Abstract
Lsc is a hematopoietic-restricted protein that functions as an effector of Gα12/13-associated G-protein coupled receptors that activates RhoA. In the absence of Lsc leukocytes exhibit impaired migration and B lymphocytes inefficiently resolve integrin-mediated adhesion. Here, we demonstrate that Lsc exists physiologically in primary B lymphocytes as a large molecular weight complex resembling a homo-tetramer. Interfering with the assembly of this large molecular weight Lsc oligomer results in the activation of both Lsc functional activities and leads to cell rounding and inhibition of integrin-mediated adhesion. During cell migration on integrin ligands we find Lsc localizes predominantly toward the rear of migrating cells where we suggest it activates RhoA to resolve integin-mediated adhesion. Together these data demonstrate that Lsc regulates integrin-mediated adhesive events at the trailing edge of migrating cells.
Keywords: Lsc, oligomerization, RhoA, integrin, adhesion
1. Introduction
Cellular migration is fundamental to a variety of biological processes that include innate and adaptive immunity and is accomplished through repeated cycles of membrane protrusion, attachment, cytoskeletal contraction, and detachment. Analysis of cell migration in diverse model systems has revealed that this process is accomplished by chemoattractant receptors signaling the activation of Rho GTPase family members in a spatially-restricted manner (Burridge and Wennerberg, 2004; Etienne-Manneville and Hall, 2002; Nobes and Hall, 1999; Parent, 2004; Ridley et al., 2003; Van Haastert and Devreotes, 2004; Xu et al., 2003). In migrating neutrophils, activation of Rac occurs at the leading edge and results in actin polymerization and lamellipodia formation whereas Rho activation at the uropod leads to actin-myosin contraction and trailing edge release.
As cells extend a leading edge protrusion, this extension, or lamellipodium, attaches via integrin-mediated adhesive contacts with integrin ligands that are either on the extracellular matrix or expressed by other cells. Forward cell movement, however, ultimately requires that integrin-mediated contacts disengage at the trailing edge. Thus, the coordinate regulation of establishing and resolving cell adhesion is critical for optimal cell movement. For relatively slow migrating cells such as fibroblasts, resolution of trailing edge adhesive events is often accomplished by severing integrin-cytoskeletal linkages through proteolysis (Franco et al., 2004; Huttenlocher et al., 1997) or cytoskeletal contractile forces that leave a significant fraction of integrins with the extracellular matrix substratum (Cox and Huttenlocher, 1998; Palecek et al., 1996; Regen and Horwitz, 1992). For more rapidly moving cells such as neutrophils, integrins at the trailing edge appear not to be shed but rather are endocytosed (Cox and Huttenlocher, 1998; Lawson and Maxfield, 1995) indicating that termination of adhesion in these cells is accomplished by releasing integrin from ligand. Therefore, dissolution of trailing edge adhesion in leukocytes likely proceeds by different regulatory mechanisms than those identified in fibroblasts. The mechanism(s) that facilitates integrin release in motile leukocytes is still undefined although likely requires RhoA activity. Inhibition of this GTPase in neutrophils, eosinophils and lymphocytes impairs trailing edge release during migration on integrin substrates (Alblas et al., 2001; Liu et al., 2002; Smith et al., 2003; Worthylake et al., 2001; Yoshinaga-Ohara et al., 2002). As RhoA can be activated through a number of pathways, the specific mediators of RhoA activation that lead to trailing edge release upon chemoattractant stimulation of leukocytes are not yet identified. In a neutrophil-like cell line, however, Gα12/13 associated receptors have been shown to signal RhoA activation toward the cell rear (Xu et al., 2003) although the effectors of this signaling have not been identified.
Lsc, or Arhgef1 (Human Genome Organization nomenclature), is a hematopoietic-restricted intracellular signaling molecule that acts as a G-protein coupled receptor (GPCR) effector to signal RhoA activation (Chen et al., 2003; Hart et al., 1998; Kozasa et al., 1998; Wells et al., 2002). Similar to the human ortholog p115RhoGEF, Lsc harbors a regulator of G-protein signaling (RGS) domain (Ross and Wilkie, 2000) that attenuates signaling by GPCRs associating with Gα12/13-containing heterotrimeric G-proteins (Kozasa et al., 1998). Lsc also contains tandem Dbl- and pleckstrin-homology (DH-PH) domains that encode Rho guanine nucleotide exchange factor (GEF) activity (Schmidt and Hall, 2002) leading to RhoA activation (Hart et al., 1998). In the absence 5 of Lsc, leukocytes are impaired in migration (Brown et al., 2007; Francis et al., 2006; Girkontaite et al., 2001; Rubtsov et al., 2005) and we have recently shown that lsc−/−marginal zone B lymphocytes remain adherent to integrin ligands for extended periods and migrate inappropriately in vivo and in vitro due to inefficient trailing edge release from integrin ligands (Rubtsov et al., 2005).
In this study we have investigated the regulation of Lsc activity and its role in integrin-mediated adhesion and migration. We demonstrate that Lsc exists physiologically in primary B lymphocytes and a B cell lymphoma cell line as a homo-oligomer, most likely tetramer, and interfering with the assembly of endogenous Lsc tetramer stimulates both RGS and RhoGEF activities, inhibits polarization and prevents integrin-mediated adhesion. We further show that during lymphocyte migration on integrin ligands Lsc localizes predominantly towards the trailing edge of migrating cells similar to that previously shown for RhoA (Xu et al., 2003). Thus, we propose that Lsc RhoGEF-mediated activation of RhoA at the cell trailing edge is required during migration for release of integrin-mediated adhesive contacts.
2. Materials and methods
2.1. Antibodies
Antibodies used were anti-FLAG (M2; Sigma), anti-HA (Covance), BD Living Colors A.v. monoclonal antibody (JL-8), anti-PRK1 (42) and anti-ERK2 (G263-7) were obtained from B&D Biosciences. Rabbit anti-mouse phospho-PRK1/2 and anti-phospho-p44/42 (E10) were from Cell Signaling Technology, HRP-labeled goat anti-mouse IgG and 6 donkey anti-rabbit IgG were from Santa Cruz Biotechnology, and AP- or HRP-labeled goat anti-hamster IgG (H+L) from Southern Biotechnology Associates.
Rabbit anti-LscCT antisera was prepared by standard immunization of New Zealand White rabbits (Jackson Laboratory) using purified LscCT protein (carboxy-terminal 162 amino acids of Lsc) and further purified by affinity chromatography using Protein A. The specificity of the anti-LscCT antisera was confirmed by western blot analysis of HEK293 cells transfected with an epitope-tagged Lsc expression vector (data not shown). To generate anti-Lsc monoclonal antibody, Armenian hamsters were immunized with purified LscCT and hybridomas established by standard protocols. Positive clones were identified with ELISA using LscCT as antigen. All positive clones were expanded and subsequently sub-cloned. Clone, JH-1 was expanded, supernatant harvested and monoclonal antibody purified by affinity chromatography and directly labeled with Alexa488 (Molecular Probes/Invitrogen)
2.2. Cell culture and transfection
All cell lines were maintained in DMEM (Mediatech, Herndon, VA) supplemented with 10% FBS, Glutamax (Gibco), antibiotics, and sodium pyruvate (Gibco). HEK293 cells were (co-) transfected by standard calcium phosphate precipitation methods, harvested 24 hours after transfection, lysed in RIPA buffer (50mM Tris, 150mM NaCl, 1mM EDTA, 1% NP-40, and 0.25% Sodium deoxycholate; pH7.2 and supplemented with 1mM Na3VO4, and 1mM NaF protease inhibitors,) and cell extracts prepared. Phoenix cells (G. Nolan, UCSF) were transfected with retroviral constructs using Lipofectamine Plus (Invitrogen) and retroviral-containing supernatant collected 48 hours after transfection. In experiments evaluating RGS and RhoGEF activity, A20 transfectants were serum-starved 2–4 hours prior to lysophosphatidic acid (Avanti Polar Lipids) stimulation and/or harvest.
2.3. DNA constructs
The plasmids (and source) used in these experiments were pGEX-4T-2 (Amersham Pharmacia), pCDNA3.1(−) (Invitrogen), pMSCV-puro-IRES-YFP and pMSCV-neo (BD Biosciences). CFP (A206K) cDNAs were provided by Roger Tsien. pGEX4T-2-LscCT was generated by inserting the last 486 bp of Lsc cDNA into the BamHI site of pGEX-4T-2. Lsc and LscCT were epitope-tagged at the carboxy terminus with FLAG, respectively, by PCR and inserted into pCDNA3.1 vector. pMSCV-neo-CFP were constructed by inserting the CFP (A206K variants (Zacharias et al., 2002) coding sequence, respectively, into the multiple cloning sites of the pMSCV-neo vector. The LscCT coding sequence was introduced upstream of CFP (A206K) to generate the fusion protein expressing plasmid: pMSCV-neo-LscCT-CFP. In addition, pMSCV-LscFLAG-puro-IRES-YFP, was generated through introducing coding sequence of Lsc-FLAG into multiple cloning site of pMSCV-puro-IRES-YFP vector.
2.4. Isolation of primary splenic B cells and construction of stable cell lines
Single cell suspensions were prepared from the spleens of 6–8 week old C57BL/6 mice (Jackson Laboratory) using standard procedures and splenic B cells were isolated by positive enrichment using anti-CD19 magnetic microbeads and autoMACS (Miltenyi Biotec). B cell purity as determined by flow cytometric analysis was at all times >90%. Animal manipulations were performed in accordance with the Institutional Animal Care and Use Committee. Stable A20 transfectants were produced by retroviral infection using Phoenix cell supernatants previously transfected with retroviral constructs and selected with 2 µg/ml puromycin (Invivogen) or G418 (Invivogen). Stable transfectants were expanded, distributed in aliquots and frozen. Thawed cells were passaged 3 times or less and then discarded.
2.5. Size exclusion chromatography
A20 parental cells, transfectants and primary splenic B cells were lysed in RIPA buffer and cell lysate extracts concentrated using Amicon ultra MW 10 KD columns (Millipore) and subsequently filtered through 0.22 µm filters. After column (Superdex 200, Amersham) equilibration, 200 µl of the sample was loaded and run with elution buffer (PBS + 0.1% Triton + Protease inhibitors) at 0.4 ml/min and 0.2 ml fractions collected. All fractions collected were subjected to SDS-PAGE and Western Blotting.
2.6. Immunoprecipitation
For immunoprecipitation, 1–2 µg of immunoprecipitating antibody was added to 500–800 µl cell extract and mixed by rotating at 4 °C for 1–2 hours. Subsequently, 30 µl protein-A/protein-G conjugated Sepharose beads (Amersham) were added rotated an additional hour. Immunoprecipitates were spun down and washed with RIPA buffer 3 times. To detect target proteins by Western blotting or Comassie staining, immunoprecipitate was mixed with 50ul 2X SDS loading buffer, and run on SDS-PAGE after boiled at 90–95°C for 5 min.
2.7. Western blotting and Coomassie blue staining
After SDS-PAGE, proteins were transferred to nitrocellulose membrane (Bio-Rad) and blocked with 5% nonfat milk-TBS solution at room temperature for 30 minutes. Membrane was then incubated with primary antibody in 5% nonfat milk-TBS solution for 1 hour at room temperature, washed 3 times with TTBS, incubated with HRP-labeled secondary antibody in 5% nonfat milk-TBS solution for 45 minutes, and washed 4 times with TTBS. Target proteins were detected by chemical fluorescence method. For Comassie blue staining, after SDS-PAGE, the gel was incubated with 30% Comassie Blue ethanol solution for 30 min, and subsequently washed with 70% methanol solution.
2.8. RhoA ELISA
GTP-bound RhoA in stable cell lines was measured by with an ELISA-based assay using the G-LISA™ RhoA Activation Assay kit (Cytoskeleton, CO) as per manufacture instructions. Briefly, stable cell lines were starved for 6–8 hours in FBS-free DMEM at 37° C and whole cell lysates prepared. Equal volume of whole cell lysates with equivalent protein concentration was added to RhoA-GTP affinity plate and incubated at 4° C for 30 min. After washing, RhoA-GTP binding to the plate was determined with anti-RhoA primary antibody and HRP-labeled secondary antibody, and RhoA-GTP content measured at O.D490.
2.9. Cell adhesion assay
For cell adhesion high protein binding plated (Nunc) were coated with 2 ug/ml soluble murine Fc-ICAM-1 or Fc-VCAM-1 (R&D Systems) or 1%BSA in DPBS without Ca2+ and Mg2+ at 4° C overnight, blocked with 1% BSA DPBS w/o Ca2+ and Mg2+ at 37° C for 2 hours, and washed once with DPBS with Ca2+ and Mg2+. Cells (1 × 106/ml) were added to the plate (100 µl/well) and incubated at 37° C, 5% CO2 for 30 min. Non-adherent cells were gently washed away with PBS, and adherent cells counted. Percent adhesion was calculated as: % adhesion = adherent cells / total cells × 100.
2.10. Cell polarization and microscopy
Briefly, 8-chamber glass slides were coated with Cell-Tak (BD Biosciences) for 20 minutes at room temperature and subsequently with murine Fc-ICAM-1 and Fc-VCAM-1 (3.0 µg/ml each; R&D Systems) or BSA (fatty-acid free; Sigma) for two hours room temperature. 200 µl of cells (3 × 105 cells/ml) were added to individual chambers and incubated at 37°C for 30 min. For evaluation of cell polarization cellular morphology was analyzed by light microscopy using a Leitz DM-IL microscope equipped with a Nikon Coolpix 5000 digital camera and 40X Leica objective. For Lsc localization during cell migration, cell movement was record by time-lapse photography (for 15min, 30s/exposure) using an inverted Zeiss Axiovert 200M microscope with a 175 watt xenon lamp within a 37° C heated chamber and driven by Slidebook 4.0 software (Intelligent Imaging Innovations Inc.). Cells were subsequently fixed with 3% paraformaldehyde, 3% sucrose PBS solution, and stained with the same cocktail of Alexa488-labeled hamster anti-LscCT (JH-1) and rhodamine phalloidin (Molecular Probes). Subsequently, cells were photographed at the same exposure times and images digitally deconvolved using Slidebook software. Alexa488-labeled hamster anti-CD3 (500A2) was used as isotype control.
3. Results
3.1. Endogenous Lsc homo-oligomerizes in a large molecular weight complex
Both Lsc and its human ortholog, p115RhoGEF, have been characterized as 115 kDa intracellular proteins whose expression is restricted to hematopoietic cells (Aasheim et al., 1997; Hart et al., 1996; Su et al., 2004; Whitehead et al., 1996) gnf.symatlas.org). Using rabbit antisera generated against the carboxy-terminal region of Lsc, we find that Lsc exists in bone marrow B lineage cells and splenic B cells predominantly as an ~125 kDa species whereas in the A20 B cell line and splenic non-B cells, Lsc is found as two species of ~125 kDa and ~107 kDa (Figure 1). Although the nature of these two species is not definitively established, we propose that the 125 kDa Lsc species represents an alternatively-spliced product relative to that previously characterized (Whitehead et al., 1996). Specifically, sequencing of splenocyte cDNA obtained by RT-PCR reveals an additional 183 bp encoding 61 amino acids with unknown function and that reside within the region between the RGS and RhoGEF domains (data not shown). Analysis of the genomic sequence further indicates this additional sequence to be contained within two exons inserted between exons 13 and 14 of the originally described locus. These alternatively-spliced exons are expressed in the A20 B cell line (data not shown) and were included in the coding sequence of the full-length Lsc-FLAG transgene. Indeed, Lsc-FLAG migrates precisely with the larger ~125 kDa endogenous Lsc protein when expressed in A20 cells (Figure 2A) providing further support that the larger Lsc species is an alternatively splice product. A cDNA harboring these additional exons has also been isolated from splenocytes of non-obese diabetic mice (GenBank accession no. AK157056).
Figure 1.
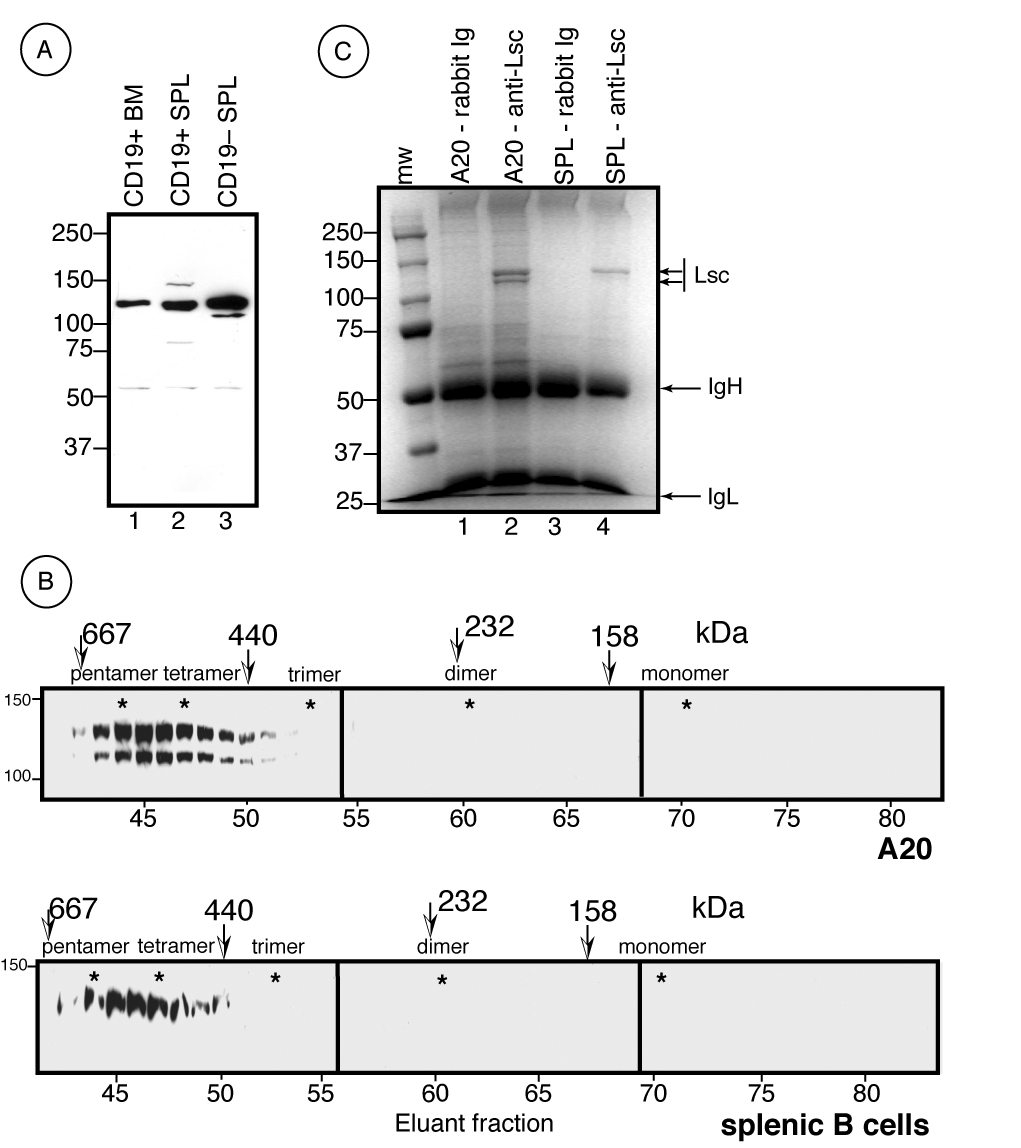
Primary B lymphocytes and the A20 B cell line express Lsc as a large molecular weight homo-oligomer. A) Western analysis of Lsc expression in primary CD19+ B lineage bone marrow cells (lane 1) and CD19+ (lane 2) and CD19−(lane 3) splenocytes as revealed with rabbit anti-mouse Lsc antisera. B) Whole cell lysates from the A20 cell line (top) or purified splenic B lymphocytes (bottom) were size fractionated as described for Figure 1 and Lsc content revealed using anti-Lsc antisera. C) Whole cell lysates from A20 and primary B lymphocytes were immunoprecipitated with either pre-immune rabbit sera (rabbit Ig) or anti-Lsc antisera (anti-Lsc) in the same buffer as that used for size fractionation, resolved by SDS/PAGE, and immunoprecipitated proteins stained with Coomassie Blue.
Figure 2.
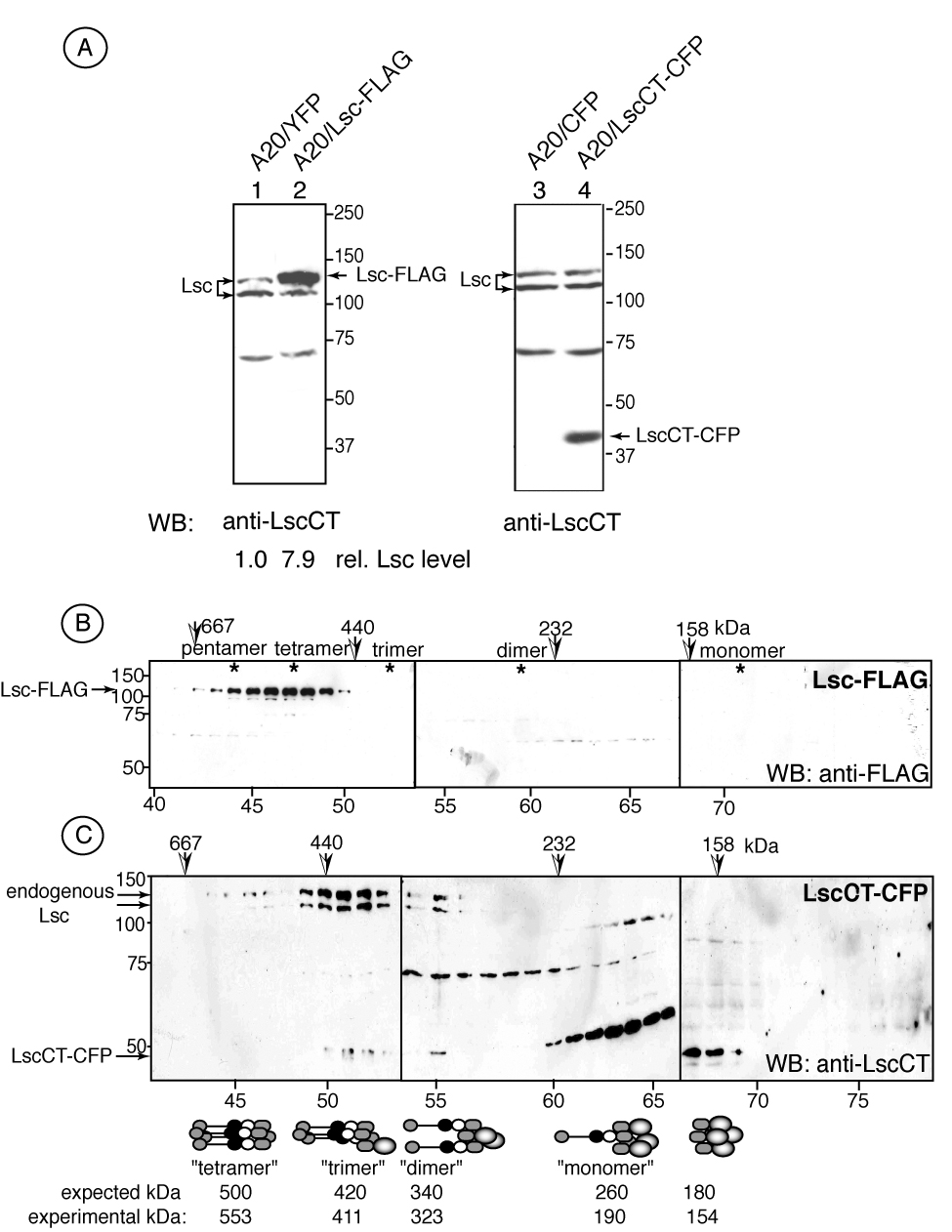
A carboxy-terminal Lsc protein interferes with the assembly of large molecular weight Lsc oligomers. A) Transgene expression in A20 transfectants as determined by Western analysis of transfectants expressing either YFP (lane 1), Lsc-FLAG (lane 2), CFP (lane 3) or LscCT-CFP (lane 4). Blots were revealed with anti-LscCT antisera against the carboxy-terminal Lsc domain. Size exclusion chromatography and SDS/PAGE of A20 transfectants reveal B) Lsc-FLAG is predominantly associated with complexes displaying 500 kDa molecular weight as determined by Western blotting with anti-FLAG antibody and C) LscCT associates with endogenous Lsc in distinct molecular weight complexes as determined using anti-Lsc antisera. Putative oligomeric complexes are shown with predicted and experimentally determined molecular weights.
Both Lsc and p115RhoGEF are capable of intermolecular interaction via sequences within the carboxy-terminus and most likely a coiled-coil domain (Chikumi et al., 2004; Eisenhaure et al., 2003) and unpublished data). To determine whether physiologically expressed Lsc also exists in an oligomeric complex, whole cell lysates from A20 cells and purified splenic B cells were subjected to size exclusion chromatography and fractions eluted from the column were evaluated by Western analysis. These data show that the endogenous Lsc expressed by both A20 and primary B lymphocytes was resident in a large complex of ~550–570 kDa molecular weight (Figure 1B). The composition of this Lsc-containing complex was examined by immunoprecipitating Lsc and any other potentially associated proteins from whole cell lysates followed by SDS-PAGE and Coomassie Blue staining. In these experiments the buffer conditions used for immunoprecipitation were identical to those used for size exclusion chromatography of whole cell lysates. Therefore, assuming that any protein capable of associating with Lsc in whole cell lysates would maintain this association during immunoprecipitation, these data (Figure 1C) reveal that the only proteins specifically immunoprecipitated were identical to Lsc in molecular weight. However, we cannot exclude the possibility that additional proteins associate with Lsc in vivo but dissociate upon isolation or are present at concentrations that are undetectable by this stain. Nevertheless, these data provide strong evidence that Lsc exists in vivo predominantly as a large molecular weight homo-oligomer.
3.2. An Lsc carboxy-terminal domain interferes with endogenous Lsc oligomerization
Lsc carboxyl terminal sequence(s) mediate intermolecular association (Chikumi et al., 2004; Eisenhaure et al., 2003) to promote assembly of a large molecular weight homo-oligomer in B lineage cells. Thus, we predicted that (over-) expression of an Lsc carboxy-terminal domain in B cells would disrupt the formation of these large Lsc-associated complexes by associating with endogenous Lsc. To test this, stable A20 transfectants were generated (Figure 2A) that expressed either full-length Lsc-FLAG, at levels 7.9-fold increased relative to endogenous Lsc, or the Lsc carboxy-terminus as a fusion protein with CFP (LscCT-CFP). Aequorea-derived fluorescent protein family members can spontaneously dimerize (Yang et al., 1996), therefore we used a CFP variant (A206K) as fusion protein partner engineered to eliminate spontaneous dimerization (Zacharias et al., 2002). Control A20 transfectants expressed only CFP or YFP A206K variants.
Size exclusion chromatography of A20 Lsc-FLAG transfectant whole cell lysates revealed that the 125 kDa epitope-tagged protein was present in a complex with a peak molecular weight of approximately 500 kDa compatible with a homo-tetrameric endogenous Lsc complex in B lineage cells (Figure 2B). An A20 stable transfectant expressing the LscCT-CFP fusion protein was also examined by size exclusion chromatography and eluted fractions were assessed for content using the anti-Lsc rabbit antisera that recognizes both the endogenous Lsc 125 kDa and 107 kDa species and the 45 kDa LscCT-CFP fusion protein. Figure 2C shows that A20 transfectants expressing the LscCT fusion protein harbor five discernible complexes that contain Lsc species and that are compatible in molecular weight to distinct predicted tetrameric complexes of full-length and carboxy-terminal Lsc proteins. The approximate 70 kDa species observed in fractions 55–65 is likely a cross-reactive protein also found in control A20 cells (Figure 2A).
These data demonstrate that ectopically-expressed full-length Lsc forms a high molecular weight oligomeric complex in the A20 B cell line and similar to endogenous Lsc. In contrast, expression of the Lsc carboxyl-terminus interferes with the formation of endogenous Lsc homo-oligomers and promotes the assembly of discrete oligomeric structures containing endogenous and LscCT proteins and reduced in molecular weight relative to endogenous Lsc homo-oligomers.
3.3. Disrupting the formation of large molecular weight Lsc homo-oligomers activates both Lsc RGS and RhoGEF activities
As protein oligomerization can positively (Chene, 2001; Marianayagam et al., 2004) or negatively (Zhu et al., 2001) regulate protein activity, we next evaluated how oligomerization status influenced Lsc RGS and RhoGEF activities. Lsc RGS activity is specific for Gα12/13 subunits and it functions to inhibit signaling by associated GPCRs (Chen et al., 2003; Kozasa et al., 1998; Majumdar et al., 1999; Mao et al., 1998; Wells et al., 2002). For example, heterologous overexpression of the p115RhoGEF RGS domain profoundly inhibits signaling by lysophosphatidic acid (LPA; (Johnson et al., 2003). LPA receptors are known to signal via Gα12/13-associated heterotrimeric G-proteins (Ishii et al., 2004) and activate ERK1/2 and other members of the MAP kinase pathway (Lai et al., 2005; Sato et al., 2004; Yu et al., 2004). Moreover, overexpression of the Lsc RGS domain inhibits ERK1/2 activation (Snabaitis et al., 2005). In consideration of these data, we assessed Lsc RGS activity in A20 transfectants by measuring ERK1/2 phosphorylation in response to LPA stimulation. In particular, we predicted that elevated RGS activity would more efficiently terminate signaling through Gα12/13-associated receptors and lead to reduced ERK1/2 phosphorylation relative to the wild type.
Figure 3A shows that upon LPA stimulation, ERK1/2 is rapidly phosphorylated in A20/YFP and A20/CFP control cells with peak phosphorylation occurring by 1 minute of stimulation and diminishing over the next 30 minutes. Stimulated Lsc-FLAG transfectants displayed comparable kinetics and magnitude of ERK1/2 phosphorylation. In contrast, when LscCT-CFP transfectants were stimulated with LPA phosphorylation of ERK1/2 was diminished by more than 50% at all time points compared to A20/CFP control or Lsc-FLAG transfectants and was essentially extinguished after 8 minutes (Figure 3A). These data, while indirect, indicate that transfectants over-expressing Lsc-FLAG display similar RGS activity compared with control transfectants whereas ectopic over-expression of the carboxy-terminal Lsc domain, LscCT, increases the inhibition of Gα-mediated signaling after stimulation and presumably by enhancing endogenous Lsc RGS activity.
Figure 3.
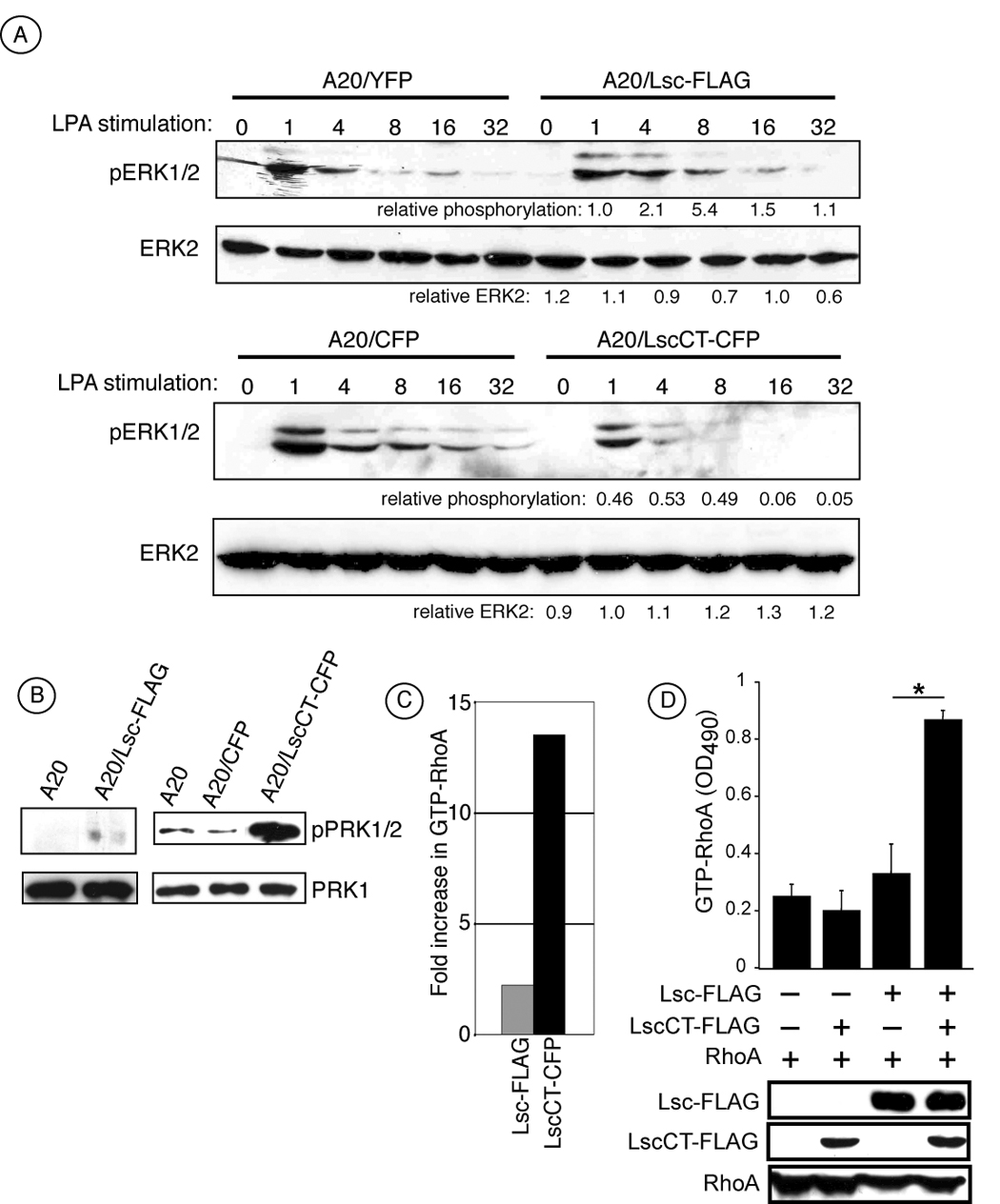
LscCT expression activates endogenous Lsc RGS and RhoGEF activity. A) A20 stable transfectants expresssing either YFP or CFP alone as controls, full-lenth Lsc (Lsc-FLAG) or LscCT (LscCT-CFP) were stimulated with 20 µM LPA for the indicated time in minutes and phosphorylated ERK1/2 and total ERK2 measured by Western blot analysis. Numbers below blots indicate relative expression of pERK1/2 or ERK2 in Lsc tranfectants compared to controls. B) Level of phosphorylated PRK1/2 (pPRK1/2) and total PRK1 in cell lysates from A20 parental cells and tranfectants expressing CFP (control transfectants), Lsc-FLAG or LscCT. C) Stable A20 cell transfectants expressing LscCT harbor significantly more active RhoA compared to control transfectants relative to the amount in Lsc-FLAG transfectants and as measured by a GTP-RhoA specific ELISA. Data in panels A, B, and C are representative of 4 experiments from at least two independent stable transfectants of each transgene. D) 293 cells transiently expressing either RhoA-YFP alone or RhoA-YFP together with either LscCT or full length Lsc or both were evaluated for level of RhoA, LscCT and Lsc by Western analysis (bottom) or level of GTP-RhoA by ELISA (top). GTP-RhoA measurement by ELISA in (D) includes data from 3 independent transient transfections. A20 parental cells and transfectants were serum-starved for 3–12 hours prior to cell harvest and evaluating RhoA-GTP, PRK1/2 phosphorylation, and total PRK1 levels.
Lsc RhoGEF activity was also measured in these same stable A20 Lsc transfectants by two assays. As an initial approach, we measured PRK phosphorylation in non-stimulated transfected cells as a surrogate marker for constitutive RhoA activity. PRK1 is an immediate effector of RhoA and is phosphorylated only when associated with active (GTP-bound) RhoA (Amano et al., 1996; Bishop and Hall, 2000; Flynn et al., 2000; Zhao and Manser, 2005). As shown in Figure 3B, LscCT transfectants displayed a considerable increase in the levels of phosphorylated PRK1/2 compared to either the A20 parental or control transfectants. In contrast, the Lsc-FLAG transfectants exhibited minimal PRK1/2 phosphorylation and that was only modestly increased over the parental A20 cell line. Thus, using this indirect measurement of RhoA activity, these data indicate that A20 cells over-expressing LscCT have increased RhoGEF activity relative the same cells expressing full-length Lsc.
To directly measure the level of active RhoA in cell lysates, we used an ELISA-based assay specific for GTP-bound RhoA. In these experiments (Figure 3C), Lsc-FLAG transfectants harbor an approximate 2-fold increase in RhoA activity over control cells whereas LscCT transfectants were increased greater than 13-fold over control transfectants (Figure 3B) and consistent with the relative amounts of phosphorylated PRK1/2 in these transfectants. These results are similar to those examining Lsc RGS activity in that the LscCT transfectants have constitutive elevated levels of activated RhoA, presumably due to increased RhoGEF activity, whereas Lsc-FLAG transfectants exhibit RhoGEF activity comparable to parental cells.
Together, these data indicate that Lsc functional activity correlates with oligomerization status. Specifically, Lsc-FLAG transfectants express about 8-fold more full-length Lsc relative to the endogenous Lsc expressed by control cells (Figure 3A), although exclusively as a homo-tetramer (Figure 2B). Despite this increased expression, the RGS and RhoGEF activity displayed by these Lsc-FLAG transfectants is only modestly elevated relative to control cells that only express endogenous Lsc (Figure 3). In contrast, LscCT transfectants demonstrate substantial RGS and RhoGEF activity although the LscCT transgene does not contain either the RGS or RhoGEF domain. Rather, these cells only express endogenous Lsc whose oligomeric nature differs significantly from control and A20 Lsc-FLAG transfectants in that the expression of LscCT diminishes the proportion of endogenous Lsc that is found in the large molecular weight ‘tetramer’ (Figure 2C). We interpret these results to indicate that disruption of the high molecular weight endogenous Lsc oligomeric complex leads to increased Lsc functional activity and is consistent with previous reports suggesting that oligomeric Lsc is inactive (Chikumi et al., 2004; Eisenhaure et al., 2003; Wells et al., 2001) and more recent reports that demonstrate disrupting oligomerization of a homologous protein, AKAP-Lbc, also leads to increased RhoGEF activity (Baisamy et al., 2005).
To gain further support for this hypothesis, we measured RhoA activity in 293 cells after transient expression of RhoA alone or together with either Lsc-FLAG, LscCT-FLAG, or both Lsc constructs (Figure 3D). These experiments demonstrate that the amount of GTP-bound RhoA expressed by cells transiently expressing RhoA alone is similar to that compared with cells expressing RhoA and LscCT (Figure 3D). Furthermore, this level of active RhoA is only slightly increased when RhoA is co-expressed together with full-length Lsc indicating that simply over-expressing the full length protein, that exists as homo-tetramer in these cells (data not shown), does not lead to substantial RhoGEF activity. In contrast, when full-length Lsc is expressed in the presence of LscCT, RhoA activity is significantly increased above that observed by similar levels of full-length Lsc alone. These data provide further strong support that Lsc activity is regulated by oligomerization and that disrupting homo-tetramers of Lsc leads to increased functional activity.
3.4. Activation of endogenous Lsc inhibits cell polarization and integrin-mediated adhesion
Because Lsc is a RhoA-specific GEF and RhoA has been implicated in integrinmediated adhesion (Giagulli et al., 2004; Laudanna et al., 1996; Vielkind et al., 2005; Wright et al., 2002) and resolution of adhesion (Alblas et al., 2001; Liu et al., 2002; Smith et al., 2003; Worthylake et al., 2001; Yoshinaga-Ohara et al., 2002), we next assessed how Lsc oligomerization and functional activity correlated with cell morphology and adhesion to integrin ligands. Figure 4A shows that parental and A20 transfectants exhibit a round non-stellate morphology when incubated on BSA-coated substrate. However, when the same cells were incubated on an integrin ligand- (ICAM-1 and VCAM-1) coated substrate, a considerable fraction of A20 control and Lsc-FLAG transfectants adopted a relatively polarized phenotype resembling primary T lymphocytes placed on ICAM-1 (Smith et al., 2003). LscCT transfectants, in contrast, clearly remained round and appeared morphologically similar to incubation on BSA-coated substrate (Figure 4A). The constitutive RhoGEF activity displayed by LscCT transfectants coupled with their inability to polarize on integrin ligands is consistent with previous findings that constitutive RhoA activation in a neutrophil cell line inhibits polarization (Xu et al., 2003).
Figure 4.

Elevated endogenous Lsc activity inhibits polarization and integrin-mediated adhesion. A) A20 transfectants were incubated on BSA- (top row) or ICAM-1 and VCAM-1-coated plates for 30 minutes at 37° C and photographed. Micrographs are at 400× magnification. B) A20/YFP (open), A20/CFP (light gray), Lsc-FLAG (dark gray) 34 and LscCT (black) transfectants were incubated for 30 minutes on BSA, ICAM-1, or VCAM-1 and percent cells adherent determined. % adhesion calculated from 6–9 independent experiments; A20/YFP (n=6), A20/CFP (n=9), Lsc-FLAG (n=6), LscΔC (n=6) and LscCT (n=9). Values are mean ± s.d. of 6 or 9 independent experiments. *, p < 0.00001 by Student’s unpaired t test.
The ability of these transfectants to adhere to the same integrin ligands was also measured in a static adhesion assay. On average, approximately 25% of control A20 cells and Lsc-FLAG transfectants reproducibly bound to ICAM-1 and VCAM-1 integrin ligands (Figure 4B). In contrast, A20 cells overexpressing LscCT displayed only minimal adhesion to these same integrin ligands that was significantly reduced compared to A20 controls and Lsc-FLAG transfectants. All transfectants express similar levels of CD11a, CD49d, and CD29 (unpublished data) indicating that the decrease in LscCT transfectant adhesion cannot be attributed to integrin expression levels.
In leukocytes, RhoA can promote either integrin-mediated adhesion (Giagulli et al., 2004; Laudanna et al., 1996; Vielkind et al., 2005) or resolution of integrin adhesive contacts (Alblas et al., 2001; Liu et al., 2002; Smith et al., 2003; Worthylake et al., 2001; Yoshinaga-Ohara et al., 2002). We have previously demonstrated that Lsc functions in marginal zone B cells to terminate integrin-mediated adhesion (Rubtsov et al., 2005). LscCT transfectants display constitutive Lsc RhoA GEF activity (Figure 3B) and diminished polarization and adhesion (Figure 4). Thus, we interpret these data to indicate that Lsc RhoGEF activation of RhoA is important in signaling the dissolution of integrin-mediated adhesive events. In this model, LscCT transfectants are unable to adhere to integrin ligands as they continuously signal integrin release due to constitutive Lsc-mediated RhoA activation.
3.5. Lsc is predominantly found at the trailing edge during migration
On integrin ligand-coated substrate, lymphocytes polarize and migrate in a random manner by a process that requires RhoA for cell detachment (Semmrich et al., 2005; Smith et al., 2003). Therefore, to further support a role for Lsc in regulating release of integrin adhesion, an anti-Lsc monoclonal antibody was used to examine Lsc localization during migration on integrin ligands. A20 parental cells were incubated for 30 minutes on ICAM-1 and VCAM-1 and subsequently monitored for an additional 15–30 minutes by time-lapse microscopy to determine direction of cell movement. Cells were then immediately fixed, permeabilized and stained for endogenous Lsc and filamentous actin (F-actin) to identify the leading edge. These data (Figure 5A) demonstrate that endogenous Lsc is found predominantly toward the rear of migrating cells and similar to that previously characterized for RhoA in migrating neutrophils (Xu et al., 2003).
Figure 5.

Lsc localizes toward the rear of migrating cells and is excluded from the leading edge. A20 cells were incubated on ICAM-1 and VCAM-1 coated substrate for 20 minutes to identify migrating cells and subsequently fixed and stained with phalloidin to identify F-actin (red) and FITC-anti-LscCT monoclonal antibody (green). F-actin staining marks leading edge lamellipodium. Differential interference contrast (DIC) images are provided to identify cell outline. Staining with a FITC-isotype control hamster antibody was negative (data not shown).
A previous investigation of Lsc localization in neutrophils (Francis et al., 2006) has suggested Lsc is mostly found at the leading edge within minutes after stimulation with N-formyl-methionyl-leucyl-phenylalanine (fMLP). Although we occasionally also find weak Lsc staining coincident with actin polymerization (Figure 5A, rows a and c), after 30–60 minutes of migration on ICAM-1/VCAM-1 integrin ligands, Lsc is mostly excluded from sites of actin polymerization in A20 B cells and localizes toward the cell rear. This spatially-restricted pattern of Lsc expression was typically not at the outer perimeter of the trailing edge but rather was at a region slightly more centrally located and resembling the recently described focal zone in migrating T lymphocytes, a region demonstrated to be enriched in integrin adhesive structures (Smith et al., 2005).
These data demonstrate that during cell migration endogenous Lsc appears largely excluded from regions of actin polymerization and is found predominantly at regions toward the rear of migrating cells thus further supporting a role for Lsc in regulating integrin release and through RhoA activation.
4. Discussion
The appropriate migration of motile leukocytes depends on differential signaling to the leading and trailing edge of the cell. Lsc is a hematopoietic-restricted intracellular protein that harbors both RGS and RhoGEF domains that function to attenuate Gα12/13 activity and stimulate RhoA activity, respectively. In this study we provide data that strongly suggest Lsc exists physiologically as a homo-tetramer in B lineage cells and that preventing the oligomerization of endogenous Lsc promotes both its RGS and RhoGEF activity. We further show that endogenous Lsc localizes toward the trailing edge of A20 cells migrating on integrin ligands and that activation of Lsc RhoGEF activity inhibits integrin-mediated cell spreading and adhesion. Thus, these data demonstrate that Lsc activity is regulated by oligomerization and is required for appropriate regulation of integrin-mediated adhesion.
The capacity of Lsc to associate intermolecularly through carboxyl terminal sequences has previously been shown (Chikumi et al., 2004; Eisenhaure et al., 2003). Our data confirm these findings and further demonstrate that ectopically- and endogenously-expressed Lsc exists in vivo as a large molecular weight homo-oligomers resembling tetramers. Evidence for this is provided by size exclusion chromatography of a B cell line expressing a full-length 125 kDa epitope-tagged Lsc transgene that elutes in a complex of 500 kDa, the predicted molecular weight for a homo-tetramer (Figure 2B). Further evidence suggesting that endogenous Lsc exists as a tetramer is found in the analysis of transfectants that express a carboxy-terminal Lsc protein (LscCT). In these cells LscCT associates with endogenous Lsc to form distinct complexes with molecular weights that closely correlate with predicted combinations of LscCT and endogenous Lsc ‘tetramers’ (Figure 2C). However, endogenous Lsc homo-oligomers display a range in molecular weights corresponding to larger than a tetramer but smaller than that of pentamer (Figure 1B). Although the basis for this difference is not yet clear, we attribute this discrepancy to both the heterogeneity of endogenous Lsc due to alternative splicing (Eisenhaure et al., 2003) and/or post-translation modifications (Holinstat et al., 2003).
Interfering with the assembly of high molecular weight Lsc oligomers in B lineage cells clearly leads to increased inducible RGS activity and constitutive RhoGEF activity. However, our efforts to isolate and measure the Lsc activity within artificial “trimers”, “dimers” and “monomers” from A20 LscCT transfectants have not been successful. In attempt to better establish the relationship between oligomerization status and Lsc activity, full-length Lsc was transiently expressed in 293 cells at a relatively constant level in the presence of increasing amounts of LscCT. In these experiments we found RhoA activity to increase with increasing amounts of LscCT (data not shown) suggesting a correlation between Lsc activity and monomer formation. Nevertheless, the oligomeric status of active Lsc remains to be definitively established.
Based on these data, a clear prediction would posit that stimuli that activate Lsc activity would do so by disrupting oligomerization. However, we have been unsuccessful in detecting endogenous Lsc dimers or monomers by size exclusion chromatography in A20 cells or primary B lymphocytes stimulated with LPA, PMA, the antigen receptor or after altering the cell phosphorylation status with pharmacological inhibitors of phosphatases or kinases (unpublished data). We consider that our inability to detect Lsc monomers (or dimers) may simply reflect the sensitivity of Western blot analysis after size exclusion chromatography as the fraction of active Lsc may be relatively minor compared with total Lsc levels. Regardless, it will be important in the future to determine the relationship between oligomerization, post-translational modification, and intracellular location.
Regulation of Lsc RhoGEF activity has previously been shown to occur by several mechanisms. For example, association of the p115RhoGEF RGS domain with Gα13 directly stimulates RhoGEF activity (Hart et al., 1998). Additionally, mutational studies, that include the initial characterization of both Lsc and p115RhoGEF (Hart et al., 1996; Whitehead et al., 1996), have demonstrated that sequences in the carboxy- (and amino-) terminus negatively regulate RhoGEF activity (Chikumi et al., 2004; Eisenhaure et al., 2003; Wells et al., 2001). These data have led to models in which the carboxy terminal domain negatively regulates Lsc RhoGEF activity by either steric hindrance of the RhoGEF domain through intramolecular associations (i.e., carboxy-terminus folds onto the RhoGEF domain) or through facilitating the association of a putative inhibitory molecule (Eisenhaure et al., 2003; Schmidt and Hall, 2002; Wells et al., 2001; Zheng, 2001). Our data demonstrate that interfering with oligomerization leads to constitutive Lsc RhoGEF activity and is most compatible with the exposure of the GEF catalytic domain upon disruption of oligomeric complexes. Thus, inactive Lsc appears to exist as a homo-tetramer and preventing the assembly of this homo-oligomer stimulates Lsc activity although the precise nature of the active Lsc complex remains to be established.
RhoA has been shown to facilitate both the establishment (Giagulli et al., 2004; Laudanna et al., 1996; Vielkind et al., 2005; Wright et al., 2002) and resolution (Alblas et al., 2001; Liu et al., 2002; Smith et al., 2003; Worthylake et al., 2001; Yoshinaga-Ohara et al., 2002) of integrin-mediated adhesive events. Whether RhoA promotes adhesion or resolution of adhesion likely depends on which of the numerous signaling pathways that lead to RhoA activation is active. Accordingly, a strength of these data is that we are able to determine how RhoA regulates integrin adhesion upon activation by endogenousLsc. We show here that constitutive activation of endogenous Lsc RhoGEF activity inhibits B cell polarization and adhesion to integrin ligands. In a subpopulation of Lsc-deficient B lymphocytes, cells inefficiently release from integrin ligands in vitro and in vivo and display elongated uropods when migrating on integrin ligands (Rubtsov et al., 2005) similar to that observed with leukocytes in which RhoA or ROCK are inhibited (Alblas et al., 2001; Liu et al., 2002; Smith et al., 2003; Worthylake et al., 2001; Yoshinaga-Ohara et al., 2002) or T lymphocytes expressing a constitutively active LFA-1 integrin (Semmrich et al., 2005). Using time-lapse microscopy to identify B lineage cells migrating on integrin ligands, we find that Lsc localizes toward the cell rear and similar to RhoA upon stimulation of a neutrophil-like cell line (Xu et al., 2003). Moreover, this localization is reminiscent to a recently described focal zone in lymphocytes enriched in an area of integrin-integrin ligand adhesive events (Smith et al., 2005). Together these data lead to a model in which Lsc is required for RhoA activation at the trailing edge of migrating B lineage cells and active RhoA subsequently leads to resolution of integrin-mediated adhesion. Given that we document Lsc is a homo-oligomer in A20 cells, it is not evident how intracellular trafficking of Lsc is facilitated in cells migrating on integrin ligands. Of potential interest, a yeast two-hybrid screen performed by the Alliance for Cellular Signaling using Lsc as a bait has suggested it to associate with both Ranbp5 and Macf1 (www.signaling-gateway.org/data/Y2H/cgibin/y2h_int.cgi?id=14722), two intracellular proteins that participate in intracellular protein transport and actin binding.
Recently, it has been shown that Lsc localizes predominantly at sites of actin polymerization and only weakly at the cell trailing edge in fMLP-stimulated primary neutrophils and the HL-60 cell line (Francis et al., 2006), whereas we find the majority of Lsc at the cell rear and only low levels at the leading edge (Figure 5). Although the basis for the disparity in Lsc localization is not clear we note that, in contrast to B lineage cells, neutrophils appear to express at least five distinct Lsc isoforms (Francis et al., 2006) and is relevant given that our monoclonal antibody recognizes an epitope in the carboxy-terminal region that has been identified to undergo alternative splicing (Eisenhaure et al., 2003). Regardless, these data demonstrate that, similar to the Rho family of GTPases, RhoGEFs also compartmentalize to distinct cellular localizations during cell migration.
5. Conclusions
In summary, these data demonstrate that the endogenous Lsc expressed by primary B lymphocytes and a B lymphoma cell line exists as a homo-oligomer and that the functional activity of this endogenous protein is regulated by oligomerization. Furthermore, our findings demonstrate that Lsc activity regulates integrin adhesion and during cell migration this protein localizes to the trailing edge where we suggest it activates RhoA and regulates integrin mediated adhesion. As such, these data extend the concept of divergent signaling of spatially activated Rho GTPases to B lymphocytes and identifies Lsc as a RhoA-specific GEF that during cell migration is restricted in its intracellular location similarly to the RhoA GTPase on which it acts.
Acknowledgements
We acknowledge Hong-Bing Shu, Gongyi Zhang, Philippa Marrack, John Cambier, Xia Hong, Qilong Mao, Kejun Han, Liangguo Xu, and the NJC Flow Cytometry Lab for their generous advice and support. This work was supported by the Sandler Program in Asthma Research, Cancer League of Colorado, Inc., (R.T.), NIH (AI052310 to R.P. and AI052157 to R.T.) and the Cancer Research Institute Predoctoral Emphasis Pathway in Tumor Immunology (J.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aasheim HC, Pedeutour F, Smeland EB. Characterization, expression and chromosomal localization of a human gene homologous to the mouse Lsc oncogene, with strongest expression in hematopoetic tissues. Oncogene. 1997;14:1747–1752. doi: 10.1038/sj.onc.1200994. [DOI] [PubMed] [Google Scholar]
- Alblas J, Ulfman L, Hordijk P, Koenderman L. Activation of Rhoa and ROCK are essential for detachment of migrating leukocytes. Mol Biol Cell. 2001;12:2137–2145. doi: 10.1091/mbc.12.7.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K. Identification of a putative target for Rho as the serinethreonine kinase protein kinase N. Science. 1996;271:648–650. doi: 10.1126/science.271.5249.648. [DOI] [PubMed] [Google Scholar]
- Baisamy L, Jurisch N, Diviani D. Leucine zipper-mediated homo-oligomerization regulates the Rho-GEF activity of AKAP-Lbc. J Biol Chem. 2005;280:15405–15412. doi: 10.1074/jbc.M414440200. [DOI] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Taube C, Miyahara N, Koya T, Pelanda R, Gelfand EW, Torres RM. Arhgef1 is Required by T Cells for the Development of Airway Hyperreactivity and Inflammation. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200702-270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Chen Z, Singer WD, Wells CD, Sprang SR, Sternweis PC. Mapping the Galpha13 binding interface of the rgRGS domain of p115RhoGEF. J Biol Chem. 2003;278:9912–9919. doi: 10.1074/jbc.M212695200. [DOI] [PubMed] [Google Scholar]
- Chene P. The role of tetramerization in p53 function. Oncogene. 2001;20:2611–2617. doi: 10.1038/sj.onc.1204373. [DOI] [PubMed] [Google Scholar]
- Chikumi H, Barac A, Behbahani B, Gao Y, Teramoto H, Zheng Y, Gutkind JS. Homo- and hetero-oligomerization of PDZ-RhoGEF, LARG and p115RhoGEF by their C-terminal region regulates their in vivo Rho GEF activity and transforming potential. Oncogene. 2004;23:233–240. doi: 10.1038/sj.onc.1207012. [DOI] [PubMed] [Google Scholar]
- Cox EA, Huttenlocher A. Regulation of integrin-mediated adhesion during cell migration. Microsc Res Tech. 1998;43:412–419. doi: 10.1002/(SICI)1097-0029(19981201)43:5<412::AID-JEMT7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Eisenhaure TM, Francis SA, Willison LD, Coughlin SR, Lerner DJ. The Rho guanine nucleotide exchange factor Lsc homo-oligomerizes and is negatively regulated through domains in its carboxyl terminus that are absent in novel splenic isoforms. J Biol Chem. 2003;278:30975–30984. doi: 10.1074/jbc.M303277200. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Flynn P, Mellor H, Casamassima A, Parker PJ. Rho GTPase control of protein kinase C-related protein kinase activation by 3-phosphoinositide-dependent protein kinase. J Biol Chem. 2000;275:11064–11070. doi: 10.1074/jbc.275.15.11064. [DOI] [PubMed] [Google Scholar]
- Francis SA, Shen X, Young JB, Kaul P, Lerner DJ. Rho GEF Lsc is required for normal polarization, migration, and adhesion of formyl-peptide-stimulated neutrophils. Blood. 2006;107:1627–1635. doi: 10.1182/blood-2005-03-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- Giagulli C, Scarpini E, Ottoboni L, Narumiya S, Butcher EC, Constantin G, Laudanna C. RhoA and zeta PKC control distinct modalities of LFA-1 activation by chemokines: critical role of LFA-1 affinity triggering in lymphocyte in vivo homing. Immunity. 2004;20:25–35. doi: 10.1016/s1074-7613(03)00350-9. [DOI] [PubMed] [Google Scholar]
- Girkontaite I, Missy K, Sakk V, Harenberg A, Tedford K, Potzel T, Pfeffer K, Fischer KD. Lsc is required for marginal zone B cells, regulation of lymphocyte motility and immune responses. Nat Immunol. 2001;2:855–862. doi: 10.1038/ni0901-855. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Sharma S, elMasry N, Qiu RG, McCabe P, Polakis P, Bollag G. Identification of a novel guanine nucleotide exchange factor for the Rho GTPase. J Biol Chem. 1996;271:25452–25458. doi: 10.1074/jbc.271.41.25452. [DOI] [PubMed] [Google Scholar]
- Holinstat M, Mehta D, Kozasa T, Minshall RD, Malik AB. Protein kinase Calpha-induced p115RhoGEF phosphorylation signals endothelial cytoskeletal rearrangement. J Biol Chem. 2003;278:28793–28798. doi: 10.1074/jbc.M303900200. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Palecek SP, Lu Q, Zhang W, Mellgren RL, Lauffenburger DA, Ginsberg MH, Horwitz AF. Regulation of cell migration by the calcium-dependent protease calpain. J Biol Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- Johnson EN, Seasholtz TM, Waheed AA, Kreutz B, Suzuki N, Kozasa T, Jones TL, Brown JH, Druey KM. RGS16 inhibits signalling through the G alpha 13-Rho axis. Nat Cell Biol. 2003;5:1095–1103. doi: 10.1038/ncb1065. [DOI] [PubMed] [Google Scholar]
- Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- Lai YJ, Chen CS, Lin WC, Lin FT. c-Src-mediated phosphorylation of TRIP6 regulates its function in lysophosphatidic acid-induced cell migration. Mol Cell Biol. 2005;25:5859–5868. doi: 10.1128/MCB.25.14.5859-5868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudanna C, Campbell JJ, Butcher EC. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981–983. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Maxfield FR. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- Liu L, Schwartz BR, Lin N, Winn RK, Harlan JM. Requirement for RhoA kinase activation in leukocyte de-adhesion. J Immunol. 2002;169:2330–2336. doi: 10.4049/jimmunol.169.5.2330. [DOI] [PubMed] [Google Scholar]
- Majumdar M, Seasholtz TM, Buckmaster C, Toksoz D, Brown JH. A rho exchange factor mediates thrombin and Galpha(12)-induced cytoskeletal responses. J Biol Chem. 1999;274:26815–26821. doi: 10.1074/jbc.274.38.26815. [DOI] [PubMed] [Google Scholar]
- Mao J, Yuan H, Xie W, Wu D. Guanine nucleotide exchange factor GEF115 specifically mediates activation of Rho and serum response factor by the G protein alpha subunit Galpha13. Proc Natl Acad Sci U S A. 1998;95:12973–12976. doi: 10.1073/pnas.95.22.12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marianayagam NJ, Sunde M, Matthews JM. The power of two: protein dimerization in biology. Trends Biochem Sci. 2004;29:618–625. doi: 10.1016/j.tibs.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek SP, Schmidt CE, Lauffenburger DA, Horwitz AF. Integrin dynamics on the tail region of migrating fibroblasts. J Cell Sci. 1996;109(Pt 5):941–952. doi: 10.1242/jcs.109.5.941. [DOI] [PubMed] [Google Scholar]
- Parent CA. Making all the right moves: chemotaxis in neutrophils and Dictyostelium. Curr Opin Cell Biol. 2004;16:4–13. doi: 10.1016/j.ceb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Regen CM, Horwitz AF. Dynamics of beta 1 integrin-mediated adhesive contacts in motile fibroblasts. J Cell Biol. 1992;119:1347–1359. doi: 10.1083/jcb.119.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- Rubtsov A, Strauch P, Digiacomo A, Hu J, Pelanda R, Torres RM. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity. 2005;23:527–538. doi: 10.1016/j.immuni.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Sato M, Shegogue D, Hatamochi A, Yamazaki S, Trojanowska M. Lysophosphatidic acid inhibits TGF-beta-mediated stimulation of type I collagen mRNA stability via an ERK-dependent pathway in dermal fibroblasts. Matrix Biol. 2004;23:353–361. doi: 10.1016/j.matbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Semmrich M, Smith A, Feterowski C, Beer S, Engelhardt B, Busch DH, Bartsch B, Laschinger M, Hogg N, Pfeffer K, Holzmann B. Importance of integrin LFA-1 deactivation for the generation of immune responses. J Exp Med. 2005;201:1987–1998. doi: 10.1084/jem.20041850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Bracke M, Leitinger B, Porter JC, Hogg N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J Cell Sci. 2003;116:3123–3133. doi: 10.1242/jcs.00606. [DOI] [PubMed] [Google Scholar]
- Smith A, Carrasco YR, Stanley P, Kieffer N, Batista FD, Hogg N. A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J Cell Biol. 2005;170:141–151. doi: 10.1083/jcb.200412032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snabaitis AK, Muntendorf A, Wieland T, Avkiran M. Regulation of the extracellular signal-regulated kinase pathway in adult myocardium: differential roles of G(q/11), Gi and G(12/13) proteins in signalling by alpha1-adrenergic, endothelin-1 and thrombin-sensitive protease-activated receptors. Cell Signal. 2005;17:655–664. doi: 10.1016/j.cellsig.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert PJ, Devreotes PN. Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol. 2004;5:624–634. doi: 10.1038/nrm1435. [DOI] [PubMed] [Google Scholar]
- Vielkind S, Gallagher-Gambarelli M, Gomez M, Hinton HJ, Cantrell DA. Integrin regulation by RhoA in thymocytes. J Immunol. 2005;175:350–357. doi: 10.4049/jimmunol.175.1.350. [DOI] [PubMed] [Google Scholar]
- Wells CD, Gutowski S, Bollag G, Sternweis PC. Identification of potential mechanisms for regulation of p115 RhoGEF through analysis of endogenous and mutant forms of the exchange factor. J Biol Chem. 2001;276:28897–28905. doi: 10.1074/jbc.M102913200. [DOI] [PubMed] [Google Scholar]
- Wells CD, Liu MY, Jackson M, Gutowski S, Sternweis PM, Rothstein JD, Kozasa T, Sternweis PC. Mechanisms for reversible regulation between G13 and Rho exchange factors. J Biol Chem. 2002;277:1174–1181. doi: 10.1074/jbc.M105274200. [DOI] [PubMed] [Google Scholar]
- Whitehead IP, Khosravi-Far R, Kirk H, Trigo-Gonzalez G, Der CJ, Kay R. Expression cloning of lsc, a novel oncogene with structural similarities to the Dbl family of guanine nucleotide exchange factors. J Biol Chem. 1996;271:18643–18650. doi: 10.1074/jbc.271.31.18643. [DOI] [PubMed] [Google Scholar]
- Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147–160. doi: 10.1083/jcb.200103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N, Hidalgo A, Rodriguez-Frade JM, Soriano SF, Mellado M, Parmo-Cabanas M, Briskin MJ, Teixido J. The chemokine stromal cell-derived factor-1 alpha modulates alpha 4 beta 7 integrin-mediated lymphocyte adhesion to mucosal addressin cell adhesion molecule-1 and fibronectin. J Immunol. 2002;168:5268–5277. doi: 10.4049/jimmunol.168.10.5268. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–204. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- Yang F, Moss LG, Phillips GN., Jr The molecular structure of green fluorescent protein. Nat Biotechnol. 1996;14:1246–1251. doi: 10.1038/nbt1096-1246. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Ohara N, Takahashi A, Uchiyama T, Sasada M. Spatiotemporal regulation of moesin phosphorylation and rear release by Rho and serine/threonine phosphatase during neutrophil migration. Exp Cell Res. 2002;278:112–122. doi: 10.1006/excr.2002.5571. [DOI] [PubMed] [Google Scholar]
- Yu N, Lariosa-Willingham KD, Lin FF, Webb M, Rao TS. Characterization of lysophosphatidic acid and sphingosine-1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia. 2004;45:17–27. doi: 10.1002/glia.10297. [DOI] [PubMed] [Google Scholar]
- Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- Zhao ZS, Manser E. PAK and other Rho-associated kinases--effectors with surprisingly diverse mechanisms of regulation. Biochem J. 2005;386:201–214. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci. 2001;26:724–732. doi: 10.1016/s0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]
- Zhu K, Debreceni B, Bi F, Zheng Y. Oligomerization of DH domain is essential for Dbl-induced transformation. Mol Cell Biol. 2001;21:425–437. doi: 10.1128/MCB.21.2.425-437.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


