Abstract
There is considerable evidence from animal studies that gonadal steroid hormones modulate neuronal activity and affect behavior. To study this in humans directly, we used H215O positron-emission tomography to measure regional cerebral blood flow (rCBF) in young women during three pharmacologically controlled hormonal conditions spanning 4–5 months: ovarian suppression induced by the gonadotropin-releasing hormone agonist leuprolide acetate (Lupron), Lupron plus estradiol replacement, and Lupron plus progesterone replacement. Estradiol and progesterone were administered in a double-blind cross-over design. On each occasion positron-emission tomography scans were performed during (i) the Wisconsin Card Sorting Test, a neuropsychological test that physiologically activates prefrontal cortex (PFC) and an associated cortical network including inferior parietal lobule and posterior inferolateral temporal gyrus, and (ii) a no-delay matching-to-sample sensorimotor control task. During treatment with Lupron alone (i.e., with virtual absence of gonadal steroid hormones), there was marked attenuation of the typical Wisconsin Card Sorting Test activation pattern even though task performance did not change. Most strikingly, there was no rCBF increase in PFC. When either progesterone or estrogen was added to the Lupron regimen, there was normalization of the rCBF activation pattern with augmentation of the parietal and temporal foci and return of the dorsolateral PFC activation. These data directly demonstrate that the hormonal milieu modulates cognition-related neural activity in humans.
Keywords: frontal cortex, regional cerebral blood flow, estrogen, progesterone, Wisconsin Card Sorting Test
An extensive body of data from animal studies indicates that gonadal steroid hormones modulate neuronal activity and affect behavior. In humans, however, the behavioral and cognitive evidence has not been conclusive, and direct neurophysiological data are scant. Effects of gonadal steroids in the human central nervous system have been inferred largely from behavioral changes during the menstrual cycle, during administration of ovarian hormones, or in a gender-specific context. These inferences are, by definition, indirect and associational and, further, are incapable of disentangling the effects of hormones, such as estrogen and progesterone, which are simultaneously present in women of reproductive age. We directly tested the central nervous system effects of gonadal steroid hormones in young women by measuring regional cerebral blood flow (rCBF) as a marker of local neuronal activity and by controlling their hormonal states with the gonadotropin-releasing hormone (GnRH) agonist leuprolide acetate (Lupron).
GnRH is produced by the hypothalamus and causes the anterior pituitary to release follicle-stimulating hormone and leuteinizing hormone. Lupron is a synthetic nonapeptide used clinically when suppression and/or control of gonadal steroid secretion is the goal, such as in infertility and endometriosis in women and prostate cancer in men. It is some 80–100 times more potent than native GnRH at anterior pituitary receptors (1), and during the first week of Lupron treatment, there is a transient increase in gonadal steroid levels in premenopausal women (2). However, with longer treatment there is down-regulation of GnRH receptors and inhibition of pituitary release of gonadotropins, resulting in postmenopausal levels of endogenous gonadal steroid hormones by the second to fourth week of administration. In this context, we could isolate the effects of estrogen and progesterone by administering them individually. We examined rCBF during cognition that targeted the frontal lobes because animal studies (3–5) and previous brain imaging work (for review, see ref. 6) have suggested that hormonal state may have particular relevance for this brain area.
METHODS
Subjects.
Eleven young women [age, 35 ± 7 years (mean ± SD); range, 27–49 years], six entirely healthy and five with menstrually related mood disorder (MRMD), 10 right-handed, signed informed consent in accordance with National Institutes of Health Institutional Review Board and Radiation Safety Committee requirements. They were screened to rule out past and present neurological, psychiatric (except MRMD), or substance-abuse problems, and were also excluded for medical illnesses or treatment relevant to cerebral metabolism and blood flow. Subjects had an average of 18 years of schooling (range, 14–23 years). They were instructed to refrain from nicotine and caffeine for 4 h and over-the-counter medications for 24 h prior to positron-emission tomography (PET) scans.
Hormone Manipulation Protocol.
As part of a larger study of the effects of gonadal steroids on brain physiology and behavior (P.J.S., unpublished data), PET scans were obtained for each woman over 4–5 months during each of three different treatment conditions: (i) ovarian suppression induced by Lupron, (ii) Lupron plus estrogen replacement, and (iii) Lupron plus progesterone replacement. Subjects received depot Lupron, 3.75 mg i.m., every 4 weeks for the entire duration of the study. Lupron alone was administered for 8–12 weeks. Subjects then received, in addition to Lupron, in counterbalanced sequence and in a double-blind cross-over design (i) transdermal patches containing the primary ovarian estrogen 17β-estradiol (0.1 mg daily) and (ii) progesterone suppositories (400 mg daily in two divided doses) each for 4–5 weeks. Each of the two “add-back” regimens was separated by a 1 week washout period. Subjects administered both patches and suppositories (active or placebo, depending upon the treatment phase) daily throughout the entire add-back period to ensure the double blind. Every 2 weeks throughout the study, subjects completed symptom self-rating scales [Beck Depression Inventory (7) and Spielberger State Anxiety Inventory (8)] and had blood samples drawn to measure plasma gonadotropins (with specific double antibody radioimmunoassays) and gonadal steroids (by radioimmunoassay after extraction chromatography). Hormone levels and behavioral ratings were analyzed by using a one-way ANOVA and post hoc paired t tests. PET sessions occurred during the last 2 weeks of each of the three treatment phases.
Cognitive Conditions.
At each of the three scanning sessions, after an initial resting eyes-closed scan for acclimation (data not reported), each subject underwent two rCBF PET measurements during two different cognitive conditions, the Wisconsin Card Sorting Test (WCS) and a no-delay matching-to-sample sensorimotor control task (WCScon), administered as reported (9). Before the PET scans, the correct strategy for performing the tasks was explained and practiced to criterion to obviate a learning effect. Subjects were instructed to work efficiently and rapidly, but otherwise proceeded at their own pace. Tasks began 1 min prior to injection of the radiotracer and continued throughout the ensuing 4- to 4.5-min scan period. Performance was recorded as described (9, 10).
WCS.
The WCS is an abstract reasoning and problem solving task involving the use of working memory to form a cognitive set and apply a conceptual strategy but also necessitating maintenance and then shifting of the set when appropriate. Despite controversy as to its specificity, the test is a sensitive indicator of the integrity of the human frontal lobes (11), particularly of the dorsolateral aspect. By using our computerized WCS (9), subjects were told to match each target stimulus to one of the four possible unchanging answer stimuli on the basis of color, number, or shape; that the rule rotated through these three parameters; and that the rule would change after a series of 10 correct answers. Before each of the three PET scans, subjects were retaught the test and practiced it to criterion. There is no guide for making the match in the immediate external environment, and subjects must use internal representations of the results of previous trials with working memory and the rules that they have been taught. We have previously shown that, in comparison to the WCScon, the WCS reliably produces physiological activation in a network of regions including dorsolateral prefrontal cortex (DLPFC), the inferior parietal lobule, and the posterior portion of the inferior temporal cortex (9).
Sensorimotor Control Task (WCScon).
The sensorimotor control task for the WCS was a no-delay matching-to-sample task designed to be similar to the WCS in visual stimulation and motor response requirements (9) but without the abstract reasoning and working memory components of the WCS. Subjects matched the target stimulus to one of four unchanging answer stimuli.
PET.
PET scans were performed on a Scanditronix PC2048–15B brain tomograph (15 contiguous slices; reconstructed in-plane and axial resolution of 6–6.5 mm) after an i.v. bolus administration of approximately 50 mCi of oxygen-15-labeled water. The time course of regional cerebral radiation concentration was determined simultaneously for each voxel by collecting a total of 16 dynamic emission scans (12 10-sec scans and 4 30-sec scans) during the 4 min after arrival of the tracer in the brain. Scan data were reconstructed with corrections for attenuation (measured with transmission scans), scatter, random coincidences, and dead time. The arterial time–activity curve was used with the 16 reconstructed emission scans and a least squares method (12) on a voxel-by-voxel basis to produce quantitative images of rCBF in ml per 100 g per min. Arterial partial pressure of carbon dioxide (pCO2) was measured at the end of each scan.
Image Processing and Statistical Analyses.
The three PET data sets for each subject were coregistered by using the automated image registration program (13). Global CBF was determined for each scan as the mean value of all voxels common to all registered scans of an individual. By using statistical parametric mapping (14), the registered data were resized and reshaped to the stereotaxic atlas of Talairach and Tournoux (15), smoothed with a filter of 20, 20, and 12 mm in the x, y, and z planes, respectively, and adjusted for individual differences in global blood flow by using analysis of covariance.
Finally, on a voxel-by-voxel basis for all planes common to all subjects (from 20 mm below to 48 mm above the intercommissural line), within-subject between-task (WCS minus WCScon) comparisons were performed for each drug condition separately and between each of the three possible drug pairs. For each planned analysis, the value of t for each voxel was calculated and transformed to a normal standard distribution, maps of the z statistic showing all voxels significantly activated at the P < 0.01 level were made, and stereotaxic coordinates of the epicenters (i.e., maxima) of areas of significant change were determined. Only maxima significant at the P < 0.001 level for within-treatment analyses and the P < 0.01 level for between-treatment analyses were tabulated. Lastly, activation data for the five women with MRMD were compared with those for the six entirely well subjects.
RESULTS
Hormone Levels and Behavioral Rating Scales.
Suppression of pituitary gonadotropin secretion was confirmed by plasma measurements (Table 1). Plasma gonadal steroid levels were consistent with menopause throughout the duration of the study, except during estrogen and progesterone replacement when physiological levels of the appropriate hormone were documented. Behavioral ratings did not change systematically across drug conditions.
Table 1.
Plasma hormone levels, behavioral/cognitive ratings, and global cerebral blood flow
| Lupron | Estrog | Progest | F | P | |
|---|---|---|---|---|---|
| Estradiol, pg/ml | 6.25 ± 1.7 | 96.1 ± 30* | 7.49 ± 3.9 | 96.12 | 0.0001 |
| Progesterone, ng/ml | 0.36 ± 0.2 | 0.49 ± 0.5 | 13.4 ± 5.7* | 57.49 | 0.0001 |
| Spielberger state anxiety | 34.2 ± 12 | 32.9 ± 9.9 | 34.9 ± 13 | 0.13 | 0.88 |
| Beck depression inventory | 2.9 ± 3.7 | 3.3 ± 4.8 | 4.3 ± 6.9 | 0.27 | 0.76 |
| WCS, % correct | 91.8 ± 3.6 | 91.8 ± 6.6 | 95.1 ± 3.5† | 2.78 | 0.09 |
| WCS, % perseverative errors | 6.1 ± 2.1 | 5.6 ± 3.8 | 3.4 ± 3.1† | 4.61 | 0.02 |
| WCS global CBF, ml per 100 g per min | 52.6 ± 9.9 | 48.5 ± 6.1 | 53.4 ± 5.9 | 1.62 | 0.22 |
| WCScon global CBF, ml per 100 g per min | 50.4 ± 9.2 | 46.7 ± 6.2 | 51.2 ± 6.4 | 1.41 | 0.27 |
Lupron, Lupron alone; Estrog, Lupron plus estrogen; Progest, Lupron plus progesterone. Values are the mean ± SD; n = 11 for all measures except the Speilberger scale and Beck inventory for which only 9 and 10 values, respectively, were available for all three study phases. For WCS, percent correct and percent perserverative errors, normative values for healthy subjects trained on the WCS and studied under the same conditions in our laboratory. WCS percent correct = 92.0 ± 4.7; WCS percent perseverative errors = 4.9 ± 2.8. Spielberger scale is on a scale of 20–80; Beck inventory is on a scale of 0–63, ∗P = 0.0001 compared to each of the other two conditions, †, P = 0.01 compared to Lupron alone; ‡, P < 0.05 compared to each of the other two conditions.
Neuropsychological Performance During the PET Scans.
Performance on the WCScon was perfect for all individuals. On the WCS, as expected given our extensive prescan task training sessions, most subjects performed at or near ceiling (Table 1). Performance on all three phases was similar to our values collected in the same way in our laboratory on healthy volunteers. Despite this practice and training and the resulting excellent performance, there was a tendency for subjects to perform best during the progesterone phase. This tendency reached statistical significance for percent correct compared with the Lupron alone condition and for the percent perseverative errors score compared with both of the other hormonal states.
Global Flow.
There were no differences in global CBF between the three treatment conditions (Table 1). Measurements of pCO2 indicated no significant changes across treatment conditions or cognitive tasks.
Regional Activation.
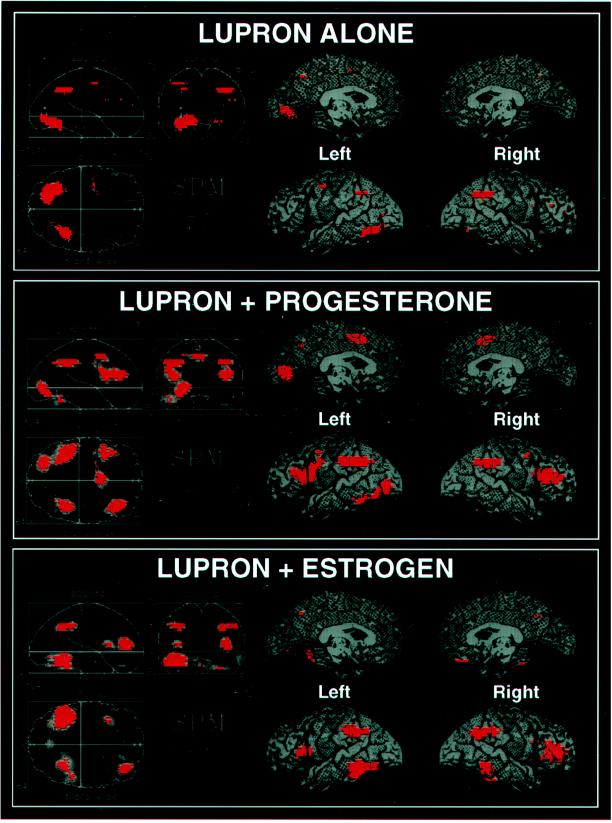
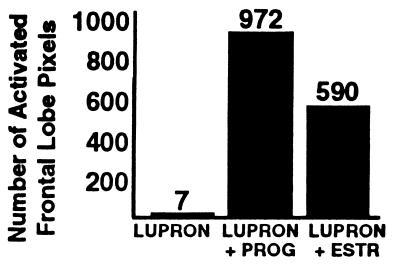
During treatment with Lupron alone (i.e., in the virtual absence of gonadal steroid hormones), there was little significant activation for the group as a whole (Figs. 1 Top and 2). Most strikingly, there was little or no activation of dorsolateral prefrontal cortex (DLPFC; P > 0.2). This attenuated activation pattern was in marked contrast to previous studies of normal volunteers, which have repeatedly shown that performance of the WCS causes robust activation of prefrontal cortex (PFC) (9, 16–18) as well as of the inferior parietal lobule and the posterior portion of the inferior temporal lobe (9). Addition of either progesterone or estrogen to the Lupron regimen normalized the rCBF activation pattern, producing augmentation of the parietal and temporal foci and a return of the DLPFC activation (Fig. 2), especially on the right (Table 2; Fig. 1 Middle and Bottom). The activation maps during the two add-back conditions not only were similar to each other but also closely resembled the pattern previously reported in normal subjects (14).
Figure 1.
Statistical parametric maps showing areas, in red, where cerebral blood flow during the WCS exceeded that during the sensorimotor control task (P < 0.01) for three hormonal conditions: hypogonadism induced by Lupron, Lupron plus progesterone replacement, and Lupron plus estrogen replacement. (Left) Results projected onto three orthogonal two-dimensional planes. (Right) Results for lateral and medial quadrants of the left and right hemispheres projected onto lateral and medial views of a brain.
Figure 2.
Number of frontal lobe pixels in which cerebral blood flow during the WCS exceeded that during the sensorimotor control task (P < 0.01) for three hormonal conditions: hypogonadism induced by Lupron, Lupron plus progesterone replacement, and Lupron plus estrogen replacement. PROG, progesterone; ESTR, estrogen.
Table 2.
Points of maximum activation (WCS − WCScon) for each hormonal condition
| Brain region (Brodmann area) | L/R | Stereotaxic coordinate
|
Z value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Activation during Lupron alone | |||||
| Inferior parietal lobule (40) | R | 34 | −46 | 40 | 3.24 |
| Fusiform gyrus (19) | L | −20 | −68 | −8 | 3.56 |
| Fusiform gyrus (37) | L | −30 | −54 | −12 | 3.13 |
| Activation during Lupron plus progesterone replacement | |||||
| Inferior frontal gyrus (46) | R | 36 | 30 | 20 | 4.09 |
| Inferior frontal gyrus (46) | L | −40 | 24 | 20 | 3.09 |
| Inferior frontal gyrus (44/45) | L | −40 | 16 | 16 | 3.16 |
| Medial frontal gyrus (6) | L | −4 | 8 | 48 | 3.33 |
| Inferior parietal lobule (40) | R | 38 | −40 | 36 | 3.54 |
| Inferior parietal lobule (40) | L | −44 | −36 | 40 | 3.67 |
| Inferior parietal lobule (40) | L | −46 | −44 | 36 | 3.61 |
| Inferior parietal lobule (40) | L | −34 | −50 | 36 | 3.49 |
| Inferior temporal/lingual gyri (37/18) | L | −24 | −76 | 0 | 3.54 |
| Inferior temporal/fusiform gyri (37/19) | L | −30 | −72 | −8 | 3.42 |
| Activation during Lupron plus estrogen replacement | |||||
| Inferior frontal gyrus (46) | R | 36 | 38 | 12 | 3.74 |
| Frontal insular cortex (44/45) | L | −34 | 16 | 12 | 3.16 |
| Inferior parietal lobule (40) | R | 28 | −52 | 36 | 3.57 |
| Inferior parietal lobule (40) | R | 48 | −35 | 40 | 3.34 |
| Inferior parietal lobule (40) | L | −40 | −40 | 40 | 3.57 |
| Inferior parietal lobule (40) | L | −30 | −52 | 40 | 3.29 |
| Inferior parietal lobule (40) | L | −48 | −36 | 40 | 3.21 |
| Inferior temporal/fusiform gyri (37) | L | −40 | −48 | −12 | 4.02 |
| Fusiform gyrus/cerebellum (37) | L | −46 | −48 | −20 | 4.31 |
Stereotaxic coordinates (in mm) refer to ref. 15; x, medial-to-lateral distance from the middle (positive = right); y, anterior–posterior distance relative to the anterior commissure (positive = anterior); z, superior–inferior distance from the intercommissural line (positive = superior). L, left; R, right.
Direct statistical comparisons between the progesterone and the Lupron alone change maps (Table 3) reached statistical significance in the three areas known to be important for this task: left and right DLPFC, left inferior parietal lobule, and left inferior temporal cortex. There were significant differences between the estrogen and the Lupron alone change maps (Table 3) in DLPFC bilaterally and in several left inferior temporal cortical foci. In contrast, there were no differences between the progesterone and estrogen conditions in these three areas, with the only significant difference between the two seen as greater activation in the left hippocampal area during estrogen. The PFC findings were largely confirmed with a region of interest analysis in which irregular regions of interest were drawn on each subject’s coplanar MRI and then projected onto the PET images.
Table 3.
Points of maximum difference between pairs of activation maps
| Brain region (Brodmann area) | L/R | Stereotaxic coordinate
|
Z values | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Estrogen activation minus Lupron activation* | |||||
| Inferior frontal gyrus (46) | R | 42 | 36 | 8 | 2.45 |
| Inferior frontal gyrus (46) | R | 32 | 34 | 12 | 2.44 |
| Inferior frontal gyrus (47) | L | −22 | 28 | −20 | 2.71 |
| Fusiform gyrus (20) | L | −42 | −36 | −16 | 2.79 |
| Fusiform gyrus (20/36) | L | −48 | −34 | −20 | 2.73 |
| Fusiform/hippocampal gyri (36) | L | −26 | −24 | −20 | 2.67 |
| Inferior temporal lobe/cerebellum | L | −32 | −34 | −20 | 2.76 |
| Progesterone activation minus Lupron activation* | |||||
| Inferior frontal gyrus (46) | R | 32 | 28 | 16 | 2.52 |
| Inferior frontal gyrus (47) | L | −22 | 26 | −16 | 2.45 |
| Inferior temporal gyrus (20) | L | −50 | −32 | −16 | 2.39 |
| Inferior parietal lobule (40) | L | −44 | −38 | 36 | 2.74 |
| Inferior parietal lobule (40) | L | −50 | −32 | 36 | 2.74 |
| Estrogen activation minus progesterone activation* | |||||
| Middle and inferior temporal gyri | L | −32 | −44 | −4 | 2.58 |
| Hippocampus | L | −20 | −2 | −20 | 2.56 |
| Hippocampus | L | −34 | −40 | 4 | 2.50 |
| Hippocampus | L | −34 | −38 | −4 | 2.50 |
There were no significant differences between women with and without MRMD for this relatively small sample. Post hoc analyses were, thus, performed on the entire group—across hormonal states for WCS rCBF alone and for WCScon alone—to determine whether the differences in rCBF activation (i.e., WCS minus WCScon) between drug conditions occurred because of rCBF changes in the WCS, the WCScon sensorimotor control task, or both. PFC changes between the lupron alone condition and both add-back conditions were seen only during the WCS and were predominantly in the right PFC.
DISCUSSION
To our knowledge, these data represent the first use of a PET activation paradigm to observe hormonal modulation of regional neurophysiological activity in response to cognition. One of the most surprising and intriguing findings is that the subjects, as a group, when treated with Lupron (i.e., in the virtual absence of gonadal steroid hormones) showed virtually no statistically significant activation of PFC during the WCS. This lack of activation, which reversed with estrogen or progesterone replacement, is in striking contrast to previous rCBF work that has documented robust and reliable participation of the PFC with this classically PFC-related task (9, 16–18). Because the present subjects were clearly performing the task and were, in fact, performing it well, there appears to be a puzzling dissociation between cognitive/behavioral output and neural activity. An important issue in considering this apparent dissociation is our use of training and practice sessions before each scan to minimize performance differences across conditions that could confound interpretation of the neurophysiological data. Our use of overlearned relatively easy cognitive material likely limited our ability to detect performance changes because of ceiling effects. Thus, on the basis of the present data, we cannot rule out the possibility that cognitive impairments during Lupron-induced hypogonadism would have been seen with other cognitive tasks that are not overlearned.
Nevertheless, taken at face value, this apparent dissociation raises questions about the source and meaning of the rCBF activation changes. It is tempting to conclude that the present findings reflect true alterations in neural activity in the face of a hormonal perturbation, but because rCBF is an indirect (albeit tightly coupled) indicator of neuronal function, mechanisms other than altered neural activity, such as changes in vascular response per se or hormonally induced dissociation between rCBF and neural activity, must be considered.
(i) Could these findings reflect purely vascular changes? The fact that significant changes were seen in all three major regions typically activated by this task is, at first glance, consistent with the notion of a widespread phenomenon such as vascular tone. However, because all three areas affected by the hormonal manipulation are known to be portions of an interconnected anatomical circuit (19), and all three have been shown to participate in the circuit activated by the WCS (9) and other tasks with similar cognitive components (20), the effects may well not be generalized throughout the brain, but only throughout the relevant circuit. The next question is whether there are any brain areas in which the functional response remains unaltered. If such a purely vascular mechanism were operational in this experiment, we would expect all rCBF increases, regardless of the behavior used to invoke them, to be blunted during the Lupron alone phase. To test this possibility, we compared the rCBF maps during the sensorimotor control task (when subjects had their eyes open and were making motor responses) to the rCBF maps during the initial resting state scan (when subjects had their eyes closed and were making no movements). Robust activation was demonstrated in both primary motor (Z values >3.15; P values <0.001) and visual (Z >4.6; P <0.0001) cortical areas during all three hormonal states. Moreover, direct statistical comparison between each possible pair of hormonal states revealed no significant hormonal effects in these areas (P values >0.2), and the effect sizes were not smaller during Lupron alone. This analysis proves that the brain’s responses to visual input and motor output remain unaltered by the hormonal manipulations in this experiment and suggests that our findings are not generalized but, rather, are in some way related to the cognitive circuit recruited by the WCS. This is supported by the results of another post hoc analysis that showed that hypogonadism mainly altered PFC rCBF during the WCS, itself, with little effect during WCScon.
(ii) The second important question, could the hormonal manipulation in some way have disrupted the normally tight coupling that has been documented to exist among rCBF, neuronal metabolism, and neuronal activity (21), is also addressed by the analyses detailed above. The facts that the hormonally induced changes occur in brain areas that are important to the cognitive task but not in others, that the response in areas subserving motor response and visual stimulation remain intact during Lupron, and that changes occur during the WCS and not the control task, collectively provide compelling evidence against this second possibility. It should also be noted that the few instances in which uncoupling between rCBF and neuronal metabolism has been demonstrated involve cases of extreme pathology, such as cerebrovascular disease, increased intracranial pressure, or stroke (22), whereas the hormonal manipulations in this study simulate conditions occurring naturally in normal healthy women. For these reasons, the most likely explanation is that these rCBF changes reflect true alterations in neural activity.
If these findings do represent hormonal modulation of neuronal activity patterns, we might have expected this to be manifest not just by loss of the normal PFC activation pattern during the Lupron condition but also by demonstration of alternative neural circuits called upon to carry out the task in the face of a functionally perturbed frontal lobe. Why isn’t such a new pattern revealed in the Lupron condition? The answer may lie in the fact that these results were derived from statistical averaging across the group and that statistical significance in these, as all statistical results, depends as much upon the error term, or variability, of the data as upon the magnitude of the average change, or difference, in question. Therefore, one explanation for the lack of significant activations during Lupron treatment could lie not in the absence of an average change but, rather, in the existence of increased physiological variability across the group during this condition compared with the other two hormonal states. This would be evident as differential variability of the rCBF change scores themselves and could derive from differential variability in the subjects’ behavior or their functional neuroanatomy. If either were the case, we would expect to see considerably more spread across the individual activation values for critical voxels in the Lupron condition than in the other two. Examination of the maxima listed in Table 3 did not support this notion and, moreover, further confirmed that the cross-hormonal activation differences in this study occurred because of rCBF differences during the WCS rather than the control condition.
Potential behavioral sources of increased variation could include changes in mood or cognitive performance. Table 2 shows that behavioral rating scores were quite small for the group as a whole, that the ratings did not change on average with hormonal state, and that the variance of these measures was likewise similar across the three conditions. Similarly, differential variance in the WCS performance measures does not appear to provide an obvious explanation because performance changes and increased variation, if seen at all, tended to occur not during the Lupron phase but, rather, during the progesterone and estrogen phases, respectively.
The most important potential source of differential variance in this study lies in the functional anatomy of the individuals, in other words in the location of the neural pathways used to perform the task. We have shown (9) that during the WCS subjects activate the same pathways even with repeated testing and that this pattern is remarkably similar across different cohorts of normal subjects. The existence of a common PFC activation pattern that is typically recruited by the WCS is further borne out in the present experiment by the similarity of the estrogen and progesterone replacement maps to each other and to these previous cohorts. If the concept of increased variance in functional anatomy played a role in the present data, this would mean that the neural circuitry typically recruited by the WCS (including the portion of the PFC utilized) was, indeed, recruited and similar from woman to woman during both estrogen and progesterone replacement (thus, allowing rCBF changes in these common areas to reach significance in group average statistics) but that during Lupron alone the PFC (or other) areas alternatively activated may have differed from woman to woman (thus, canceling out in group averaging). The ability of such alternate circuits to perform the WCS may have been facilitated by the overlearned nature of the task. We cannot evaluate the presence or absence of this potential source of variability because the PET method used does not allow activation patterns for individual subjects to be discerned, but, if present, it could represent a switch from the primary neural systems typically used to secondary neural circuits that differ between individuals in the context of less efficient neural processing. Recent advances in “three-dimensional” PET and in functional MRI that allow activation patterns of individual subjects to be discerned should provide more information about these questions.
Further work will also be necessary to fully explore the neuropsychological implications of these rCBF data. At least one previous study (23) has suggested neuropsychological changes with Lupron treatment, but in a larger cohort of women studied with a battery of neuropsychological tests during the same hormonal treatment regimen as this PET study, no neuropsychometric alterations across hormonal condition were found (P.J.S. and D.R.R., unpublished data). If cognitive changes did occur in the present experiment, they would appear to lie in improved WCS performance during progesterone treatment (which occurred despite our attempts to equate performance across the three PET sessions via training and practice and despite the excellent scores in all sessions) rather than in performance decrement during the Lupron alone phase. Interestingly enough, although there was no statistically significant difference in PFC activation between the estrogen and progesterone conditions, the spatial extent of the PFC activation was greatest during progesterone add-back (Fig. 2), especially on the left (Fig. 1). The exact relationship between neurophysiological activation and cognitive performance level is, however, complicated, varying with task and brain region. It is, moreover, unlikely that the small improvement in performance during progesterone replacement is clinically meaningful in view of the near ceiling levels throughout the study. The major rCBF difference between the progesterone and estrogen replacement was that the hippocampal area was more activated with estrogen treatment. This is consistent with observations that estradiol increases brain excitability, whereas progesterone appears to have the opposite effect (24, 25).
There is congruent indirect animal and functional neuroimaging evidence that gender differences and the hormonal milieu have particular relevance for metabolism and cognition related to the frontal lobe system. Esposito et al. (6) found that male–female differences in global CBF were best demonstrated during tasks traditionally considered to reflect PFC function and that regional differences in absolute blood flow between the sexes were best demonstrated in the frontal lobes. Few other investigators have specifically tested for sex effects in brain activity during DLPFC-related cognitive activities, but studies during rest and during nonspecific activities have also emphasized differences between men and women in frontal lobe physiology (for review, see ref. 6). These neuroimaging data are particularly interesting in light of frontal lobe findings in nonhuman primates. McDowell et al. (3) found that, compared with male monkeys, females learned quicker and performed better a classic PFC task, delayed response, that shares with the WCS an important working memory component. Also, the age at which cognitive deficits emerged after early orbital PFC lesions depended on the sex of the animal, with males being impaired earlier than females (4) and cognitive deficits being seen in male monkeys and in female monkeys given androgens but not in untreated female monkeys (5). One interpretation of these data, and our data, is that female hormones may have a facilatory effect on PFC function that could play a protective role in human neuropsychiatric diseases involving the frontal lobe in which male–female differences in expression of the illness may occur.
It is likely that these hormonal effects are indirect and mediated by other factors, including neurotransmitters. One possible candidate that is known to be modulated by these hormones is dopamine. Depending upon the dose and experimental conditions, estrogens can either facilitate or inhibit central dopaminergic tone, but with doses at physiological levels (as they were in this experiment), the effects appear to be facilatory (26, 27). A relationship between dopamine and progesterone, including regulation of gene expression via cross-talk between membrane receptors for this neurotransmitter and intracellular steroid receptors (28), has also been demonstrated but is less well studied. Extrahypothalamic interactions between female gonadal steroid hormones and dopamine have been most extensively explored in the striatum, but there is evidence in rodents, at least for estrogen, that interactions also occur in the cortex (29).
Such hormonal modulatory effects on the cortical dopamine system, if they also exist in humans, could provide an explanation for the present findings since behavioral (30, 31) and electrophysiological (32) studies have demonstrated that dopamine is an important modulator of primate PFC function. In humans it has been shown that cerebrospinal fluid levels of the dopamine metabolite homovanillic acid predict the level of PFC response when patients with schizophrenia perform the WCS (33) and that dopamine agonists augment PFC activation during the WCS (34, 35). The present study, by offering direct neurophysiological evidence in humans that the hormonal milieu modulates neurophysiological response to cognition, provides impetus for future work to determine the exact mechanism underlying these findings and their implications for cognitive function.
ABBREVIATIONS
- WCS
Wisconsin Card Sorting Test
- PET
positron-emission tomography
- rCBF
regional cerebral blood flow
- PFC
prefrontal cortex
- DLPFC
dorsolateral PFC
- GnRH
gonadotropin-releasing hormone
- MRMD
menstrually related mood disorder
- WCScon
no-delay matching-to-sample sensorimotor control task
References
- 1.Santen R J, Warner B. Urology. 1985;25:53–57. [PubMed] [Google Scholar]
- 2.Conn P M, Crowley W F. Annu Rev Med. 1994;45:391–405. doi: 10.1146/annurev.med.45.1.391. [DOI] [PubMed] [Google Scholar]
- 3.McDowell A A, Brown W L, McTee A C. J Comp Physiol Psychol. 1960;53:429–432. [Google Scholar]
- 4.Goldman P S, Crawford H T, Stokes L P, Galkin T P, Rosvold H E. Science. 1974;186:540–542. doi: 10.1126/science.186.4163.540. [DOI] [PubMed] [Google Scholar]
- 5.Clark, Goldman-Rakic P S. Behav Neurosci. 1989;103:1287–1295. doi: 10.1037//0735-7044.103.6.1287. [DOI] [PubMed] [Google Scholar]
- 6.Esposito G, Van Horn J D, Weinberger D R, Berman K F. J Nucl Med. 1996;37:559–564. [PubMed] [Google Scholar]
- 7.Beck A T, Ward C H, Mendelson M, Mock J, Erbawgh J. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 8.Spielberger C D. Manual for State-Trait Anxiety. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 9.Berman K F, Ostrem J L, Randolph C, Gold J, Goldberg T E, Coppola R, Carson R E, Herscovitch P, Weinberger D R. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- 10.Heaton R K, Cheloune G J, Tally J L, Kay G G, Curtis G. Wisconsin Card Sorting Test Manual Revised and Expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- 11.Milner B. Arch Neurol. 1963;9:100–110. [Google Scholar]
- 12.Koeppe R A, Holden J E, Ip W R. J Cereb Blood Flow Metab. 1985;5:224–234. doi: 10.1038/jcbfm.1985.29. [DOI] [PubMed] [Google Scholar]
- 13.Woods R P, Cherry S R, Mazziotta J C. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. , 1992. [DOI] [PubMed] [Google Scholar]
- 14.Friston K J, Frith C D, Liddle P F, Frackowiak R S J. J Cereb Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- 15.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 16.Weinberger D R, Berman K F, Zec R F. Arch Gen Psychiatry. 1986;43:114–125. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 17.Berman K F, Zec R F, Weinberger D R. Arch Gen Psychiatry. 1986;43:126–135. doi: 10.1001/archpsyc.1986.01800020032005. [DOI] [PubMed] [Google Scholar]
- 18.Marenco S, Coppola R, Daniel D G, Zigun J R, Weinberger D R. Psychiatry Res. 1993;50:177–192. doi: 10.1016/0925-4927(93)90029-h. [DOI] [PubMed] [Google Scholar]
- 19.Selemon L D, Goldman-Rakic P S. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold J M, Berman K F, Randolph C, Goldberg T E, Weinberger D R. Neuropsychology. 1996;10:3–10. [Google Scholar]
- 21.Sokoloff L. Fed Proc Fed Am Soc Exp Biol. 1981;40:2311–2316. [PubMed] [Google Scholar]
- 22.Herscovitch P. In: Head Injury and Post-Concussive Syndrome. Rizzo M, Tranel D, editors. New York: Churchill Livingstone; 1996. pp. 89–118. [Google Scholar]
- 23.Varney N R, Syrop C, Kubu C S, Struchen M, Hahn S, Franzen K. J Assist Reprod Genet. 1993;10:53–57. doi: 10.1007/BF01204441. [DOI] [PubMed] [Google Scholar]
- 24.Woolley D E, Timiras P S. Endocrinology. 1962;70:196–209. doi: 10.1210/endo-70-2-196. [DOI] [PubMed] [Google Scholar]
- 25.Backstrom T. Acta Neurol Scand. 1976;54:321–347. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- 26.McDermott J L, Liu B, Dluzen D E. Exp Neurol. 1994;125:306–311. doi: 10.1006/exnr.1994.1034. [DOI] [PubMed] [Google Scholar]
- 27.Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V. J Neurochem. 1995;65:1651–1657. doi: 10.1046/j.1471-4159.1995.65041651.x. [DOI] [PubMed] [Google Scholar]
- 28.Mani S K, Allen M C, Clark J H, Blaustein J D, O’Malley B W. Science. 1994;265:1246–1249. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- 29.Woolley D E, Hope W G, Thompson-Reece M A, Gietzen D W, Conway S B. Recent Prog Horm Res. 1994;49:383–92. doi: 10.1016/b978-0-12-571149-4.50029-5. [DOI] [PubMed] [Google Scholar]
- 30.Sawaguchi T, Goldman Rakic P S. J Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- 31.Brozoski T J, Brown R N, Rosvold H E, Goldman P S. Science. 1979;31:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 32.Williams G V, Goldman-Rakic P S. Nature (London) 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 33.Weinberger D R, Berman K F, Illowsky B J. Arch Gen Psychiatry. 1988;45:609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- 34.Daniel D G, Weinberger D R, Jones D W, Zigun J R, Coppola R, Handel S, Bigelow L B, Goldberg T E, Berman K F, Kleinman J E. J Neurosci. 1991;11:1907–1917. doi: 10.1523/JNEUROSCI.11-07-01907.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattay V S, Berman K F, Ostrem J L, Esposito G, Van Horn J D, Bigelow L B, Weinberger D R. J Neurosci. 1996;16:4816–4822. doi: 10.1523/JNEUROSCI.16-15-04816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]