Abstract
γ-Aminobutyric acid (GABA) type A receptors mediate fast inhibitory synaptic transmission and have been implicated in responses to sedative/hypnotic agents (including neuroactive steroids), anxiety, and learning and memory. Using gene targeting technology, we generated a strain of mice deficient in the δ subunit of the GABA type A receptors. In vivo testing of various behavioral responses revealed a strikingly selective attenuation of responses to neuroactive steroids, but not to other modulatory drugs. Electrophysiological recordings from hippocampal slices revealed a significantly faster miniature inhibitory postsynaptic current decay time in null mice, with no change in miniature inhibitory postsynaptic current amplitude or frequency. Learning and memory assessed with fear conditioning were normal. These results begin to illuminate the novel contributions of the δ subunit to GABA pharmacology and sedative/hypnotic responses and behavior and provide insights into the physiology of neurosteroids.
Inhibitory ion currents in the vertebrate central nervous system primarily are carried by Cl− ions conducted via γ-aminobutyric acid type A receptors (GABAA-Rs). These ligand-gated receptors are believed to be pentamers, with subunits selected from (at least) 15 possible variants (α1–6, β1–4, γ1–3, δ, and ɛ) (1). GABAA-Rs are modulated by many drugs, including ethanol, benzodiazepines, various anesthetics, and neuroactive steroids (2–5). Specific roles for various GABAA-R subunits in mediating anesthetic responses are beginning to be elucidated (6–9). Additionally, GABAA-Rs have been shown to be involved in epilepsy (4, 10, 11), various behavioral states such as depression and anxiety (12), and learning and memory (11, 13).
Several studies have shown that the δ subunit participates in GABAA-Rs that exhibit a unique pharmacology. Such receptor isoforms are benzodiazepine-insensitive (14), neuroactive steroid-insensitive (15), and Zn2+-sensitive (16). Nearly 30% of cerebellar GABAA-Rs appear to contain the δ subunit; levels of δ mRNA are highest in the cerebellar granule cells, secondarily in hippocampus and thalamus (17, 18).
Neuroactive steroids are naturally occurring metabolites of endogenous steroid hormones, or synthetic analogs, which exert very rapid, nongenomic effects on membrane-bound GABAA-Rs (19, 20). Those synthesized in the brain, termed neurosteroids, are believed to regulate anxiety, stress, and neuronal excitability by modulating GABAA-R function in vivo (5, 21). Examples include metabolites of progesterone and corticosterone that enhance GABA inhibition and pregnenolone (3α-hydroxy-5β-pregnan-20-one) sulfate that inhibits GABA action (13, 19–21). The synthetic analog alphaxalone (3α-hydroxy-5α-pregnan-11,20-dione) enhances GABAA-R function and has been used clinically as an i.v. anesthetic. The chemical analog ganaxalone (3α-hydroxy-3β-methyl-5α-pregnan-20-one) was developed for improved bioavailability and potential anxiolytic and anticonvulsant activity (22).
To investigate the contribution of δ-containing GABAA-R isoforms to behavior and various drug responses, we used gene targeting in embryonic stem (ES) cells to create a strain of mice lacking a functional δ-subunit gene. Here we report the characterization of the δ knockout mice.
Materials and Methods
Generation of Mutant Mice.
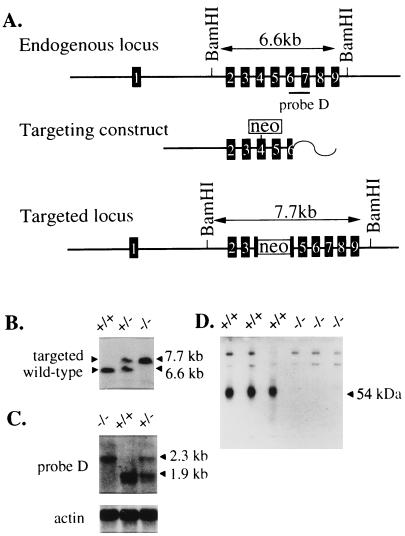
A 9.5-kb PstI restriction fragment was isolated from a strain 129/SvJ P1 phage library (Genome Systems, St. Louis) with δ-specific primers from exon 2 (5′-CAGGGCAATGAATGACATTG-3′) and exon 3 (5′-CAAGCGCCACATTCACAG-3′). A replacement-type DNA targeting vector was constructed in which the MC1Neo gene (Stratagene) was blunt-end ligated into a blunted HindIII site in exon 4 (Fig. 1A). The targeting vector was linearized with ClaI before electroporation into R1 ES cells (23). G418-resistant ES cell clones were screened for targeting by Southern blot analysis of BamHI-digested genomic DNA hybridized with a 3′ probe (probe D) (Fig. 1A).
Figure 1.
Gene targeting and molecular characterization. (A) Structure of δ locus. Numbered black boxes represent exons in genomic DNA. Probe D is 830-bp PCR product used for genotyping. The neo gene was flanked on the 5′ side by 8.3 kb of genomic DNA and 1.2 kb of 3′ genomic DNA. Probe D hybridizes to an ≈7.7-kb BamHI restriction fragment at a correctly targeted locus compared with a 6.6-kb BamHI fragment from the endogenous locus. (B) Southern blot analysis of BamHI digested DNA illustrating mice of all three genotypes. (C) Northern blot analysis using adult whole brain total RNA hybridized with probe D or human β-actin probe for loading control. (D) Western blot analysis of total cerebellar protein from δ+/+ and δ−/− with δ(1–44) polyclonal antibody. The 54-kDa δ protein present in wild-type lanes is completely absent from δ−/− lanes. Higher molecular mass bands of nonspecific binding show equal loading of samples in all lanes.
Three correctly targeted ES cell lines were microinjected into C57BL/6J blastocysts, two of which produced chimeric mice. Highly chimeric males were mated to C57BL/6J females (The Jackson Laboratory). Agouti offspring that were heterozygous for the targeted allele (δ+/−) were interbred to produce mice that were wild type (δ+/+), δ+/−, and homozygous null (δ−/−). The mice used for the studies reported here were derived primarily from the δ#767 ES cell line. A more limited analysis of the δ#773 ES cell line yielded similar results. The genetic background of all mice was C57BL/6J X strain 129Sv/SvJ, F2-F5 generation.
Northern Blot Analysis.
Total RNA was isolated from adult mouse brain by using Trizol reagent (Life Technologies, Grand Island, NY). Approximately 10 μg of total RNA was electrophoresed in a 1.9% formaldehyde/1% agarose gel, blotted to Hybond-N (Amersham Pharmacia) and hybridized as described (24). After the membrane was hydridized with probe D, it was rehybridized with a human β-actin cDNA probe (CLONTECH) as a control for RNA loading in each lane.
Western Blot Analysis.
Antibody production, gel electrophoresis, and blotting were performed as in ref. 25. Briefly, the δ(1–44)R5 polyclonal antibody (26) was prepared by immunizing rabbits with a maltose binding protein-δ(1–44)-7His fusion protein and purifying by affinity chromatography. The antibody is specific for the δ subunit and does not precipitate α1β3γ2 receptors (26). Equal amounts of cerebellar membrane proteins were subject to SDS/PAGE and immunoblotted onto poly(vinylidene difluoride) membranes. Membranes were incubated with digoxigenin-labeled antibodies and treated with anti-digoxigenin-alkaline phosphatase Fab fragments (Boehringer Mannheim). Proteins were detected by fluorescence using the CSPD substrate (Tropix, Bedford, MA). Blots were exposed to Kodak X-Omat S film and recorded with a DocuGel 2000i gel system using rflpscan software (MWG Biotec, Ebersberg, Germany).
GABAA-R Ligand Binding.
[3H]Ro15–4513 and [3H]muscimol binding to whole brain homogenates was determined exactly as described (7). To visualize brain regional distribution of GABA and benzodiazepine binding sites, 14-μm horizontal sections were cut from δ+/+ and δ−/− brains by using a Microm cryostat, thaw-mounted onto gelatin-coated object glasses, and used for [3H]Ro 15–4513 (a benzodiazepine site ligand) and [3H]muscimol (a GABA site ligand) autoradiography as described in detail by Mäkelä et al. (27).
Electrophysiology.
Transverse slices of dorsal hippocampus were obtained by using standard techniques (28). Pharmacologically isolated miniature inhibitory postsynaptic currents (mIPSCs) were recorded from cells located in the upper blade of the dentate gyrus at 34.5°C during perfusion with artificial cerebrospinal fluid (ACSF) composed of 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 26 mM NaHCO3, and 10 mM dextrose. The ACSF was continuously bubbled with a 95%/5% mix of O2/CO2 to ensure adequate oxygenation of slices and a pH of 7.4. Patch pipettes contained 135 mM Cs gluconate, 2 mM MgCl2, 1 mM CaCl2, 11 mM EGTA, 10 mM Pipes, 2 mM K2ATP, and 0.2 mM Na2GTP, pH to 7.5 with CsOH. mIPSCs were recorded in the presence of 0.5 mM tetrodotoxin, 40 mM d(-)-2-amino-5-phosphonopentanoate, 10 mM 6-cyano-7-nitroquinoxaline-2,3-dione, and 1 mM CGP 54,626. Signals were recorded with an amplifier (Axoclamp 2A, Axon Instruments, Foster City, CA). Data were acquired with pclamp 7 (Axon Instruments) software, digitized at 20 kHz (Digidata 1200B, Axon Instruments), and analyzed by using the Mini Analysis Program (version 4.1.1, Jaejin Software, Leonia, NJ).
Behavioral Characterization.
All animal experiments were approved by the Institutional Animal Care and Use Committee. Mice were grouped together by age, weight, and generation such that they were all between 8 and 16 weeks old and 15–35 g. Mice of both sexes were used for all studies.
Sleep Time Assay.
Alphaxalone (8 mg/kg and 16 mg/kg), pregnanolone (16 mg/kg), midazolam (25 mg/kg), and propofol (50 mg/kg) were administered i.v. Etomidate (20 mg/kg), pentobarbital (45 mg/kg), and ketamine (150 mg/kg) were given by i.p. injection. Injection volumes were 5 μl/g body weight for i.v. and 20 μl/g for i.p. Neuroactive steroids [alphaxalone (Research Biochemicals, Natick, MA) and pregnanolone (Sigma)] were dissolved in a 22.5% (wt/vol) solution of 2-hydroxypropyl-β-cyclodextrin (Research Biochemicals). Pentobarbital (Abbott) and ketamine (Sigma) were dissolved in sterile saline. Midazolam HCl (Roche Clinical Laboratories, Burlington, NC) was dissolved in 0.8% NaCl, 0.01% EDTA, and 1% benzyl alcohol.
Sleep times were determined as follows: after drug injection and loss of the righting reflex, mice were placed on their backs in a V-shaped trough and a timer was started. The sleep time period ended when the animal was able to flip over six times in 45 sec (neuroactive steroids) or three times in 30 sec (all other drugs). Normothermia was maintained with the aid of a heat lamp. All assays were performed by an investigator who was unaware of the genotypes of the individual mice being tested. Effect of genotype was compared by Student’s t test.
Loss of Righting Reflex (LORR) and Tail-Clamp/Withdrawal Assay.
LORR and tail-clamp/withdrawal using volatile anesthetics were performed as described (7). Briefly, mice were placed in small, cylindrical wire-mesh cages attached to a carousel housed in a sealed Plexiglas box. Anesthetics diluted with oxygen were delivered from an anesthetic-specific vaporizer. After 15-min equilibration at each concentration, the carousel was rotated five times while the mice were observed. Scoring was quantal; mice that passively rolled over twice were scored as positive for LORR. For tail-clamp, the carousel was removed and a 10-compartment divider was installed. After a 15-min exposure to each concentration of anesthetic, a tail-clamp stimulus was given. If any motor activity occurred as a result of the stimulus, the concentration was scored as one that permitted a positive response.
Elevated Plus-Maze Test.
The anxiolytic effect of the synthetic neuroactive steroid ganaxolone (CoCensys, Irvine, CA) was tested by using the elevated plus-maze. The plus-maze was constructed as described (29). A stock solution of ganaxolone was made in DMSO and diluted with saline (pH 7.1) before systemic administration. The final concentration of DMSO was 0.1%, a concentration known not to interfere with GABAA-R function (30).
Ganaxolone (10 mg/kg, i.p.) or an equal volume of saline was injected 10 min before testing (n = 9/group). To start the 5-min test session, a mouse was placed on the central platform of the maze, facing an open arm. Open-arm and closed-arm entries and the cumulative time spent on the open and closed arms was recorded. A mouse was considered to be on the central platform when all four paws were within its perimeter. The ratio of open/total arm entries (% open-arm entries) was calculated and expressed as mean % open-arm entries ± SEM. Data were analyzed by using one-way ANOVA, and ganaxolone-treated group means were compared with their respective saline-treated controls by using Bonferroni’s post-hoc test.
Low-Dose Pentylenetetrazol (PTZ) Seizures.
Pretreatment of δ+/+ and δ−/− mice with ganaxolone (10 mg/kg, i.p.) or saline occurred 10 min before PTZ (Sigma) (20 mg/kg, i.p.) injection. The behavior induced by saline/PTZ versus ganaxolone/PTZ was observed and recorded for 2–3 h post-PTZ treatment. Data are expressed as mean duration of hypoactivity ± SEM (n = 9/group). One-way ANOVA followed by Bonferroni’s post-hoc test was used to compare multiple group means.
Pavlovian Fear Conditioning.
Associative memory was examined with context and tone-dependent fear conditioning (31, 32). Briefly, a tone conditional stimulus was paired with an aversive unconditional foot shock stimulus in a novel context. Mice trained in this manner develop a fear of both tone and context, which was measured as freezing, an adaptive defense reaction. One day after conditioning, mice were returned to the training context for an 8-min context test. One day after the context test, mice were placed in a novel context for an 8-min tone fear test. After a 2-min baseline period, the training tone was played for 6 min. Freezing (% time ± SEM) was scored continuously during both tests. To assess exploratory activity, crossovers were scored during the 3-min period before tone-shock pairing on the conditioning day. To assess pain sensitivity, velocity was computer-tracked for 2 sec immediately before and for 2 sec during the first foot shock (33). Consolidation and retention of contextual fear was examined by repeating the context test 50 days after training.
Results
Production of Mice.
Of 307 ES cell clones analyzed for gene targeting, three displayed the predicted BamHI restriction fragment length polymorphism indicative of correct gene targeting at the δ locus. Two cell lines (δ#767 and δ#773) yielded germ-line competent chimeric males. As indicated in Fig. 1B, probe D hybridized to a 6.6-kb BamHI fragment from the wild-type δ gene and a 7.7-kb BamHI fragment from the targeted allele. The δ locus was examined with 12 restriction enzymes, three unique genomic probes, and a neo probe. All results (data not shown) were consistent with the targeting event depicted in Fig. 1A.
Northern and Western blots were used to examine δ gene expression. Hybridization of adult cerebellar total RNA with probe D showed an abundant 1.9-kb band in the δ+/+ mice. In contrast, samples from δ−/− mice revealed the absence of the wild-type 1.9-kb mRNA (Fig. 1C). In addition, in mice bearing a targeted allele, a novel 2.3-kb band was observed. Rehybridization of the same blot with a neo probe revealed that only the 2.3-kb message from δ+/− and δ−/− mice contained neo-homologous sequences (data not shown). These results suggest that the targeting event resulted in a δ allele that was transcribed as a chimeric δ/neo mRNA.
Western blots from cerebellar membranes (the neuronal tissue with the highest level of δ expression) of three different δ+/+ and three different δ−/− mice were probed with the rabbit polyclonal antibody δ(1–44) (26). The δ subunit exhibits an apparent molecular mass of 54 kDa (Fig. 1D) and is readily apparent in the δ+/+ lanes. In contrast, samples from the δ−/− mouse cerebellums were completely devoid of the δ protein. Together, these results demonstrated that the gene targeting event disrupted the δ locus and prevented production of δ protein, i.e., a true null allele.
The size, histological appearance, and folding of the folia in the cerebellum all appeared normal and indistinguishable between δ+/+ and δ−/− mice, as did the staining patterns of toluidine blue, RT97, and antisynaptophysin (data not shown).
Of 1,030 F2 and F5 pups genotyped at weaning from heterozygote mating pairs, only 211 (20.5%) were null. This percent of null pups is significantly below the expected Mendelian frequency distribution of 25% (P < 0.005). Thus it appears that ≈5% of δ−/− pups die before weaning. To determine whether the δ knockout had any effect on fecundity, true breeding F3 lines were established. These breeding experiments revealed that δ−/− breeding pairs produced statistically fewer pups per litter than the δ+/+ breeding pairs [δ+/+ matings: 7.6 ± 0.4 pups/litter (n = 47 litters) vs. δ−/− matings: 6.3 ± 0.3 pups/litter (n = 50 litters)] (P < 0.01).
Pharmacological Characterization.
Ligand binding in whole brain homogenates from δ+/+ and δ−/− mice was studied with [3H]Ro15–4513 and [3H]muscimol. The slopes of the binding isotherms were comparable (Table 1), allowing valid comparison of the Kd values. Maximal receptor number and binding affinity for [3H]muscimol in homogenates from δ+/+ brains were similar to previous reports (7, 34). However, the maximal binding for muscimol was markedly decreased in δ−/− homogenates (Table 1). The binding affinity for [3H]Ro15–4513 in homogenates from δ+/+ was similar to previous reports (7). The maximal receptor number and affinity constants for [3H]Ro15–4513 were not different between δ+/+ and δ−/− brain homogenates (Table 1).
Table 1.
Binding of [3H]Ro15-4513 and [3H]muscimol to whole brain homogenates
| Wild type | Null | |
|---|---|---|
| [3H]Ro15-4513 binding* | ||
| Apparent Kd (nM) | 8.9 ± 1.2 | 9.1 ± 1.0 |
| Maximal binding (pmol/mg) | 1.4 ± 0.1 | 1.2 ± 0.1 |
| Slope | 1.2 ± 0.2 | 1.3 ± 0.3 |
| [3H]Muscimol binding† | ||
| Apparent Kd (nM) | 37.0 ± 9.9 | 21.0 ± 10.9 |
| Maximal binding (pmol/mg) | 4.5 ± 0.4 | 2.4 ± 0.4‡ |
| Slope | 1.1 ± 0.2 | 1.1 ± 0.4 |
These data were pooled from four separate binding experiments for each genotype. Each experiment consisted of five specific binding determinations for each [3H]Ro15-4513 concentration tested.
† These data were pooled from three separate binding experiments for each genotype. Each experiment consisted of five specific binding determinations at each [3H]muscimol concentration tested.
‡P < 0.02.
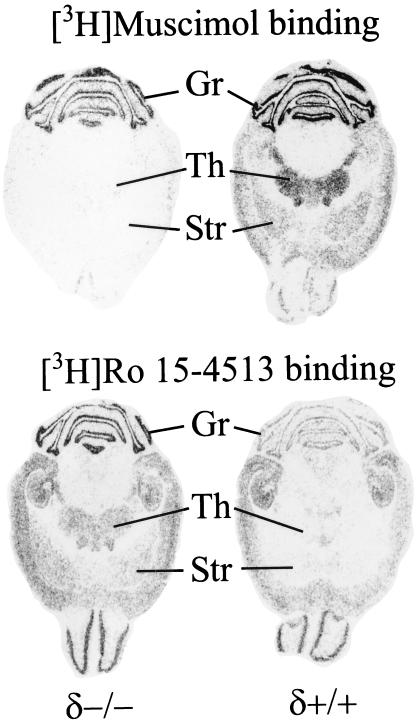
Using in situ autoradiography to examine the abundance and distribution of GABA and benzodiazepine sites, it was observed that [3H]muscimol binding was drastically reduced in most regions of δ−/− brains (Fig. 2). In contrast, the [3H]Ro 15–4513 binding was slightly elevated in several regions of the δ−/− brain, including the thalamus, striatum, and the cerebellar granule cell layer.
Figure 2.
Autoradiographic distribution of [3H]muscimol and [3H]Ro 15–4513 binding sites in horizontal brain sections of GABAA-R δ+/+ and δ−/− mice. Representative images illustrate the reduction of [3H]muscimol binding and region-specific elevation of [3H]Ro 15–4513 binding. Gr, cerebellar granule cell layer; Th, thalamus; Str, striatum.
Delta Knockout Mice Exhibit Altered Synaptic GABAA Currents.
mIPSCs were recorded in hippocampal slices from adult δ+/+ and δ−/− mice to directly investigate the possibility of altered GABAA-R current kinetics in mutant mice. Table 2 illustrates that the average amplitude, rise time, and frequency of mIPSCs recorded at 0 mV did not differ between genotypes. However, the decay time (τ) of mIPSCs from δ−/− mice was significantly faster than that of δ+/+ controls.
Table 2.
Hippocampal electrophysiology
| Genotype | Wild type | Null |
|---|---|---|
| Rise time (msec) | 1.53 ± 0.11 | 1.49 ± 0.10 |
| Amplitude (mV) | 11.30 ± 1.05 | 9.92 ± 0.65 |
| Frequency (Hz) | 3.04 ± 0.42 | 2.85 ± 0.26 |
| Decay, τ (msec) | 7.05 ± 0.39 | 5.74 ± 0.18* |
| # Cells/mice | 16/14 | 12/6 |
τ is the time from 90% to 37% of decay phase of mIPSC.
*P < 0.05.
Delta Knockout Mice Are Resistant to Neuroactive Steroids: Reduced Sleep Time.
Using a sleep time assay, significant differences were found in response to i.v. injections of alphaxalone and pregnanolone (Table 3). The δ−/− mice had a 54% and 38% reduction in sleep time duration compared with δ+/+ at the two concentrations of alphaxalone used (8 mg/kg and 16 mg/kg), respectively. Comparison of δ+/+ and δ−/− after an 8 mg/kg injection of pregnanolone revealed a 42% reduction in sleep time in δ−/− mice. Intraperitoneal injection of alphaxalone (75 mg/kg) also revealed a similar difference (data not shown). Similar effects were observed in δ−/− mice derived from the δ#773 cell line (data not shown). Wild-type and δ−/− mice responded similarly to etomidate, pentobarbital, midazolam, propofol, and ketamine (an NMDA receptor antagonist) (Table 3).
Table 3.
Sleep time assays
| Drug (dose) | n | Genotype | Sleep time, min (mean ± SEM) |
|---|---|---|---|
| Alphaxalone (8 mg/kg) | 18 | Wild type | 3.5 ± 0.5 |
| 11 | Null | 1.6 ± 0.4* | |
| Alphaxalone (16 mg/kg) | 17 | Wild type | 8.6 ± 0.8 |
| 13 | Null | 5.3 ± 0.7† | |
| Pregnanolone (8 mg/kg) | 18 | Wild type | 24.1 ± 1.8 |
| 12 | Null | 14.0 ± 2.2‡ | |
| Pentobarbital (45 mg/kg) | 25 | Wild type | 56.4 ± 3.2 |
| 21 | Null | 52.2 ± 3.6 | |
| Propofol (50 mg/kg) | 16 | Wild type | 14.1 ± 1.8 |
| 11 | Null | 13.7 ± 2.5 | |
| Midazolam (25 mg/kg) | 22 | Wild type | 14.4 ± 4.0 |
| 16 | Null | 13.2 ± 4.8 | |
| Etomidate (20 mg/kg) | 19 | Wild type | 55.3 ± 5.0 |
| 20 | Null | 42.0 ± 4.5 | |
| Ketamine (150 mg/kg) | 6 | Wild type | 40.1 ± 3.8 |
| 6 | Null | 37.5 ± 2.6 |
*, P < 0.02; †, P < 0.01; ‡, P < 0.002.
A trivial explanation for the change in neuroactive steroid response could be an unexpected pharmacokinetic effect. To investigate this possibility, pregnanolone concentration in brain tissue was determined (35) in a limited number of mice at waking from a 16 mg/kg i.v. injection. After an average sleep time of 45.3 ± 7.4 min, δ+/+ brains had 5.5 ± 0.8 (n = 2) μg/g pregnanolone, and after an average sleep time of 33.2 ± 4.9 min, δ−/− brains had 7.3 ± 1.2 (n = 3) μg/g pregnanolone. The level of endogenous allopregnanolone also was measured in the same mice: δ+/+, 514 ± 48 pg/g (n = 2) and δ−/−, 394 ± 118 pg/g (n = 3). These preliminary results indicate no gross differences between genotypes in neuroactive steroid pharmacokinetics or endogenous levels.
A second explanation for the difference in sleep times is that they result from the influence of genetic background. Therefore, the two strains used to make the δ−/− mice, C57BL/6J and strain 129/SvJ mice, were tested with alphaxalone (16 mg/kg) and pregnanolone (16 mg/kg). Sleep times were not statistically different between strains for either compound: alphaxalone, C57BL/6J (8.1 ± 1.3 min., n = 10) and strain 129/SvJ (9.1 ± 2.7 min., n = 10) (P = 0.34) and pregnanolone, C57BL/6J (38.4 ± 3.5 min., n = 9) and strain 129/SvJ (43.8 ± 1.6 min., n = 10) (P = 0.16). Thus, the phenotype observed in the δ−/− mice is not likely caused by genetic background effects from the parental strains.
Reduced Anxiolysis.
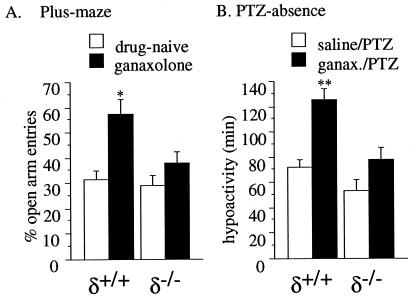
Using an elevated plus-maze assay, the tendency of mice to enter or remain on the open arm of the maze is increased by anxiolytic drugs (36). Basal levels of anxiety (i.e., anxiety levels in drug-naive mice) were not statistically different between genotypes. With percent open-arm entries as a measure of anxiolysis, a 10 mg/kg i.p. dose of ganaxolone in δ+/+ mice caused open-arm entries to increase nearly 2-fold (Fig. 3A; *, P < 0.01). No significant increase in open-arm entries occurred in δ−/− mice. Thus, the well-characterized anxiolytic effect of neuroactive steroids was absent in δ−/− mice.
Figure 3.
Anxiolytic (A) and pro-absence (B) effects of ganaxolone in δ+/+ and δ−/− mice. (A) Mice were injected with ganaxolone (10 mg/kg, i.p.) 10 min before testing on the elevated plus-maze assay (n = 9/group). Ganaxolone produced an ≈2-fold increase in the number of open-arm entries in δ+/+ (*, P < 0.01, Bonferroni’s post-hoc test), whereas no significant change was observed in δ−/−. (B) Mice were treated with ganaxolone (10 mg/kg, i.p.) or saline (n = 9/group) 10 min before an absence seizure-producing dose of PTZ (20 mg/kg). Behavior was observed for 2–3 h posttreatment. Ganaxolone significantly prolonged absence-like behavior in δ+/+ (**, P < 0.001, Bonferroni’s post-hoc test), but not in δ−/− mice (mean ± SEM).
Reduced Pro-Absence Seizure Effect.
Absence seizures are exacerbated by GABAA-R agonists, including neuroactive steroids (37). Using a low-dose PTZ absence seizure model, ganaxolone (10 mg/kg, i.p.) failed to prolong PTZ-induced absence-like freezing in δ−/− mice, whereas it increased absence-like freezing 74% in δ+/+ mice (Fig. 3B; **, P < 0.001). PTZ-induced hypoactivity was not statistically different between genotypes.
Normal Behavioral Responses to Volatile Anesthetics.
Wild-type and δ−/− mice were compared for their sensitivity toward the obtunding (LORR) and pain suppression (tail-clamp/withdrawal) effects of two halogenated volatile anesthetics. There was no difference statistically in the tail-clamp response between δ+/+ and δ−/− mice in the EC50 value for either anesthetic or for LORR with halothane (Table 4). Similar results were obtained in our analyses of the δ#773 mice (data not shown).
Table 4.
Behavioral responses to volatile anesthetics
| Genotype | n | Anesthetic | EC50 (atm % ± SEM) | Slope (mean ± SEM) |
|---|---|---|---|---|
| Tail clamp | ||||
| Wild type | 30 | Halothane | 1.55 ± 0.03 | 10.71 ± 1.66 |
| Null | 29 | Halothane | 1.50 ± 0.04 | 11.11 ± 1.72 |
| Wild type | 20 | Enflurane | 2.43 ± 0.05 | 11.11 ± 2.03 |
| Null | 20 | Enflurane | 2.32 ± 0.05 | 11.86 ± 2.09 |
| LORR assay | ||||
| Wild type | 19 | Halothane | 0.72 ± 0.04 | 8.83 ± 2.74 |
| Null | 15 | Halothane | 0.71 ± 0.04 | 10.65 ± 1.47 |
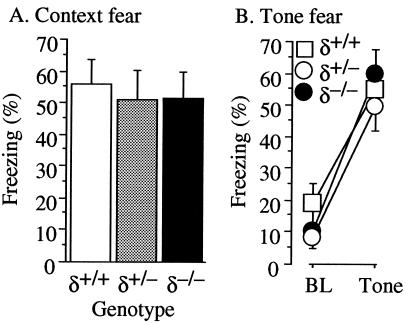
Normal Fear Conditioning, Exploratory Activity, and Pain Sensitivity.
Fig. 4A depicts freezing during the 8-min context test. There were no significant differences in context conditioning. Fig. 4B depicts cued conditioning, during the 2-min baseline period before and during the 6-min tone: there were no significant differences. Mutant mice showed comparable levels of activity to δ+/+ during the baseline period on the conditioning day and showed robust foot shock reactivity equivalent to controls (data not shown). Finally, δ−/− mice showed robust context freezing during the 50-day posttraining memory test, not significantly different than δ+/+ (data not shown).
Figure 4.
Pavlovian fear conditioning. Mice were given three tone-shock pairings in a distinctive context. (A) Context fear. One day after training, mice were returned to conditioning chambers and contextual freezing (% time, mean ± SEM) was assessed for 8 min. Mutant mice exhibited normal levels of contextual fear. (B) Tone fear. One day after the context fear test, the mice were brought to a novel context, and after a 2-min baseline (BL) period, the conditioning tone was played for 6 min. Freezing (%time, mean ± SEM) was assessed for both periods.
Discussion
The absence of the δ subunit of the GABAA-R resulted in a significant decrease in the sensitivity to neuroactive steroids. The duration of alphaxalone/pregnanolone anesthesia and the anxiolytic effect and pro-absence seizure effect of ganaxolone all were reduced/ablated. The reduced response of δ−/− mice to neuroactive steroids reveals a potential role of δ-containing GABAA-Rs in modulating behavioral responses to endogenous neuroactive steroids. This dramatic reduction in whole animal neuroactive steroid sensitivity was specific, as deletion of δ had no effect on response to several other sedative/hypnotic agents in the sleep time assay or on the LORR or tail clamp/withdrawal response after exposure to volatile anesthetics. The observed difference does not appear to be confounded by pharmacokinetic or genetic background influences. These results show an animal model that demonstrates a selective alteration in behavioral responses to neuroactive steroids. If responses to endogenous neuroactive steroids are similarly attenuated, these mice will be invaluable for elucidating the physiological mechanisms of these enigmatic compounds.
These results support the findings that neuroactive steroids have unique, noninteracting binding requirements on GABAA-Rs distinct from those of benzodiazepines and barbiturates (19, 21). Additionally, the differential behavioral response to sedative/hypnotic agents indicates that different drugs have different molecular targets as suggested by Eger et al. (38) and illustrated by the GABAA-R β3 knockout (9). It remains to be determined whether δ subunit-containing GABAA-Rs represent actual targets of steroid action or whether the behavioral sensitivity is indirectly altered in the δ−/− mice.
Although these results (i.e., reduced behavioral response to neuroactive steroids in δ−/− mice) may appear at odds to those with Zhu et al. (15) (i.e., enhanced sensitivity to neuroactive steroids by GABAA-Rs lacking δ), one must keep in mind that in vivo behavioral changes may not necessarily be directly related to in vitro findings using recombinant receptors, because of the complexity of the nervous system, the circuits involved, pleiotropic changes in a chronic model (lifetime null mutation), etc. Detailed in vitro studies will be needed to analyze the possible mechanism(s) responsible for the altered neuroactive steroid sensitivity. One possibility would be that the GABAA-Rs remaining in δ−/− mice have reduced sensitivity to neuroactive steroids.
The dentate gyrus granule cell electrophysiology (chosen because it is one of three major cell types that express δ) suggests that there is no drastic reduction in synaptic inhibition in these cells because of the lack of decrease in mIPSC amplitude and frequency. Rather, there may be a change in the molecular composition of the GABAA-Rs involved. The observed faster decay of mIPSCs is consistent with such a possibility. The electrophysiological data also suggest δ-subunit involvement in normal synaptic transmission in the dentate gyrus.
Analysis of the expected Mendelian ratio of pups and the fecundity of wild-type versus null breeding pairs indicated that deletion of the δ subunit impacted reproduction: litters from δ+/− parents had fewer than the expected number of null pups and δ−/− parents had fewer pups per litter than δ+/+ parents. The reduced sensitivity to neuroactive steroids may affect multiple aspects of reproduction and development, an intriguing possibility that warrants further investigation.
In conclusion, we have shown that a global deletion of the δ subunit of the GABAA-R resulted in a decrease in the sensitivity of mice to the sedative/hypnotic, anxiolytic, and pro-absence effects of neuroactive steroids, and this change was remarkably specific. Together, these results reveal a central involvement of δ-containing GABAA-Rs in neuroactive steroid action in multiple behavioral modalities.
Acknowledgments
We thank Carolyn Ferguson, JoAnn Steinmiller, Frank Kist, Janey Whalen, and Jodi Daggett for expert technical assistance. This work was supported by the University Anesthesiology and Critical Care Medicine Foundation, the National Institutes of Health (Grants AA10422 to G.E.H., GM52035 to L.L.F., and NS28772 to R.W.O.), and the National Science Foundation (Grant IBN 9723295 to M.S.F.).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GABA, γ-aminobutyric acid; GABAA-R, GABA type A receptor; mIPSC, miniature inhibitory postsynaptic current; PTZ, pentylenetetrazol; LORR, loss of righting reflex; ES, embryonic stem.
References
- 1.Barnard E A, Skolnick P, Olsen R W, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson A N, Langer S Z. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 2.Homanics G E, Quinlan J J, Mihalek R, Firestone L L. Toxicol Lett. 1998;100–101:301–307. doi: 10.1016/s0378-4274(98)00199-4. [DOI] [PubMed] [Google Scholar]
- 3.Lüddens H, Korpi E R. Neuroscientist. 1996;2:15–23. [Google Scholar]
- 4.Olsen R W, DeLorey T M, Gordey M, Kang M-H. In: Jasper’s Basic Mechanisms of the Epilepsies: Advances in Neurology. 3rd Ed. Delgado-Escueta A V, Wilson W A, Olsen R W, Porter R J, editors. Vol. 79. Philadelphia: Lippincott; 1999. pp. 499–510. [Google Scholar]
- 5.Olsen R W, Sapp D W. Adv Biochem Psychopharmacol. 1995;48:57–74. [PubMed] [Google Scholar]
- 6.Günther U, Benson J, Benke D, Fritschy J, Reyes G, Knoflach F, Crestani F, Aguzzi A, Arigoni M, Lang Y, et al. Proc Natl Acad Sci USA. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homanics G E, Ferguson C, Quinlan J J, Daggett J, Snyder K, Lagenaur C, Mi Z P, Wang X H, Grayson D R, Firestone L L. Mol Pharmacol. 1997;51:588–596. doi: 10.1124/mol.51.4.588. [DOI] [PubMed] [Google Scholar]
- 8.Mihic S, Ye Q, Wick M, Koltchine V, Finn S, Krasowski M, Hanson K, Mascia M, Valenzuela C, Greenblatt E, et al. Nature (London) 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 9.Quinlan J J, Homanics G E, Firestone L L. Anesthesiology. 1998;88:775–780. doi: 10.1097/00000542-199803000-00030. [DOI] [PubMed] [Google Scholar]
- 10.Huntsman M M, Porcello D M, Homanics G E, DeLorey T M, Huguenard J R. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- 11.DeLorey T M, Handforth A, Anagnostaras S G, Homanics G E, Minassian B A, Asatourian A, Fanselow M S, Delgado-Escueta A, Ellison G D, Olsen R W. J Neurosci. 1998;18:8505–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent J P, Belzung C, Fritschy J-M, Luscher B, Mohler H. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- 13.Flood J F, Morley J E, Roberts E. Proc Natl Acad Sci USA. 1992;89:1567–1571. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shivers B D, Killisch I, Sprengel R, Sontheimer H, Kohler M, Schofield P R, Seeburg P H. Neuron. 1989;3:327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhu W J, Wang J F, Krueger K E, Vicini S. J Neurosci. 1996;16:6648–6656. doi: 10.1523/JNEUROSCI.16-21-06648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxena N, Macdonald R. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- 17.Laurie D J, Seeburg P H, Wisden W. J Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurie D J, Wisden W, Seeburg P H. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert J J, Belelli D, Hill-Venning C, Callachan H, Peters J A. Cell Mol Neurobiol. 1996;16:155–174. doi: 10.1007/BF02088174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison N L, Simmonds M A. Brain Res. 1984;323:287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- 21.Morrow A L, Pace J R, Purdy R H, Paul S M. Mol Pharmacol. 1990;37:263–270. [PubMed] [Google Scholar]
- 22.Gasior M, Carter R B, Goldberg S R, Witkin J M. J Pharmacol Exp Ther. 1997;282:543–553. [PubMed] [Google Scholar]
- 23.Nagy A, Cocza E, Merenties Diaz E, Prideaux V R, Ivanyi E, Markkula M, Rossant J. Development (Cambridge, UK) 1990;110:815–821. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- 24.Homanics G E. Dev Genet. 1991;12:371–379. doi: 10.1002/dvg.1020120506. [DOI] [PubMed] [Google Scholar]
- 25.Jones A, Korpi E R, McKernan R M, Pelz R, Nusser Z, Makela R, Mellor J R, Pollard S, Bahn S, Stephenson F A, et al. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jechlinger M, Pelz R, Tretter V, Klausberger T, Sieghart W. J Neurosci. 1998;18:2449–2457. doi: 10.1523/JNEUROSCI.18-07-02449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mäkelä R, Uusi-oukari M, Homanics G E, Quinlan J J, Firestone L L, Wisden W, Korpi E R. Mol Pharmacol. 1997;52:380–388. doi: 10.1124/mol.52.3.380. [DOI] [PubMed] [Google Scholar]
- 28.Spigelman I, Zhang L, Carlen P L. J Neurophysiol. 1992;68:55–69. doi: 10.1152/jn.1992.68.1.55. [DOI] [PubMed] [Google Scholar]
- 29.Pellow S, Chopin P, File S E, Briley M. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 30.Nakahiro M, Arakawa O, Narahashi T, Ukai S, Kato Y, Nishinuma K, Nishimura T. Neurosci Lett. 1992;138:5–8. doi: 10.1016/0304-3940(92)90459-k. [DOI] [PubMed] [Google Scholar]
- 31.Anagnostaras S G, Maren S, Fanselow M S. J Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva A J. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 33.DeLorey T M, Handforth A, Homanics G E, Minassian B A, Anagnostaras S G, Asatourian A, Ellison G, Fenslow M S, Delgado- Escueta A V, Olsen R W. J Neurosci. 1998;18:8505–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Salvaterra P, Roberts E. Biochem Pharmacol. 1979;28:1123–1128. doi: 10.1016/0006-2952(79)90316-2. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto K, Uzunova V, Pinna G, Taki K, Uzunov D, Mienville J, Guidotti A, Costa E. J Neuropharmacol. 1999;38:955–963. doi: 10.1016/s0028-3908(99)00018-0. [DOI] [PubMed] [Google Scholar]
- 36.Lister R G. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 37.Snead O C., III Ann Neurol. 1998;44:688–691. doi: 10.1002/ana.410440417. [DOI] [PubMed] [Google Scholar]
- 38.Eger E I, Koblin D D, Harris R A, Kendig J J, Pohorille A, Halsey M J, Trudell J R. Anesth Analg. 1997;84:915–918. doi: 10.1097/00000539-199704000-00039. [DOI] [PubMed] [Google Scholar]