Abstract
Blood circulation is dependent on heart valves to direct blood flow through the heart and great vessels. Valve development relies on epithelial to mesenchymal transition (EMT), a central feature of embryonic development and metastatic cancer. Abnormal EMT and remodeling contribute to the etiology of several congenital heart defects. Leptin and its receptor were detected in the mouse embryonic heart. Using an ex vivo model of cardiac EMT, the inhibition of leptin results in a signal transducer and activator of transcription 3 and Snail/vascular endothelial cadherin–independent decrease in EMT and migration. Our data suggest that an Akt signaling pathway underlies the observed phenotype. Furthermore, loss of leptin phenocopied the functional inhibition of αvβ3 integrin receptor and resulted in decreased αvβ3 integrin and matrix metalloprotease 2, suggesting that the leptin signaling pathway is involved in adhesion and migration processes. This study adds leptin to the repertoire of factors that mediate EMT and, for the first time, demonstrates a role for the interleukin 6 family in embryonic EMT.
Introduction
The delicate leaflets of the heart valves act as gatekeepers to navigate blood flow through the heart and great vessels. As the embryonic heart begins the morphogenic process of looping, endocardial cushions, protrusions of complex extracellular matrix and proteoglycans, develop between the layers of the bilaminar heart tube and serve as the primordial cardiac valves. After formation, endocardial (cardiac endothelial) cells that overlie the cushions undergo epithelial to mesenchymal transition (EMT) in response to inductive factors secreted by the juxtaposing myocardium. The newly formed mesenchymal cells disperse within the cushions and undergo terminal differentiation into valvular fibroblasts. Subsequently, remodeling events resolve the cushions into slender valve leaflets and the membranous portion of the chamber septa. Disturbances during these morphogenetic events contribute to the etiology of a spectrum of anomalies (ventricular septal defect; tetralogy of Fallot; pulmonic and aortic valve atresia, or stenosis; and double outlet right ventricle) affecting ∼1% of live births and carrying a significant mortality (for review see Hoffman, 1995).
Experiments in vertebrate models and the atrioventricular canal (AVC) explant assay revealed that TGF-β, Notch, nuclear factor of activated T cells, VEGF, and Wnt/β-catenin are involved at discrete stages of valvulogenesis (Person et al., 2005). However, the pathogenetic mechanisms leading to the structural abnormalities observed in human congenital heart defects remain largely unknown. To date, little information regarding the role of the interleukin 6 (Il-6) superfamily in EMT has been published except for a handful of studies. In mononuclear cells, oncostatin M (OSM) induces epithelial cell to myofibroblast transdifferentiation, a process similar to EMT, and in human breast carcinoma cells, OSM results in acquisition of a mesenchymal morphology and an invasive phenotype (Nightingale et al., 2004; Jorcyk et al., 2006). A role for Il-6 in breast cancer cell motility via the down-regulation of epithelial cadherin (a necessary and sufficient step in EMT) and the acquisition of fibroblastoid morphology has been reported (Asgeirsson et al., 1998). To our knowledge, studies involving other Il-6 members (cardiotropin, leukemia inhibitory factor, granulocyte colony-stimulating factor, and leptin) have not been reported in any developmental or pathological EMT event.
In this study, we explore the role of the pleiotropic cytokine leptin in development of the endocardial cushion during valvulogenesis. Of the Il-6 family members, leptin became an attractive target because of its known roles in endothelial cell biology and induction during cardiovascular diseases. Leptin guides endothelial cell migration and organization into vascular structures (Sierra-Honigmann et al., 1998; Park et al., 2001; Goetze et al., 2002). Additionally, leptin elicits several physiological responses in the cardiovascular system that influence vascular tone, blood pressure, and heart rate (Haynes et al., 1997; Shek et al., 1998; Nickola et al., 2000). ob/ob mice (a spontaneous mutation in leptin) develop left ventricular hypertrophy independently of body weight that is completely reversed with leptin treatment, suggesting antihypertrophic properties for leptin (Barouch et al., 2003). However, plasma leptin levels increase after acute myocardial infarction and during advanced congestive heart failure, indicating pathological roles of leptin in the cardiovascular system (Meisel et al., 2001; Schulze et al., 2003). The precise functions of leptin may be varied and complex, as leptin administration after ischemia reperfusion is cardioprotective via an Akt–phosphoinositide-3 kinase (PI3K) pathway (Smith et al., 2006). Collectively, these data underscore the pleiotropic characteristic of this cytokine.
A diverse array of biological actions for leptin has been revealed by the ob/ob mouse. These mice display a phenotype of obesity, hypothermia, hyperinsulinemia, hyperglycemia, and sterility, but reproductive function, body weight, and metabolic status return to normal with the administration of recombinant leptin (Coleman, 1978; Campfield et al., 1995; Chehab et al., 1996). Additional defects occur in immune response, inflammation, hematopoiesis, angiogenesis, and bone growth/development (Fantuzzi and Faggioni, 2000; Murad et al., 2003; Thomas, 2004). Leptin-deficient mice are viable, although the administration of recombinant leptin is required during conception, pregnancy, and nursing for fertility, maintenance of pregnancy, and lactation (Mantzoros and Moschos, 1998; Malik et al., 2001). Thus, recombinant leptin from the maternal circulation may mask the effects of leptin deficiency on early postimplantation embryonic development in ob/ob mice. Alternatively, given the propensity for compensation in mice, other Il-6 members such as cardiotrophin or OSM may be induced, or pathways downstream of leptin may be hyperactivated, resulting in normal development. db/db mice carry a mutation that results in a truncated leptin receptor incapable of Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling and display a similar phenotype to ob/ob mice. However, endothelial and mesangial cells from db/db mice are capable of responding to leptin via alternative signaling pathways, raising the possibility that leptin engagement of the short form of the receptor occurs during in utero development in this strain (Han et al., 2001).
Of note, oocytes and preimplantation embryos express leptin and leptin receptor, which function in oocyte polarity, preimplantation development, and implantation (Antczak and Van Blerkom, 1997; Kawamura et al., 2002; Cervero et al., 2005; Herrid et al., 2006; Yang et al., 2006). The influence of leptin on postimplantation mammalian embryonic development has not been fully explored. Accordingly, this study was designed to determine leptin involvement in cardiac EMT and to explore the underlying cell signaling pathways engaged. Furthermore, known mediators of differentiation and migration were evaluated to identify the downstream participants targeted by the leptin cascade. In this study, we demonstrate the presence of leptin and leptin receptor in the cardiac endocardial cushion throughout the process of valvulogenesis. Neutralization of leptin or inhibition of Akt in the AVC explant assay resulted in decreased total cell number, inhibition of EMT, increased cell death, and attenuated migration. Furthermore, these treatments result in the failure of αvβ3 integrin receptor induction and decreased matrix metalloprotease 2 (MMP2), suggesting a leptin signaling pathway in endocardial adhesion, EMT, and migration processes in the developing heart.
Results
Spatiotemporal distribution of leptin and leptin receptor during endocardial cushion morphogenesis
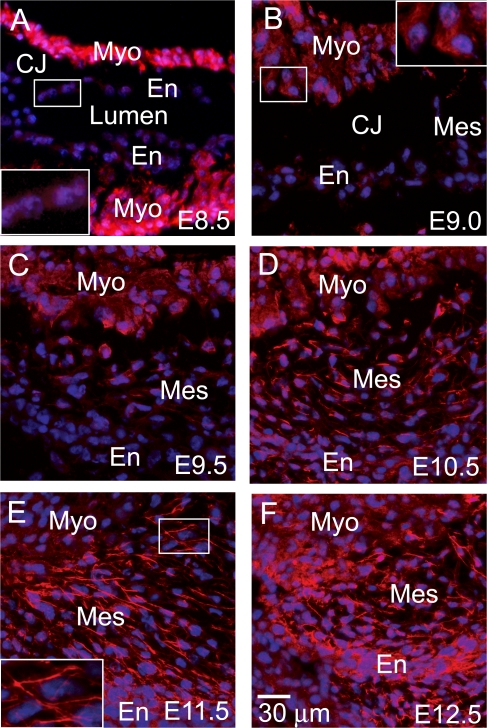
A series of immunofluorescence images from embryonic day (E) 8.5–12.5 hearts, representing stages through the formation of endocardial cushion protrusions to the remodeling of endocardial mesenchyme, was captured to evaluate leptin distribution during valvulogenesis. At E8.5, as the myocardium secretes matrix molecules, swelling the extracellular space between the myocardial and endocardial layers, leptin presence is strong in the myocardium throughout the heart, including the trabeculating myocardium in the common ventricle (Fig. 1 A). The endocardium over the endocardial cushions and throughout the heart possesses a weakly detectable level of leptin (Fig. 1 A, inset). Induction of EMT begins shortly after formation of the acellular endocardial cushions. As the first few cells delaminate from the endocardial layer (E9.0), the punctuate pattern of leptin decreases in the myocardium (Fig. 1 B, inset), but expression begins to appear in the newly formed mesenchymal cells (Fig. 1 C). At the later stages of endocardial cushion morphogenesis (E9.5–12.5), myocardial expression continues to abate; however, expression in the endocardium and mesenchymal cells increases during stages of active EMT (Fig. 1, C and D), proliferation (Fig. 1 E), and early remodeling (Fig. 1 F), displaying an increasingly filamentous pattern (Fig. 1 E, inset). The dynamic expression of leptin throughout endocardial cushion morphogenesis suggests regulatory roles in early EMT events such as myocardial induction of EMT and maintenance of EMT as well as in later migratory roles in the remodeling cushion.
Figure 1.
Spatiotemporal localization of leptin during endocardial cushion morphogenesis. Immunofluorescence staining (red) for leptin was performed on embryonic hearts excised at E8.5 (A), E9.0 (B), E9.5 (C), E10.5 (D), E11.5 (E), and E12.5 (F). DAPI, blue. Myo, myocardium; En, endocardium; Mes, mesenchyme; CJ, cardiac jelly. Insets are enlargements of the endothelium overlaying the endocardial cushion (A), the myocardium adjacent to the endocardial cushion (B), and the mesenchymal cells within the endocardial cushion (E).
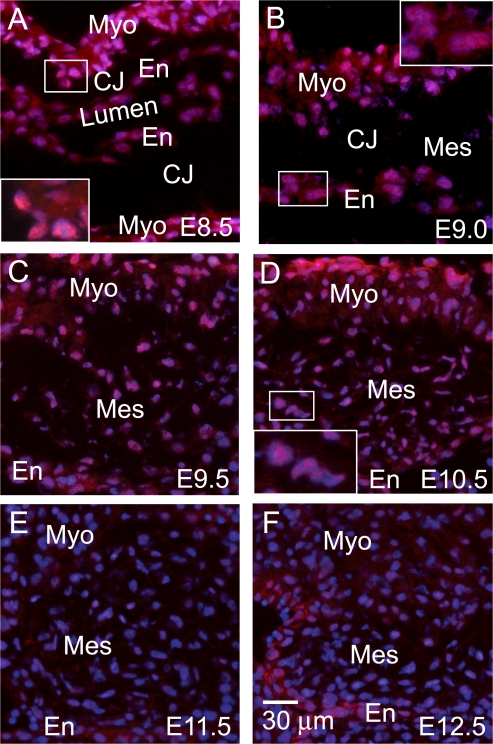
Leptin receptor was evaluated using an antibody directed toward the intracellular domain of the long form of the receptor. Although the short forms of the receptor have signaling capabilities, they are generally thought to mediate leptin internalization for degradation, leptin transport across cell barriers, such as the blood brain barrier, or to serve as a soluble form of the receptor upon proteolytic cleavage of the extracellular domain (Uotani et al., 1999; Hileman et al., 2000; Ge et al., 2002). In the acellular expanding endocardial cushion (Fig. 2, A and B), there is cytoplasmic staining and strong perinuclear expression in the myocardium (Fig. 2 A, inset) and endocardium (Fig. 2 B, inset). This pattern is consistent with a published study on leptin receptor cellular localization (Lundin et al., 2000). In Ob-R–transfected COS cells, the majority of leptin receptors (75–95%) localize intracellularly, residing within the endoplasmic reticulum and Golgi (Lundin et al., 2000). Similar to leptin ligand, leptin receptor decreases in the myocardium as morphogenesis progresses (Fig. 2, C–F).
Figure 2.
Spatiotemporal localization of leptin receptor during endocardial cushion morphogenesis. Immunofluorescence staining (red) for leptin receptor was performed on embryonic hearts excised at E8.5 (A), E9.0 (B), E9.5 (C), E10.5 (D), E11.5 (E), and E12.5 (F). DAPI, blue. Myo, myocardium; En, endocardium; Mes, mesenchyme; CJ, cardiac jelly. Insets are enlargements of the myocardium adjacent to the endocardial cushion (A), the endothelium overlaying the endocardial cushion (B), and the mesenchymal cells within the endocardial cushion (D).
Endocardial leptin receptor is detected at E8.5 (Fig. 2 A) and peaks at E9.0 (Fig. 2 B, inset) just as the first few mesenchymal cells appear, suggesting a role for leptin receptor engagement in the initiation of EMT. During active EMT (Fig. 2, C and D; inset), leptin receptor expression abates, whereas in the later stages of morphogenesis (Fig. 2, E and F), leptin receptor is only faintly present in the mesenchymal and endocardial cells. The weak expression of leptin receptor and strong expression of leptin in the mesenchymal cells at E11.5–12.5 suggests a mechanism to limit leptin signaling. In contrast to other Il-6 members, leptin has not been reported to bind structurally similar cytokine receptors, although this possibility remains.
Sequestration of leptin abrogates endocardial commitment to and completion of EMT
Progress in our understanding of the molecular events that underlie cardiac EMT has been derived from an ex vivo model that mimics the spatiotemporal, molecular, and biochemical events that are observed during in vivo valvulogenesis and provides a system amenable to experimental manipulation (Bernanke and Markwald, 1982; Runyan and Markwald, 1983). In this model, the AVC segment is excised before EMT; the cushion tissue is exposed and placed onto the surface of a collagen gel matrix. Endocardial cells migrate onto the surface of the collagen gel and away from the explants. Subsequently, in response to inductive myocardial-derived signals, a subset of the endothelial cells undergoes EMT and invades the collagen gel. Cells undergoing EMT follow a semilinear progression of morphological changes, including (1) lateral cell–cell separation from epithelial sheets, (2) single, rounded cells subsequently elongate on the surface of the collagen gel, and (3) elongated cells invade into and migrate through the collagen gel.
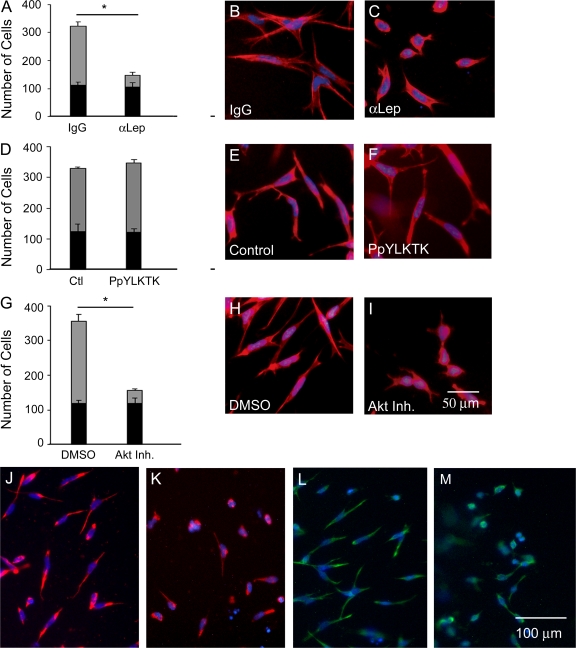
The effects of leptin neutralization on EMT were evaluated by the addition of 5–20 μg/ml leptin antibody or isotype control IgG to the media bathing the explants. Leptin sequestration resulted in a significant decrease (∼60%) in the total number of cells on and in the collagen gel compared with IgG control (136.0 ± 18.2 vs. 320.4 ± 53.6; P = 0.001; Fig. 3 A), suggesting a defect in the commitment to undergo EMT. These cells display phenotypic activation (loss of cell–cell junctions and separation between cells). However, the percentage of total cells that undergo EMT decreased to 23% with leptin antibody treatment compared with 67% of IgG control (number of mesenchymal cells = 32.6 ± 8.6 vs. 214.0 ± 30.9; P = 0.0002; Fig. 3 A, gray bars).
Figure 3.
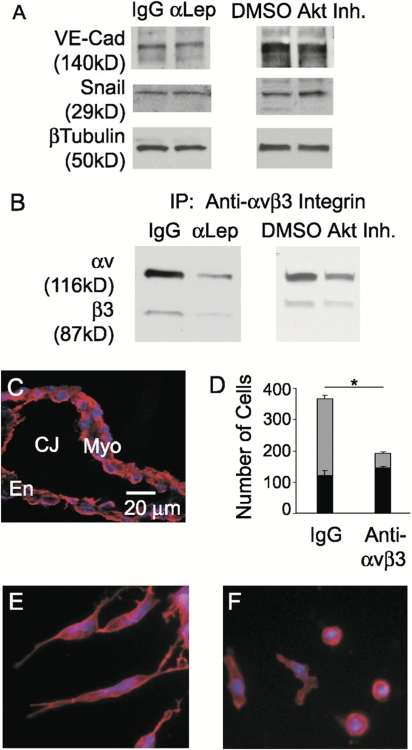
Effects of leptin, STAT3, and Akt inhibition on EMT in the endocardial cushions. Quantification of endocardial (black bars) and mesenchymal cell numbers (gray bars) after AVCs were cultured with 5 μg/ml IgG or α-leptin antibody (A), vehicle or 1 mM PpYLKTK (D), and DMSO or 5 μM Akt inhibitor XI (G). Cellular morphology was evaluated by F-actin staining (red): IgG (B), α-leptin antibody (C), vehicle (E), PpYLKTK (F), DMSO (H), and Akt inhibitor XI (I). Vimentin staining after treatment with IgG (J), α-leptin antibody (K), DMSO (L), and Akt inhibitor XI (M). DAPI, blue. Error bars represent SD. *, P < 0.05.
Cells undergoing EMT display a distinct cellular morphology that can be evaluated by filamentous actin (F-actin) distribution. Hallmarks of EMT include increased stress fibers and focal adhesions concomitant with decreased cell–cell junctions. Treatment with leptin antibody resulted in a failure of induction of changes in cell morphology normally associated with EMT. The majority of cells appeared rounded with a cortical expression of F-actin (Fig. 3 C) compared with the elongated cells with a filamentous pattern of F-actin present in control cells (Fig. 3 B). Accordingly, 76% of leptin antibody–treated cells maintained an epithelial morphology compared with 33% of IgG-treated cells (Fig. 3 A, black bars). Compared with the control, mesenchymal cells in the leptin antibody group displayed decreased cellular elongation.
The morphological appearance of the cells present on the collagen is activated (i.e., the endocardial cells have undergone lateral cell–cell separation and appear rounded but have not transformed). In cultures treated with leptin antibody, immunofluorescent staining for vimentin, a marker of mesenchyme, detected reduced levels of vimentin in a cortical expression pattern (Fig. 3 K) compared with the control (Fig. 3 J). This phenotype suggests an inability to complete or an early arrest of the EMT program.
Leptin induces Akt phosphorylation, and AVCs cultured with Akt inhibitors phenocopy leptin inhibition
To further understand the abrogation of EMT induced by leptin sequestration, signaling mechanisms downstream of leptin receptor engagement were considered. The leptin receptor, a member of the class I cytokine receptor family with sequence and structural homology to glycoprotein 130, leukemia inhibitory factor, and OSM receptors, is encoded by the db gene. All six isoforms contain a JAK-binding site, but only the long form of the receptor Ob-R contains the motifs necessary for STAT signaling, providing the mechanism by which leptin signals in the majority of tissues studied (Hegyi et al., 2004). The exquisite specificity of STAT recruitment is thought to lie in the JAK2-phosphorylated tyrosines and flanking amino acid residues of the SH2-binding domain of the cytokine receptor (Ghilardi et al., 1996; Ihle, 1996). Leptin signal transduction through JAK2/STAT3 has been demonstrated to regulate many of leptin's effects on endothelial cell biology (Heim et al., 1995).
To establish whether STAT3 plays a role in cardiac EMT, a peptide inhibitor of STAT3 (PpYLKTK-mts, a cell-permeable analogue of the STAT3-SH2 domain that prevents dimerization of STAT3 proteins and subsequent translocation into the nucleus) was used. Surprisingly, STAT3 inhibition (Fig. 3 F) did not recapitulate the phenotypic effects of leptin inhibition. There was no significant difference in the total number of cells or in the number of mesenchymal cells between PpYLKTK and the control (Fig. 3 D).
Leptin's downstream signaling events are complex. In cell lines, leptin activates multiple signaling pathways, including the mitogen-activated protein kinase, PI3K, PKC, phosphodiesterase, PLC, and JNK pathways (Sweeney, 2002; Frühbeck, 2006). Based on these studies, other leptin targets were considered, in particular the PI3K–Akt pathway, which has gained much attention in cancer EMT, migration, and cardiovascular survival. In cancer, aberrant Akt signaling has been implicated in cell transformation via induction of Snail and down-regulation of epithelial cadherin (Grille et al., 2003). To date, the role of Akt in EMT is based on experiments in cell lines, which do not provide the appropriate physiological context. Unfortunately, Akt knockout mice provided little information concerning their role in embryonic EMT (Cho et al., 2001a,b; Peng et al., 2003; Larue and Bellacosa, 2005; Yang et al., 2005; Dummler et al., 2006). However, ex vivo assays provide a system to conduct functional experiments on mammalian embryos.
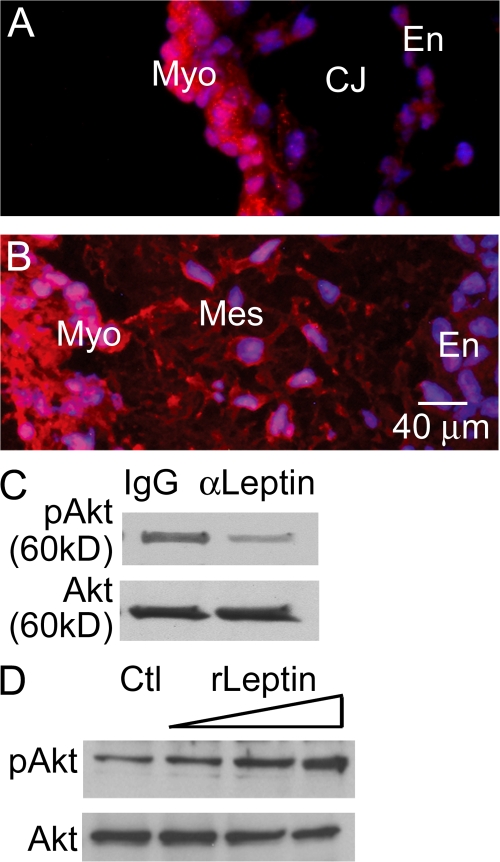
To determine whether the Akt pathway may be present in the embryonic heart, immunofluorescent localization of Akt (antibody detects Akt1, 2, and 3) was performed on embryonic hearts excised from timed pregnant matings at stages of endocardial cushion morphogenesis before EMT (E8.5) and during EMT (E9.5). The presence of Akt in the endocardial cells before EMT (Fig. 4 A) suggests recruitment of Akt signaling in the endocardial response to EMT inductive stimuli. The expression of Akt in mesenchymal cells (Fig. 4 B) signifies a potential role in maintenance of the mesenchymal phenotype after EMT or participating in invasion-associated cellular activities. The expression patterns of leptin receptor and Akt prompted the investigation of the Akt pathway as a downstream mediator of leptin action. Inhibition of leptin in the AVC explants resulted in decreased Akt phosphorylation at serine 473 after 2 h (Fig. 4 C). The intensity of the bands was measured using Quantity One software to determine relative phosphorylation levels. In the displayed blot, 89% of total Akt was phosphorylated in control hearts compared with 62% after leptin antibody treatment for 2 h (Fig. 4 C), resulting in an ∼30% decrease in pAkt. The potential role of Akt was further supported by the observation that dose-dependent induction of Akt phosphorylation at serine 473 occurs in AVCs treated with exogenous recombinant leptin (50, 100, and 500 pM) for 30 min (Fig. 4 D). In Fig. 4 D, initial relative phosphorylation levels in control hearts was 16%. After the addition of 50, 100, and 500 pM of recombinant leptin for 30 min, the percent total Akt that was phosphorylated increased to 26, 57, and 80%, respectively. At the highest dose of recombinant leptin, there was a fivefold increase in relative pAkt levels compared with the control. These doses did not induce the phosphorylation of STAT3 at serine 727 and tyrosine 705 (unpublished data).
Figure 4.
Akt localization in the endocardial cushion and phosphorylation by exogenous leptin. (A and B) Akt immunofluorescence staining (red) was performed on embryonic hearts excised just before EMT (A) and during EMT (B). (C and D) Representative Western blots for pAkt (serine 473) and Akt in AVCs treated with 5 μg/ml α-leptin antibody (C) or recombinant leptin (50, 100, and 500 pM for 30 min; D). DAPI, blue. Myo, myocardium; En, endocardium; Mes, mesenchyme; CJ, cardiac jelly.
Functional experiments were performed using pharmacological inhibitors of Akt1–3 (Akt inhibitor IV or XI) in the ex vivo AVC assay. Treatment with Akt inhibitor XI, which inhibits the kinase domain of Akt, resulted in a significant decrease in the total number of cells present on and in the gel compared with the DMSO control (156.4 ± 18.2 vs. 356.4 ± 21.5; P < 0.0001; Fig. 3 G). The number of mesenchymal cells was significantly less after Akt inhibition compared with the DMSO control (38.2 ± 5.3 vs. 238.4 ± 20.0; P < 0.0001; Fig. 3 G, gray bars). The majority of cells (75%) remained epithelial compared with the control (33%), and an epithelial pattern of F-actin was observed (Fig. 3, compare I with H). Similar results were obtained with Akt inhibitor VI and PI3K inhibitor LY294002 (unpublished data). Vimentin staining of cultures treated with Akt inhibitor XI detected reduced levels of vimentin in a cortical expression pattern (Fig. 3 M) compared with the control (Fig. 3 L).
Akt inhibition (Fig. 3 I) phenocopied the morphological defects of leptin inhibition (Fig. 3 C). Furthermore, quantification of the defects revealed similar effects on total cell number and EMT, which supports the hypothesis of an Akt pathway downstream of leptin receptor engagement. However, the involvement of other signaling pathways downstream of leptin receptor engagement and activation remained possible. Therefore, the mitogen-activated protein kinase, PKC, and protein phosphatase 1/protein phosphatase 2A pathways were evaluated using pharmacological inhibitors (PD98059, Gö6983, and okadaic acid, respectively) at a range of doses. All of these resulted in normal cell number and EMT (unpublished data). Of note, high doses of Gö6983 and okadaic acid affected myocardial survival and resulted in death of the explant tissue.
Perturbation of leptin or Akt pathways does not affect snail or VE-cadherin levels
TGF-β ligands and receptors display distinct and dynamic spatiotemporal patterns in the embryonic heart and induce cardiac EMT (Camenisch et al., 2002; Dünker and Krieglstein, 2002). After induction of endothelial cells to the EMT pathway by myocardial-derived factors such as TGF-β, cell–cell junctions must be dissociated. Snail-induced degradation, relocalization, and transcriptional repression of cadherins has been intensely studied and is widely appreciated in multiple model organisms and cell lines (Cano et al., 2000; Carver et al., 2001; Nieto, 2002). This repression is believed to be the mechanism by which Snail participates in EMT and/or maintenance of the mesenchymal/migratory phenotype. TGF-β acting upstream of Snail in this pathway has become one of the most studied models of EMT (Frid et al., 2002; Peinado et al., 2003; Nawshad et al., 2005; Zavadil and Bottinger, 2005). To further elucidate the function of leptin, the interaction of leptin in this established pathway was assessed by determining the levels of Snail and vascular endothelial (VE) cadherin by Western blotting 48–72 h after treatment. A failure of Snail induction would result in the persistence of VE-cadherin and the inability to undergo EMT. However, the inhibition of leptin and Akt resulted in similar levels of Snail and VE-cadherin as control cultures actively undergoing EMT (Fig. 5 A), suggesting that this pathway is intact. Furthermore, a monolayer of cells was not observed on the collagen gel after leptin or Akt inhibition (Fig. 3, C and I, respectively), which would be expected in the event of the persistence of VE-cadherin. Instead, the cells appeared separated from each other, which is consistent with a loss of cell–cell junctions. Additionally, supplementation of recombinant TGF-β2 into the media bathing the explants did not rescue the defects induced by leptin antibody (unpublished data), nor did leptin inhibition phenocopy TGF-β2 inhibition in which there is outgrowth of endothelial cells, retention of cell–cell contacts, and near-complete inhibition of EMT (Camenisch et al., 2002). Collectively, these data suggest that a pathway downstream of or parallel to TGF-β leads to the defects induced by leptin inhibition.
Figure 5.
Inhibition of leptin or Akt does not affect Snail or VE-cadherin but alters αvβ3 integrin levels. (A and B) Representative Western blots of VE-cadherin and Snail (A) after treatment with either 5 μg/ml IgG or α-leptin antibody and either DMSO or 5 μM Akt inhibitor XI. β-Tubulin was used as a loading control. Representative Western blot of αv and β3 integrins (B) after immunoprecipitation of αvβ3 integrins in AVCs treated with 5 μg/ml IgG compared with 5 μg/ml α-leptin antibody or DMSO compared with 5 μM Akt inhibitor XI. (C) αvβ3 Integrin immunofluorescence staining (red) was performed on embryonic hearts excised just before EMT (C). (D) Quantification of endocardial (black bars) and mesenchymal cell numbers (gray bars) after AVCs were cultured with 2 μg/ml IgG or LM609 antibody. (E and F) Cellular morphology was evaluated by F-actin staining (red): 2 μg/ml IgG (E) or LM609 antibody (F). DAPI, blue. Myo, myocardium; En, endocardium; Mes, mesenchyme; CJ, cardiac jelly. Error bars represent SD. *, P < 0.0001.
Perturbation of leptin or Akt pathways alters αvβ3 integrin levels
Explants from TGF-β and endothelial β-catenin mutants as well as explants treated with cyclosporine A (to inhibit calcineurin) all exhibit the outgrowth of endocardial sheets, leading to normal total cell numbers but a lack of EMT (Camenisch et al., 2002; Chang et al., 2004; Liebner et al., 2004). After dissolution of cadherin junctions within the monolayer, the freed endocardial cells must interact with and adhere to the surrounding matrix. Therefore, the substantial defect in total cell number may reflect an endothelial cell adhesion defect. To a large extent, endothelial cells mediate adhesion to extracellular matrix through cell surface integrin receptors (Stupack and Cheresh, 2002). Upon initiation of endothelial migration, the distribution and levels of integrins change. In particular, αvβ3 integrin becomes dramatically induced and binds to the provisional matrix facilitating adhesion and migration (Stupack and Cheresh, 2002).
Just before EMT (E8.5), embryonic hearts express αvβ3 integrin (Fig. 5 C). Inhibition of leptin or Akt resulted in decreased αvβ3 integrin protein levels (Fig. 5 B). Further hearts cultured with a function-blocking antibody to αvβ3 integrin (Fig. 5 F) phenocopied the leptin inhibition morphological defects (Fig. 3 C), suggesting decreased adhesion as one of the consequences of the loss of leptin. Functional inhibition of αvβ3 integrin resulted in a significant decrease in the total number of cells that migrated onto and into the gel compared with IgG control (191 ± 1.0 vs. 366.7 ± 15.1; P < 0.0001; Fig. 5 D). Fewer cells underwent EMT compared with the control (number of mesenchymal cells = 45.0 ± 5.3 vs. 243 ± 12.3; P = 0.0008; Fig. 5 D, gray bars), with the majority of total cells remaining epithelial after functional inhibition of αvβ3 integrin (76.4 vs. 33.7%) and displaying a cortical distribution of F-actin (Fig. 5, compare F with E). These data demonstrate an adhesion defect possibly mediated via αvβ3 integrin; however, endothelial cells express several other integrins that may contribute to the adhesion defect induced by leptin inhibition.
Inhibition of leptin or Akt reduces invasion and MMP2 enzymatic activity
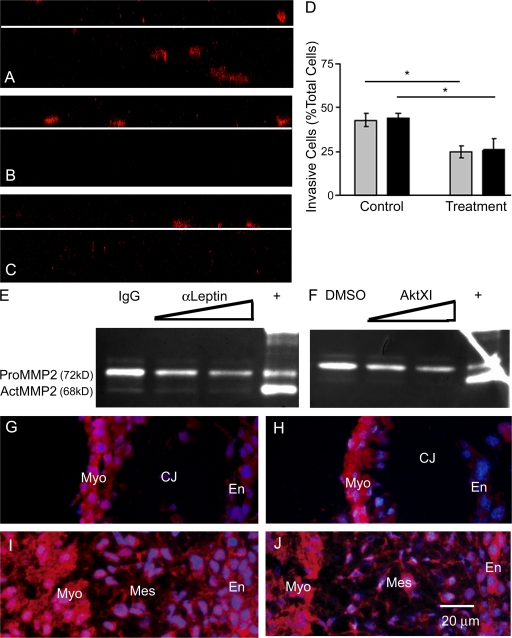
Confocal z-plane images of F-actin–stained cultures revealed decreased invasion after leptin or Akt inhibition (Fig. 6, B and C) compared with the control (Fig. 6 A). In control AVC cultures, ∼50% of total cells invade into the collagen gel, whereas after leptin or Akt inhibition, 25% of total cells invade (P < 0.0001; Fig. 6 D). Mesenchymal cell migration into the endocardial cushion in vivo or into the collagen gel ex vivo requires coordinated dissolution of adhesion molecules followed by activation of pericellular protease activity (Person et al., 2005). Proteolysis of extracellular matrix components facilitates cellular travel through the complex matrices of the endocardial cushion and may release cytokines/chemokines bound to the cardiac jelly. The MMP family of proteases modifies cell–matrix interactions by digesting extracellular matrices to facilitate cell spreading/migration. Inhibition of MMPs with the broad spectrum inhibitor GM6001 in murine AVC cultures does not block EMT but inhibits the migration of mesenchymal cells into the collagen gel (Enciso et al., 2003).
Figure 6.
Inhibition of leptin or Akt reduces invasion and MMP2 enzymatic activity. (A–C) Confocal z-plane slices through F-actin–stained cultures demonstrating cell migration from the surface of the collagen gel (white lines) in control (A), α-leptin– (B), and Akt inhibitor XI (C)–treated cultures. (D) Percentage of total cells that invade the collagen gel (controls on left, treatment on right): 5 μg/ml IgG versus α-leptin antibody (gray bars) and DMSO versus 5 μM Akt inhibitor XI (black bars). (E and F) Zymography of the media bathing the explants 48 h after treatment: 5 μg/ml IgG, 5 and 10 μg/ml α-leptin antibody (E); and DMSO, 1 and 5 μM Akt inhibitor XI (F). (G–J) Immunofluorescence (red) for MMP2 (G and I) and MMP14 (H and J) was performed on embryonic hearts excised at E8.5, just before EMT (G and H), and at E9.5, during EMT (I and J). DAPI, blue. Myo, myocardium; En, endocardium; Mes, mesenchyme; CJ, cardiac jelly. Error bars represent SD. *, P < 0.0001.
The profile of latent and active gelatinases (MMP2 and 9) secreted into the media by the AVCs was assessed by zymography. Culture media was collected 48 h after addition of the indicated treatment. Leptin antibody and Akt XI inhibitor decreased both pro-MMP2 (72 kD) and active MMP2 (62 kD; Fig. 6, E and F). Quantity One software was used to measure the band intensities from digital images of the zymograms. Treatment with 10 μg/ml leptin antibody resulted in a 38% decrease in total MMP2 levels (control = 7,316,033 ± 1,332,255 pixels; leptin antibody = 4,475,740 ± 970,559 pixels; n = 3; P < 0.05). Treatment with 5 μM Akt inhibitor XI resulted in a 40% decrease in total MMP2 levels (control = 7,193,127 ± 905,809 pixels; Akt inhibitor XI = 4,247,400 ± 350,421 pixels; n = 3; P < 0.05). Heart/gel lysates displayed a similar pattern of decreased MMP2 (unpublished data). As a negative control, the addition of EDTA, an MMP inhibitor, to the incubation buffer of a duplicate zymogram completely abolished the degradation of gelatin (unpublished data).
Presumably, the MMP2 detected in the media is generated initially in greater part by the myocardium and modestly by the endocardium. A series of immunofluorescently labeled embryonic hearts from developmental time points during endocardial cushion morphogenesis revealed MMP2 expression to be strongly present in the myocardium and weakly present in the endocardium (Fig. 6 G). During EMT and migration, the mesenchyme intensely expresses MMP2 (Fig. 6 I). In accordance with the idea of MMP2 enzymatic activity in multiple cell types in the embryonic heart, MMP14, a key regulator of MMP2 activity that acts by cleaving and activating pro-MMP2 at the membrane, is present in an overlapping pattern as MMP2 (Fig. 6, H and J).
Inhibition of leptin or Akt induces increased rates of cell death
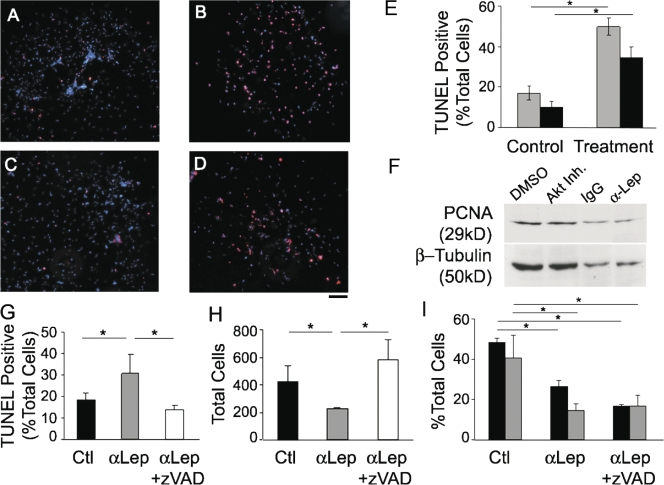
The cell survival properties of leptin have been described in multiple cell types in the vascular, bone, and nervous systems (Artwohl et al., 2002; Najib and Sanchez-Margalet, 2002; Gordeladze and Reseland, 2003; Guo et al., 2007). Therefore, an alternative explanation for changes in total cell number may be leptin's effects on proliferation and apoptosis. To evaluate this hypothesis, TUNEL staining was performed on cultures treated with leptin antibody (Fig. 7 B) or Akt inhibitor XI (Fig. 7 D). Inhibition of leptin resulted in 49.8 ± 4.3% of total cells displaying TUNEL reactivity compared with 17 ± 3.4% in the control group (n = 3; P < 0.0001; Fig. 7 E). Similarly, inhibition of Akt resulted in 34.4 ± 5.3% of total cells displaying TUNEL reactivity compared with 10 ± 2.8% in the control group (n = 3; P < 0.0009; Fig. 7 E). A 2.8- or 3.4-fold induction in cell death was detected after treatment with α-leptin antibody or Akt inhibitor XI, respectively, suggesting that leptin/Akt signaling plays a role in cell survival during cardiac EMT or that the failure of normal differentiation results in cell death.
Figure 7.
Inhibition of leptin or Akt induces apoptosis. (A–D) TUNEL staining (red) after treatment with 5 μg/ml α-leptin antibody (B) or 5 μM Akt inhibitor XI (D) compared with the control (A and C, respectively). DAPI, blue. (E) Percentage of total cells that are TUNEL positive (controls on left, treatment on right): IgG versus α-leptin antibody (gray bars) and DMSO versus Akt inhibitor XI (black bars). (F) Representative Western blot of PCNA after treatment with either 5 μg/ml IgG or α-leptin antibody and either DMSO or 5 μM Akt inhibitor XI. β-Tubulin was used as a loading control. (G–I) Percentage of total cells that are TUNEL positive (G), total cells present in and on the gel (H), and percentage of total cells undergoing EMT (black bars) or migrating into the collagen gel (gray bars; I) after treatment with IgG, α-leptin antibody, or α-leptin antibody plus 40 mM z-VAD. Error bars represent SD. Bar, 40 μm. *, P < 0.05.
Proliferation was evaluated by Western blotting for proliferating cell nuclear antigen (PCNA) 48 h after treatment with either leptin antibody or Akt inhibitor XI. The data were normalized to β-tubulin (n = 5). There was not a significant change in control versus leptin antibody treatment or control versus Akt XI inhibitor treatment (Fig. 7 F).
To differentiate between the effects of leptin on EMT versus cell survival, the caspase inhibitor Z-VAD was added to the cultures. Hearts treated with α-leptin antibody plus Z-VAD had reduced levels of TUNEL-positive cells compared with leptin antibody alone (14.0 ± 2.0 vs. 30.6 ± 8.9%; n = 3; P < 0.05), similar to the control (18.6 ± 3.4%; Fig. 7 G). Concomitantly, the total number of cells present in and on the gel after treatment with leptin antibody plus Z-VAD was increased compared with leptin antibody alone (584 ± 143 vs. 226 ± 9; n = 3; P < 0.05) and surpassed that of the control (423 ± 112; Fig. 7 H). The decrease in EMT and migration observed after leptin inhibition was not rescued by the addition of Z-VAD, suggesting that the decrease in differentiation and migration is independent of the induction of apoptosis (Fig. 7 I).
Discussion
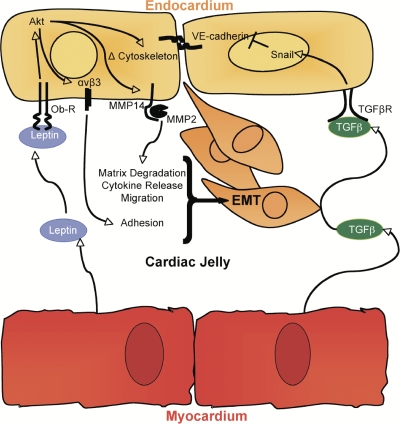
Leptin affects diverse cellular processes that modulate angiogenesis, metabolism, and reproduction. In this study, modulation of cardiac EMT and migration were found to be novel roles of leptin, demonstrating a functional role of the first Il-6 member in cardiac EMT. Robust EMT is regulated by the interplay between differential responses of distinct cell types. Leptin and leptin receptor localization patterns suggest a pathway of EMT by which myocardial-derived ligand acts on endocardial receptors to drive EMT, which is consistent with the known cell-autonomous pathway of cardiac EMT (Fig. 8). Interestingly, the increasing expression of leptin as mesenchymal cells migrate toward the myocardium (Fig. 1 C) and the strong presence of leptin in the mesenchyme later in cushion morphogenesis (Fig. 1, E and F) may suggest that leptin is a marker of mature mesenchyme. The spatial expression pattern of leptin and leptin receptor in the myocardium also suggests a role as an autocrine mediator of myocardial biology. This finding is consistent with the observation that ob/ob mice develop cardiac hypertrophy (Barouch et al., 2003). Furthermore, STAT3 may mediate these aspects of leptin, as cardiomyocyte-targeted STAT3 deletion causes postnatal death by heart failure (Jacoby et al., 2003). Leptin appears to play a role not only in myocardial plasticity but in endothelial biology, as ob/ob mice display attenuated angiogenesis in response to injury (Suganami et al., 2004). Immunofluorescence experiments in embryonic hearts failed to demonstrate STAT3 in the endocardium (unpublished data), and pharmacological inhibition of STAT3 did not affect cardiac EMT. Concomitantly, STAT3 endothelial-targeted transgenic mice are viable and healthy, with no apparent cardiac defect (Kano et al., 2003). Therefore, the effects of the leptin-STAT3 axis in the myocardium are likely independent of leptin's effects on the endocardium.
Figure 8.
Summary of leptin's roles in the endocardial cushion. The spatiotemporal pattern of leptin and leptin receptor depicts a pathway by which myocardial-derived leptin ligand engages with endocardial leptin receptors to drive EMT and migration, which is consistent with the known cell-autonomous pathway of cardiac EMT. In an ex vivo functional assay, loss of leptin abrogates adhesion, EMT, cell survival, and migration, potentially via Akt. The leptin pathway is independent of the TGF-β/Snail-driven dissolution of VE-cadherin. Interestingly, the specific effector molecules disrupted by loss of leptin in the endocardial cushion are known mediators of adhesion, migration, and differentiation. Leptin's role in adhesion of endocardial cells may occur through αvβ3 integrin receptor, whereas leptin-driven proteolytic activity and invasion of mesenchymal cells may be mediated by MMP2. Furthermore, MMPs modulate growth factor release and receptor availability and may affect morphogen gradients across the cardiac jelly and, thus, signaling cascades. The potential branch points in the leptin signaling pathway and cross talks with other pathways remain to be uncovered. Ultimately, leptin signaling affects adhesion, EMT, cell survival, and migration within the embryonic heart.
Leptin (ob/ob) and leptin receptor (db/db) mutant mice have not been reported to have developmental defects. However, such experiments are confounded by the requirement of recombinant leptin during pregnancy or heterozygous matings. Therefore, the ex vivo AVC explant assay was used to overcome the previously stated complications of studying the role of leptin in early postimplantation embryonic development. Using this assay, we demonstrated that leptin depletion in the media bathing the explants results in attenuated cardiac EMT. Our results demonstrate that leptin is required for but is not sufficient for EMT.
Leptin interacts with a plethora of cell signaling elements (Bjorbaek et al., 1997; Sweeney, 2002; Frühbeck, 2006). We were particularly interested in leptin's interaction with the PI3K–Akt pathway because this system has been associated with migration, cancer EMT, and cardiovascular survival. In many human cancers, Akt is activated, endowing tumor cells with motile and invasive properties (Altomare and Testa, 2005). The definitive study demonstrating the role of Akt in EMT is based on experiments using squamous cell carcinoma lines, which do not provide the appropriate physiological context (Grille et al., 2003). Several developmental milestones rely on EMT, including gastrulation, neural crest formation, cardiac morphogenesis, and sclerotome formation. Unfortunately, Akt knockout mice provided little information concerning their role in embryonic EMTs. To overcome the difficulties of performing functional experiments on mammalian embryos in utero, we used ex vivo AVC assays that provide a system to conduct functional experiments on complex, intact mammalian embryonic tissue. We demonstrate that inhibition of Akt in AVC cushions inhibits EMT, which provides the first direct evidence of the involvement of Akt in embryonic EMT. The defects induced by Akt inhibition phenocopied leptin inhibition, suggesting that Akt may be downstream of leptin receptor engagement. Furthermore, recombinant leptin induced Akt phosphorylation, whereas leptin inhibition reduced Akt phosphorylation. Given the lack of the phenotypic similarity of inhibition of several other major cell signaling pathways (mitogen-activated protein kinase, PKC, and phosphatase 1/protein phosphatase 2A), Akt is suspected to be the primary intracellular pathway mediating leptin's actions in the endocardial cushion. However, given that inhibition at the ligand level often disrupts numerous signaling components, the participation of other intracellular signaling pathways cannot be definitively excluded.
Targets downstream of or parallel to TGF-β/Snail-driven dissolution of VE-cadherins are speculated to underlie the EMT defect induced by leptin inhibition. After cell–cell detachment, signals transmitted between the endocardial cell and its extracellular environment within the cardiac jelly or collagen gel guide cell behaviors. In endothelial cells, these interactions are largely mediated by integrin receptors (Stupack and Cheresh, 2004). The integrin family is comprised of a multitude of α and β subunits that noncovalently associate in various heterodimeric combinations to produce 24 different αβ-heterodimeric receptors on the cell surface that participate in bidirectional signaling. The balance between cell adhesion and migration relies on this bidirectional signaling between the cytosol and extracellular environment. However, little is known regarding heart development and integrins, although α3, α6, α7, and β1 integrins are expressed in the embryonic heart (Hescheler and Fleischmann, 2000). Furthermore, integrin-linked kinase mediates EMT in multiple epithelia potentially via the phosphorylation of β integrins, Akt, and GSK3β (Li et al., 2003; Ahmed et al., 2006; Shimizu et al., 2006; Weaver et al., 2007).
After leptin or Akt inhibition, only a few cells are able to delaminate from the explant tissue. These cells are able to disperse from each other but appear rounded or short rather than elongated with filopodia. This phenotype suggests that the cells are not able to interact with their environment in a normal manner, presumably via integrin engagement. The cardiac jelly consists of a complex network of matrix molecules and proteoglycans, including laminins, collagens, and fibronectin, whereas the ex vivo assay consists of the matrix secreted by the explant and the type I collagen gel the explant is planted on. Although several integrins could potentially mediate endocardial attachment, αvβ3 integrin became an attractive candidate because of its known roles in endothelial cell migration and its repertoire of ligands (including fibronectin, vitronectin, and proteolysed forms of collagen and laminin; Stupack and Cheresh, 2004).
Our data illustrating a reduction in αvβ3 integrin expression in explants after leptin or Akt inhibition combined with the data demonstrating a reduction of total cell numbers after addition of a function-blocking αvβ3 antibody are all consistent with the hypothesis that leptin signaling modulates αvβ3 expression via an Akt pathway during cardiac EMT. Supporting this hypothesis is a recent study documenting leptin-driven blastocyst adhesion via the up-regulation of αvβ3 expression (Yang et al., 2006). Therefore, the significant decrease in total cell number concomitant with the dissolution of cell–cell junctions after leptin inhibition suggests that leptin may affect the adhesion of endocardial cells to matrix. Decreased adhesiveness to the collagen gel or cardiac jelly may cause decreased EMT and migration and may thus affect subsequent valvular development. However, further evaluation of additional adhesion receptors and matrix molecules are required to fully appreciate the role of leptin in endocardial adhesion.
An alternative hypothesis to explain differences in total cell number is the substantial negative effect of leptin and Akt inhibition on cell survival. However, supplementation with Z-VAD to inhibit apoptosis also resulted in decreased EMT. Cell death may be the default pathway in a population of cells in which differentiation is unable to occur. Therefore, we speculate that the primary effects are impaired differentiation, which subsequently leads to cell death, and reduced total cell number.
After adhesion and differentiation, mesenchymal cells elongate and migrate. Recently, leptin has been reported to modulate actin cytoskeleton dynamics via RhoA/Rock (Zeidan et al., 2007). Consistent with this study, we demonstrate a filamentous expression pattern of leptin in the mesenchymal cells of the remodeling endocardial cushion. Elongated mesenchymal cells invade the collagen gel or cardiac jelly via MMP2 (Song et al., 2000; Enciso et al., 2003). Inhibition of leptin reduces MMP2 levels and activity, leading to decreased invasion into the collagen gel. Leptin is known to increase the levels and activity of MMP2 and MMP9 in human umbilical endothelial cells and the rat corneal angiogenesis assay, suggesting a role for leptin in matrix remodeling and migration (Park et al., 2001; Lee et al., 2005; Moon et al., 2007). Furthermore, Akt, a major signaling pathway in cell migration and known activator of MMP2, mediates migration in leptin-treated endothelial cells (Goetze et al., 2002; Jin et al., 2007). Therefore, in the embryonic heart, the requirement of MMPs in the migratory events during cardiac EMT may be fulfilled by leptin acting upstream of Akt and, thereby, contributing to the invasive phenotype of cardiac mesenchymal cells. Interestingly, leptin's ability to modulate the expression of αvβ3 integrin and MMP2 may also influence the binding of MMP2 to the cell surface by providing the increased expression of αvβ3 integrin, a known MMP2 tethering molecule (Brooks et al., 1996, 1998; Silletti et al., 2001). This possibility coupled with the ability of MMPs to release ECM-bound growth factors and to proteolyse a broad range of cytokines and chemokines further broadens leptin's downstream effects (Overall et al., 2002; Dean and Overall, 2007). Collectively, our observations define several roles for leptin in valvular morphogenesis (Fig. 8); determining the range of leptin-induced effector molecules that elicit entry into EMT and how leptin integrates into the complex network that confers successful EMT remains to be deciphered.
Materials and methods
Mice
10-wk-old CD1 mice (Charles River Laboratories) were maintained under standard conditions. Timed matings were performed and detected by the presence of a vaginal plug (designated day 0.5 postconceptual). The Yale University Animal Care and Use Committee approved all animal protocols, and experimentation was performed in accordance with National Institutes of Health regulations.
Immunofluorescence
Tissues were excised and frozen in optimal cutting temperature medium. Cryosections were stained with antibodies for F-actin (Invitrogen), leptin (Rocio Honigmann), leptin receptor (Rocio Honigmann), Akt (Cell Signaling Technology), MMP2 (Chemicon), αvβ3 (David Cheresh, University of California, San Diego, San Diego, CA), and MMP14 (Robert Black, Amgen Inc., Seattle, WA). Immunoreactivity was visualized with AlexaFluor594 antibody (Invitrogen). Nuclei were stained with DAPI.
Image acquisition and processing
Immunohistochemistry was performed on frozen sections using AlexaFluor594 secondary antibody. Slides were mounted in aqueous mounting media. AVC collagen gel cultures were imaged in PBS on plastic tissue culture wells (Thermo Fisher Scientific). Samples were visualized at room temperature on an inverted fluorescent microscope (IX71-S1F; Olympus) with CPlan 10× NA 0.25 and LCPlanFL 20× NA 0.40 objectives (Olympus) and captured with a digital camera (S97809; Olympus). The acquisition software used was Picture Frame (Optronics). Confocal images were obtained using a laser-scanning confocal microscope (Fluoview; Olympus) integrated with a microscope (IX70-S1F2; Olympus) with UplanFl 20× NA 0.50 and LCPlanFl 40× NA 0.60 objectives (Olympus) and were acquired using Fluoview software (Olympus). Digital images were processed (cropped, brightness/contrast adjusted, and color balanced) using Photoshop (Adobe).
AVC explant assay
AVCs were isolated from E9.0 embryos and placed onto a 1-mg/ml type I rat tail collagen gel (BD Biosciences). The explants were allowed to adhere before the addition of media (medium 199, 1% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.1% each of insulin, transferrin, and selenium [Invitrogen]; Enciso et al., 2003). Pharmacological inhibitors were purchased from EMD. Five explants were plated per well. Cultures were stopped at 48–72 h. All experiments were repeated at least five times. Some gels were fixed with 4% PFA and stained with F-actin. En face and z-plane confocal images were obtained using FluoView software, and the number of total cells, cells that have undergone EMT (mesenchymal vs. epithelial phenotype), and cells that have migrated into the gel were counted based on morphology and distance from the surface of the collagen gel.
Zymography
The FBS for these experiments was run through a gelatin-packed column to remove MMP2 and MMP9 before use in cultures. Lysates of hearts/gels were obtained using 50 mM Tris/1% Triton X-100. Under nonreducing conditions, 25 μg of tissue lysate or media from cultures was run on a 10% zymogram gel (Bio-Rad Laboratories). The gels were washed in 2.5% Triton X-100 and water and incubated for 72 h at 37°C in a 50-mM Tris-HCl buffer, pH 8.0, containing 5 mM calcium chloride (10 mM EDTA was used as a negative control). Gels were stained with Coomassie blue R250 and imaged.
Western blotting
Explanted hearts plus the collagen gels containing cells were harvested from culture conditions and lysed in radioimmunoprecipitation assay buffer (Millipore) supplemented with 1% SDS and inhibitor cocktails (Calbiochem). 25 μg of protein was subjected to SDS-PAGE using 4–15% Tris gels (Bio-Rad Laboratories) under reducing conditions followed by transfer to polyvinylidene difluoride membranes. Primary antibodies used included Akt, pAkt (serine 473 and threonine 308), STAT3, pSTAT3 (serine 727 and tyrosine 705; Cell Signaling Technology), VE-cadherin (BD Biosciences), Snail (Santa Cruz Biotechnology, Inc.), and PCNA (Abcam). Blots were stripped and reprobed with β-tubulin (Invitrogen) as a loading control. For immunoprecipitation of αvβ3 integrin, 100 μg of lysate (prepared in radioimmunoprecipitation assay buffer) was precleared with normal serum and precipitated with protein A/G Sepharose. The supernatant was incubated with the primary antibody (anti–αvβ3 integrin), washed with decreased salt conditions, and electroblotted for αv and β3 integrins.
In situ cell death detection
Cell death was evaluated using the In Situ Cell Death Detection kit (TMR red; Roche) according to the manufacturer's instructions. Subsequently, cells were counterstained with DAPI, and the percentage of apoptotic to total cells was calculated. To inhibit apoptosis, 40 μM Z-VAD–fluoromethylketone (BD Biosciences) was added every 24 h.
Statistics
The data were analyzed by t test (StatView; SAS Institute, Inc.) and reported as the mean ± SD.
Acknowledgments
This study was supported by National Institutes of Health grant R37HL28373 to J.A. Madri, grant HG02357 to M. Snyder, and grant 5T32 GM07499 to A.K. Nath.
Abbreviations used in this paper: AVC, atrioventricular canal; EMT, epithelial to mesenchymal transition; F-actin, filamentous actin; Il-6, interleukin 6; JAK, Janus kinase; MMP, matrix metalloprotease; OSM, oncostatin M; PCNA, proliferating cell nuclear antigen; PI3K, phosphoinositide-3 kinase; STAT, signal transducer and activator of transcription; VE, vascular endothelial.
References
- Ahmed, N., S. Maines-Bandiera, M.A. Quinn, W.G. Unger, S. Dedhar, and N. Auersperg. 2006. Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. Am. J. Physiol. Cell Physiol. 290:C1532–C1542. [DOI] [PubMed] [Google Scholar]
- Altomare, D.A., and J.R. Testa. 2005. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464. [DOI] [PubMed] [Google Scholar]
- Antczak, M., and J. Van Blerkom. 1997. Oocyte influences on early development: the regulatory proteins leptin and STAT3 are polarized in mouse and human oocytes and differentially distributed within the cells of the preimplantation stage embryo. Mol. Hum. Reprod. 3:1067–1086. [DOI] [PubMed] [Google Scholar]
- Artwohl, M., M. Roden, T. Holzenbein, A. Freudenthaler, W. Waldhausl, and S.M. Baumgartner-Parzer. 2002. Modulation by leptin of proliferation and apoptosis in vascular endothelial cells. Int. J. Obes. Relat. Metab. Disord. 26:577–580. [DOI] [PubMed] [Google Scholar]
- Asgeirsson, K.S., K. Olafsdottir, J.G. Jonasson, and H.M. Ogmundsdottir. 1998. The effects of IL-6 on cell adhesion and e-cadherin expression in breast cancer. Cytokine. 10:720–728. [DOI] [PubMed] [Google Scholar]
- Barouch, L.A., D.E. Berkowitz, R.W. Harrison, C.P. O'Donnell, and J.M. Hare. 2003. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 108:754–759. [DOI] [PubMed] [Google Scholar]
- Bernanke, D.H., and R.R. Markwald. 1982. Migratory behavior of cardiac cushion tissue cells in a collagen-lattice culture system. Dev. Biol. 91:235–245. [DOI] [PubMed] [Google Scholar]
- Bjorbaek, C., S. Uotani, B. da Silva, and J.S. Flier. 1997. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J. Biol. Chem. 272:32686–32695. [DOI] [PubMed] [Google Scholar]
- Brooks, P.C., S. Stromblad, L.C. Sanders, T.L. von Schalscha, R.T. Aimes, W.G. Stetler-Stevenson, J.P. Quigley, and D.A. Cheresh. 1996. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 85:683–693. [DOI] [PubMed] [Google Scholar]
- Brooks, P.C., S. Silletti, T.L. von Schalscha, M. Friedlander, and D.A. Cheresh. 1998. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 92:391–400. [DOI] [PubMed] [Google Scholar]
- Camenisch, T.D., D.G. Molin, A. Person, R.B. Runyan, A.C. Gittenberger-de Groot, J.A. McDonald, and S.E. Klewer. 2002. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev. Biol. 248:170–181. [DOI] [PubMed] [Google Scholar]
- Campfield, L.A., F.J. Smith, Y. Guisez, R. Devos, and P. Burn. 1995. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 269:546–549. [DOI] [PubMed] [Google Scholar]
- Cano, A., M.A. Perez-Moreno, I. Rodrigo, A. Locascio, M.J. Blanco, M.G. del Barrio, F. Portillo, and M.A. Nieto. 2000. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2:76–83. [DOI] [PubMed] [Google Scholar]
- Carver, E.A., R. Jiang, Y. Lan, K.F. Oram, and T. Gridley. 2001. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol. Cell. Biol. 21:8184–8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero, A., J.A. Horcajadas, F. Dominguez, A. Pellicer, and C. Simon. 2005. Leptin system in embryo development and implantation: a protein in search of a function. Reprod. Biomed. Online. 10:217–223. [DOI] [PubMed] [Google Scholar]
- Chang, C.P., J.R. Neilson, J.H. Bayle, J.E. Gestwicki, A. Kuo, K. Stankunas, I.A. Graef, and G.R. Crabtree. 2004. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell. 118:649–663. [DOI] [PubMed] [Google Scholar]
- Chehab, F.F., M.E. Lim, and R. Lu. 1996. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 12:318–320. [DOI] [PubMed] [Google Scholar]
- Cho, H., J. Mu, J.K. Kim, J.L. Thorvaldsen, Q. Chu, E.B. Crenshaw III, K.H. Kaestner, M.S. Bartolomei, G.I. Shulman, and M.J. Birnbaum. 2001. a. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science. 292:1728–1731. [DOI] [PubMed] [Google Scholar]
- Cho, H., J.L. Thorvaldsen, Q. Chu, F. Feng, and M.J. Birnbaum. 2001. b. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276:38349–38352. [DOI] [PubMed] [Google Scholar]
- Coleman, D.L. 1978. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 14:141–148. [DOI] [PubMed] [Google Scholar]
- Dean, R.A., and C.M. Overall. 2007. Proteomics discovery of metalloproteinase substrates in the cellular context by iTRAQ labeling reveals a diverse MMP-2 substrate degradome. Mol. Cell. Proteomics. 6:611–623. [DOI] [PubMed] [Google Scholar]
- Dummler, B., O. Tschopp, D. Hynx, Z.Z. Yang, S. Dirnhofer, and B.A. Hemmings. 2006. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol. Cell. Biol. 26:8042–8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dünker, N., and K. Krieglstein. 2002. Tgfbeta2 −/− Tgfbeta3 −/− double knockout mice display severe midline fusion defects and early embryonic lethality. Anat. Embryol. (Berl.). 206:73–83. [DOI] [PubMed] [Google Scholar]
- Enciso, J.M., D. Gratzinger, T.D. Camenisch, S. Canosa, E. Pinter, and J.A. Madri. 2003. Elevated glucose inhibits VEGF-A-mediated endocardial cushion formation: modulation by PECAM-1 and MMP-2. J. Cell Biol. 160:605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantuzzi, G., and R. Faggioni. 2000. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J. Leukoc. Biol. 68:437–446. [PubMed] [Google Scholar]
- Frid, M.G., V.A. Kale, and K.R. Stenmark. 2002. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ. Res. 90:1189–1196. [DOI] [PubMed] [Google Scholar]
- Frühbeck, G. 2006. Intracellular signalling pathways activated by leptin. Biochem. J. 393:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge, H., L. Huang, T. Pourbahrami, and C. Li. 2002. Generation of soluble leptin receptor by ectodomain shedding of membrane-spanning receptors in vitro and in vivo. J. Biol. Chem. 277:45898–45903. [DOI] [PubMed] [Google Scholar]
- Ghilardi, N., S. Ziegler, A. Wiestner, R. Stoffel, M.H. Heim, and R.C. Skoda. 1996. Defective STAT signaling by the leptin receptor in diabetic mice. Proc. Natl. Acad. Sci. USA. 93:6231–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetze, S., A. Bungenstock, C. Czupalla, F. Eilers, P. Stawowy, U. Kintscher, C. Spencer-Hansch, K. Graf, B. Nurnberg, R.E. Law, et al. 2002. Leptin induces endothelial cell migration through Akt, which is inhibited by PPARgamma-ligands. Hypertension. 40:748–754. [DOI] [PubMed] [Google Scholar]
- Gordeladze, J.O., and J.E. Reseland. 2003. A unified model for the action of leptin on bone turnover. J. Cell. Biochem. 88:706–712. [DOI] [PubMed] [Google Scholar]
- Grille, S.J., A. Bellacosa, J. Upson, A.J. Klein-Szanto, F. van Roy, W. Lee-Kwon, M. Donowitz, P.N. Tsichlis, and L. Larue. 2003. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 63:2172–2178. [PubMed] [Google Scholar]
- Guo, Z., H. Jiang, X. Xu, W. Duan, and M.P. Mattson. 2007. Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization. J. Biol. Chem. 283:1754–1763. [DOI] [PubMed] [Google Scholar]
- Han, D.C., M. Isono, S. Chen, A. Casaretto, S.W. Hong, G. Wolf, and F.N. Ziyadeh. 2001. Leptin stimulates type I collagen production in db/db mesangial cells: glucose uptake and TGF-beta type II receptor expression. Kidney Int. 59:1315–1323. [DOI] [PubMed] [Google Scholar]
- Haynes, W.G., D.A. Morgan, S.A. Walsh, A.L. Mark, and W.I. Sivitz. 1997. Receptor-mediated regional sympathetic nerve activation by leptin. J. Clin. Invest. 100:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi, K., K. Fulop, K. Kovacs, S. Toth, and A. Falus. 2004. Leptin-induced signal transduction pathways. Cell Biol. Int. 28:159–169. [DOI] [PubMed] [Google Scholar]
- Heim, M.H., I.M. Kerr, G.R. Stark, and J.E. Darnell Jr. 1995. Contribution of STAT SH2 groups to specific interferon signaling by the Jak-STAT pathway. Science. 267:1347–1349. [DOI] [PubMed] [Google Scholar]
- Herrid, M., V.L. Nguyen, G. Hinch, and J.R. McFarlane. 2006. Leptin has concentration and stage-dependent effects on embryonic development in vitro. Reproduction. 132:247–256. [DOI] [PubMed] [Google Scholar]
- Hescheler, J., and B.K. Fleischmann. 2000. Integrins and cell structure: powerful determinants of heart development and heart function. Cardiovasc. Res. 47:645–647. [DOI] [PubMed] [Google Scholar]
- Hileman, S.M., J. Tornoe, J.S. Flier, and C. Bjorbaek. 2000. Transcellular transport of leptin by the short leptin receptor isoform ObRa in Madin-Darby Canine Kidney cells. Endocrinology. 141:1955–1961. [DOI] [PubMed] [Google Scholar]
- Hoffman, J.I. 1995. Incidence of congenital heart disease: II. Prenatal incidence. Pediatr. Cardiol. 16:155–165. [DOI] [PubMed] [Google Scholar]
- Ihle, J.N. 1996. Janus kinases in cytokine signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 351:159–166. [DOI] [PubMed] [Google Scholar]
- Jacoby, J.J., A. Kalinowski, M.G. Liu, S.S. Zhang, Q. Gao, G.X. Chai, L. Ji, Y. Iwamoto, E. Li, M. Schneider, et al. 2003. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc. Natl. Acad. Sci. USA. 100:12929–12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, E.J., K.S. Park, O.S. Bang, and S.S. Kang. 2007. Akt signaling regulates actin organization via modulation of MMP-2 activity during chondrogenesis of chick wing limb bud mesenchymal cells. J. Cell. Biochem. 102:252–261. [DOI] [PubMed] [Google Scholar]
- Jorcyk, C.L., R.G. Holzer, and R.E. Ryan. 2006. Oncostatin M induces cell detachment and enhances the metastatic capacity of T-47D human breast carcinoma cells. Cytokine. 33:323–336. [DOI] [PubMed] [Google Scholar]
- Kano, A., M.J. Wolfgang, Q. Gao, J. Jacoby, G.X. Chai, W. Hansen, Y. Iwamoto, J.S. Pober, R.A. Flavell, and X.Y. Fu. 2003. Endothelial cells require STAT3 for protection against endotoxin-induced inflammation. J. Exp. Med. 198:1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura, K., N. Sato, J. Fukuda, H. Kodama, J. Kumagai, H. Tanikawa, A. Nakamura, and T. Tanaka. 2002. Leptin promotes the development of mouse preimplantation embryos in vitro. Endocrinology. 143:1922–1931. [DOI] [PubMed] [Google Scholar]
- Larue, L., and A. Bellacosa. 2005. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 24:7443–7454. [DOI] [PubMed] [Google Scholar]
- Lee, M.P., S. Madani, D. Sekula, and G. Sweeney. 2005. Leptin increases expression and activity of matrix metalloproteinase-2 and does not alter collagen production in rat glomerular mesangial cells. Endocr. Res. 31:27–37. [DOI] [PubMed] [Google Scholar]
- Li, Y., J. Yang, C. Dai, C. Wu, and Y. Liu. 2003. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J. Clin. Invest. 112:503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner, S., A. Cattelino, R. Gallini, N. Rudini, M. Iurlaro, S. Piccolo, and E. Dejana. 2004. β-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J. Cell Biol. 166:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin, A., H. Rondahl, E. Walum, and M. Wilcke. 2000. Expression and intracellular localization of leptin receptor long isoform-GFP chimera. Biochim. Biophys. Acta. 1499:130–138. [DOI] [PubMed] [Google Scholar]
- Malik, N.M., N.D. Carter, J.F. Murray, R.J. Scaramuzzi, C.A. Wilson, and M.J. Stock. 2001. Leptin requirement for conception, implantation, and gestation in the mouse. Endocrinology. 142:5198–5202. [DOI] [PubMed] [Google Scholar]
- Mantzoros, C.S., and S.J. Moschos. 1998. Leptin: in search of role(s) in human physiology and pathophysiology. Clin. Endocrinol. (Oxf.). 49:551–567. [DOI] [PubMed] [Google Scholar]
- Meisel, S.R., M. Ellis, C. Pariente, H. Pauzner, M. Liebowitz, D. David, and I. Shimon. 2001. Serum leptin levels increase following acute myocardial infarction. Cardiology. 95:206–211. [DOI] [PubMed] [Google Scholar]
- Moon, H.S., H.G. Lee, J.H. Seo, C.S. Chung, D.D. Guo, T.G. Kim, Y.J. Choi, and C.S. Cho. 2007. Leptin-induced matrix metalloproteinase-2 secretion is suppressed by trans-10,cis-12 conjugated linoleic acid. Biochem. Biophys. Res. Commun. 356:955–960. [DOI] [PubMed] [Google Scholar]
- Murad, A., A.K. Nath, S.T. Cha, E. Demir, J. Flores-Riveros, and M.R. Sierra-Honigmann. 2003. Leptin is an autocrine/paracrine regulator of wound healing. FASEB J. 17:1895–1897. [DOI] [PubMed] [Google Scholar]
- Najib, S., and V. Sanchez-Margalet. 2002. Human leptin promotes survival of human circulating blood monocytes prone to apoptosis by activation of p42/44 MAPK pathway. Cell. Immunol. 220:143–149. [DOI] [PubMed] [Google Scholar]
- Nawshad, A., D. Lagamba, A. Polad, and E.D. Hay. 2005. Transforming growth factor-beta signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs. 179:11–23. [DOI] [PubMed] [Google Scholar]
- Nickola, M.W., L.E. Wold, P.B. Colligan, G.J. Wang, W.K. Samson, and J. Ren. 2000. Leptin attenuates cardiac contraction in rat ventricular myocytes. Role of NO. Hypertension. 36:501–505. [DOI] [PubMed] [Google Scholar]
- Nieto, M.A. 2002. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 3:155–166. [DOI] [PubMed] [Google Scholar]
- Nightingale, J., S. Patel, N. Suzuki, R. Buxton, K.I. Takagi, J. Suzuki, Y. Sumi, A. Imaizumi, R.M. Mason, and Z. Zhang. 2004. Oncostatin M, a cytokine released by activated mononuclear cells, induces epithelial cell-myofibroblast transdifferentiation via Jak/Stat pathway activation. J. Am. Soc. Nephrol. 15:21–32. [DOI] [PubMed] [Google Scholar]
- Overall, C.M., G.A. McQuibban, and I. Clark-Lewis. 2002. Discovery of chemokine substrates for matrix metalloproteinases by exosite scanning: a new tool for degradomics. Biol. Chem. 383:1059–1066. [DOI] [PubMed] [Google Scholar]
- Park, H.Y., H.M. Kwon, H.J. Lim, B.K. Hong, J.Y. Lee, B.E. Park, Y. Jang, S.Y. Cho, and H.S. Kim. 2001. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp. Mol. Med. 33:95–102. [DOI] [PubMed] [Google Scholar]
- Peinado, H., M. Quintanilla, and A. Cano. 2003. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J. Biol. Chem. 278:21113–21123. [DOI] [PubMed] [Google Scholar]
- Peng, X.D., P.Z. Xu, M.L. Chen, A. Hahn-Windgassen, J. Skeen, J. Jacobs, D. Sundararajan, W.S. Chen, S.E. Crawford, K.G. Coleman, and N. Hay. 2003. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 17:1352–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person, A.D., S.E. Klewer, and R.B. Runyan. 2005. Cell biology of cardiac cushion development. Int. Rev. Cytol. 243:287–335. [DOI] [PubMed] [Google Scholar]
- Runyan, R.B., and R.R. Markwald. 1983. Invasion of mesenchyme into three-dimensional collagen gels: a regional and temporal analysis of interaction in embryonic heart tissue. Dev. Biol. 95:108–114. [DOI] [PubMed] [Google Scholar]
- Schulze, P.C., J. Kratzsch, A. Linke, N. Schoene, V. Adams, S. Gielen, S. Erbs, S. Moebius-Winkler, and G. Schuler. 2003. Elevated serum levels of leptin and soluble leptin receptor in patients with advanced chronic heart failure. Eur. J. Heart Fail. 5:33–40. [DOI] [PubMed] [Google Scholar]
- Shek, E.W., M.W. Brands, and J.E. Hall. 1998. Chronic leptin infusion increases arterial pressure. Hypertension. 31:409–414. [DOI] [PubMed] [Google Scholar]
- Shimizu, M., S. Kondo, M. Urushihara, M. Takamatsu, K. Kanemoto, M. Nagata, and S. Kagami. 2006. Role of integrin-linked kinase in epithelial-mesenchymal transition in crescent formation of experimental glomerulonephritis. Nephrol. Dial. Transplant. 21:2380–2390. [DOI] [PubMed] [Google Scholar]
- Sierra-Honigmann, M.R., A.K. Nath, C. Murakami, G. Garcia-Cardena, A. Papapetropoulos, W.C. Sessa, L.A. Madge, J.S. Schechner, M.B. Schwabb, P.J. Polverini, and J.R. Flores-Riveros. 1998. Biological action of leptin as an angiogenic factor. Science. 281:1683–1686. [DOI] [PubMed] [Google Scholar]
- Silletti, S., T. Kessler, J. Goldberg, D.L. Boger, and D.A. Cheresh. 2001. Disruption of matrix metalloproteinase 2 binding to integrin alpha vbeta 3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc. Natl. Acad. Sci. USA. 98:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C.C., M.M. Mocanu, S.M. Davidson, A.M. Wynne, J.C. Simpkin, and D.M. Yellon. 2006. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br. J. Pharmacol. 149:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W., K. Jackson, and P.G. McGuire. 2000. Degradation of type IV collagen by matrix metalloproteinases is an important step in the epithelial-mesenchymal transformation of the endocardial cushions. Dev. Biol. 227:606–617. [DOI] [PubMed] [Google Scholar]
- Stupack, D.G., and D.A. Cheresh. 2002. ECM remodeling regulates angiogenesis: endothelial integrins look for new ligands. Sci. STKE . 10.1126/stke.2002.119.pe7. [DOI] [PubMed]
- Stupack, D.G., and D.A. Cheresh. 2004. Integrins and angiogenesis. Curr. Top. Dev. Biol. 64:207–238. [DOI] [PubMed] [Google Scholar]
- Suganami, E., H. Takagi, H. Ohashi, K. Suzuma, I. Suzuma, H. Oh, D. Watanabe, T. Ojima, T. Suganami, Y. Fujio, et al. 2004. Leptin stimulates ischemia-induced retinal neovascularization: possible role of vascular endothelial growth factor expressed in retinal endothelial cells. Diabetes. 53:2443–2448. [DOI] [PubMed] [Google Scholar]
- Sweeney, G. 2002. Leptin signalling. Cell. Signal. 14:655–663. [DOI] [PubMed] [Google Scholar]
- Thomas, T. 2004. The complex effects of leptin on bone metabolism through multiple pathways. Curr. Opin. Pharmacol. 4:295–300. [DOI] [PubMed] [Google Scholar]
- Uotani, S., C. Bjorbaek, J. Tornoe, and J.S. Flier. 1999. Functional properties of leptin receptor isoforms: internalization and degradation of leptin and ligand-induced receptor downregulation. Diabetes. 48:279–286. [DOI] [PubMed] [Google Scholar]
- Weaver, M.S., N. Toida, and E.H. Sage. 2007. Expression of integrin-linked kinase in the murine lens is consistent with its role in epithelial-mesenchymal transition of lens epithelial cells in vitro. Mol. Vis. 13:707–718. [PMC free article] [PubMed] [Google Scholar]
- Yang, Y.J., Y.J. Cao, S.M. Bo, S. Peng, W.M. Liu, and E.K. Duan. 2006. Leptin-directed embryo implantation: leptin regulates adhesion and outgrowth of mouse blastocysts and receptivity of endometrial epithelial cells. Anim. Reprod. Sci. 92:155–167. [DOI] [PubMed] [Google Scholar]
- Yang, Z.Z., O. Tschopp, N. Di-Poi, E. Bruder, A. Baudry, B. Dummler, W. Wahli, and B.A. Hemmings. 2005. Dosage-dependent effects of Akt1/protein kinase Balpha (PKBalpha) and Akt3/PKBgamma on thymus, skin, and cardiovascular and nervous system development in mice. Mol. Cell. Biol. 25:10407–10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavadil, J., and E.P. Bottinger. 2005. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774. [DOI] [PubMed] [Google Scholar]
- Zeidan, A., B. Paylor, K.J. Steinhoff, S. Javadov, V. Rajapurohitam, S. Chakrabarti, and M. Karmazyn. 2007. Actin cytoskeleton dynamics promote leptin-induced vascular smooth muscle hypertrophy via Rho/ROCK and PI3K/Akt dependent pathways. J. Pharmacol. Exp. Ther. 322:1110–1116. [DOI] [PubMed] [Google Scholar]