Abstract
The adaptive immune response is initiated by the presentation of peptides bound to major histocompatibility complex molecules on dendritic cells (DCs) to antigen-specific T lymphocytes at a junction termed the immunological synapse. Although much attention has been paid to cytoplasmic events on the T cell side of the synapse, little is known concerning events on the DC side. We have sought signal transduction components of the neuronal synapse that were also expressed by DCs. One such protein is spinophilin, a scaffolding protein of neuronal dendritic spines that regulates synaptic transmission. In inactive, immature DCs, spinophilin is located throughout the cytoplasm but redistributes to the plasma membrane upon stimulus-induced maturation. In DCs interacting with T cells, spinophilin is polarized dynamically to contact sites in an antigen-dependent manner. It is also required for optimal T cell activation because DCs derived from mice lacking spinophilin exhibit defects in antigen presentation both in vitro and in vivo. Thus, spinophilin may play analogous roles in information transfer at both neuronal and immunological synapses.
Introduction
The adaptive immune response depends on the ability of antigen-presenting cells (APCs) to communicate with effector cells such as T lymphocytes about the molecular nature of invading pathogens. The most potent of APCs are dendritic cells (DCs), which are present in tissues throughout the body, where they sample antigens and process them into short peptides bound to major histocompatibility complex (MHC) class I or II molecules (Mellman, 2007). After migration to the lymph nodes, DCs activate naive T cells, thereby initiating antigen-specific responses. Elucidating the mechanisms by which DCs achieve their unique capacity for antigen presentation is of considerable interest. Although multiple specializations contributing to antigen uptake and processing have been described (Mellman, 2007), mechanisms controlling the final interaction of DCs with their target cells have been incompletely studied.
The “immunological synapse” (IS) refers to the contact site between APCs and T cells where T cell receptors (TCRs) engage their cognate peptide–MHC complexes (Norcross, 1984; Monks et al., 1998; Grakoui et al., 1999). The term “synapse” is appealing because both immunological and neuronal synapses form with great molecular specificity and for the purpose of information transfer. Much is known about the signal transduction mechanisms that contribute to the formation, maintenance, and plasticity of mature neuronal synapses (Calabrese et al., 2006). These include not only adhesion molecules and receptors but also cytoplasmic scaffolding proteins that assemble other signaling molecules and cytoskeletal components on both sides of the synapse. In contrast, much less is known about analogous factors controlling the IS, with most available information being restricted to events occurring in the T cell (Wang et al., 2004a; Lin et al., 2005; Dustin et al., 2006). After adhesion and recognition of peptide–MHC complexes, for example, TCRs and associated signaling molecules segregate to the center of the contact site, whereas adhesion proteins (e.g., LFA-1) form a peripheral ring (Lin et al., 2005). Scaffolding proteins in T cells such as Discs large-1 also accumulate at the IS and, together with cytoskeleton-regulating proteins such as ezrin and moesin, they may play a role in concentrating kinases required for TCR signaling (Wang et al., 2004a; Xavier et al., 2004; Ludford-Menting et al., 2005; Ilani et al., 2007).
Signaling events on the APC side of the synapse are even less well characterized, with many studies having used planar lipid bilayers as surrogate APCs (Mossman et al., 2005; Sims et al., 2007). To address this problem, we sought scaffolding proteins of the neuronal synapse that were also expressed by DCs. One such component is the PDZ domain protein spinophilin, whose expression is highly enriched in the brain (Allen et al., 1997; Nakanishi et al., 1997). Several characteristics of spinophilin made it an appealing candidate to function in DCs at the IS. In neurons, spinophilin is localized to dendritic spines, where it binds to and organizes the actin cytoskeleton, directs protein phosphatase I (PP1) toward specific targets, interacts with G protein–coupled receptors (GPCRs), and regulates the interactions of proteins (e.g., arrestin) involved in endocytosis or membrane traffic (Allen et al., 1997; Grossman et al., 2002; Brady et al., 2003; Hsieh-Wilson et al., 2003; Ouimet et al., 2004; Wang et al., 2004b; Ryan et al., 2005; Wang et al., 2005, 2007). Spinophilin knockout (KO) mice exhibit defective neuronal dendritic spine development and partial defects in glutamatergic and dopaminergic transmission (Feng et al., 2000; Allen et al., 2006). Here, we show that spinophilin also plays a role in DCs at the IS, indicating an active role for the DC in the signaling of the IS and supporting the hypothesis that signal transduction mechanisms at neuronal synapses may be informative in understanding signaling events at the IS.
Results and discussion
Spinophilin is expressed in the immune system
We found that spinophilin mRNA is expressed by a variety of immune cells in mice, including DCs, macrophages, B cells, and T cells, by RT-PCR (unpublished data). At the protein level, we could detect the same 135-kD band in lysates of brain-, spleen-, and bone marrow–derived DCs by Western blotting (Fig. 1 a). Although most abundant (relative to actin) in brain, significant amounts of spinophilin were detected in DCs (∼10–20% that of brain, normalized to actin). Purified B and T cell populations also expressed spinophilin, as equivalent 135-kD proteins were detected in Western blots of lysates (unpublished data). As expected, the neuron-specific isoform of spinophilin, neurabin I (Allen et al., 1997; Nakanishi et al., 1997), was found only in brain lysates (unpublished data).
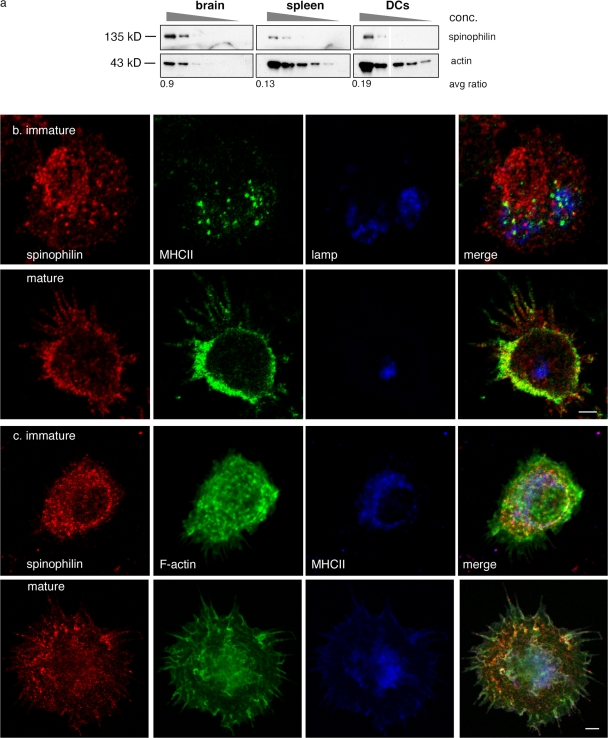
Figure 1.
Spinophilin is expressed in the immune cells and its localization in DCs is dynamic. (a) Detection of spinophilin by Western blot in serial dilutions of cell lysates from brain, spleen, or DCs. Numbers below indicate the ratio of adjusted mean density of spinophilin to actin on the Western blot. (b) Localization of spinophilin was detected by immunofluorescence in immature (top) and mature BMDCs (bottom) as indicated. DCs were stained with anti-spinophilin (RU466; Allen et al., 1997) and anti-MHCII (14.4.4 anti–IE-k FITC) antibodies and, to detect lysosomes, anti-Lamp (Lamp2) antibodies. (c) Localization of spinophilin, actin, and MHCII was detected by immunofluorescence in immature (top) and mature BMDCs (bottom) as indicated. DCs were stained with anti-spinophilin (RU466; Allen et al., 1997), TRITC-phalloidin, and anti-MHCII (14.4.4 anti–IE-k FITC) antibodies. Images in section c were pseudo-colored for illustrative purposes. Bars, 5 μM.
To localize spinophilin in DCs, immunofluorescence microscopy was performed on DCs using anti-spinophilin antibodies. In immature DCs, spinophilin exhibited a punctate pattern throughout the cell that was largely distinct from MHC class II (MHCII) and from Lamp-2+ late endosomes and lysosomes (Fig. 1 b, top). To quantify these observations, we analyzed regions of interest high in spinophilin fluorescence and compared the labeling intensity within it for MHCII and with a known binding partner of spinophilin, actin (Fig. 1 c, top). Although spinophilin intensity correlated with phalloidin labeling of F-actin (R2 = 0.54, P < 0.001), we did not observe a significant correlation with the fluorescence intensity of MHCII (R2 = 0.24, P < 0.015; n = 24 observations from 12 micrographs).
Upon maturation, spinophilin redistributed near the plasma membrane and into the dendrites, a maturation-induced specialization thought to enhance a DC's capacity to present an antigen (Fig. 1, b and c, bottom). This redistribution close to the plasma membrane was reflected in a positive correlation of labeling with both actin (R2 = 0.30, P < 0.001) and MHCII (R2 = 0.36, P < 0.001; 50 observations from 24 micrographs). The trafficking of MHCII does not appear to reflect a dependence on spinophilin, as surface levels of MHCII were normal in DCs cultured from spinophilin KO animals (see Fig. 4 a). The punctate appearance of spinophilin suggested that it was present on either membrane-bound structures or in large cytosolic complexes. Similar patterns were also observed in other immune cell types including B cells, T cells, and macrophages (unpublished data).
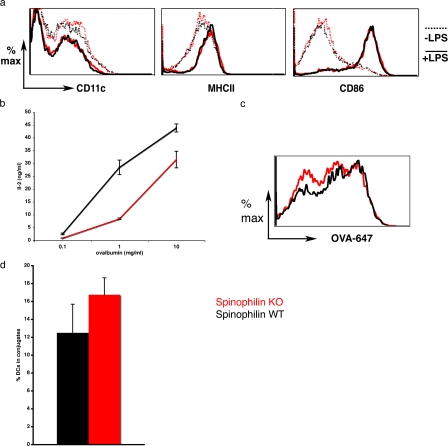
Figure 4.
Spinophilin plays a functional role in antigen presentation in vitro. (a) DCs isolated from spinophilin KO (dotted lines) and WT mice (solid lines) were stimulated in vitro and the expression of cell surface markers was analyzed by flow cytometry. WT (black) and KO (red) solid lines indicate unstimulated cells, whereas dotted lines are cells stimulated (matured) overnight at day five in culture by 30 ng/ml LPS. Expression of cell surface markers was comparable between the two cell types. Data are representative of three independent experiments. (b, left) DCs were isolated from spinophilin WT (black) or KO (red) mice and cocultured with OT-II CD4+ T cells in the presence of an antigen (ovalbumin). After 18 h, supernatants were isolated and assayed for IL-2 by ELISA. Values shown are the mean of triplicate measurements ± SD. (c) DCs were isolated from spinophilin WT (black) and KO (red) mice and cultured for 5 d. Ovalbumin-647 was incubated with DCs for 10 min at 37°C and chased for 1 h. A representative of three experiments is shown (n = 8 animals total from three independent experiments; cells are gated on CD11c+). Uptake of FITC-RNAase and FITC-dextran yielded similar results (not depicted). (d) DCs from −/− or +/+ mice were cultured for 5–6 d, matured with 30 ng/ml LPS, and pulsed with an antigen (10 μg/ml ovalbumin aa 323–339 peptide). DCs were then cultured with OT-II CD4+ T cells for 20–60 min, fixed, and labeled for immunofluorescence microscopy. The number of fixed conjugates was counted for both KO and WT cells and found to be comparable (P = 0.15; n = 4 animals per group; n = 562 and 625 cells, respectively). Error bars indicate the SEM.
Spinophilin in DCs redistributes with T cell contact
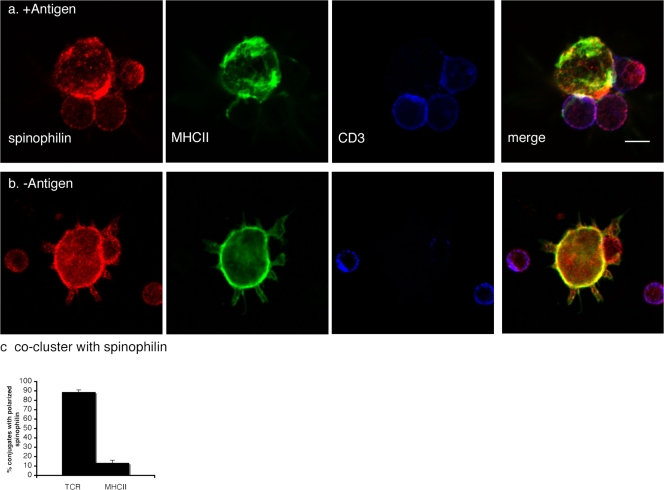
Because spinophilin is enriched at neuronal synapses, we wondered if it was similarly localized to the IS and, if so, whether its localization correlated with T cell activation. We cocultured mature DCs (B10 or C57BL/6) with antigen-specific T cells (AND or OT-II, respectively) in the presence or absence of an agonist peptide (moth cytochrome c aa 88–103 or ovalbumin aa 323–339). After 20 min, cells were fixed and stained for spinophilin, MHCII, and the TCR (CD3). A portion of conjugates were found to have spinophilin polarized toward the T cell. Strikingly, the polarization of spinophilin within a DC toward a T cell coincided almost exclusively with clustered TCR on the T cell (Fig. 2, a and c). In contrast, MHCII was rarely polarized toward a T cell in a pattern overlapping with spinophilin (n = 48 conjugates total; Fig. 2, a and c), indicating that the recruitment of spinophilin toward contact sites did not simply reflect an accumulation or ruffling of bulk DC membrane. As shown previously, the presence of an antigen increased TCR enrichment at contacts nearly twofold (unpublished data; Revy et al., 2001). In conjugates without TCR clustering (and without antigen), spinophilin was found in the cytosol and in close apposition to the plasma membrane, as observed in mature DCs cultured without T cells (Fig. 2 b).
Figure 2.
Spinophilin is recruited to the IS. (a and b) DCs (day six) were cocultured in the presence (+Antigen) or absence (−Antigen) of agonist peptide (MCC; a and b, respectively) and splenic CD4+ T cells. Confocal sections along the z axis of conjugated cells are shown in a and b. (a) In the presence of an antigen, topographical distribution of spinophilin (red), MHCII (green), and CD3, a component of the TCR (Cy5), reveals the polarization of spinophilin on the DC toward activated T cells. (b) In the absence of antigen, spinophilin remains localized close to the plasma membrane, as seen in mature DCs cultured in the absence of T cells (Fig. 1). Bar, 5 μm. (c) Correlation of spinophilin polarization in DCs with TCR clustering within T cells. Within DCs, the polarization of spinophilin toward contacting T cells correlated with TCR clustering to the contact site but not with MHCII. Error bars indicate the SEM.
Spinophilin in DC localizes to T cell contact persistently
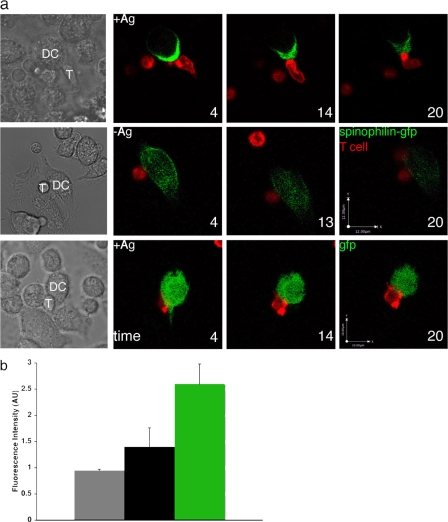
Within T cells, many proteins have been found to localize transiently to the contact site with APCs (Dustin et al., 2006). Far less is known, however, about the dynamics of protein trafficking to the IS from the APC side. To understand the relationship between contact duration and spinophilin polarization, we next used video confocal microscopy to examine the distribution of spinophilin fused to GFP or GFP alone in living DCs allowed to interact with antigen-specific T cells. Immature DCs were transduced with a recombinant retrovirus expressing spinophilin-GFP or GFP alone and maintained in culture. Mature DCs were then cultured in the presence (n = 6 conjugates) or absence (n = 3 conjugates) of an agonist peptide (moth cytochrome c, aa 88–103), adhered to coverslips, and cocultured with antigen-specific CD4+ T cells. In fixed cells, spinophilin-GFP was distributed similarly to the endogenous protein (detected by polyclonal antisera) and could be found polarized toward the T cell contact sites in the presence of an agonist peptide (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200711149/DC1). In live cells, this localization in conjugates with polarized spinophilin was even more dramatic, with spinophilin-GFP rapidly (<5 min) polarizing toward the T cell contact for the duration of the contact (Fig. 3 a, top; Fig. 3 b; and Videos 1 and 2). The polarization was barely evident in those few conjugates that formed in the absence of the antigen (Fig. 3 a, middle; Fig. 3 b; and Video 3). Free GFP, a cytosolic marker reporting overall cell shape, did not exhibit any detectable polarization in the presence of the antigen (n = 3 conjugates; Fig. 3 a, bottom; and Fig. 3 b). The distribution of GFP with the antigen or spinophilin-GFP with and without the antigen was quantified in a region of interest (ROI) both toward and away from the contact site at 0, 4, 14, and 20 min after imaging began. The ratio of fluorescence intensity in an ROI toward/away from the contact site was determined and averaged over time. As shown in Fig. 3 b, spinophilin-GFP with the antigen was 2.6-fold enriched at the contact site and 1.4-fold enriched without the antigen. There was no enrichment at the contact site of GFP alone with the antigen.
Figure 3.
Real-time imaging of spinophilin polarizing at the IS. (a) DCs expressing spinophilin-GFP that were cultured in the presence (+Ag; top, n = 6 conjugates) or absence (−Ag; middle, n = 3 conjugates) of agonist peptide together with antigen-specific CD4+ T cells that had been labeled red by the lipophilic PKH-26 dye (Sigma-Aldrich). In the presence of the antigen, spinophilin was polarized minutes after contact and remained polarized throughout the duration of the contact (30–60 min; a, top; and b). In the absence of antigen, spinophilin was distributed throughout the cytoplasm (a, middle; and b). As a control, the distribution of GFP alone in DCs contacting T cells (n = 3 conjugates) was imaged in the presence of the antigen and localized evenly throughout the cytoplasm (a, bottom). Time is indicated in minutes after imaging began and is rounded to the nearest minute. The bar in section a (middle) applies to the spinophilin-GFP +/− antigen. T, T cell. (b) The distribution of fluorescence signal for GFP alone with an antigen (gray) and spinophilin-GFP both with (green) and without an antigen (black) in live cells was analyzed at 0, 4, 14, and 20 min after imaging began (n = 9, 12, and 22 observations from 3, 3, and 6 conjugates, respectively, for GFP alone, spinophilin-GFP –Ag, and spinophilin-GFP +Ag). Spinophilin-GFP was most polarized toward the T cell in the presence of the antigen (P < 0.05 with respect to spinophilin-gfp –Ag; P < 0.002 with respect to GFP alone +Ag by Student's t test).
Spinophilin is necessary for efficient antigen presentation
Because spinophilin was expressed at high levels in DCs and recruited to the IS upon antigen-specific encounters with T cells, we next asked if it plays a functional role in antigen presentation and subsequent T cell activation. To do so, we prepared DCs from spinophilin wild-type (WT) or KO mice. Spinophilin KO DCs expressed normal amounts of surface MHCII, CD86, and the DC marker CD11c both before and after lipopolysaccharide (LPS)-induced maturation (Fig. 4 a). DCs derived from WT and KO mice were loaded with antigen (ovalbumin protein, ovalbumin peptide aa 323–339, SIINFEKL, or I-Eα 52–68) and then cocultured with CD4+ T cells transgenic for the appropriate TCR (OT-I, OT-II, or DO.11 for ovalbumin or 1H3.1 for I-Eα, respectively). As summarized in Table I and as shown by a representative experiment for ovalbumin presentation to OT-II CD4+ T cells (Fig. 4 b), spinophilin KO DCs were defective in activating naive T cells 24 h after coculture as measured by interleukin-2 (IL-2) secretion.
Table I. Summary of in vitro antigen presentation experiments.
| Peptide dose | Ratio KO/WT | SEM | n |
|---|---|---|---|
| μg/ml | |||
| 1 | 0.82 | 0.24 | 5 |
| 0.3 | 0.41 | 0.21 | 3 |
| 0.1 | 0.51 | 0.26 | 3 |
| 0.03 | 0.22 | 0.22 | 2 |
| 0.01 | 0 | 0 | 3 |
DCs derived from spinophilin WT and KO mice were loaded with peptide antigen (ovalbumin peptide aa 323–339 or I-Eα 52–68) and then cocultured with CD4+ T cells transgenic for the appropriate TCR (OT-II or DO.11 for ovalbumin or 1H3.1 for I-Eα, respectively). After 18 h, supernatants were isolated and assayed for IL-2 by ELISA. Values shown are the ratio of the response from T cells cocultured with KO/WT DCs. One animal in each group was used for each experiment. The number of experiments (n) is indicated.
These data indicate that spinophilin enhances the ability of DCs to present peptide–MHCII complexes to T cells. Two possible explanations for these data could be a lower capacity of spinophilin KO DCs for uptake of the antigen itself or their establishment of fewer contacts with T cells. We tested both of these possibilities. Endocytosis by CD11c+ cells was comparable between the spinophilin WT or KO DCs as measured by uptake of ovalbumin-647 or FITC-dextran (Fig. 4 c and not depicted). There was no measurable difference in the ability of DCs from WT or KO littermates to form conjugates with antigen-specific T cells (Fig. 4 d), although the duration of their contact was not assessed.
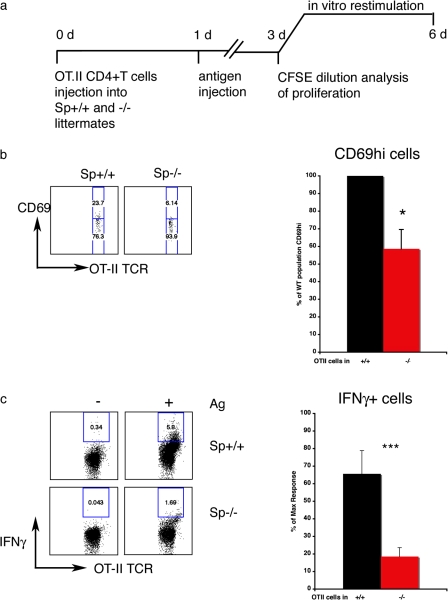
Given the diminished capacity of spinophilin KO DCs to present antigen in vitro, we next assayed the efficiency of antigen presentation in vivo by using naive antigen-specific T cells adoptively transferred into spinophilin KO and WT mice (Fig. 5 a). Mice were injected i.v. with 106 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE)-labeled CD4+ T cells isolated from mice expressing an ovalbumin-specific TCR (OT-II). 24 h later, mice were injected i.v. with ovalbumin and LPS. After 2 d, spleens were isolated and the proliferation of adoptively transferred CD4+ T cells was measured by CFSE dilution. No significant defect in proliferation was observed (unpublished data). However, in the presence of an antigen, fewer CD4+ T cells adoptively transferred to spinophilin KO mice had elevated levels of CD69 than those transferred to WT littermates (Fig. 5 b), which suggests that spinophilin KO DCs are defective in activating T cells in vivo. As expected, there was no T cell activation in the absence of an antigen in either group of mice (unpublished data).
Figure 5.
Spinophilin plays a functional role in antigen presentation in vivo. (a) Protocol: CD4+ T cells were isolated form TCR-transgenic mice (OT-II) and labeled with CFSE. 106 labeled cells were injected i.v. into WT and KO littermates. 1 d later, animals were injected i.v. with 10 μg ovalbumin + 100 ng LPS or LPS alone. On day three, spleen cells from WT and KO mice were isolated and analyzed for proliferation as measured by CFSE dilution or restimulated with ovalbumin (0, 10, and 20 μg/ml) for an additional 3 d. Intracellular cytokine staining was then performed. (b) The CD69hi population of adoptively transferred T cells was smaller in spinophilin KO (red) than in spinophilin WT (black) mice. Representative flow cytometry plots (left) and the data pooled from four independent experiments (right) is shown (n = 14 WT and 15 KO mice total; *, P < 0.05 by Student's t test). (c) The relative abundance of IFNγ-producing effector T cells was significantly greater in WT than in KO, as measured by flow cytometry of intracellular cytokine staining. (left) Representative flow cytometry plots. (right) Data pooled from three independent experiments (n = 8 animals total for WT and KO; ***, P < 0.001 by Student's t test).
A functional consequence of successful activation of naive CD4+ T cell activation by DCs at the IS is the development of IFNγ-producing effector T cells. Therefore, we next asked whether spinophilin KO DCs were defective in triggering the development of IFNγ-producing effector T cells in an in vivo model of antigen challenge (Fig. 5 c). 106 CFSE-labeled antigen-specific T cells were injected i.v. into spinophilin KO and WT littermates. 1 d later, mice were exposed to an antigen (ovalbumin) and LPS. 3 d later, spleen cells were isolated and rechallenged with the same antigen in vitro. In WT mice, more adoptively transferred CD4+ T cells became IFNγ-producing effector T cells than in KO littermates (Fig. 5 c). A representative plot (Fig. 5 c, left) and data pooled from three independent experiments (Fig. 5 c, right) are shown. As only the host cells were deficient in spinophilin and not the adoptively transferred antigen-specific T cells, these data reflect a defect in the host APC's capacity to induce an antigen-specific immune response. Collectively, these data support a regulatory role for spinophilin in immune function, most likely in antigen presentation by DCs.
Spinophilin was originally discovered in neurons as a targeting subunit for PP1 that recruits PP1 to synaptic substrates. It also interacts directly with neurotransmitter receptors and GPCRs and competes with arrestin to direct receptor localization (Allen et al., 1997; Brady et al., 2003, 2005; Wang et al., 2004b). At many types of synapses, the regulation of synaptic strength is thus influenced by spinophilin, which is able to promote and inhibit biochemical interactions between first and second messengers as well as interact with the cytoskeleton (Allen, 2004, 2006; Wang et al., 2005, 2007; Liu et al., 2006).
The presence of spinophilin in DCs provides insight into the existence of intracellular signal transduction or other organizational events occurring in DCs during antigen presentation to T cells. The presence of some of spinophilin's known binding partners in APCs (PP1, actin, arrestin, and GPCRs) supports the hypothesis that these interactions, which are crucial to efficient neurotransmission, may play analogous roles at the IS. Increasingly, studies are reporting an influence of neurotransmitters on immune cells directly, opening up the possibility that spinophilin's role in immune cells may directly mirror some of its functions in neurons (Tracey, 2002; Flierl et al., 2007). Spinophilin has been shown to direct the trafficking and endocytosis of adrenergic receptors via competition with arrestins (Wang and Limbird, 2002; Wang et al., 2004b; Brady et al., 2005), which are present in DCs, and their expression may be modulated by pathogen stimulation (Maestroni, 2005). Our preliminary results suggest that PP1 can also polarize toward the contact site between DCs and T cells, although its distribution does not exactly mirror that of spinophilin (unpublished data).
In neurons, spinophilin regulates the actin cytoskeleton and microtubules by direct and indirect interactions (Grossman et al., 2004; Ryan et al., 2005; Bielas et al., 2007). Although in DCs, actin and its regulation by Rac regulate DC–T cell interactions (Benvenuti et al., 2004), Rac does not seem to be a target for spinophilin or the Rho–guanine nucleotide exchange factor with which spinophilin interacts (Ryan et al., 2005). Nevertheless, there are several events during antigen presentation during which spinophilin could influence the efficiency of communication between immune cells. These include: local membrane domain reorganization such as actin assembly at a contact site, recycling of MHCII and costimulatory molecules at the synapse, directed secretion of cytokines (Molon et al., 2005) and possibly neurotransmitters (O'Connell et al., 2006; Flierl et al., 2007), and stabilization of membrane-associated scaffolds that organize an optimal platform for immunological synaptic transmission. Our data indicate that the APC does not play a passive role during antigen presentation and that signal transduction cascades in DCs may prove to be as complex as in T cells and on both sides of the neuronal synapse.
Materials and methods
Cells
Mouse bone marrow–derived DCs were grown as described previously (Delamarre et al., 2005). Single cell suspensions of DCs, B cells, and T cells were isolated from mouse spleen and lymph node with liberase for 5 min at RT. CD11c+ cells were purified by positive selection, whereas B cells and T cells were purified by negative selection with MACS reagent kits (Miltenyi Biotec). Transgenic CD4+ T cells were prepared from lymph nodes or spleen of OT-I, OT-II (ovalbumin), and AND (MCC peptide) mice, respectively, using negative selection (Miltenyi Biotec). 1H3.1 T cell hybridomas were used in combination with IE-α peptide experiments.
Antigen presentation assays
In vitro antigen presentation assays were performed by adding bone marrow–derived dendritic cells (BMDCs), B cells, or CD11c+ splenic DCs to CD4+ T cells from OT-II mice in flat-bottom 96-well plates (105 cells per well of each type or diluted serially from that value). At 24 h, supernatants were harvested and tested for the presence of IL-2 by sandwich ELISA. All antibodies for ELISA were obtained from BD Biosciences. In vivo assays were performed by injecting spinophilin WT and KO littermates retro-orbitally with 106 OT-II CD4+ T cells that had been labeled at 5 × 106 cells/ml with 5 μM CFSE (Invitrogen) for 10 min at 37°C, and, after 24 h, inoculating mice retro-orbitally with ovalbumin (10 μg ovalbumin + 100 ng LPS/mouse). Proliferation of primed CD4+ T cells was monitored 40–48 h later by isolating spleen from injected mice. Division of cells was measured by FACS (FACSCalibur; Becton Dickinson). As described previously (Feng et al., 2000), spinophilin KO mice were on average 30% smaller than their WT littermates, so in vivo experiments were confirmed using weight-adjusted doses of ovalbumin (0.6–1.2 μg/g of body weight) of antigens, with weight- and sex-matched mice in each group. For analysis of CD69 within each division, gates were set to 0% on cells harvested from animals without an antigen.
Restimulation assays were performed as in the previous paragraph until day three, when spleen cells were isolated and incubated for an additional 3 d in vitro at 5 × 106 or 10 × 106 cells per well in a 96-well flat bottom plate in the presence of 0, 10, or 20 μg/ml ovalbumin peptide (aa 323–338) with 30 ng/ml LPS. Intracellular cytokine staining was performed using BD Cytoperm/CytoFix Plus kit (BD Biosciences) using Brefeldin A (Epicentre Biotechnologies) at 5 μg/ml for 6 h to trap intracellular cytokines. Cytokines and cell surface markers were labeled using antibodies obtained from BD Biosciences. 106 cells were collected by flow cytometry using a FACSCalibur and analyzed using FlowJo software (Treestar, Inc.). For analysis of IFNγ-producing cells, gates were set to 0% on cells harvested from animals without antigen. Because of fluctuations in the maximal response from among three independent experiments, the data are expressed as a percentage of the maximum; in WT mice, the mean response was 65% of the maximum response, whereas in KO mice, the mean response was 18.5% of the maximum.
Mice
Adult C57BL/6 (B6) and B10Br mice were obtained from Jackson ImmunoResearch Laboratories. Ovalbumin-specific, TCR-transgenic OT-I and OT-II mice as well as MCC-specific AND mice were bred in our animal facilities and used as described previously (Unternaehrer et al., 2007). Spinophilin WT and KO littermates were derived as described previously (Feng et al., 2000) and bred in our animal facilities.
Antibodies
Rabbit anti-spinophilin antibody (Allen et al., 1997) was used to label spinophilin at a dilution of 1:5–10,000. Mouse monocolonal 14.4.4 and I-Ab were used at a dilution of 1:300 and obtained from BD Biosciences. Rat monoclonal anti–mouse Lamp2 was used at a dilution of 1:200. Secondary antibodies conjugated to various fluorophores were obtained from Invitrogen and used at a dilution of 1:300. Coverslips were mounted on slides using Gel Mount (Biomedia Corp.).
Spinophilin GFP and retrovirus construct
Spinophilin-GFP was subcloned into the LZRS retroviral vector as described previously (Chow et al., 2002). DCs used for spinophilin-GFP experiments were cultured from bone marrow of B10Br mice and cocultured with T cells from AND mice, which have a TCR specific for MCC peptide.
Western blot
Cell lysates from the brain or spleen or from isolated single cell suspensions of immune cells were prepared as described previously (Allen et al., 1997; Unternaehrer et al., 2007) separated by SDS-PAGE, blotted to polyvinylidene fluoride membrane (Immobilon-P; Millipore) and developed with the indicated antibodies. To compare the relative expression levels of spinophilin in different tissue lysates, bands were quantified using the histogram function in Photoshop (Adobe). A background band from the same blot was quantified and subtracted from the mean density value of all the bands. Subsequently, the adjusted mean density of the spinophilin band was divided by that of the actin band from the same sample.
Flow cytometry
Data were collected on a FACSCalibur flow cytometer. Quantitative analysis was performed using Cellquest (BD Biosciences) and FlowJo software.
Qualitative and quantitative confocal microscopy
All images (live and fixed) were acquired on a confocal microscope (LSM-510 Meta; Carl Zeiss, Inc.) using an Apochromat 40× 1.2 NA water-immersion objective (Carl Zeiss, Inc.) at RT (fixed imaging) or in an environmental chamber at 37°C (live imaging) using LSM software (Carl Zeiss, Inc.) as described previously (Chow et al., 2002). Images were analyzed using Photoshop and National Institutes of Health ImageJ software. For fixed imaging, cells were mounted onto coverslips (Fisherbrand) using Gel Mount Aqueous mounting media (BioMeda). For live cell imaging, cells were observed in MatTek dishes in RPMI (Invitrogen) without phenol red with 1% FCS.
For characterization of spinophilin distribution in immature and mature DCs, ImageJ software was used to analyze confocal micrographs. The fluorescence intensity of each marker in an ROI high in spinophilin labeling was measured and an identical ROI in an area low in spinophilin labeling was also measured. The ratio of fluorescence intensity was determined from the IntDens values in high versus low ROIs. This number was plotted versus identical measurements performed for actin and MHCII in the same ROIs.
Using Excel software (Microsoft), a regression was performed with line fit plots drawn to statistically evaluate the relationship between labeling patterns of spinophilin, actin, and MHCII. n = 24 observations from 12 micrographs of immature DCs and 50 observations from 24 micrographs of mature DCs.
For analysis of spinophilin distribution in fixed DC–T cell conjugates shown in Fig. 2, stacks of confocal images of conjugates were viewed using ImageJ. T cell activation was defined by polarization of the TCR. Conjugates that had apparent spinophilin polarization to the contact site were chosen and the distributions of the TCR and MHCII were determined to be clustered manually (n = 48 conjugates).
For live cell video microscopy, BMDCs (isolated from B10Br mice) were infected on day two in culture with a retrovirus expressing spinophilin-GFP or GFP alone and maintained in culture until day five to six. Immature BMDCs were stimulated with LPS in the presence or absence of an agonist peptide (moth cytochrome c aa 88–103). BMDCs were then adhered to coverslips and cocultured with TCR transgenic CD4+ T cells (from AND mice) that were labeled red by a nonspecific lipophilic dye. Allowing 1–5 min for T cells to settle onto the coverslip, conjugates were imaged for a minimum of 20 min, with images acquired every ∼30 s (spinophilin-GFP + antigen) or every ∼2 min (spinophilin-GFP–Ag and GFP alone + Ag). LSM files were imported into the Volocity software program (Improvision), which was used to assemble QuickTime (Apple) movies at 15 frames per second.
Analysis of distribution of spinphilin-GFP or GFP alone at 0, 4, 14, and 20 min after contact was performed as on fixed images; an ROI close to the contact site was chosen and the fluorescence intensity was measured. The same ROI away from the contact site was chosen and the fluorescence intensity was measured. A ratio of the fluorescence intensity was calculated toward/away from the contact site. The values of observations over time were pooled for each group and graphed as shown in Fig. 3 b.
Online supplemental material
Fig. S1 shows a primary BMDC matured with LPS, fed an agonist peptide, expressing spinophilin-GFP, and interacting with an antigen-specific T cell. The cells were fixed, permeabilized and labeled with anti-spinophilin antibodies and anti-CD3 antibodies. Video 1 corresponds to Fig. 3 (top) and shows a primary BMDC matured with LPS, fed an agonist peptide, and expressing spinophilin-GFP interacting with an antigen-specific T cell. Video 2 corresponds to Fig. 3 (middle) and shows a primary BMDC matured with LPS and expressing spinophilin-GFP interacting with an antigen-specific T cell. Video 3 corresponds to Fig. 3 (bottom) and shows a primary BMDC matured with LPS, fed an agonist peptide, and expressing GFP alone interacting with an antigen-specific T cell. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200711149/DC1.
Supplemental Material
Acknowledgments
The authors wish to thank Rachael Couture for excellent technical assistance and Jacob Appelbaum for assistance in data analysis. We thank Drs. Philipp Alberts, Angus Nairn, Jennifer Morgan, Ellen Lumpkin, and members of the Mellman laboratory for helpful discussions of the manuscript.
This work was supported by a Sandler Foundation SPAR award, a postdoctoral fellowship from the Cancer Research Institute to O. Bloom, and a MERIT award from the National Institutes of Health (R37AI34098) to I. Mellman. This investigation was partly supported by the National Institutes of Health National Research Service Award (5-T32-AI-07019). I. Mellman is an Affiliate Member of the Ludwig Institute for Cancer Research.
O. Bloom's present address is The Feinstein Institute for Medical Research, North Shore Long Island Jewish Health System, Manhasset, NY 11030.
J.J. Unternaehrer's present address is Division of Hematology/Oncology, Children's Hospital, Harvard Medical School, Boston, MA 02115.
A. Jiang's present address is Department of Immunobiology, Yale School of Medicine, New Haven, CT 06520.
J.-S. Shin's present address is Department of Immunobiology, University of California, San Francisco, San Francisco, CA 94143.
Abbreviations used in this paper: APC, antigen-presenting cell; BMDC, bone marrow–derived dendritic cell; CFSE, 5(6)-carboxyfluorescein diacetate N-succinimidyl ester; DC, dendritic cell; GPCR, G protein–coupled receptor; IL-2, interleukin-2; IS, immunological synapse; KO, knockout; LPS, lipopolysaccharide; MHCII, major histocompatibility complex type II; PP1, protein phosphatase I; ROI, region of interest; TCR, T cell receptor; WT, wild type.
References
- Allen, P.B. 2004. Functional plasticity in the organization of signaling complexes in the striatum. Parkinsonism Relat. Disord. 10:287–292. [DOI] [PubMed] [Google Scholar]
- Allen, P.B., C.C. Ouimet, and P. Greengard. 1997. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc. Natl. Acad. Sci. USA. 94:9956–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, P.B., V. Zachariou, P. Svenningsson, A.C. Lepore, D. Centonze, C. Costa, S. Rossi, G. Bender, G. Chen, J. Feng, et al. 2006. Distinct roles for spinophilin and neurabin in dopamine-mediated plasticity. Neuroscience. 140:897–911. [DOI] [PubMed] [Google Scholar]
- Benvenuti, F., S. Hugues, M. Walmsley, S. Ruf, L. Fetler, M. Popoff, V.L. Tybulewicz, and S. Amigorena. 2004. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 305:1150–1153. [DOI] [PubMed] [Google Scholar]
- Bielas, S.L., F.F. Serneo, M. Chechlacz, T.J. Deerinck, G.A. Perkins, P.B. Allen, M.H. Ellisman, and J.G. Gleeson. 2007. Spinophilin facilitates dephosphorylation of doublecortin by PP1 to mediate microtubule bundling at the axonal wrist. Cell. 129:579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady, A.E., Q. Wang, R.J. Colbran, P.B. Allen, P. Greengard, and L.E. Limbird. 2003. Spinophilin stabilizes cell surface expression of alpha 2B-adrenergic receptors. J. Biol. Chem. 278:32405–32412. [DOI] [PubMed] [Google Scholar]
- Brady, A.E., Q. Wang, P.B. Allen, M. Rizzo, P. Greengard, and L.E. Limbird. 2005. Alpha 2-adrenergic agonist enrichment of spinophilin at the cell surface involves beta gamma subunits of Gi proteins and is preferentially induced by the alpha 2A-subtype. Mol. Pharmacol. 67:1690–1696. [DOI] [PubMed] [Google Scholar]
- Calabrese, B., M.S. Wilson, and S. Halpain. 2006. Development and regulation of dendritic spine synapses. Physiology (Bethesda). 21:38–47. [DOI] [PubMed] [Google Scholar]
- Chow, A., D. Toomre, W. Garrett, and I. Mellman. 2002. Dendritic cell maturation triggers retrograde MHCI class I transport from lysosomes to the plasma membrane. Nature. 418:988–994. [DOI] [PubMed] [Google Scholar]
- Delamarre, L., M. Pack, H. Chang, I. Mellman, and E.S. Trombetta. 2005. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 307:1630–1634. [DOI] [PubMed] [Google Scholar]
- Dustin, M.L., S.Y. Tseng, R. Varma, and G. Campi. 2006. T cell-dendritic cell immunological synapses. Curr. Opin. Immunol. 18:512–516. [DOI] [PubMed] [Google Scholar]
- Feng, J., Z. Yan, A. Ferreira, K. Tomizawa, J.A. Liauw, M. Zhuo, P.B. Allen, C.C. Ouimet, and P. Greengard. 2000. Spinophilin regulates the formation and function of dendritic spines. Proc. Natl. Acad. Sci. USA. 97:9287–9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flierl, M.A., D. Rittirsch, B.A. Nadeau, A.J. Chen, J.V. Sarma, F.S. Zetoune, S.R. McGuire, R.P. List, D.E. Day, L.M. Hoesel, et al. 2007. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 449:721–725. [DOI] [PubMed] [Google Scholar]
- Grakoui, A., S.K. Bromley, C. Sumen, M.M. Davis, A.S. Shaw, P.M. Allen, and M.L. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science. 285:221–227. [DOI] [PubMed] [Google Scholar]
- Grossman, S.D., L.C. Hsieh-Wilson, P.B. Allen, A.C. Nairn, and P. Greengard. 2002. The actin-binding domain of spinophilin is necessary and sufficient for targeting to dendritic spines. Neuromolecular Med. 2:61–69. [DOI] [PubMed] [Google Scholar]
- Grossman, S.D., M. Futter, G.L. Snyder, P.B. Allen, A.C. Nairn, P. Greengard, and L.C. Hsieh-Wilson. 2004. Spinophilin is phosphorylated by Ca2+/calmodulin-dependent protein kinase II resulting in regulation of its binding to F-actin. J. Neurochem. 90:317–324. [DOI] [PubMed] [Google Scholar]
- Hsieh-Wilson, L.C., F. Benfenati, G.L. Snyder, P.B. Allen, A.C. Nairn, and P. Greengard. 2003. Phosphorylation of spinophilin modulates its interaction with actin filaments. J. Biol. Chem. 278:1186–1194. [DOI] [PubMed] [Google Scholar]
- Ilani, T., C. Khanna, M. Zhou, T.D. Veenstra, and A. Bretscher. 2007. Immune synapse formation requires ZAP-70 recruitment by ezrin and CD43 removal by moesin. J. Cell Biol. 179:733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, J., M.J. Miller, and A.S. Shaw. 2005. The c-SMAC: sorting it all out (or in). J. Cell Biol. 170:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W., E.Y. Yuen, P.B. Allen, J. Feng, P. Greengard, and Z. Yan. 2006. Adrenergic modulation of NMDA receptors in prefrontal cortex is differentially regulated by RGS proteins and spinophilin. Proc. Natl. Acad. Sci. USA. 103:18338–18343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludford-Menting, M.J., J. Oliaro, F. Sacirbegovic, E.T. Cheah, N. Pedersen, S.J. Thomas, A. Pasam, R. Iazzolino, L.E. Dow, N.J. Waterhouse, et al. 2005. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 22:737–748. [DOI] [PubMed] [Google Scholar]
- Maestroni, G.J. 2005. Adrenergic modulation of dendritic cells function: relevance for the immune homeostasis. Curr. Neurovasc. Res. 2:169–173. [DOI] [PubMed] [Google Scholar]
- Mellman, I. 2007. Private lives: reflections and challenges in understanding the cell biology of the immune system. Science. 317:625–627. [DOI] [PubMed] [Google Scholar]
- Molon, B., G. Gri, M. Bettella, C. Gomez-Mouton, A. Lanzavecchia, A.C. Martinez, S. Manes, and A. Viola. 2005. T cell costimulation by chemokine receptors. Nat. Immunol. 6:465–471. [DOI] [PubMed] [Google Scholar]
- Monks, C.R., B.A. Freiberg, H. Kupfer, N. Sciaky, and A. Kupfer. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 395:82–86. [DOI] [PubMed] [Google Scholar]
- Mossman, K.D., G. Campi, J.T. Groves, and M.L. Dustin. 2005. Altered TCR signaling from geometrically repatterned immunological synapses. Science. 310:1191–1193. [DOI] [PubMed] [Google Scholar]
- Nakanishi, H., H. Obaishi, A. Satoh, M. Wada, K. Mandai, K. Satoh, H. Nishioka, Y. Matsuura, A. Mizoguchi, and Y. Takai. 1997. Neurabin: a novel neural tissue-specific actin filament-binding protein involved in neurite formation. J. Cell Biol. 139:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcross, M.A. 1984. A synaptic basis for T-lymphocyte activation. Ann. Immunol. (Paris). 135D:113–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell, P.J., X. Wang, M. Leon-Ponte, C. Griffiths, S.C. Pingle, and G.P. Ahern. 2006. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood. 107:1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet, C.C., I. Katona, P. Allen, T.F. Freund, and P. Greengard. 2004. Cellular and subcellular distribution of spinophilin, a PP1 regulatory protein that bundles F-actin in dendritic spines. J. Comp. Neurol. 479:374–388. [DOI] [PubMed] [Google Scholar]
- Revy, P., M. Sospedra, B. Barbour, and A. Trautmann. 2001. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat. Immunol. 2:925–931. [DOI] [PubMed] [Google Scholar]
- Ryan, X.P., J. Alldritt, P. Svenningsson, P.B. Allen, G.Y. Wu, A.C. Nairn, and P. Greengard. 2005. The Rho-specific GEF Lfc interacts with neurabin and spinophilin to regulate dendritic spine morphology. Neuron. 47:85–100. [DOI] [PubMed] [Google Scholar]
- Sims, T.N., T.J. Soos, H.S. Xenias, B. Dubin-Thaler, J.M. Hofman, J.C. Waite, T.O. Cameron, V.K. Thomas, R. Varma, C.H. Wiggins, et al. 2007. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 129:773–785. [DOI] [PubMed] [Google Scholar]
- Tracey, K.J. 2002. The inflammatory reflex. Nature. 420:853–859. [DOI] [PubMed] [Google Scholar]
- Unternaehrer, J., A. Chow, M. Pypaert, K. Inaba, and I. Mellman. 2007. The tetraspanin CD9 mediates lateral association of MHC class II molecules on the dendritic cell surface. Proc. Natl. Acad. Sci. USA. 104:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q., and L.E. Limbird. 2002. Regulated interactions of the alpha 2A adrenergic receptor with spinophilin, 14-3-3zeta, and arrestin 3. J. Biol. Chem. 277:50589–50596. [DOI] [PubMed] [Google Scholar]
- Wang, H., F.E. McCann, J.D. Gordan, X. Wu, M. Raab, T.H. Malik, D.M. Davis, and C.E. Rudd. 2004. a. ADAP-SLP-76 binding differentially regulates supramolecular activation cluster (SMAC) formation relative to T cell-APC conjugation. J. Exp. Med. 200:1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q., J. Zhao, A.E. Brady, J. Feng, P.B. Allen, R.J. Lefkowitz, P. Greengard, and L.E. Limbird. 2004. b. Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science. 304:1940–1944. [DOI] [PubMed] [Google Scholar]
- Wang, X., W. Zeng, A.A. Soyombo, W. Tang, E.M. Ross, A.P. Barnes, S.L. Milgram, J.M. Penninger, P.B. Allen, P. Greengard, and S. Muallem. 2005. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat. Cell Biol. 7:405–411. [DOI] [PubMed] [Google Scholar]
- Wang, X., W. Zeng, M.S. Kim, P.B. Allen, P. Greengard, and S. Muallem. 2007. Spinophilin/neurabin reciprocally regulate signaling intensity by G protein-coupled receptors. EMBO J. 26:2768–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier, R., S. Rabizadeh, K. Ishiguro, N. Andre, J.B. Ortiz, H. Wachtel, D.G. Morris, M. Lopez-Ilasaca, A.C. Shaw, W. Swat, and B. Seed. 2004. Discs large (Dlg1) complexes in lymphocyte activation. J. Cell Biol. 166:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.