induce active EAE.
RESULTS AND DISCUSSION
We generated a peptide analog of the encephalitogenic peptide PLP-(139–151) by replacing the two principal TCR contact residues within the peptide with leucine (L) at position 144 and arginine (R) at position 147. This peptide analog (L144/R147) acted as a TCR antagonist for encephalitogenic Th1 clones, blocking their activation in vitro. Furthermore, coimmunization of SJL mice with a mixture of the encephalitogenic PLP-(139–151) peptide and the TCR antagonist peptide L144/R147 resulted in inhibition of disease (17). It was assumed that the mechanism by which L144/R147 peptide inhibited autoimmune disease depended upon antagonism preventing the generation of encephalitogenic T cells in vivo. However, the observation that L144/R147 plus PLP-(139–151) coimmunized mice that are protected from developing EAE have T cells which invade the central nervous system suggested that L144/R147 may inhibit EAE by mechanisms other than simply antagonizing the induction and activation of PLP-(139–151)-specific T cells. Two experiments were performed to determine whether L144/R147 inhibited EAE only by acting as a TCR antagonist in vivo. The L144/R147 peptide was tested for its ability to inhibit the induction of PLP-(139–151)-specific T cells and to prevent EAE induced in vivo with other myelin antigens.
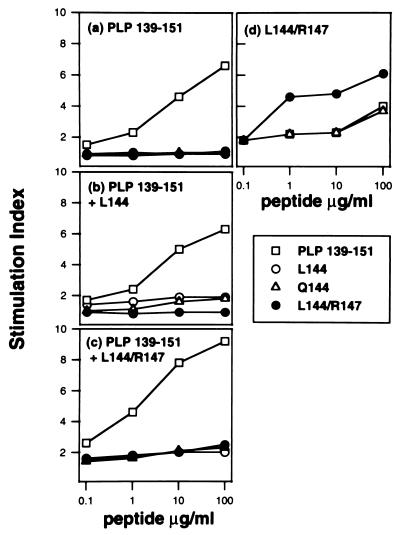
To determine if the L144/R147 antagonist peptide could block the generation of PLP-(139–151)-specific T cells, mice were coimmunized with L144/R147 and PLP-(139–151). L144 peptide, which is also a TCR antagonist, was used as a control in these experiments. LNC were derived from animals immunized with PLP-(139–151), PLP-(139–151) plus L144, and PLP-(139–151) plus L144/R147 (Fig. 1 a–c). The proliferative response of LNC to PLP-(139–151) was not significantly reduced by coimmunization with either of the antagonists. On the other hand, immunization with L144/R147 alone induced T cells which were activated by L144/R147 and could also respond to Q144 and PLP-(139–151) at high concentrations (Fig. 1d). Therefore L144/R147 does not prevent the induction of PLP-(139–151)-specific cells, nor does it mediate MHC blockade in vivo, but it induces T cells that are crossreactive with the PLP-(139–151) peptide.
Figure 1.
The L144/R147 TCR antagonist does not block the generation of PLP-(139–151)-specific T cells. Groups of mice were immunized with 100 μg of PLP-(139–151) (a), 100 μg of PLP-(139–151) plus 300 μg of L144 (b), 100 μg of PLP-(139–151) plus 300 μg of L144/R147 (c), or 100 μg of L144/R147 (d). Twelve days after immunization LNC were removed and activated in vitro with the PLP-(139–151) native or analog peptides shown or with a control peptide (data not shown). Proliferation was assessed by adding [methyl-3H]thymidine after 48 hr and harvesting 18 hr later, and a stimulation index was calculated. The background proliferation (cpm) of LNCs without antigen was 8,550 (a), 11,000 (b), 4,900 (c), and 1,970 (d).
It was of interest that following immunization with a mixture of PLP-(139–151) with either the L144 or L144/R147 peptide, proliferative responses in vitro to L144 or L144/R147 alone were much lower than responses to PLP-(139–151) (Fig. 1 b and c). However, T cells specific for L144/R147 were generated in this coimmunization protocol, since we could derive L144/R147-specific T cell clones from mice immunized with PLP-(139–151) plus L144/R147 which responded specifically to L144/R147 (data not shown). A number of factors may contribute to the low responses to L144/R147 and L144 when mice are immunized with these peptides together with PLP-(139–151). The frequency of T cells able to respond to PLP-(139–151) may be greater than the frequency of T cells able to respond to L144/R147. In addition, the native PLP-(139–151) peptide may act as an antagonist for the induction of L144/R147- or L144-specific cells which may reduce the proliferative response in vitro. This effect is not due to differential binding of PLP-(139–151) over L144/R147 or L144, since all three altered peptides bind with similar affinity to IAs (17).
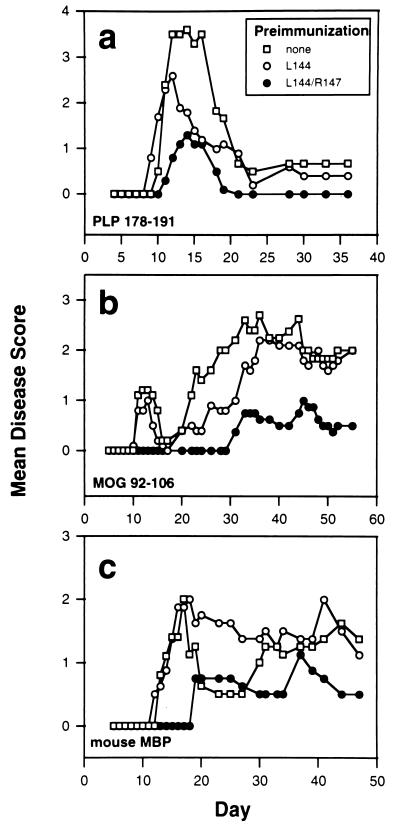
To investigate whether the L144/R147 antagonist peptide affected disease induced with other myelin antigens or whether its effects were restricted to disease induced with the related peptide PLP-(139–151), mice were preimmunized with L144/R147 and disease was induced with a number of unrelated encephalitogens (Fig. 2 and Table 1). First we utilized a second encephalitogenic region within the PLP molecule, PLP-(178–191), and we found that L144/R147 protected mice against the development of EAE induced with this unrelated PLP peptide (Fig. 2a). The peptide had only a slight effect on the disease incidence but had a significant effect on the disease severity (Table 1). Mice preimmunized with L144 or the non-pre-immunized control mice developed disease that was significantly more severe than mice preimmunized with L144/R147. Disease following preimmunization with the native PLP-(139–151) developed earlier and was less severe than that of the mice preimmunized with L144 or not preimmunized, although these differences were not statistically significant (Table 1 and Fig. 2a). These data suggest that the protective effects of L144/R147 did not depend on immunization with PLP-(139–151) and were not restricted to disease induced by that peptide.
Figure 2.
Preimmunization with L144/R147 protects animals from EAE induced with PLP-(178–191) (a), MOG-(92–106) (b), or mouse MBP (c). Mice (four or five per group, 100–200 μg of peptide per mouse) were preimmunized with L144, L144/R147, or PLP-(139–151) or were not immunized 3 weeks before immunization with PLP-(178–191) (25 μg per mouse), MOG-(92–106) (200 μg per mouse), or mouse MBP (200 μg per mouse, 2 times). Mice immunized with mouse MBP received two immunizations with antigen 1 week or 1 month apart. In the latter case the second immunization was taken as day 0.
Table 1.
Preimmunization of mice with the antagonist peptide L144/R147 protects mice from the induction of EAE with unrelated encephalitogens
| Preimmunization | Immunization | Acute disease
|
All disease
|
||||
|---|---|---|---|---|---|---|---|
| Incidence | Mean day of onset | Mean maximum severity | Incidence | Mean day of onset | Mean maximum severity | ||
| None | PLP-(178–191) | 14/14 | 11.0 ± 0.2 | 4.1 ± 0.2 | |||
| L144 | 12/15 | 12.3 ± 1.6 | 3.8 ± 0.3 | ||||
| PLP-(139–151) | 4/5 | 9.3 ± 1.0a | 3.1 ± 0.3 | ||||
| L144/R147 | 11/15 | 12.3 ± 0.4a | 2.6 ± 0.3b | ||||
| None | MOG-(92–106)c | 9/11 | 13.9 ± 1.0 | 1.8 ± 0.3 | 10/11 | 14.9 ± 1.7 | 2.7 ± 0.5 |
| L144 | 8/11 | 14.9 ± 1.3 | 1.5 ± 0.2 | 10/11 | 17.2 ± 2.1 | 2.0 ± 0.3 | |
| PLP-(139–151) | 4/4 | 15 ± 1.7 | 1.5 ± 0.3 | 4/4 | 15 ± 1.7 | 2.0 ± 0.4 | |
| L144/R147 | 1/9d | 20 | 1 | 4/9e | 34.6 ± 5.8a | 2.5 ± 0.5 | |
| None | MBPf | 7/9 | 16.9 ± 1.3 | 2.6 ± 0.6 | 8/9 | 18.5 ± 2.0 | 2.3 ± 0.5 |
| L144 | 7/9 | 16.3 ± 0.9 | 2.0 ± 0.3 | 7/9 | 16.2 ± 0.9 | 2.0 ± 0.3 | |
| L144/R147 | 1/9e | 19 | 3 | 3/9g | 34.3 ± 8.2h | 1.8 ± 0.6 | |
Mice were preimmunized with the indicated antigens (100–200 μg per mouse in CFA) and challenged with encephalitogenic peptides/proteins as described. Results are mean ± SEM (except for incidence).
P < 0.05 by t test compared with no preimmunization.
P < 0.005 by t test compared with no preimmunization and P < 0.05 compared with L144.
Acute disease is taken as disease onset on or before day 20.
P < 0.01 by Fisher’s exact test compared with no preimmunization or preimmunization with PLP-(139–151), P < 0.05 compared with preimmunization with L144.
P < 0.05 by Fisher’s exact test compared with no preimmunization or preimmunization with L144.
Acute disease is taken as disease onset on or before day 25.
P < 0.05 by Fisher’s exact test compared with no preimmunization.
P < 0.05 by Mann–Whitney U test compared with no preimmunization or preimmunization with L144.
To define whether the L144/R147 peptide would confer protection from disease induced with unrelated myelin antigens, groups of animals were preimmunized with various peptides and challenged with either the encephalitogenic peptide MOG-(92–106) or intact MBP. Mice preimmunized with L144 or PLP-(139–151) or unimmunized controls experienced an initial episode of mild disease after immunization with MOG-(92–106) which remitted spontaneously by day 20. This remission was followed by a much more severe relapse and chronic disease which was maintained through day 55 (Fig. 2b). In comparison to unimmunized mice, animals preimmunized with the control peptide L144 or the native PLP peptide 139–151 showed no changes in the disease incidence, severity, or day of onset [Table 1, MOG-(92–106) group]. By contrast, the L144/R147 preimmunized mice were almost completely protected against the initial acute episode of disease induced with MOG-(92–106) [P < 0.001 compared with no preimmunization or preimmunization with the native PLP-(139–151) peptide, P < 0.05 compared with preimmunization with L144; Table 1] and showed a significantly delayed onset of disease (Table 1). Mice immunized with MBP also developed EAE, but the disease was significantly delayed and occurred in significantly fewer animals pretreated with L144/R147 compared with no preimmunization or preimmunization with L144 or a control IAs binding peptide Nase-(101–120) (Fig. 2c and Table 1).
If the L144/R147 peptide acted in vivo only as an antagonist of PLP-(139–151)-specific T cells, it should have inhibited EAE induced with this peptide but should not have affected disease induced with the other myelin antigens. Because the antagonist L144/R147 peptide did inhibit disease induced with other unrelated myelin antigens, we concluded that immunization with the L144/R147 peptide can initiate bystander suppression. Furthermore, coimmunization with PLP-(139–151) was not necessary for this to occur and therefore TCR antagonism was not the only mechanism by which the L144/R147 peptide mediated protection in vivo. We then considered two other possible mechanisms: L144/R147 may selectively antagonize the generation of encephalitogenic PLP-(139–151) T cells, allowing the development of PLP-(139–151)-specific nonpathogenic regulatory cells, or it may itself induce regulatory cells which are crossreactive with both L144/R147 and PLP-(139–151) and which suppress disease.
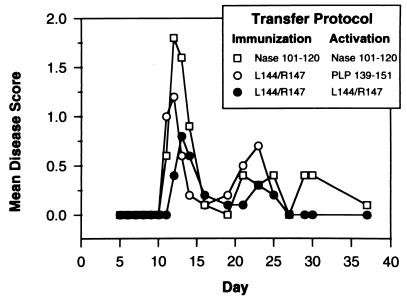
Previous studies have shown that immunization with a tolerogenic form of the antigen induces effector suppressor cells which, when activated either by the antigen or by anti-idiotype-bearing transducer suppressor cells, mediate bystander suppression of responses to other antigens present with the immunogen (21). Although effector suppressor cells are antigen specific, once activated they produce factors that nonspecifically suppress immune responses to other antigens in the vicinity (22). This suggests that the final molecule that suppresses immune responses is probably an antigen-nonspecific regulatory molecule (probably a suppressive cytokine). Bystander suppression also occurs after oral administration of antigens (oral tolerance) (23). In that system it is due to transforming growth factor β and Th2 cytokine-producing regulatory T cells which suppress EAE and other autoimmune diseases in a tissue-specific manner (24, 25). To test whether the L144/R147 peptide induces regulatory T cells which transfer protection, animals were immunized with L144/R147 or an irrelevant IAs-binding peptide, Nase-(101–120) or pigeon cytochrome c residues 92–104 [PCC-(92–104)]. LNC were harvested, reactivated in vitro for 5 days, and then transferred into mice that were simultaneously immunized with PLP-(178–191) to induce disease. Before transfer the LNC from mice immunized with L144/R147 were reactivated in vitro with either L144/R147 or PLP-(139–151) peptide and the LNC from control immunized mice were reactivated with the appropriate peptide. As an additional control in these experiments we included groups of mice that were not transferred LNC. In two experiments 7/10 mice that did not receive cells developed disease. This occurred 1–2 days later than for mice that received cells, and it suggests that cell transfer itself can change the pattern of disease onset. The adoptive transfer of cells from mice immunized with L144/R147 protected mice from disease, and the protection was greatest in the group of mice given cells activated in vitro with L144/R147 (Fig. 3). Data from two independent experiments show that adoptive transfer of short term T cell lines activated with the antagonist L144/R147 peptide lowers the incidence of disease to 4/10 compared with the group of mice transferred with T cell lines activated with the control peptide (8/10). Therefore the incidence of disease in the mice transferred with L144/R147 peptide-specific T cell line was half of that seen in the mice transferred T cell lines specific for the control peptide, although this difference did not reach statistical significance. There is no difference in the mean maximum severity between groups. It should be noted that in these experiments the T cell lines being tested for the protective effects are specific for the antagonist peptide of PLP-(139–151) and the mice are immunized with an unrelated encephalitogenic peptide PLP-(178–191) for the development of EAE. Thus transfer of antagonist peptide-specific T cells has to regulate the autopathogenic effects of encephalitogenic T cells that are continuously being generated and are specific for an unrelated encephalitogenic epitope. This demonstrates directly that L144/R147 peptide in vivo does not mediate its protective effects by antagonizing the generation of encephalitogenic T cells but induces regulatory cells that mediate the bystander suppression of EAE induced with a structurally unrelated encephalitogen.
Figure 3.
Transfer of short term T cell lines from mice immunized with L144/R147 but not control peptide protects animals from disease. T cell lines were restimulated in vitro with L144/R147 or PLP-(139–151) (LNC from mice immunized with L144/R147) or with Nase-(101–120) or PCC-(92–104) [LNC from mice immunized with Nase-(101–120) or PCC-(92–104), respectively] and transferred i.v. (5 × 106 cells per mouse) at the time of immunization to induce active disease with a low dose of PLP-(178–191) (10–50 μg per mouse).
To analyze the mechanism further, T cell lines and clones specific for the antagonist peptide L144/R147 were generated and tested for proliferative responses and cytokine production. Three independent lines were generated against the L144/R147 peptide. All of the T cell lines specific for the L144/R147 peptide generally produced cytokines of the Th0 phenotype, which included IFN-γ, IL-10, and IL-4 (data not shown). A panel of 16 T cell clones was established from one of the lines. Seven of these were crossreactive with PLP-(139–151) (data not shown). Eight stable clones that showed a significant antigen-specific response to L144/R147 were analyzed in detail. All responded to L144/R147 (but not to a control peptide, data not shown) by secreting cytokines of a Th2 or Th0 phenotype. Five of the eight clones showed crossreactivity with the peptide PLP-(139–151) and four of the five clones that proliferated in response to this peptide also secreted detectable levels of the same cytokines (Table 2). None of the clones secreted significant levels of transforming growth factor β (data not shown). Analysis of TCR Vβ expression by five clones demonstrated a diverse repertoire with the expression Vβ6, Vβ14, and an unidentified TCR Vβ, showing that they were not sister clones (data not shown), although there was possibly an over-representation of Vβ6-bearing clones. Whereas the encephalitogenic PLP-(139–151) peptide induces Th1 (IFN-γ, tumor necrosis factor α) cells, our data demonstrate that immunization with the TCR antagonist peptide L144/R147 can induce crossreactive T cells which are of a Th2/Th0 phenotype and that lines derived from immunization with L144/R147 can regulate tissue destruction within the central nervous system and prevent clinical paralysis.
Table 2.
The cytokines produced by T cell clones specific for the L144/R147 antagonist peptide are predominantly Th2/Th0
| T cell clone | Activating antigen L144/R147
|
Activating antigen PLP-(139–151)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Δ cpm | Δ cytokine, pg/ml
|
Δ cpm | Δ cytokine pg/ml
|
|||||||
| IFN-γ | IL-2 | IL-4 | IL-10 | IFN-γ | IL-2 | IL-4 | IL-10 | |||
| 1A1 | 186,800 | — | — | 7,500 | 4,900 | 9,200 | — | 100 | — | — |
| 1B2 | 333,800 | 300 | 400 | 8,400 | 7,300 | 135,400 | 200 | — | 2,400 | 1,700 |
| 1B3 | 295,400 | — | 500 | 8,850 | 6,500 | 20,800 | — | — | — | — |
| 1D2 | 197,400 | — | 100 | 8,300 | 8,100 | <100 | — | — | — | — |
| 1E3 | 220,600 | — | 1,200 | 3,700 | 6,200 | 28,500 | — | 1,100 | 700 | — |
| 1F1 | 212,300 | — | 5,800 | 3,400 | 6,800 | 200 | — | — | — | — |
| 1F2 | 266,800 | — | 800 | 4,000 | 11,000 | 34,900 | — | — | 100 | 400 |
| 1F3 | 264,500 | — | — | 3,900 | 9,900 | 69,700 | — | — | 1,100 | 1,300 |
T cell clones were activated with L 144/R147, PLP-(139–151) (20 μg/ml) or a control antigen (data not shown), and the proliferation and cytokine production were measured. Results are presented as the cpm and cytokine production after background values were subtracted.
The altered peptide L144/R147 was originally designed as a TCR antagonist and was effective in blocking the activation of Th1 clones specific for PLP-(139–151) in vitro, in spite of the diversity of TCR αβ chains utilized by the clones. This broad inhibitory capacity could be rationalized on the basis of the finding that all T cell clones analyzed recognized the peptide/MHC in very similar ways and, crucially, all utilized the same primary TCR contact residue (W144). The L144/R147 peptide was also found to be an effective inhibitor of EAE in vivo when given either at the time of immunization or at the onset of clinical disease (17). However, the mechanisms by which a TCR antagonist peptide mediates protection against autopathogenic T cell responses in vivo have not been determined (17, 26, 27). Our results illustrate the complexity of the mechanisms underlying this phenomenon. Direct antagonism of PLP-(139–151)-specific T cells probably accounts for some of the ability of L144/R147 to ameliorate EAE when given at the onset of disease (17), but as the data presented here indicate, immunization with L144/R147 in CFA can also induce regulatory T cells of a Th2/Th0 phenotype which transfer protection even to unrelated myelin antigens (MBP and MOG). It has previously been demonstrated that suppressor cells may have a mixed cytokine phenotype, producing both Th1 and Th2 cytokines. Whereas Th1 cytokines can inhibit Th2 cells and Th2 cytokines can antagonize Th1 cells, a cell producing both Th1 and Th2 cytokines is capable of inhibiting both Th1 and Th2 cells by the virtue of cytokines it produces (28). It is interesting to speculate that in infection with human immunodeficiency and hepatitis B viruses, which elaborate variant antagonistic epitopes (29, 30), this mechanism may contribute to chronic infection both by antagonizing virus-specific T cells and also by causing immune deviation toward a Th2 phenotype which will further inhibit the generation of protective Th1 cells. Using another peptide analog of PLP-(139–151), Q144, which protects SJL mice from EAE and is not a TCR antagonist, we have shown that protection is in this case due to immune deviation (20). Other studies have suggested that administration of altered peptides results in a decrease in the proinflammatory cytokines tumor necrosis factor α and IFN-γ and an efflux of inflammatory cells from brain lesions (31). Similar observations have been reported in an experimental model using altered peptides derived from human collagen IV (32). However, the present study brings together two important concepts: (i) The TCR antagonist peptides do not prevent autoimmunity solely by antagonism in vivo, but induce regulatory T cells. (ii) The regulatory T cells mediate bystander suppression and inhibit autoimmunity initiated by T cells specific for a number of autoantigens present in the target organ.
The mechanism by which altered peptides induce T cells that regulate immune responses is unknown. It is noteworthy that a predominantly Th0/Th2 response develops even after immunization with TCR antagonist peptide emulsified in CFA, which is known to strongly favor the development of a Th1 response (33). One possibility is that altered peptide ligands engage the TCRs of naive Thp cells, which would go on to be PLP-(139–151) specific, with a lower avidity that is sufficient to alter their differentiation to a Th2/Th0 pathway as has been described in TCR transgenic systems (34, 35). If this were the case then one would predict that the TCR specificity of the clones for PLP-(139–151) and L144/R147 peptide analog would be unchanged, and we find that this is not the case (M. Prabhu Das, L.B.N., and V.K.K., unpublished results). It therefore seems likely that immunization with the L144/R147 altered peptide recruits a different population of cells and that these cells are also of a different phenotype. If differences in TCR/MHC/peptide avidity are responsible for the differences in phenotype at the population level, then this predicts that the average avidity of the population of T cells which recognizes PLP-(139–151) is different from the average avidity of the population which recognizes the altered peptide. These differences could arise because the repertoire of T cells in the periphery available to respond to PLP-(139–151) is affected by mechanisms of central and peripheral tolerance to a greater extent than the repertoire of cells available to respond to altered self peptides. Another possibility is that L144/R147 selectively antagonizes encephalitogenic Th1 precursors but expands regulatory T cells directly by acting as an agonist for them. This model is consistent with the differences we have observed between encephalitogenic Th1 and regulatory T cells (M. Prabhu Das, L.B.N., and V.K.K., unpublished results).
The demonstration that bystander suppression induced with a peptide antagonist is effective in ameliorating disease caused by structurally unrelated myelin antigens argues for its therapeutic importance. Clearly, if protective immunization can be established with a single TCR antagonist peptide which itself is nonpathogenic, antagonizes specific pathogenic Th1 cells, and induces regulatory T cells, it may provide a desirable therapeutic option for use in the treatment of many autoimmune diseases.
Acknowledgments
We thank Deepak Kaul and Edward Howard for their technical assistance and Byron Waksman and Raymond Sobel for thoughtful review of the manuscript. This work was supported in part by grants from the National Institutes of Health, NS-30843, NS-35685, and NS-P01AI39671, and by the National Multiple Sclerosis Society, RG2571A2 and RG2320B3. L.B.N. is a Postdoctoral Fellow of the National Multiple Sclerosis Society.
ABBREVIATIONS
- MHC
major histocompatibility complex
- TCR
T cell receptor
- PLP
myelin proteolipid protein
- EAE
experimental autoimmune encephalomyelitis
- MBP
myelin basic protein
- MOG
myelin oligodendrocyte protein
- Nase
neuraminidase
- CFA
complete Freund’s adjuvant
- LNC
lymph node cells
- IFN
interferon
- IL
interleukin
- PCC
pigeon cytochrome c
References
- 1.Sercarz E, Krzych U. Immunol Today. 1991;12:111–118. doi: 10.1016/0167-5699(91)90094-A. [DOI] [PubMed] [Google Scholar]
- 2.Kapp J A, Pierce C W, Schlossman S, Benacerraf B. J Exp Med. 1974;140:648–659. doi: 10.1084/jem.140.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz M, Waltenbaugh C, Dorf M E, Cesla R, Sela M, Benacerraf B. Proc Natl Acad Sci USA. 1976;73:2862–2866. doi: 10.1073/pnas.73.8.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wraith D C, McDevitt H O, Steinman L, Acha-Orbea H. Cell. 1989;57:709–715. doi: 10.1016/0092-8674(89)90786-1. [DOI] [PubMed] [Google Scholar]
- 5.Smilek D E, Wraith D C, Hodgkinson S, Dwivedy S, Steinman L, McDevitt H O. Proc Natl Acad Sci USA. 1991;88:9633–9637. doi: 10.1073/pnas.88.21.9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakai K, Zamvil S S, Mitchell D J, Hodgkinson S, Rothbard J B, Steinman L. Proc Natl Acad Sci USA. 1989;86:9470–9474. doi: 10.1073/pnas.86.23.9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamont A L, Sette A, Fujinami R, Colon S A, Miles C, Grey H M. J Immunol. 1990;145:1687–1693. [PubMed] [Google Scholar]
- 8.Janeway C A. Nature (London) 1989;341:342. [Google Scholar]
- 9.Wauben M H M, Boog C J P, Van der Zee R, Joosten I, Schlief A, Van Eden W. J Exp Med. 1992;176:667–677. doi: 10.1084/jem.176.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evavold B D, Allen P M. Science. 1991;252:1308–1310. [PubMed] [Google Scholar]
- 11.Racioppi L, Ronchese F, Matis L A, Germain R N. J Exp Med. 1993;177:1047–1060. doi: 10.1084/jem.177.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Magistris M T, Alexander J, Coggeshall M, Altman A, Gaete F C A, Grey H M, Sette A. Cell. 1992;68:625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- 13.Sloan-Lancaster J, Evavold B D, Allen P M. Nature (London) 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 14.Tuohy V K, Lu Z, Sobel R A, Laursen R A, Lees M B. J Immunol. 1989;142:1523–1527. [PubMed] [Google Scholar]
- 15.Sobel R A, Greer J A, Kuchroo V K. Neurochem Res. 1994;19:915–921. doi: 10.1007/BF00968701. [DOI] [PubMed] [Google Scholar]
- 16.Greer J M, Sobel R A, Sette A, Southwood S, Lees M B, Kuchroo V K. J Immunol. 1996;156:371–379. [PubMed] [Google Scholar]
- 17.Kuchroo V K, Greer J M, Kaul D, Ishioka G, Franco A, Sette A, Sobel R A, Lees M B. J Immunol. 1994;153:3326–3336. [PubMed] [Google Scholar]
- 18.Zamvil S S, Steinman L. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 19.Amor S, Groome N, Linnington C, Morris M M, Dornmair K, Gardinier M V, Matthieu J, Baker D. J Immunol. 1994;153:4349–4356. [PubMed] [Google Scholar]
- 20.Nicholson L B, Greer J M, Sobel R A, Lees M A, Kuchroo V K. Immunity. 1995;3:397–405. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 21.Dorf M E, Benacerraf B. Annu Rev Immunol. 1984;2:127–158. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- 22.Meyers C M, Kelly C J. J Clin Invest. 1994;94:2093–2104. doi: 10.1172/JCI117564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller A, Lider O, Weiner H L. J Exp Med. 1991;174:791–798. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller A, Lider O, Roberts A B, Sporn M B, Weiner H L. Proc Natl Acad Sci USA. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Kuchroo V K, Inobe J, Hafler D A, Weiner H L. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 26.Franco A, Southwood S, Arrhenius T, Kuchroo V K, Grey H M, Sette A, Ishioka G Y. Eur J Immunol. 1994;24:940–946. doi: 10.1002/eji.1830240424. [DOI] [PubMed] [Google Scholar]
- 27.Karin N, Mitchell D J, Brocke S, Ling N, Steinman L. J Exp Med. 1994;180:2227–2237. doi: 10.1084/jem.180.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nanda N K, Sercarz E E, Hsu D, Kronenberg M. Int Immunol. 1994;6:731–737. doi: 10.1093/intimm/6.5.731. [DOI] [PubMed] [Google Scholar]
- 29.Klenerman P, Rowland-Jones S, McAdam S, Edwards J, Daenke S, Lalloo D, Köppe B, Rosenberg W, Boyd D, Edwards A, Glangrande P, Phillips R E, McMichael A J. Nature (London) 1994;369:403–407. doi: 10.1038/369403a0. [DOI] [PubMed] [Google Scholar]
- 30.Bertoletti A, Sette A, Chisari F V, Penna A, Levrero M, De Carli M, Fiaccadori F, Ferrari C. Nature (London) 1994;369:407–410. doi: 10.1038/369407a0. [DOI] [PubMed] [Google Scholar]
- 31.Brocke S, Gijbels K, Allegretta M, Ferber I, Piercy C, Blankenstein T, Martin R, Utz U, Karin N, Mitchell D J, Veromaa T, Waisman A, Gaur A, Conlon P, Ling N, Fairchild P J, Wraith D C, O’Garra A, Fathman C G, Steinman L. Nature (London) 1996;379:343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- 32.Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. J Exp Med. 1995;181:1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janeway C A J, Carding S, Jones B, Murray J, Portoles P, Rasmussen R, Saizawa K, West J, Bottomly K. Immunol Rev. 1988;101:39–80. doi: 10.1111/j.1600-065x.1988.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 34.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosken N A, Shibuya K, Heath A W, Murphy K M, O’Garra A O. J Exp Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]