Abstract
In man the brain accounts for about 20% of the body's free cholesterol, most of which is synthesised de novo in brain. To maintain cholesterol balance throughout life, cholesterol becomes metabolised to 24S-hydroxycholesterol principally in neurons. In mouse, rat, and probably human, metabolism to 24S-hydroxycholesterol accounts for about 50% of cholesterol turnover, however, the route by which the remainder is turned over has yet to be elucidated. Here we describe a novel liquid chromatography (LC) – multi-stage fragmentation mass spectrometry (MSn) methodology for the identification, with high sensitivity (low pg), of cholesterol metabolites in rat brain. The methodology includes derivatisation to enhance ionisation, exact mass analysis at high-resolution to identify potential metabolites, and LC-MS3 to allow their characterisation. 24S-Hydroxycholesterol was confirmed as a major oxysterol in rat brain, while other oxysterols identified for the first time in brain included 24,25-, 24,27-, 25,27-, 6,24, 7α,25-, and 7α,27-dihydroxycholesterols. In addition, 3β-hydroxy-5-oxo-5,6-secocholestan-6-al and its aldol, two molecules linked to amyloidogenesis of proteins, were characterised in rat brain.
Keywords: Sterol, Cholesterol, 24S-hydroxycholesterol, dihydroxycholesterol, secosterol, derivatisation, Girard P, LTQ Orbitrap, Liver X Receptor, Alzheimer's disease
Introduction
Cholesterol in brain is synthesised de novo throughout life (1,2). The majority of experimental evidence suggests it cannot cross the blood brain barrier (BBB) (2), and to maintain cholesterol balance in brain it is converted to 24S-hydroxycholesterol, a transport form of cholesterol which can pass the BBB (3). In mouse, rat, and probably human, metabolism of cholesterol to 24S-hydroxycholesterol accounts for about 50% of cholesterol turnover (2,4,5), however, the mechanism by which the remaining cholesterol is turned over has yet to be elucidated. 24S-Hydroxycholesterol is a member of a class of oxidised cholesterol metabolites collectively known as oxysterols, it not only acts as a transport form of cholesterol, but is a biologically active molecule, activating the liver X receptors (LXR) (6). Interestingly, both cholesterol 24-hydroxylase (CYP46A1), the enzyme responsible for hydroxylation of cholesterol at C-24 (7), and LXRβ show similar patterns of expression in brain (8). Other oxysterols have also been identified in brain (9), including cholest-4-en-3-one (10), and 3β-hydroxy-5-oxo-5,6-secocholestan-6-al (11) (5,6-seco-sterol). Cholest-4-en-3-one has been shown to be formed in an oxidation reaction catalysed by a amyloid beta (Aβ) peptide – Cu2+ complex, while 5,6-seco-sterol is suggested to be formed as a result of cholesterol ozonolysis, and is proposed to covalently modify Aβ and initiate amyloidogenesis. This suggestion is at present under debate (12).
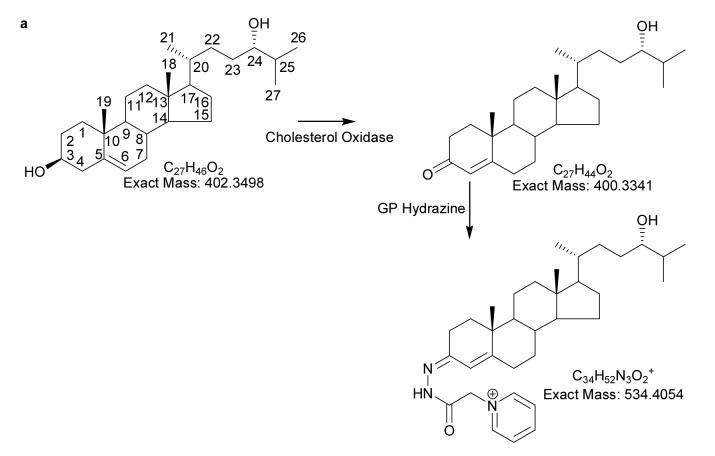
Oxysterol analysis by mass spectrometry (MS) has traditionally been performed in combination with gas-chromatography (GC) following derivatisation (13) i.e. by GC-MS. However, identification of unknown metabolites is hampered by elimination of derivatising groups in the ion-source (14), making molecular weight determination difficult, if not impossible. As an alternative, atmospheric pressure ionisation (API) methods are used. Oxysterol analysis by electrospray (ES) suffers from their poor ionisation cross-section, while atmospheric pressure chemical ionisation (APCI) and atmospheric pressure photoinisation (APPI) both lead to dehydrated protonated molecules and an absence of molecular weight information (15-17). To improve the analysis of oxysterols by ES, we have introduced a derivatisation method which involves: (i) conversion of the 3β-hydroxy-5-ene (or 3β-hydroxy-) to a 3-oxo-4-ene (or a 3-oxo) group, (ii) and the reaction of the resultant oxo group with the Girard P (GP) hydrazine reagent to give a GP hydrazone (Fig. 1a). As the GP hydrazone contains a quaternary nitrogen, the derivatised oxysterol gives a high ion-yield in the ES ion-source (18).
Fig. 1.
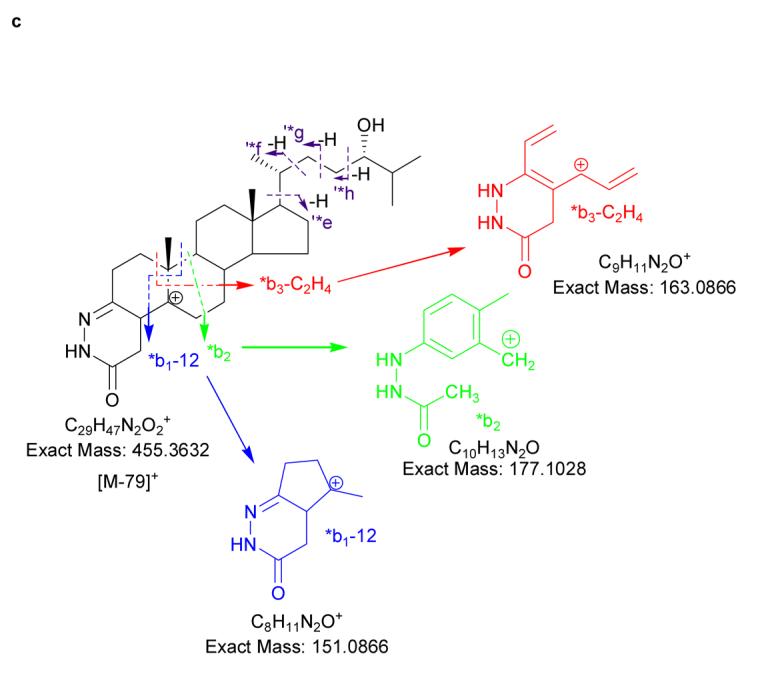
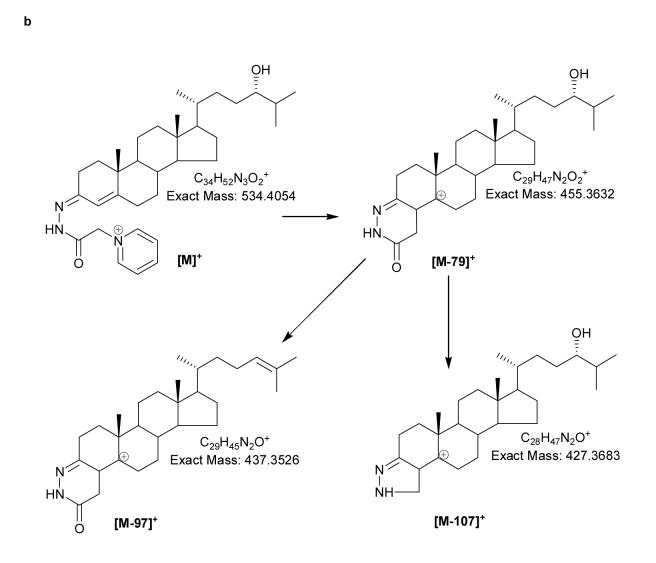
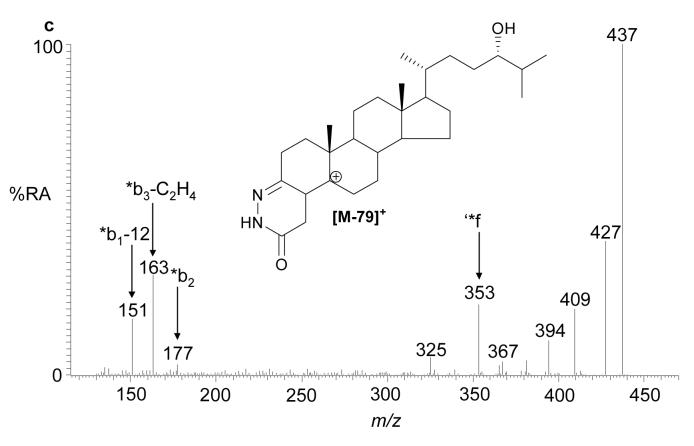
(a) Oxidation and derivatisation of sterols to give GP hydrazones. (b) MS2 fragmentation of sterol GP hydrazones. (c) MS3 ([M]+→[M-79]+→) fragmentation of 3-oxo-4-ene GP hydrazones. In (a), (b) and (c) 24S-hydroxycholesterol is used as an example. Elemental compositions were confirmed by exact mass measurements. An asterisk preceding a fragment-ion describing letter e.g. *b1-12, indicates that the fragment-ion has lost the pyridine moiety from the derivatising group. A prime preceding a fragment-ion describing letter e.g. ‘*f, indicates that cleavage proceeds with the transfer of a H-atom from the ion to the neutral fragment. A prime to the right of the fragment describing letter indicates that cleavage proceeds with H-atom transfer to the fragment-ion. Some confusion can arise concerning the nomenclature used to describe 27-hydroxycholesterol, which was previously denoted as 26-hydroxycholesterol. According to rules of priority of numbering the correct description of 27-hydroxycholesterol is 25R,26-hydroxycholesterol, however, as the medical community uses the name 27-hydroxycholesterol, we will use this name in this article.
An important consideration for the MS analysis of oxysterols extracted from brain is their low-abundance amongst a matrix of other lipids including phospholipids and cholesterol itself, which is present in 103 – 105 fold excess (2). To overcome this problem, oxysterols are enriched using straight-phase chromatography, derivatised specifically, and separated in their derivatised form by reversed-phase liquid chromatography (LC) prior to MS analysis. Only compounds with an oxo group, or oxidised to include one, will become derivatised with the GP reagent which provides preferential ionisation. Further, GP hydrazones give a characteristic neutral loss of 79 Da, i.e. loss of the pyridine group, which can be targeted in neutral-loss type scans, or utilised in multiple reaction monitoring (MRM) experiments, which add an extra dimension of specificity to oxysterol analysis (Fig. 1b). For the analysis of possible oxysterols in brain advantage can be taken of their predicted elemental composition (oxysterols are cholesterol metabolites with additional oxygen functionalities and/or changed carbon skeleton), and use can be made of a knowledge of their exact-mass to profile LC-MS ion-chromatograms for potential compounds.
The benefit provided by the abundant neutral-loss of 79 Da for the detection of GP derivatives, proves to be a disadvantage for their structural determination. To identify novel oxysterols in brain it will be necessary to maximise the structural information provided by tandem mass spectrometry (MS/MS), and when fragmentation proceeds predominantly through one or just a few reaction-channels this is difficult to achieve. Multi-stage fragmentation (MSn) can offer a benefit in this regard. By performing a MS3 experiment in which the precursor-ion, [M]+, fragments by the loss of 79 Da to give an [M-79]+ ion in a first fragmentation event (MS2), followed by activation of the [M-79]+ ion and recording of its fragmentation pattern, i.e. MS3, considerable structural information can be forthcoming. The MS3 scan also adds specificity to the conventional MS/MS experiment in which fragmentation pathways can be recorded for precursor-ions specifically giving a [M-79]+ “intermediate”. Other ions which coincidentally have the same nominal mass as derivatised oxysterols and are co-selected in the MS2 event will not give a [M-79]+ intermediate, and hence their fragment-ions will not be selected further for the MS3 scan and thus will not contaminate the final fragment-ion MS3 spectrum. This is in contrast to the situation in a simple two-stage MS/MS experiment, where, for low abundance analytes contamination of MS/MS spectra may be significant (18).
Recent developments in mass spectrometry instrumentation can take advantage of the properties of derivatised oxysterols. While tandem quadruple instruments provide the “gold standard” for recording neutral-loss (e.g. for the loss of 79 Da) and MRM scans, and quadrupole - time-of-flight instruments offer the possibility of exact mass and MS/MS analysis, ion-trap instrument have the advantage of being able to generate MSn spectra. A new type of hybrid mass spectrometer the LTQ-Orbitrap from Thermo Electron Corp (San Jose, CA) combines many of the figures of merit of the above (19), and here we demonstrate that this instrument is ideal for the identification of cholesterol metabolites in brain.
Even when employing a high-performance mass spectrometer, such as the LTQ-Orbitrap, it is desirable to incorporate on-line chromatographic separation when analysing complex biological mixtures. With respect to oxysterols, there is evidence for cholesterol 24-, 25- and 27-hydroxylase activity in mammalian brain (2,5,7,14,20,21). These enzymes generate 24S-, 25- and 27-hydroxycholesterols respectively, isomers differing only by the location of the hydroxyl group on the C-17 side-chain. Unless separated, their presence in a mixture will result in a composite MS3 spectrum, which is difficult to interpret. Oxysterols are hydrophobic molecules with limited solubility in aqueous solvents, however, derivatisation to GP hydrazones makes them more hydrophilic and increases the diversity of mobile phase compositions compatible with analysis by reversed-phase chromatography.
Here we describe a novel LC–MSn methodology for the identification, with high sensitivity (low pg), of oxysterols in brain. The methodology includes derivatisation to add selectivity and enhance ionisation, exact mass analysis at high-resolution to identify potential metabolites, and LC-MS3 to allow their characterisation.
Experimental Methods
Materials
Sterols were from Steraloids Inc Ltd (London, UK), Sigma-Aldrich Ltd (Poole, Dorset, UK), or from previous studies (see Supplementary Table 1 online). Girard P reagent {1-(carboxymethyl)pyridinium chloride hydrazide} and cholesterol oxidase from Streptomyces sp were also from Sigma-Aldrich Ltd. Unisil (activated silicic acid, 200 - 325 mesh) was from Clarkson Chromatography Products Inc. (South Williamsport, PA, USA) and SepPak C18 was from Waters Corp. (Milford, MA).
Synthesis of 3β-hydroxy-5-oxo-5,6-secocholestan-6-al and 3β,5β-dihydroxy-B-norcholestane-6β-carboxaldehyde
Synthesis of 5,6-seco-sterol and its aldol from cholesterol was accomplished essentially as described by Wentworth et al (22).
3β-Hydroxy-5-oxo-5,6-secocholestan-6-al
Cholesterol (500 mg) in chloroform-methanol (9:1, v/v; 50 mL) was treated with an excess of ozone at −78°C for 5 min. The solvents were evaporated and the residue treated with 325 mg of zinc powder suspended in 90% aqueous acetic acid (25 mL) at 23°C for 3 h. Material extracted with dichloromethane was subjected to silicic acid open column chromatography. Elution with diethyl ether-hexane 1:1 (v/v) afforded the title compound (468 mg; yield, 86%). An aliquot was treated with O-methyl hydroxylamine in pyridine at 23°C for 2 h and subsequently trimethylsilylated. Analysis by GC-MS showed two peaks of comparable magnitude due to the syn/anti isomers of the aldoxime - TMS derivative (the C-5 keto group was not derivatized). The mass spectrum showed prominent ions at m/z 488 [M+ - 31], 398 [M+ - (31+90)], and 320 [M+ - 199], (loss of the A-ring). The purity was in excess of 95%.
3β,5β-Dihydroxy-B-norcholestane-6β-carboxaldehyde
3β-Hydroxy-5-oxo-5,6-secocholestan-6-al (400 mg) in acetonitrile-water (20:1, v/v; 50 mL) was treated with 118 mg of L-proline at 23°C for 2 h. The product obtained by extraction with ethyl acetate was subjected to silicic acid open column chromatography. Elution with diethyl ether-hexane 6:4 (v/v) afforded the title compound (316 mg; yield, 79%). Treatment of an aliquot with O-methyl hydroxylamine and trimethylchlorosilane afforded the aldoxime - mono-TMS derivative (the C-5 tertiary alcohol was not derivatized) which appeared as a single but broadened peak on GC-MS. The mass spectrum showed prominent ions at m/z 488 [M+ - 31], 398 [M+ - (31+90)], 343, and 278. The purity was in excess of 95%.
Extraction of Oxysterols from Brain
In brief, oxysterols were extracted from three 100 mg portions of rat brain (Sprague Dawley, female, 306 g, 15 weeks, Harlan UK.) as follows: 100 mg of brain was homogenised in ethanol (1 mL), the ethanol extract was centrifuged and the supernatant retained. The precipitate was ultrasonicated in 1 mL of methanol/dichloromethane (1:1, v/v), centrifuged and the supernatant added to that retained previously. The supernatants were evaporated, and the residue re-dissolved in 2 mL of hexane/dichloromethane (2:8, v/v). The three brain extracts were then pooled, and one third of the total extract was applied to a Unisil column (8 × 0.8 cm, 200 - 325 mesh, activated silicic acid) prepared in hexane, and washed with 20 mL of hexane/dichloromethane (2:8, v/v) prior to sample application. Following sample application, the column was washed with 80 mL of hexane/dichloromethane (2:8, v/v, fraction 1), and eluted with 10 mL of ethyl acetate (fraction 2). The eluate was dried and re-dissolved in 50 μL isopropanol. For brain samples found to contain a significant amount of cholesterol in the ethyl acetate eluate (fraction 2), the eluate was dried and re-constituted in 2 mL of hexane/dichloromethane (2:8, v/v) and re-applied to a fresh Unisil column and chromatographed as before (see Supplementary Fig. 1 online). Note that in this initial study, the oxysterols were extracted from whole brain. The extraction, identification, and quantification of oxysterols in defined brain regions is a topic for further study.
Oxidation of 3β-Hydroxy-5-ene Sterols to 3-oxo-4-ene Sterols
The 3β-hydroxy-5-ene sterols {both reference sterols (5 μg) and those extracted from brain above} were oxidised with cholesterol oxidase essentially as described by Brooks et al (23). The 3β-hydroxy-5-ene sterols were dissolved in 50 μL of isopropanol, and 2 μL of cholesterol oxidase from Streptomyces sp (2 mg/mL, 44U/mg protein) in 1 mL of buffer (50 mM KH2PO4, pH 7) added, the mixture was incubated at 37°C for 60 min and subsequently used as the starting solution for reaction with the GP reagent as described below (Fig. 1a).
Derivatisation of 3-oxo-4-ene Sterols
The derivatisation of oxosterols to GP hydrazones was carried out essentially as described by Shackleton et al (24) (Fig. 1a). The reaction mixture from the oxidation step above was used directly after incubation with enzyme. The oxidation mixture, 1 mL (50 mM phosphate buffer, 5% isopropanol, 4 μg enzyme and reference oxosterols or those extracted from brain) was diluted with 2 mL of methanol to give a ∼ 70% methanol solution, and 150 mg of GP hydrazine and 150 μL of glacial acetic acid were added. The mixture was left at room temperature overnight.
Following overnight incubation the GP reaction mixture (∼ 3 mL 70% methanol) was directly applied to a SepPak C18 bed (1 cm × 0.8 cm in a glass column) followed by 1 mL of 70% methanol and 1 of mL 35% methanol. The combined effluent (now 5 mL) was diluted with 4 mL of water. The resulting mixture (now 9 mL in 35% methanol) was again applied to the column followed by a wash with 1 mL of 17% methanol. To the combined effluent 9 mL of water was added. The sample was then in 19 mL of about 17.5% methanol. This was again applied to the column followed by a wash with 10 mL of 10% methanol. Now all the GP derivatives are extracted by the column. They were then eluted with two portions of 1 mL of methanol followed by 1 mL of chloroform/methanol, 1:1 (v/v). The three fractions were analysed separately, and in combination by LC-ES-MSn. The GP derivatives were to be found predominantly in the second mL of methanol. The derivatisation protocol has been applied to mixtures of oxosteroids on the μg–ng level and is suitable for the low level (pg) derivatisation of neutral steroids extracted from tissue (18).
LC-MSn
Chromatographic separation of oxidised/derivatised oxysterols was performed on a Finnigan Surveyor HPLC system utilising a Hypersil GOLD reversed-phase column (1.9 μm particles, 50 × 2.1 mm) from Thermo Electron Corp (San Jose, CA). Mobile phase A consisted of 50% methanol containing 0.1% formic acid, and mobile phase B consisted of 95% methanol containing 0.1% formic acid. After 1 min at 20% B, the proportion of B was raised to 80% B over the next 7 min, and maintained at 80% B for a further 5 min, before returning to 20% B in 6 s and re-equilibration for a further 3 min 54 s, giving a total run time of 17 min. The flow rate was maintained at 200 μL min and eluent directed to the API source of a LTQ-Orbitrap mass spectrometer (19).
Mass spectrometry analysis was performed on a Finnigan LTQ-Orbitrap (Thermo Electron Corp, San Jose, CA) hybrid linear ion-trap (LIT) – Orbitrap analyser (19). Ions from the API source, operated in the positive ES mode, are initially stored in the LIT and analysed in either MS or MSn modes. Ions can be detected at the LIT detector, or ejected axially and trapped in an intermediate C-trap from which they are “squeezed” into the Orbitrap. Trapped ions in the Orbitrap assume circular trajectories around the central electrode and perform axial oscillation. The oscillating ions induce an image current into the two halves of the Orbitrap, which can be detected. The axial oscillation frequency of an ion (ω) is proportional to the square root of the inverse of m/z, {ω = (k/m/z)½}, and the frequencies of complex signals derived from many ions can be determined using a Fourier transformation (FT).
For the analysis of reference compounds (16 pg/μL) infused at 10 μL/min, the Orbitrap was operated at 60,000 resolution (full width at half maximum height, FWHM definition). MS and MSn spectra were recorded using both the LIT detector and the Orbitrap as a detector. The MS2 isolation width was set at 2 so as to allow the selection of monoisotopic precursor ions. The Orbitrap was calibrated externally prior to each analytical session, and mass accuracy was in all cases better than 5 ppm. For LC-MS and LC-MSn analysis of reference compounds each sample (30 pg/μL in 60% methanol) was injected (10 μL) onto the reversed-phase column and eluted into the LTQ-Orbitrap at a flow-rate of 200 μL/min. The Orbitrap was operated at 30,000 resolution.
For the analysis of oxidised/derivatised oxysterols extracted from rat brain, 5 μL of the methanol eluent (2 × 1 mL) from the SepPak C18 bed (equivalent to 0.25 mg of brain assuming all the oxysterols elute in the methanol fractions) was diluted with 45 μL of 60% methanol and 10 μL injected onto the LC column (equivalent to 0.05 mg brain). MS, MS2 and MS3 spectra were recorded in a data dependent mode, with the incorporation of an inclusion list for expected oxidised/derivatised oxysterols. MS3 was programmed to be performed on ions resulting from a neutral loss of 79 Da in the MS2 scan. For the acquisition of both MS2 and MS3 spectra the collision energy setting was 30 – 35.
Results and Discussion
The experimental method involves homogenisation and extraction of oxysterols from brain tissue with ethanol. Ethanol is a good solvent for oxysterols and readily penetrates cell membranes. Oxysterols are present in brain at levels of 103 - 105 lower than cholesterol, and to avoid autoxidation artefacts, cholesterol was separated from oxysterols by straight phase chromatography on a Unisil column immediately after extraction. As oxysterols possess a 3β-hydroxy-5-ene or 3β-hydroxy functionality (or a carbonyl group at C-3 or elsewhere) advantage was taken of the specificity of cholesterol oxidase from Streptomyces sp which was used to convert oxysterols to 3-oxo-4-ene or 3-oxo analogues (see Fig. 1a). The oxo group was then derivatised with GP reagent to give GP hydrazones which possess a charged quaternary nitrogen and give an improvement in ES signal of 2 - 3 orders of magnitude (18). This oxidation/derivatisation approach adds both specificity and sensitivity to the analysis of oxysterols. It should be pointed out, however, that in its present form the method does not distinguish between a 3β-hydroxy-5-ene sterol and its corresponding 3-oxo-4-ene analogue. The latter can be separately determined by deletion of the cholesterol oxidase reaction from the analytical protocol.
Oxidation and Derivatisation
We have demonstrated previously that the process of oxidising 3β-hydroxyl groups to 3-oxo groups followed by derivatisation to GP hydrazones enhances the oxysterol ion-current by at least two orders of magnitude (18). However, this method, like all derivatisation procedures, has its drawbacks, one of which is variation in the susceptibility of the 3β-hydroxyl group to oxidation by cholesterol oxidase depending on the overall sterol structure and the enzyme employed. A second consideration is the reactivity of other oxo groups present on the sterol skeleton to the GP reagent, which varies according to their location. We have optimised the methodology with brain oxysterols in mind, in that we have used cholesterol and 24S-hydroxycholesterol as the test substrates. By incubating these sterols for 60 min at 37°C with ∼ 0.2 U of cholesterol oxidase conversion to 3-oxo-4-ene sterols is maximised while the formation of oxidation artefacts is avoided. In the current study cholesterol oxidase from Streptomyces sp has been used. Cholesterol oxidase from different bacterial sources have varying activity to different 3β-hydroxysteroids and are also likely to generate different profiles of artefacts (23,25,26). Test experiments were performed with 5 μg of cholesterol and 24S-hydroxycholesterol, the sterols were supplied as 99% pure and this level of purity was maintained throughout the oxidation/derivatisation process. However, cholesterol is likely to be 103 times more abundant than any oxysterol in brain, and even as little as 0.1% formation of autoxidation products in the oxidation/derivatisation process would lead to significant artefacts when analysed as part of an oxysterols study. To minimise this eventuality, cholesterol is removed in the first stage of the sample purification by a single or double passage through Unisil columns.
To simulate closely the analytical procedure for the analysis of oxysterols extracted from brain tissue, 1 mg of cholesterol which approximates to the cholesterol content of 0.1 g of brain was subjected to the entire sample purification, oxidation and derivatisation protocol. A single passage of cholesterol through the Unisil column results in 95% elution in fraction 1, and 5% elution in fraction 2 (see Supplementary Fig. 1). Following treatment of fraction 2 with cholesterol oxidase and derivatisation with GP, desmosterol (m/z 516.3948) was found at 0.5 - 1.5% of the level of cholesterol (relative abundance, RA, 100%, m/z 518.4105) in fraction 2. A minor amount of 6-oxocholestenone (RA 0.3%, m/z 532.3898) was also identified, as were cholesterol epoxides (RA 0.2%, m/z 532.3898). Minor components with m/z 550.4003 were observed (RA 1%, 2%), both of which gave MS2 or MS3 spectra compatible with dihydroxycholesterols with at least one of the added hydroxyl groups in the A or B ring. A second separation of the material collected in fraction 2 after removal of solvents on a fresh Unisil column essentially eliminates all cholesterol and its subsequent autoxidation products.
Mass Spectrometry
The reference oxysterols listed in Supplementary Table 1 were analysed after oxidation/derivatisation by direct infusion-MS, -MSn, LC-MS and LC-MSn.
MS2 spectra of most oxysterols are dominated by a [M-79]+ fragment-ion, accompanied by lower abundance [M-97]+ and [M-107]+ ions. Exact mass analysis of these fragments performed at high-resolution (60 000) confirm that they result from the loss of pyridine (C5H5N), pyridine plus water (C5H7NO), and pyridine plus carbon monoxide (C6H5NO), respectively (Fig. 1b). Dihydroxycholesterols additionally give [M-115]+ and [M-125]+ ions which result from the loss of pyridine and two molecules of water (C5H9NO2), and pyridine, carbon monoxide and water (C6H7NO2). Although not providing a wealth of structural information, MS2 spectra complement MS3 spectra, giving information on the lability of the modified cholesterol skeleton. For example, for most oxidised/derivatised monohydroxycholesterols (m/z 534.4054) the MS2 spectra show [M-97]+ ions with relative abundance (RA) of ∼1%, however, for 25-hydroxycholesterol the lability of the hydroxyl group on tertiary C-25 results in an [M-97]+ ion with RA of ∼15% (See Supplementary Table 2 and Supplementary Figs. 2 – 7 online). In contrast to MS2 spectra, MS3 spectra of oxidized/derivatised sterols provide a wealth of structural information, where different functional groups provide different fragmentation patterns according to their location.
3-Oxo-4-ene Sterols
Cholesterol oxidase converts 3β-hydroxy-5-ene sterols to their 3-oxo-4-ene analogues. The simplest sterol to undergo this conversion is cholesterol, which is oxidised to cholest-4-en-3-one. The GP derivative of cholest-4-en-3-one, and other 3-oxo-4-ene sterols, which are unmodified by additional substituents in the A and B rings, give informative MS3 spectra containing a triad of fragment ions at m/z 151, 163 and 177. These fragment ions are formed by cleavage in the B ring and are described as *b1-12, *b3-C2H4 (or *b2-CH2) and *b2 ions respectively (18) (Fig. 1c, and see Supplementary Fig. 2b) Modification of the B ring by introduction of a hydroxyl group at C-7 changes the pattern of B-ring fragment ions. The comparative abundance of the *b1-12 ion at m/z 151 is enhanced, while the *b3-C2H4 fragment is shifted in m/z from 163 to 179 (see Supplementary Fig. 3b). Introduction of a 6-hydroxyl group attenuates the *b1-12 and *b3-C2H4 fragment-ions, leaving an ion m/z 177. The observation of an ion at m/z 177 rather than 193 indicates that the C-6 hydroxyl is no longer present in the *b2 ion (See Supplementary Table 2). The presence of an oxo group at C-6 eliminates the pattern of B-ring fragment ions, although a low intensity *b2 ion is observed at m/z 191 (a carbonyl group at C-6 may dictate the fragment-ion at m/z 191 has a structure different from *b2). An oxo group at C-7 attenuates the *b1-12 fragmentation and shifts *b3-C2H4 up in mass to m/z 177, leaving a *b2-CH2 ion at m/z 163. A detailed description of how the location of additional hydroxyl and oxo groups to the steroid skeleton and C-17 side-chain are determined is given in Supplementary Results and Supplementary Figs. 2 – 11.
Oxysterols in Rat Brain
Following oxidation/derivatisation, oxysterols extracted from brain were separated by reversed-phase chromatography and analysed on an LTQ-Orbitrap hybrid tandem mass spectrometer. In an initial run the instrument was used to record mass spectra at high resolution in the Orbitrap. Reconstructed exact mass (5 ppm) ion chromatograms (RIC) were plotted for all potential oxysterols (Table 1). In a second LC-MS run, MS2 and MS3 spectra were recorded in a data dependent mode with the incorporation of an inclusion list for all potential oxysterols suggested to be present from the initial LC-MS run. MS3 was programmed to be performed on ions resulting from a neutral loss of 79 Da in the MS2 scan i.e [M]+→[M-79]+→. The MS3 scans provide added specificity to the method as this final product-ion spectrum is only generated from precursor-ions of the selected mass giving an [M-79]+ intermediate. The exact mass capability of the LTQ-Orbitrap instrument adds further specificity to the method in that only precursor-ions with an exact mass and chromatographic retention time compatible with an oxysterol will be selected for MSn.
Table 1.
Potential oxysterols and those identified in brain
| Sterol/Oxysterola,b | Structure after oxidation with cholesterol oxidase |
Formula of GP | Mass of GP | Identified in current work (concentration wet brain) |
Identified by others in brain (concentration wet brain) |
|---|---|---|---|---|---|
| C5,x-3β-ol | C4,x-3-one | C34H50N3O+ | 516.3948 | Desmosterolc (Not quantified) |
Rat (0.1% of total sterol)d Mouse (50 ng/mg)e |
| C5-3β-ol | C4-3-one | C34H52N3O+ | 518.4105 | Cholesterolc (Not quantified) |
Human (7-8 μg/mg)f Mouse (20 μg/mg)e |
| 5α-C-3β-ol | 5α-C-3-one | C34H54N3O+ | 520.4261 | Not detected | |
| C5-3β-ol-x-one/ C5,x-3β,y-diol |
C4-3,x-dione/ C4,x-y-ol-3-one |
C34H50N3O2+ | 532.3898 | 24-Oxocholesterol (∼0.5 ng/mg) Not detected |
|
| C5-3β,x-diol | C4-x-ol-3-one | C34H52N3O2+ | 534.4054 | 24S-Hydroxycholesterolg (15-25 ng/mg) |
Human (5-15 ng/mg)f,h Mouse (40-50 ng/mg)e,i |
| 5α-C-3β,x-diol | 5α-C-x-ol-3-one | C34H54N3O2+ | 536.4211 | Not detected | |
| C5-3β-ol-x,y-dione/ C5,x-3β,y-diol-z-one |
C4-3,x,y-trione/ C4,x-y-ol-3,z-dione |
C34H48N3O3+ | 546.3690 | Not detected Not detected |
|
| C5-3β,x-diol-y-one/ C5,x-3β,y,z-triol/ CA5-3β-ol |
C4-x-ol-3,y-dione/ C4,x-y,z-diol-3-one/ CA4-3-one |
C34H50N3O3+ | 548.3847 | 24-Oxohydroxycholesterol (∼125 pg/mg) Not detected Not detected |
CYP46A1j Astrocytesk |
| C5-3β,x,y-triol | C4-x,y-diol-3-one | C34H52N3O3+ | 550.4003 | 24,25-Dihydroxycholesterol (∼125 pg/mg) 24,27-Dihydroxycholesterol (∼30pg/mg) 25,27-Dihydroxycholesterol (∼75pg/mg) 6,24-Dihydroxycholesterol (∼150pg/mg) 7α,25-Dihydroxycholesterol (∼30pg/mg) 7α,27-Dihydroxycholesterol (∼30pg/mg) |
Astrocytesk/CYP46A1j CYP46A1j Astrocytesk CYP46A1j Astrocytesk Astrocytesj |
| 5α-C-3β,x,y-triol/ 5,6-Seco-sterolL/ AldolL |
C-x,y-diol-3-one/ 5,6-Seco-sterolL/ AldolL |
C34H54N3O3+ | 552.4160L | Not detected 5,6-seco-sterol (∼100pg/mg) Aldol (∼300pg/mg) |
Human (150 pg/mg)m |
| C5-3β-ol-x,y,z-trione/ C5,w-3β,x-diol-y,z-dione/ |
C4-3,x,y,z-tetraone/ C4,w-x-ol-3,y,z-trione |
C34H46N3O4+ | 560.3483 | Not detected Not detected |
|
| C5-3β,x-diol-y,z-dione/ C5,w-3β,x,y-triol-z-one |
C4-x-ol-3,y,z-trione/ C4,w-x,y-diol-3,z-dione |
C34H48N3O4+ | 562.3639 | Not detected Not detected |
|
| C5-3β,x,y-triol-z-one/ C5,w-3β,x,y,z-tetraol/ CA5-3β,x-diol |
C4-x,y-diol-3,z-dione/ C4,w-x,y,z-triol-3-one/ CA4-x-ol-3-one |
C34H50N3O4+ | 564.3796 | Detected, not characterised |
Astrocytesk |
| C5-3β,x,y,z-tetraol | C4-x,y,z-triol-3-one | C34H52N3O4+ | 566.3952 | Detected, not characterised | CYP46A1j/Astrocytesk |
| 5α-C-3β,x,y,z-tetraol | 5α-C-x,y,z-triol-3-one | C34H54N3O4+ | 568.41088 | Detected, not characterised |
Cholestane (C), cholestanoic acid (CA), 3β-hydroxy-5-oxo-5,6-secocholestan-6-al (5,6-Seco-sterol), 3,5-dihydroxy-B-norcholestane-6-carboxyaldehyde (Aldol). See below for structures. Superscripts indicate location of double bonds.
Oxysterols with more than four oxygen atoms were not detected.
Cholesterol and its precursors, not oxysterols. Found in fraction 1 from Unisil column.
Other precursors of cholesterol, besides desmosterol, have been identified in rat brain (33,34). Desmosterol concentration from ref. (33).
From ref. (35)
From ref. (36).
Only 24S-hydroxycholesterol was identified although chromatographic performance was sufficient to resolve it from closely eluting isomers, i.e. 25-hydroxycholesterol and 27-hydroxycholesterol.
Other oxysterols identified in human brain include 27-hydroxycholesterol (1 – 3 ng/mg) (35), 22R-hydroxycholesterol (45 – 90 pg/mg) (37), 7-hydroxycholesterol (234 – 745 ng/mg Cerebellar cortex) (38) and 7-oxocholesterol (170 – 640 ng/mg Cerebellar cortex) (38).
Other oxysterols identified in mouse or rat brain include 27-hydroxycholesterol (5 ng/mg mouse) (35), 20S-hydroxycholesterol (50 pg/mg rat) (39).
Recombinant CYP46A1 shows 25- and 27-hydroxylase activity when incubated with 24S-hydroxycholesterol. HEK293 cells transfected with CYP46A1 can also hydroxylate the steroid nucleus (14).
Astrocytes show 25-hydroxylase activity when incubated with 27- and 24-hydroxycholesterol, and 7α-hydroxylase activity when incubated with 25- and 27-hydroxycholesterol (21).
5,6-Seco-sterol and its adol are not oxidised by cholesterol oxidase.
Concentration of combined 5,6-seco-sterol and its aldol (11).
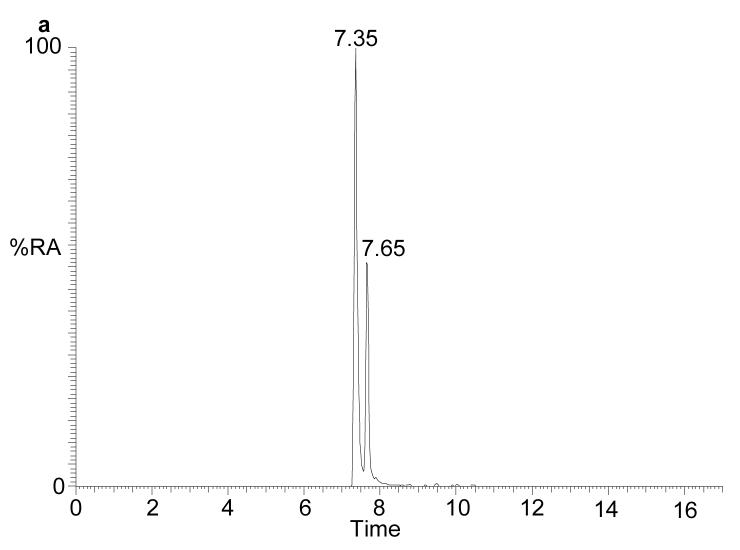
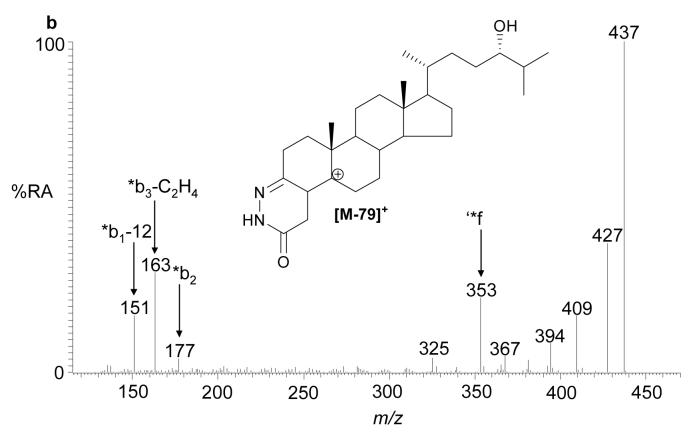
Passing the brain extract twice through the Unisil column effectively removes all the cholesterol and desmosterol from the final oxysterol fraction. The RA of cholesterol and desmosterol in these samples amount to <2% and <0.1% of the major oxysterol in the fraction. The major sterol in the oxysterol fraction has a m/z of 534.4054 (RA 100%) and appears with retention time (7.35 and 7.65 min, syn/anti forms of the GP hydrazone) identical to that of oxidized and derivatised 24S-hydroxycholesterol (Supplementary Table 1). Both the MS2 and MS3 spectra are identical to those from the authentic compound, thereby confirming 24S-hydroxycholesterol to be the major oxysterol in rat brain (2) (Fig. 2). With the use of an internal standard the level of 24S-hydroxycholesterol in rat brain was found to vary between 15 and 25 μg/g (data not shown), in good agreement with values for other mammals (9,27). Evidence from experiments with recombinant CYP46A1 and HEC 293 cells transfected with CYP46A1, suggest that this enzyme can, in addition to 24S-hydroxylation of cholesterol, further metabolise the resulting oxysterol to 24,25- and 24,27-dihydroxycholesterols (14). Additional products of the incubation of 24S-hydroxycholesterol with CYP46A1 transfected HEC 293 cells were partially characterised as 24,X-dihydroxycholesterol, 24,25,X-trihydroxycholesterol and 24,27,X-trihydroxycholesterol, where the extra hydroxyl group as signified by X is in the steroid nucleus (14). 24-Oxocholesterol with an extra hydroxyl group in the steroid nucleus, which is presumably formed via 24-oxocholesterol, was also identified (14). Exact mass RIC for the m/z values of these oxysterols were generated (Table 1). Although its RA was <2% of that of 24S-hydroxycholesterol (RA of all oxysterols are measured relative to 24S-hydroxycholesterol), the major component with m/z 532.3898 appeared at a retention time (7.60 min) and gave MS2 and MS3 spectra identical to those of authentic oxidized/derivatised 24-oxocholesterol (see Supplementary Table 2 online). The additionally hydroxylated version of this compound has a m/z of 548.3847, and gave a peak (RA <0.5%) which appears in the RIC with a retention time (5.49 min), and gives MS2 and MS3 spectra compatible with an oxidized/derivatised oxohydroxycholesterol.
Fig. 2.
(a) RIC for m/z 534.4054 (±5 ppm) corresponding to oxidised/derivatised monohydroxycholesterols extracted from rat brain. (b) MS3 ([M]+→[M-79]+→) spectrum of the peak eluting at 7.35 min, and (c) of authentic oxidised/derivatised 24S-hydroxycholesterol with a similar retention time. The peaks eluting at 7.35 and 7.65 min in (a) give identical MS2 and MS3 spectra and correspond to syn and anti isomers of the derivative. The RIC was recorded by FT in the Orbitrap analyser while MS3 spectra were recorded by the LTQ detector.
The MS2 and MS3 spectra of oxidized/derivatised dihydroxycholesterols (m/z 550.4003) from brain gave informative fragmentation patterns. As can be seen in the RIC in Fig. 3a nine distinct peaks are observed, but compared to that of 24S-hydroxycholesterol all are of low abundance (RA <1%). The MS2 and MS3 spectra of the first eluting component at 4.31 min indicate a dihydroxy derivative of a 3-oxo-4-ene sterol. In the MS3 spectrum (Fig. 3b) the pattern of fragment ions at m/z 151, 163 and 177 confirms the 3-oxo-4-ene structure (derived from cholesterol oxidase treatment of the 3β-hydroxy-5-ene sterol), while the significant fragment ion at m/z 353 (‘*f, see Fig. 1c and cf. Fig. 2b) defines the location of one hydroxyl group at C-24 with the second necessarily on C-25 or C-27. Based on the ratio of [M-97]+, [M-107]+, [M-115]+ and [M-125]+ (m/z 453, 443, 435, 425) fragment ions in both the MS2 (1.5:10.0:0.5:<0.5) (data not shown) and MS3 (10.0:3.5:1.0:2.0) spectra (Fig. 3b), and the greater stability of the 27-hydroxyl group in comparison to the 25-hydroxyl group, the second hydroxyl group is located to C-27. The chromatographic peak eluting at 4.82 min gave similar MS2 and MS3 spectra to that eluting at 4.31 min and it is concluded that these are syn and anti isomers of the GP derivative of 24,27-dihydroxycholesterol. The chromatographic peak at m/z 4.47 min also gave MS2 and MS3 spectra compatible with an oxidised/derivatised dihydroxycholesterol, where one of the additional hydroxyl groups is on C-24 and the second on C-25 or C-27. A characteristic of MS3 spectra of oxidized/derivatised oxysterols with a 25-hydroxyl group, is that the [M-97]+ ion dominates the spectrum at the expense of all other fragment ions (see Supplementary Table 2 online). This is also evident in the MS3 spectrum of the peak eluting at 4.47 min (Fig. 3c) with the consequence that the fragment ions at m/z 151, 163, 177 and 353 are of lower abundance than in the spectrum of the 24,27-dihydroxy isomer (Fig. 3b). The abundance of the [M-107]+ fragment is also reduced, but not the abundance of [M-115]+ or [M-125]+ fragments which is reflected in the ratio of ions of m/z 453, 443, 435, 425 being 10.0:1.5:1.0:1.5. Further evidence for the location of the second hydroxyl group at C-25 comes from the abundance ratio of this series of ions in the MS2 spectrum i.e 2.5:10.0:0.5:0.5 (data not shown), which is compatible of the enhanced lability of the C-25 hydroxyl group as compared to that at C-27. This data leads to the conclusion that the peaks eluting at 4.31 and 4.82 min correspond to oxidized/derivatised 24,27-dihydroxycholesterol and that at 4.47 min to 24,25-dihydroxycholesterol. The MS2 and MS3 spectra of the peak eluting at 5.06 min again indicate an oxidized/derivatised dihydroxycholesterol. In the MS3 spectrum (Fig. 3d) the presence of a peak at m/z 325 (‘*e), but the absence of one at 353 (‘*f) indicate that both hydroxyl groups are on the C-17 side-chain, but not at C-24. The spectrum does not show fragment ions characteristic of hydroxylation at C-20, 22, 24 (see Supplementary Table 2 online), but the ratio of ions of m/z 453, 443, 435, 425 in the MS2 and MS3 spectra of 2.5:10.0:<0.5:0.5 (data not shown) and 10.0:1.5:1.5:1.5 does strongly suggest a 25,27-diol. 25,27-Dihydroxycholesterol has been identified by Zhang et al in incubations of 27-hydroxycholesterol with rat brain astrocytes, and has been suggested to be an intermediate in cholesterol metabolism in astrocytes (21). It should be noted that microchemical reactions, such as treatment with periodate, or the Jones oxidation, could be attempted to confirm the structures of the above oxysterols. However, their presence at low abundance (ng/g) and as part of a mixture has precluded such a study as part of the present work.
Fig. 3.
(a) RIC for m/z 550.4003 corresponding to oxidised/derivatised dihydroxycholesterols extracted from brain. MS3 ([M]+→[M-79]+→) spectra of chromatographic peaks eluting at (b) 4.31 min corresponding to 24,27-dihydroxycholesterol, (c) 4.47 min corresponding to 24,25-dihydroxycholesterol, (d) 5.06 min corresponding to 25,27-dihydroxycholesterol, and (e) 5.45 min corresponding to 6,24-dihydroxycholesterol. The RIC was recorded by FT in the Orbitrap analyser and the MS3 spectra recorded by the LTQ detector.
The presence of a 6-hydroxyl group on the cholesterol skeleton alters the MS3 spectrum of the oxidised derivative, in that instead of a triad of fragment ions at m/z 151,163, and 177, only the ion at 177 is preserved. Also, in the MS3 spectrum of oxidised/derivatised 6-hydroxycholesterol the normal arrangement of [M-97]+ ions being more abundant than [M-107]+ ions is reversed, and an intense ion is observed at m/z 383 corresponding to [M-79-C4H8O] (see Supplementary Table 2 online). The MS3 spectrum of the peak eluting at 5.45 min gives a fragment ion at m/z 177, and the [M-107]+ fragment is more abundant than that [M-97]+ ion signifying hydroxylation at C-6 (Fig. 3e). Further evidence is provided by the presence of a fragment ion at 381 corresponding to [M-97-C4H8O]+. A fragment ion at m/z 341 (‘*e) indicates that the second hydroxyl group is on the C-17 side-chain and a fragment ion at m/z 369 (‘*f) locates it to C-24. Thus the spectrum is compatible with that of oxidized/derivatised 6,24-dihydroxycholesterol. Significantly when Mast et al incubated 24-hydroxycholesterol with HEK293 cells transfected with CYP46A1 a dihydroxycholesterol with one hydroxyl group at C-24 and the second in the steroid nucleus was identified (14). Furthermore, CYP7B1 an enzyme with 6-hydroxylase activity is expressed in brain (28,29).
The chromatographic peaks at 5.91 and 6.29 give MS2 and MS3 spectra identical to those of authentic standards of oxidized/derivatised 7α,25- and 7α,27-dihydroxycholesterol. The 7-hydroxyl groups are characterized by the fragment ions at m/z 151 and 179 in the MS3 spectra, while their α- positioning is indicated by the heightened abundance of fragment ions at m/z 231 (see Supplementary Fig. 12). The latter eluting peak shows enhanced intensity of ions at m/z 443 [M-107]+ as a consequence of the stability of the 27-hydroxyl group. Both 7α,25- and 7α,27-dihydroxycholesterols have been suggested as intermediates in the cholesterol metabolic pathways in rat brain (21). Thus, rat astrocyte cultures convert them by oxidation with 3β-hydroxy-Δ5-C27-steroid oxidoreductase/isomerase (HSD3B7, specific for 7α-hydroxylated sterols and bile acids) into the 3-oxo-Δ4 analogues (included in the chromatographic peaks at 5.91 and 6.29), and 27-hydroxysterols can be converted by astrocytes into cholestenoic acids (21). The latter are intermediates in the biosynthesis of C24 bile acids, which have recently been identified in rat brain (30). The enzyme with 7α-hydroxylase activity in brain, i.e. CYP7B1, will 7α-hydroxylate both 25- and 27-hydroxycholesterols, but has much lower activity against 24S-hydroxycholesterol (28,29). Significantly 24-hydroxycholesterol was not 7α-hydroxylated by rat astrocyte, Schwann cell or neuron cultures (21), and 7α,24-dihydroxycholesterol was not observed in the present study.
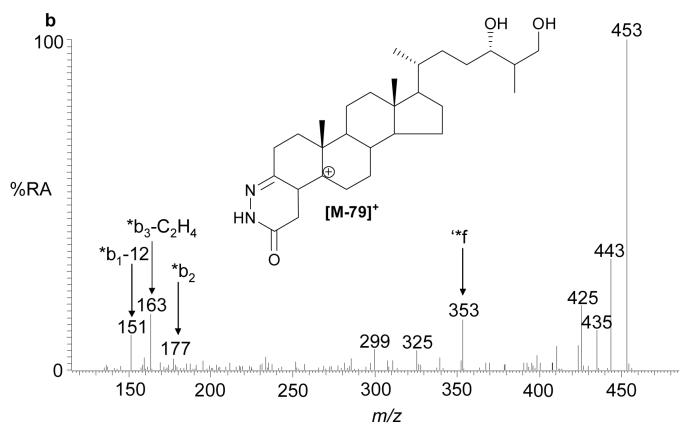
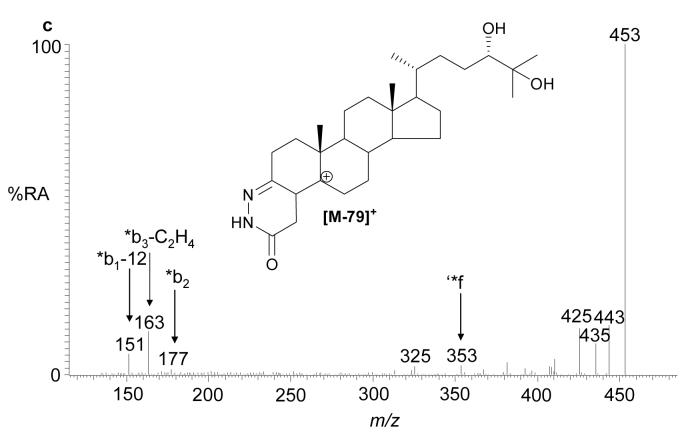
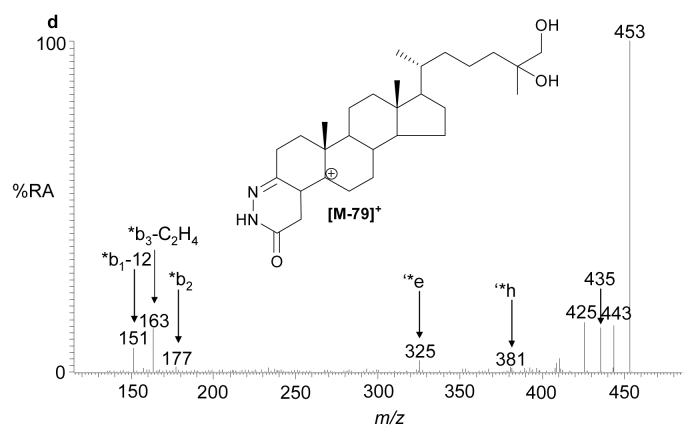
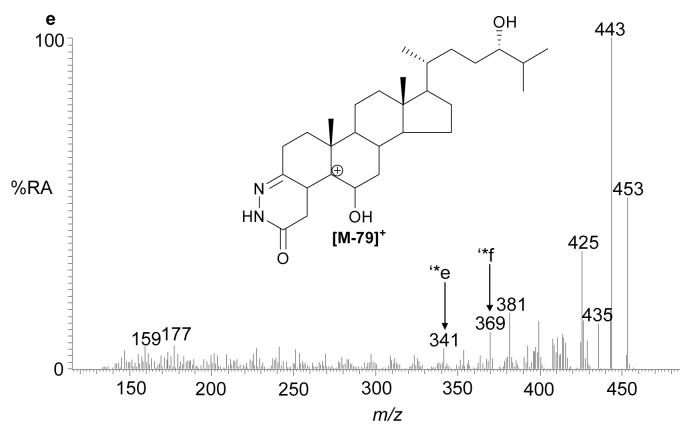
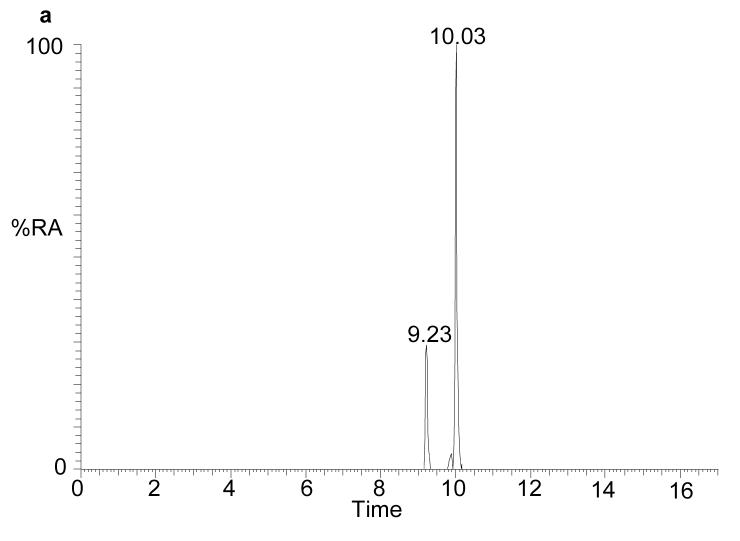
The recent interest in seco-sterols inspired us to search for 5,6-seco-sterol and its aldol condensation product, 3,5-dihydroxy-B-norcholestane-6-carboxyaldehyde, which have been previously suggested to be present in human brain (11,31). The RIC for m/z 552.4160 revealed two components with retention times of 9.23 and 10.03 min. The MS2 spectra were rich in fragment ions and gave spectra identical to GP hydrazones of 5,6-seco-sterol and its aldol respectively (Fig. 4). The abundance of these isomers was less than 2% of that of 24S-hydroxycholesterol.
Fig. 4.
(a) RIC for m/z 552.4160 corresponding to derivatised 3β-hydroxy-5-oxo-5,6-secocholestan-6-al and 3,5-dihydroxy-B-norcholestane-6-carboxyaldehyde extracted from brain. The MS2 spectra of chromatographic peaks eluting at (b) 9.23, and (c) authentic 3β-hydroxy-5-oxo-5,6-secocholestan-6-al; and (d) 10.03 min and (e) authentic 3,5-dihydroxy-B-norcholestane-6-carboxyaldehyde. The RIC was recorded by FT in the Orbitrap analyser and MS2 spectra recorded by the LTQ detector.
The analytical method described offers sensitivity and selectivity for the determination of oxysterols in brain. On-column detection limits were of the order of 1.5 pg (data not shown), and at this level MS2 and MS3 spectra were sufficiently informative to allow structure determination. At this level of sensitivity it was possible to identify novel oxysterols in brain from only 50 μg of brain injected on-column, opening up avenues of investigation which will allow oxysterol analysis from distinct brain regions. The informative fragmentation, particularly in MS3, of oxidised and GP derivatised sterols contrasts with their untreated analogues (15-17), and has allowed the identification of novel oxysterols in rat brain. Although previously observed in incubations of 24S-hydroxycholesterol with recombinant CYP46A1 or cells transfected (14), this study provides the first identification and approximate quantification of 24,25-, 24,27, and 6,24-dihydroxycholesterols in rat brain (Table 1). Furthermore, while 24,25-, 25,27- 7α,25- and 7α,27- dihydroxycholesterols have been observed in incubations of 24-, 27-, 25- and 27-hydroxycholesterols with astrocytes (21), the current study is the first to identify these oxysterols in brain. Of particular interest is the high relative abundance of 24,25-dihydroxycholesterol which is consistent with the importance of 25-hydroxylation in astrocyte cultures (21). Additionally, here we have detected 5,6-seco-sterol and its aldol in rat brain. The presence of these two oxysterols in human has been under debate (11,12,31), but their association to neurodegenerative disease (11,31) highlights the importance of their analysis. With this in mind, the analytical methodology described here can easily be used for oxysterol analysis in other tissues, and also plasma or cerebrospinal fluid. The identification of novel oxysterols in brain raises questions as to their function. Their low abundance (<2%) compared to 24S-hydroxycholesterol does not suggest they act as transport forms of cholesterol from brain to liver, however, their turn-over in brain or abundance in plasma or CSF has yet to be studied. Alternatively, like other oxysterols (6,32) they may behave as ligands to nuclear receptors, e.g. LXRβ which akin to CYP46A1 is expressed in neurons (7,8).
Supplementary Material
Acknowledgements
This work was supported by the UK Biotechnology and Biological Sciences Research Council (BBSRC grant no. BB/C515771/1, & BB/C511356/1), The School of Pharmacy, the Swedish Research Council (VR grant no. 03X-12551), and Karolinska Institutet. We also thank Dr Michaela Scigelova for critical reading of the manuscript.
References
- 1.Edmond J, Korsak RA, Morrow JW, Torok-Both G, Catlin DH. Dietary cholesterol and the origin of cholesterol in the brain of developing rats. J. Nutr. 1991;121:1323–1330. doi: 10.1093/jn/121.9.1323. [DOI] [PubMed] [Google Scholar]
- 2.Dietschy JM, Turley SD. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Lütjohann D, Breuer O, Ahlborg G, Nennesmo I, Sidén A, Diczfalusy U, Björkhem I. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. USA. 1996;93:9799–9804. doi: 10.1073/pnas.93.18.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lund EG, Xie C, Kotti T, Turley SD, Dietschy JM, Russell DW. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J. Biol. Chem. 2003;278:22980–22988. doi: 10.1074/jbc.M303415200. [DOI] [PubMed] [Google Scholar]
- 5.Björkhem I, Lütjohann D, Breuer O, Sakinis A, Wennmalm A. Importance of a novel oxidative mechanism for elimination of brain cholesterol. Turnover of cholesterol and 24(S)-hydroxycholesterol in rat brain as measured with 18O2 techniques in vivo and in vitro. J. Biol. Chem. 1997;272:30178–30184. doi: 10.1074/jbc.272.48.30178. [DOI] [PubMed] [Google Scholar]
- 6.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 7.Lund EG, Guileyardo JM, Russell DW. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. USA. 1999;96:7238–7243. doi: 10.1073/pnas.96.13.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teboul M, Enmark E, Li Q, Wikstrom AC, Pelto-Huikko M, Gustafsson J-Å. OR-1, a member of the nuclear receptor superfamily that interacts with the 9-cis-retinoic acid receptor. Proc. Natl. Acad. Sci. USA. 1995;92:2096–21100. doi: 10.1073/pnas.92.6.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroepfer GJ., Jr Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000;80:361–554. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
- 10.Puglielli L, Friedlich AL, Setchell KD, Nagano S, Opazo C, Cherny RA, Barnham KJ, Wade JD, Melov S, Kovacs DM, Bush AI. Alzheimer disease beta-amyloid activity mimics cholesterol oxidase. J. Clin. Invest. 2005;115:2556–2563. doi: 10.1172/JCI23610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Powers ET, Nieva J, Huff ME, Dendle MA, Bieschke J, Glabe CG, Eschenmoser A, Wentworth P, Lerner RA, Kelly JW. Metabolite-initiated protein misfolding may trigger Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2004;101:4752–4757. doi: 10.1073/pnas.0400924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith LL. Oxygen, oxysterols, ouabain, and ozone: a cautionary tale. Free Radic. Biol. Med. 2004;37:318–324. doi: 10.1016/j.freeradbiomed.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Dzeletovic S, Breuer O, Lund E, Diczfalusy U. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 1995;225:73–80. doi: 10.1006/abio.1995.1110. [DOI] [PubMed] [Google Scholar]
- 14.Mast N, Norcross R, Andersson U, Shou M, Nakayama K, Björkhem I, Pikuleva IA. Broad substrate specificity of human cytochrome P450 46A1 which initiates cholesterol degradation in the brain. Biochemistry. 2003;42:14284–14292. doi: 10.1021/bi035512f. [DOI] [PubMed] [Google Scholar]
- 15.Raith K, Brenner C, Farwanah H, Muller G, Eder K, Neubert RH. A new LC/APCI-MS method for the determination of cholesterol oxidation products in food. J. Chromatogr. A. 2005;1067:207–211. doi: 10.1016/j.chroma.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 16.Lembcke J, Ceglarek U, Fiedler GM, Baumann S, Leichtle A, Thiery J. Rapid quantification of free and esterified phytosterols in human serum using APPI-LC-MS/MS. J. Lipid Res. 2005;46:21–26. doi: 10.1194/jlr.C400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Burkard I, Rentsch KM, von Eckardstein A. Determination of 24S- and 27-hydroxycholesterol in plasma by high-performance liquid chromatography-mass spectrometry. J. Lipid Res. 2004;45:776–781. doi: 10.1194/jlr.D300036-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths WJ, Wang Y, Alvelius G, Liu S, Bodin K, Sjövall J. Analysis of oxysterols by electrospray tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2006;17:341–362. doi: 10.1016/j.jasms.2005.10.012. J. [DOI] [PubMed] [Google Scholar]
- 19.Makarov A, Denisov E, Kholomeev A, Balschun W, Lange O, Strupat K, Horning S. Performance evaluation of a hybrid linear ion trap/orbitrap mass spectrometer. Anal. Chem. 2006;78:2113–20. doi: 10.1021/ac0518811. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen JI, Oftebro H, Björkhem I. Reconstitution of C27-steroid 26-hydroxylase activity from bovine brain mitochondria. Biochem. Int. 1989;18:615–622. [PubMed] [Google Scholar]
- 21.Zhang J, Akwa Y, el-Etr M, Baulieu EE, Sjövall J. Metabolism of 27-, 25- and 24-hydroxycholesterol in rat glial cells and neurons. Biochem. J. 1997;322(Pt 1):175–184. doi: 10.1042/bj3220175. J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wentworth P, Nieva J, Takeuchi C, Galve R, Wentworth AD, Dilley RB, DeLaria GA, Saven A, Babior BM, Janda KD, Eschenmoser A, Lerner RA. Evidence for ozone formation in human atherosclerotic arteries. Science. 2003;302:1053–1056. doi: 10.1126/science.1089525. [DOI] [PubMed] [Google Scholar]
- 23.Brooks CJW, Cole WJ, Lawrie TDV, MacLachlan J, Borthwick JH, Barrett GM. Selective reactions in the analytical characterization of steroids by gas chromatography-mass spectrometry. J. Steroid Biochem. 1983;19:189–210. [PubMed] [Google Scholar]
- 24.Shackleton CHL, Chuang H, Kim J, de la Torre X, Segura J. Electrospray mass spectrometry of testosterone esters: Potential for use in doping control. Steroids. 1997;62:523–529. doi: 10.1016/s0039-128x(97)00004-4. J. [DOI] [PubMed] [Google Scholar]
- 25.MacLachlan J, Wotherspoon AT, Ansell RO, Brooks CJ. Cholesterol oxidase: sources, physical properties and analytical applications. J. Steroid Biochem. Mol. Biol. 2000;72:169–195. doi: 10.1016/s0960-0760(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 26.Teng JI, Smith LL. Sterol peroxidation by Pseudomonas fluorescens cholesterol oxidase. Steroids. 1996;61:627–633. doi: 10.1016/s0039-128x(96)00135-3. [DOI] [PubMed] [Google Scholar]
- 27.Lütjohann D, Brzezinka A, Barth E, Abramowski D, Staufenbiel M, von Bergmann K, Beyreuther K, Multhaup G, Bayer TA. Profile of cholesterol-related sterols in aged amyloid precursor protein transgenic mouse brain. J. Lipid Res. 2002;43:1078–1085. doi: 10.1194/jlr.m200071-jlr200. [DOI] [PubMed] [Google Scholar]
- 28.Rose K, Allan A, Gauldie S, Stapleton G, Dobbie L, Dott K, Martin C, Wang L, Hedlund E, Seckl JR, Gustafsson J-Å, Lathe R. Neurosteroid hydroxylase CYP7B: vivid reporter activity in dentate gyrus of gene-targeted mice and abolition of a widespread pathway of steroid and oxysterol hydroxylation. J. Biol. Chem. 2001;276:23937–23944. doi: 10.1074/jbc.M011564200. [DOI] [PubMed] [Google Scholar]
- 29.Stapleton G, Steel M, Richardson M, Mason JO, Rose KA, Morris RG, Lathe R. A novel cytochrome P450 expressed primarily in brain. J. Biol. Chem. 1995;270:29739–29745. doi: 10.1074/jbc.270.50.29739. [DOI] [PubMed] [Google Scholar]
- 30.Mano N, Goto T, Uchida M, Nishimura K, Ando M, Kobayashi N, Goto J. Presence of protein-bound unconjugated bile acids in the cytoplasmic fraction of rat brain. J. Lipid Res. 2004;45:295–300. doi: 10.1194/jlr.M300369-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Bosco DA, Fowler DM, Zhang Q, Nieva J, Powers ET, Wentworth P, Lerner RA, Kelly JW. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat. Chem. Biol. 2006;2:249–253. doi: 10.1038/nchembio782. [DOI] [PubMed] [Google Scholar]
- 32.Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, Mangelsdorf DJ. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc. Natl. Acad. Sci. USA. 1999;96:266–271. doi: 10.1073/pnas.96.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorszewska J, Adamczewska-Goncerzewicz Z. Patterns of free and esterified sterol fractions of the cerebral white matter in severe and moderate experimental hypoxia. Med. Sci. Monit. 2000;6:227–231. Z. [PubMed] [Google Scholar]
- 34.Wender M, Adamczewska-Goncerzewicz Z, Doroszewska J. Transformation of sterols pattern in course of late development of rat brain. Folia Neuropathol. 1995;33:31–34. J. [PubMed] [Google Scholar]
- 35.Heverin M, Bogdanovic N, Lütjohann D, Bayer T, Pikuleva I, Bretillon L, Diczfalusy U, Winblad B, Björkhem I. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer's disease. J. Lipid Res. 2004;45:186–193. doi: 10.1194/jlr.M300320-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Lütjohann D. Cholesterol metabolism in the brain: importance of 24S-hydroxylation. Acta Neurol. Scand. Suppl. 2006;185:33–42. doi: 10.1111/j.1600-0404.2006.00683.x. [DOI] [PubMed] [Google Scholar]
- 37.Yao ZX, Brown RC, Teper G, Greeson J, Papadopoulos V. 22R-Hydroxycholesterol protects neuronal cells from beta-amyloid-induced cytotoxicity by binding to beta-amyloid peptide. J. Neurochem. 2002;83:1110–1119. doi: 10.1046/j.1471-4159.2002.01202.x. [DOI] [PubMed] [Google Scholar]
- 38.Miyajima H, Adachi J, Kohno S, Takahashi Y, Ueno Y, Naito T. Increased oxysterols associated with iron accumulation in the brains and visceral organs of acaeruloplasminaemia patients. QJM. 2001;94:417–422. doi: 10.1093/qjmed/94.8.417. [DOI] [PubMed] [Google Scholar]
- 39.Lin YY, Welch M, Lieberman S. The detection of 20S-hydroxycholesterol in extracts of rat brains and human placenta by a gas chromatograph/mass spectrometry technique. J. Steroid Biochem. Mol. Biol. 2003;85:57–61. doi: 10.1016/s0960-0760(03)00137-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.