Abstract
We have studied GABAergic synaptic transmission in retinal ganglion cells and hippocampal pyramidal cells to determine, at a cellular level, what is the effect of the targeted disruption of the gene encoding the synthetic enzyme GAD65 on the synaptic release of γ-aminobutyric acid (GABA). Neither the size nor the frequency of GABA-mediated spontaneous inhibitory postsynaptic currents (IPSCs) were reduced in retina or hippocampus in GAD65−/− mice. However, the release of GABA during sustained synaptic activation was substantially reduced. In the retina both electrical- and K+-induced increases in IPSC frequency were depressed without a change in IPSC amplitude. In the hippocampus the transient increase in the probability of inhibitory transmitter release associated with posttetanic potentiation was absent in the GAD65−/− mice. These results indicate that during and immediately after sustained stimulation the increase in the probability of transmitter release is not maintained in GAD65−/− mice. Such a finding suggests a decrease in the size or refilling kinetics of the releasable pool of vesicles, and various mechanisms are discussed that could account for such a defect.
The major inhibitory neurotransmitter in the mammalian brain, γ-aminobutyric acid (GABA), is synthesized by two glutamic acid decarboxylase (GAD) isoforms, GAD65 and GAD67. Several studies suggest that these two enzymes may have distinct roles in neuronal function (1–3). For instance, GAD67 is a cytosolic enzyme and is distributed throughout the cell, whereas GAD65 is localized to the nerve terminal and is reversibly bound to the membrane of synaptic vesicles (2, 4–6). These findings suggest that GAD65 may play a specific role in the control of the synaptic release of GABA (7). Indeed, mice in which GAD65 has been disrupted are susceptible to seizures (8, 9) and anxiety (10), and the K+-induced release of GABA from the visual cortex is reduced (11). Furthermore, recent behavioral studies have found an increased anxiety and altered responses to anxiolytics in mice deficient in GAD65 (10). However, nothing is known about the role of GAD65 in controlling the vesicular contents and/or the release of vesicles.
To investigate the role of GAD65 in synaptic transmission at the cellular level we have examined GABAergic synaptic currents in the retina and hippocampus of mice in which GAD65 was disrupted. We find that both the quantal size and frequency of GABA-mediated miniature inhibitory postsynaptic currents (IPSCs) appear to be normal in GAD65−/− mice, but that the release of GABA is reduced during sustained stimulation. This reduction involves a decrease in the probability of synaptic vesicle release, rather than any change in quantal size, suggesting that the number of vesicles available for release is deficient in the GAD65−/− mice.
Materials and Methods
Animals.
GAD65−/− mice were generated as described (9). Both C57BL/6 and NOD/LtJ backgrounds were used without any obvious differences being noted.
Retinal Slice Recordings.
The mouse retinal slice preparation has been described in detail (12). Briefly, the eyes were enucleated and hemisected at the ora serata immediately after cervical dislocation. The retina was detached from the hemisected eyecup mechanically and put into oxygenated saline on a piece of Millipore filter paper with photoreceptors facing the filter paper. The retina was cut into 250-μm-thick slices. Single slices were mounted in a recording chamber for each experiment and continuously perfused during the experiments. All of the procedures were performed at room temperature (23°C) and in normal room light. The recording chamber was mounted on the stage of an upright microscope (Zeiss Axioskop). A 40× water immersion objective lens was used for visualizing the retina and the recording pipettes by using Nomarski differential interference contrast optics.
The extracellular solution contained 137 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 28 mM glucose, and 10 mM Hepes (pH 7.4). The neurotransmitter antagonists (-)-bicuculline methiodide (bicuculline), gabazine, and strychnine were dissolved into the extracellular solution when required and bath-applied by perfusion from a multibottle array to the recording chamber by a gravity superfusion system at a rate of 1 ml/min. The pipette solution contained 120 mM CsCl, 5 mM CaCl2, 3 mM MgCl2, 20 mM Hepes, and 28 mM glucose (pH 7.2 by using CsOH). Perforated patch recording were made by adding gramicidin to the pipette solution to give a final concentration of 100 μg/ml (0.2% of DMSO). The electrodes were tip-filled with a small volume of gramicidin-free pipette solution and then back-filled with pipette solution containing gramicidin to avoid interference of gramicidin with seal formation.
Access resistance and cell conductance were monitored every 5–10 min during the entire course of each experiment. Recordings began after the access resistance reached a stable plateau, which generally took 20–30 min. Membrane currents were recorded with an Axopatch 1D amplifier, and data were collected by using a Macintosh-based interface (ITC-16 Mac computer interface, Instrutech, Great Neck, NY) run by HEKA software (pulse+pulsefit, HEKA Elektronics, Lambrecht/Pfalz, Germany). The signals were filtered at 1 kHz.
Hippocampal Slice Recordings.
Transverse hippocampal slices were cut (400 μm) and incubated at room temperature in the standard extracellular medium containing 119 mM NaCl, 26 mM NaHCO3, 10 mM glucose, 2.5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgCl2, and 1 mM NaH2PO4, equilibrated with 95% O2 and 5% CO2. Slices then were transferred to an immersion-type recording chamber superfused with the standard extracellular medium with the addition of 10 μM 6-nitro-7-sulfamoylbenzoquinoxaline-2,3-dione (NBQX), 100 μM d(-)-2-amino-5-phosphonovaleric acid, and 500 μM p-(3-aminopropyl)-p-diethoxymethyl-phosphinic acid. Experiments were done at room temperature. Whole-cell patch-clamp recordings were made from CA1 neurons with the “blind” recording technique (30). Pipette solution contained 120 mM Cs-gluconate, 15 mM CsCl, 10 mM Hepes, 5 mM NaCl, 2 mM Mg3ATP2, 0.3 mM Na3GTP, and 0.2 mM Cs-EGTA (pH 7.2 using CsOH). Bipolar stainless steel electrodes were placed close to the cell body layer and stimulating strength was adjusted to give a mean evoked IPSC (eIPSC) of around 150 pA recorded at a membrane potential of 0 mV. Data were collected with an Axopatch 1D amplifier, filtered at 1 kHz, sampled at 5 kHz, and analyzed off-line.
Data Analysis.
Off-line data analysis was carried out by using igor (WaveMetrics, Lake Oswego, OR) with custom-written routines and as described (12). The Kolmogorov–Smirnov test was used to quantify the statistical significance of the differences among the distributions of spontaneous synaptic event amplitudes (31–35). The Student’s t test was used to examine the difference between means.
Results
The Amplitude of GABAA-Mediated Spontaneous Synaptic Events Are Similar in Control and GAD65−/− Mice.
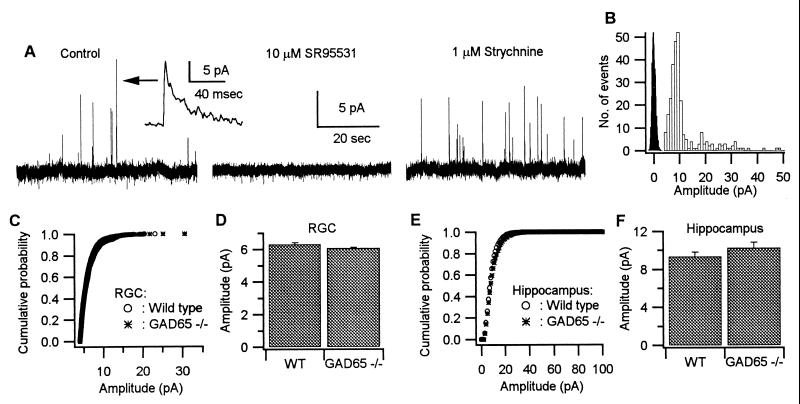
We initially examined the effects of deleting GAD65 on spontaneous GABAergic synaptic transmission in retinal ganglion cells (RGCs) and hippocampal pyramidal cells. Fig. 1A shows spontaneous outward currents obtained with perforated patch recording from a RGC held at 0 mV. In this paper we will refer to spontaneous IPSCs recorded in the absence of the sodium channel blocker tetrodotoxin (TTX) as spontaneous IPSCs (sIPSCs). Previously we found that the average size of events recorded in the presence of TTX was only slightly smaller (≈85%) than the events recorded in control conditions and thus in the absence of TTX the large majority of events represent the response to a single quantum (12). Therefore TTX was not routinely used in the rest of the experiments on the RGCs. These events were blocked by the GABAA receptor antagonist gabazine, but not by strychnine (Fig. 1A), which blocks the glycinergic spontaneous currents that are seen in approximately 50% of the cells. The amplitude of the sIPSCs either displayed as a cumulative probability distribution (Fig. 1C) or as the mean amplitude (Fig. 1D) was unaltered in the GAD65−/− mice. A similar result was obtained from hippocampal pyramidal cells. Because a high proportion of sIPSCs in the hippocampus are multiquantal, TTX was present in these experiments. No difference in the cumulative probability distribution (Fig. 1E) or the mean amplitude of these quantal IPSCs (Fig. 1F) was detected. These results indicate that deleting GAD65 has no effect on the GABA content of spontaneously released synaptic vesicles.
Figure 1.
The amplitude of GABA-mediated spontaneous synaptic events are similar in WT and GAD65−/− mice. (A) One-minute recordings of membrane current from a RGC of a GAD65−/− mouse at a holding potential of 0 mV in control saline, 10 μM gabazine (SR95531), and 1 μM strychnine, respectively. (Inset) A single sIPSC event. In this cell the outward events were completely blocked by the GABAA receptor antagonist gabazine but not by strychnine. (B) Histograms of the amplitude of spontaneous synaptic events (open bars) and an all-point plot of event-free recording (filled bars) from a RGC to show the baseline noise and event detection threshold. (C) Cumulative distributions of the amplitudes of GABA-mediated sIPSCs recorded from RGCs of WT (n = 781, four cells) and GAD65−/− mice (n = 2,233, 11 cells). (D) Average amplitude of GABA-mediated sIPSCs from events used to calculate the cumulative distribution curves shown in C (mean ± SE = 6.32 ± 0.09 pA for WT, mean ± SE 6.08 ± 0.05 pA for GAD65−/− mice). (E) Cumulative distributions of the amplitudes of GABA-mediated IPSCs recorded in TTX from hippocampal pyramidal cells of WT (n = 2,330, six cells) and GAD65−/− mice (n = 3,442, six cells). (F) Average amplitude of IPSCs from events used to calculate the cumulative distribution curves shown in E (mean ± SE = 9.34 ± 0.45 pA for WT, mean ± SE = 10.26 ± 0.56 pA for GAD65−/− mice).
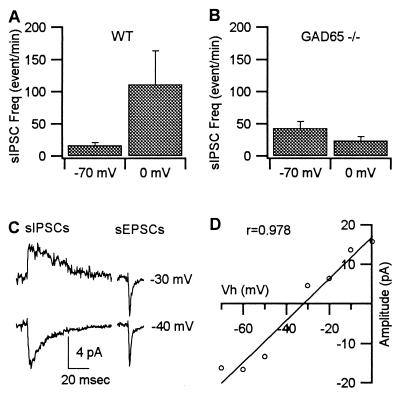
The Frequency of GABA-Mediated IPSCs in RGCs During Stimulation Is Reduced in GAD65−/− Mice.
Previous biochemical studies have suggested that GAD65 might function to provide GABA during periods of stimulated GABA release (7, 11). We therefore examined whether deleting GAD65 had any effect on the evoked release of GABA measured electrophysiologically. In the mouse retina depolarization of RGCs from −70 mV to 0 mV causes a marked increase in the frequency of sIPSCs (Fig. 2A), which is thought to occur by a depolarization of GABAergic amacrine nerve terminals via gap junctions that exist between these two cell types (13–16). The depolarization induced increase in sIPSCs was approximately 7-fold in control mice (Fig. 2A) (P < 0.05), whereas in GAD65−/− mice there was no significant difference in the frequency recorded at −70 mV and 0 mV (Fig. 2B). The sIPSCs can clearly be separated from the excitatory glutamatergic events, both by their much slower kinetics (12) and by their more negative reversal potential (Fig. 2C). However, to ensure that the sIPSC frequency increase recorded at 0 mV was not attributable to a change in either the time course of the events or to an increase in driving force that simply enabled us to detect more events at this potential, we measured the current-voltage characteristics of the sIPSCs (Fig. 2 C and D). The plot of the average sIPSC amplitude against the holding potentials indicates that at the holding potential of −70 mV the average size of the IPSC (−16.3 pA) is very close to that recorded at 0 mV (15.8 pA). The sIPSC amplitude distribution is fitted with a linear distribution with a correlation coefficient of 0.978. The fitted line crosses the voltage axes near −33 mV, indicating a reversal potential of −33 mV in this particular cell. Similar results were obtained in 17 cells tested in control animals (mean ± SD = −37 ± 12 mV) and three cells in GAD65−/− mice (mean ± SD = −35 ± 10 mV). These results rule out the possibility that the increased sIPSC frequency at 0 mV is caused by the change of driving force or voltage-dependent properties of the GABA channels. Therefore, the increased frequency at 0 mV is indicative of a depolarization-evoked increase in sIPSC frequency. The absence of a similar increase in the GAD65−/− mice indicates that, in response to depolarization, the probability of GABA release is reduced.
Figure 2.
Current-induced depolarization elevates sIPSC frequency in WT but not in GAD65−/− mice. (A) Frequency of sIPSCs recorded at holding potentials of −70 mV and 0 mV from RGCs of WT mice (n = 24). The sIPSC frequency was obtained from 10-min recordings of membrane current at each holding potential. The average sIPSC frequency is almost 7-fold higher at 0 mV versus −70 mV (mean ± SE = 111 ± 52 events/min at 0 mV, mean ± SE = 16 ± 4 events/min at −70 mV) for WT animals. The difference is significant statistically (P < 0.05). (B) Frequency of sIPSCs recorded at holding potentials of −70 mV and 0 mV from RGCs of GAD65−/− mice (mean ± SE = 24 ± 6 events/min at 0 mV, mean ± SE = 43 ± 11 events/min at −70 mV) (n = 21). The difference is not significant statistically (P > 0.1). (C) Average spontaneous events recorded at −30 mV and −40 mV. (D) Plot of amplitude-voltage relationship of sIPSCs. The average amplitudes were obtained from 1-min segments at 10-mV intervals of holding potential between −70 mV and 0 mV. These results demonstrate that the average amplitude of sIPSCs was comparable at −70 mV and 0 mV, ruling out the possibility that observed frequency changes reflected a higher detectability of sIPSCs at 0 mV.
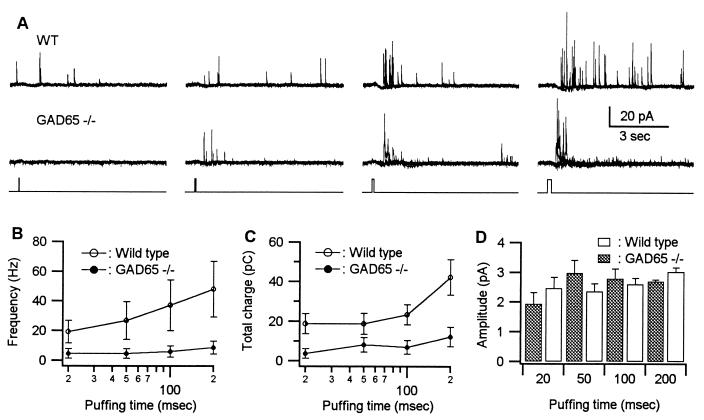
A more direct approach for studying the evoked release of transmitter is to depolarize the terminals directly with high K+. eIPSCs were produced by applying puffs of K+ (60 mM) on the amacrine somata located at the proximal edge of the inner nuclear layer, the site where the amacrine cells are localized. As shown in Fig. 3A the frequency of eIPSCs recorded from a wild-type (WT) RGC (Upper) is increased by puffing K+ and as the duration of the puff is increased the frequency of events also increases. In the GAD65−/− mouse (Fig. 3A, Lower) application of K+ was considerably less effective in increasing the frequency. The effect of increasing puff duration on the frequency of eIPSCs is summarized for a number of control (n = 5) and GAD65−/− (n = 7) cells in Fig. 3B. The frequency is reduced at all puff durations. Replotting this data as the total charge carried by the eIPSCs also showed a dramatic reduction in the K+-induced release of GABA in the GAD65−/− mice (Fig. 3C). The reduced frequency and total charge transfer are statistically significant at all of the durations in the GAD65−/− mice (P < 0.05). The mean amplitude of the individual IPSCs during K+ stimulation also was recorded in these experiments (Fig. 3D). The mean amplitudes of IPSCs evoked in WT and GAD65−/− mice by K+ puffs were statistically indistinguishable. These results, as well as the results obtained by depolarizing the RGC, indicate that in the absence of GAD65 the probability of releasing IPSCs in response to depolarization is greatly reduced. However, the postsynaptic GABAA receptor-mediated response is unchanged.
Figure 3.
Potassium-evoked GABA-mediated IPSCs are much reduced in GAD65−/− mice. (A) (Top) GABA-mediated, K+-eIPSCs recorded from a RGC of a WT mouse at a holding potential of 0 mV. The GABA release was evoked by puffing 60 mM K+ on the cell body layer of the amacrine cells. Puff durations varied from 20 msec to 200 msec. The puffing was repeated three times at each duration. (Middle) GABA-mediated eIPSCs recorded from a RGC of a GAD65−/− mouse. (Bottom) The puffing durations. (B) Frequency of GABA-mediated eIPSCs recorded from RGCs of WT (n = 5) and GAD65−/− mice (n = 7) at various puff durations. Frequency was derived as the number of IPSCs recorded over a 1-sec period after the puff. (C) The total charge carried by the GABA-mediated eIPSCs recorded from RGCs of WT and GAD65−/− mice at various puff durations. (D) Average amplitude of GABA-mediated eIPSCs recorded from RGCs of WT and GAD65−/− mice at various puff durations.
The Release of GABA in the Hippocampus Is Decreased in GAD65−/− Mice.
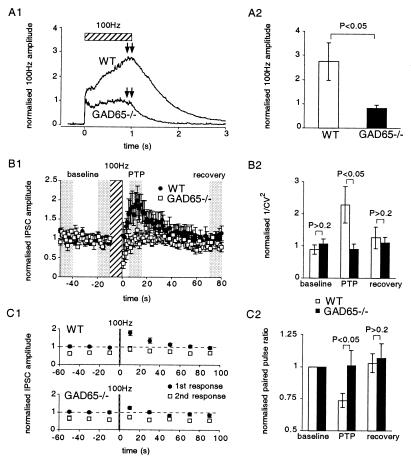
We also examined the release of GABA from inhibitory interneurons in the hippocampus. In contrast to the experiments in RGCs where release was induced by relatively prolonged depolarizations, release in the hippocampus was induced by action potentials. Monosynaptic IPSCs were evoked in the presence of the glutamate receptor blockers NBQX (6-nitro-7-sulfamoylbenzoquinoxaline-2,3-dione) and 2-amino-5-phosphonovaleric acid. Although difficult to quantify across slices, the response to single, low-frequency electrical stimulation appeared to be normal. In WT, when the frequency of stimulation of inhibitory synapses is increased from 0.1 Hz to 1 Hz, the amplitude of the IPSC is reduced to 0.56 ± 0.04 (n = 10) of control. This depression was unaltered in the GAD65−/− mice (0.58 ± 0.05, n = 9) (not illustrated). However, high-frequency stimulation revealed two abnormalities in the GAD65−/− mice. First, during high-frequency stimulation (100 Hz) an envelope of GABA current is generated, consisting of a combination of synchronous and asynchronous events. The envelopes from a number of experiments were averaged together by normalizing with respect to the amplitude of the previous 10 stimuli evoked at 0.1 Hz. (Fig. 4A1). In control cells the envelope continued to increase during the 100-Hz stimulation and then slowly returned to baseline. In contrast, in the GAD65−/− mice the envelope remained nearly constant and returned rapidly to baseline. A comparison in the envelope measured at the end of the 100-Hz stimulus (see arrows in Fig. 4A1) is shown in Fig. 4A2. The second defect was observed after high-frequency stimulation. In control mice IPSCs are potentiated for approximately a minute after 100-Hz stimulation for 10 sec (Fig. 4B1, ●). This posttetanic potentiation (PTP) is absent in GAD65−/− mice, and a transient depression immediately after the tetanus is observed. To address the mechanism underlying this defect we measured the 1/CV2 (Fig. 4B2) and paired pulse modulation (Fig. 4 C1 and C2). PTP in WT mice was associated with an increase in 1/CV2 (Fig. 4B2), as would be expected for an increase in the probability of release or in the number of release sites. In contrast, no change in 1/CV2 was detected in the GAD65−/− mice. PTP also was associated with a decrease in the paired pulse ratio (Fig. 4 C1 and C2), which supports the notion that the PTP is caused by an increase in the probability of release, because it is well established that the paired pulse ratio is altered by a variety of manipulations known to change the probability of transmitter release at both excitatory and inhibitory synapses. In contrast, in the GAD65−/− mice no change in the paired pulse ratio was observed, supporting the notion that the transient increase in the probability of transmitter release is absent in the GAD65−/− mice.
Figure 4.
GAD65−/− mouse shows reduced release of GABA in response to high-frequency stimulation in the hippocampus. (A1) IPSCs are recorded in a hippocampal CA1 pyramidal cell in response to extracellular stimulation. Upon stimulation at 100 Hz for 1 sec, the IPSCs add onto each other because of their slow time course, and an envelope is recorded. The amplitude of the envelope is normalized to the amplitude of the responses to the previous 10 stimuli evoked at low frequency (0.1 Hz), allowing an average envelope to be calculated from the different experiments. In GAD65−/− mice less GABA appears to be released during the tetanic stimulation (WT, n = 8; GAD65−/−, n = 13). (A2) Average amplitude of the last 100 ms of the envelope in response to 100-Hz stimulation indicates that there is a significant reduction in GABA release (WT, n = 8; GAD65−/−, n = 13, P < 0.05). (B1) PTP also is reduced in GAD65−/− mice after 10 sec of 100-Hz stimulation (WT, n = 10; GAD65−/−, n = 7). (B2) Variance analysis was carried out on the four shaded regions of the data around the 100-Hz stimulation. Using one baseline period to normalize the 1/CV2 we have three regions of variance measurements. 1/CV2 increases during PTP for the WT mice, suggesting that the probability of release has increased, but no such increase is apparent in the GAD65−/− mice (P < 0.05). (C1 and C2) During PTP, the paired pulse ratio for WT mice decreased, suggesting that the release probability increased. No such change is observed in the GAD65−/− mice (WT, n = 8; GAD65−/−, n = 13: different set of cells to those used for the 1/CV2 analysis).
Discussion
The hallmark of chemical synaptic transmission is that the transmitter is packaged into synaptic vesicles and released as packets, or quanta, upon stimulation. The integrated amount of transmitter released when a synapse is activated for a period of time normally is determined by the total number of vesicles released. However, there are a number of instances in which alterations in the filling of synaptic vesicles can change the total amount of transmitter released (17). The factors controlling the packaging and release of GABA are not well understood. However, it is known that two enzymes are responsible for the synthesis of GABA, GAD65 and GAD67. The specific anchoring of GAD65 to the membrane of synaptic vesicles (2, 5, 6) suggests that it may play an important role in the packaging and/or release of GABA. GAD67 appears to be primarily responsible for the synthesis of GABA, because the brains of mice lacking GAD67 contain approximately 10% of the normal levels of GABA (18, 19), whereas in mice lacking GAD65 there is only a small and variable decrease in total GABA content (8, 9, 11). Despite this small defect in GABA content, GAD65−/− mice are prone to seizures (8, 9), have altered visual cortical plasticity during the critical period (11), and have reduced release of GABA in the visual cortex in response to high K+ (11). These results suggest that GAD65 plays an important role in GABAergic synaptic transmission. We have, therefore, investigated at a cellular level the consequences of deleting GAD65 on synaptic transmission.
The results in both the retina and hippocampus suggest that there is a very selective defect at GABA synapses in GAD65−/− mice. Basal synaptic transmission appears to be essentially normal in that there is no significant decrease in the frequency and amplitude of miniature IPSCs recorded in the absence of stimulation in GAD65−/− mice compared with the WT mice. Furthermore, in the hippocampus there was no obvious impairment in the response to low-frequency stimulation of inhibitory interneurons. However, during sustained stimulation a marked defect in transmitter release is revealed. In the retina this defect is observed as a decrease in the frequency of sIPSCs without a change in their amplitude. In the hippocampus the release during a tetanus is reduced and the transient potentiation that occurs after the tetanus is absent in the GAD65−/−. This transient potentiation, referred to as PTP, is observed as an increase in the probability of transmitter release, because it is associated with an increase in 1/CV2 and a decrease in the paired pulse ratio, two parameters that are sensitive to changes in the probability of transmitter release. Thus in the hippocampus, as well as in the retina, an impairment in the ability to release synaptic vesicles during sustained stimulation appears to be the primary defect present in GAD65−/− mice.
These findings presumably indicate that the mobilization of vesicles and/or replenishment of vesicles at release sites that normally occurs during the sustained activation of a synapse is impaired in the GAD65−/− mice. This defect is, at least on the surface, similar to that found when synapsins are either deleted genetically (20) or inactivated by the injection of antibodies into the presynaptic terminal (21). In these studies it was found that whereas synaptic transmission was normal for low-frequency stimulation, release was markedly impaired during repetitive stimulation. There are a number of possible explanations for such a defect in the GAD65−/− mice. First, the number of vesicles in the presynaptic terminal could be reduced and, indeed, this appears to be the case when synapsins are either deleted (20) or inactivated (21). There is no electronmicroscopic data of inhibitory synapses available for GAD65−/− mice. However, it recently has been reported that deletion of GAD in Caenorhabditis elegans, if anything, causes a slight increase in the number of synaptic vesicles in GABAergic terminals (22). Second, it is possible that GAD65, which is bound to the membrane of synaptic vesicles, facilitates the movement of the vesicles to the release site or facilitates the docking of vesicles, although there is no experimental evidence that addresses this possibility. Finally, an intriguing possibility is that in the absence of GAD65 the filling of vesicles with GABA is defective and that a mechanism exists that allows only fully filled vesicles to be released. A great deal of work has been carried out on the effects of altering vesicle filling on the release of transmitter. In many cases changing the transmitter content of synaptic vesicles does change quantal size, indicating that the release mechanism can allow vesicles with altered content to be released (17, 23, 24). However, there are also a number of reports that suggest that there may be a change in the probability of release associated with changes in vesicle filling (24–28). A more direct approach for determining whether changes in the transmitter content of vesicles affects exocytosis is to monitor vesicle recycling with FM1–43. It has been reported that bafilomycin A1, a drug that is known to deplete synaptic vesicles of GABA and glutamate, has little effect on the activity-dependent destaining of cerebellar granule cell synapses of the dye FM1–43 (29). Although it was shown in this study that the release of radioactive aspartate was reduced by bafilomycin A1, biophysical measurements of quantal size and frequency were not performed. Further studies on the possible interactions between the process of vesicle filling and vesicle release would be of considerable interest.
Acknowledgments
We thank T.N. Hwang for developing the algorithms for detecting synaptic events in the retina and Helen Czerwonka for secretarial assistance. This study was supported by grants from the National Institutes of Health (R.N. and D.C), Bristol-Myers Squibb Company (R.N.), National Research Service Award (N.T.), the Wellcome Trust (C.P.), That Man May See at the University of California at San Francisco (D.C.), and Research to Prevent Blindness (D.C.). R.N. is a member of the Keck Center for Integrative Neuroscience and the Silvio Conte Center for Neuroscience Research.
Abbreviations
- GABA
γ-aminobutyric acid
- GAD
glutamic acid decarboxylase
- IPSC
inhibitory postsynaptic current
- RGC
retinal ganglion cell
- TTX
tetrodotoxin
- sIPSC
spontaneous IPSC
- eIPSC
evoked IPSC
- WT
wild type
- PTP
posttetanic potentiation
References
- 1.Erlander M G, Tobin A J. Neurochem Res. 1991;16:215–226. doi: 10.1007/BF00966084. [DOI] [PubMed] [Google Scholar]
- 2.Christgau S, Aanstoot H J, Schierbeck H, Begley K, Tullin S, Hejnaes K, Baekkeskov S. J Cell Biol. 1992;118:309–320. doi: 10.1083/jcb.118.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dirkx R, Jr, Thomas A, Li L, Lernmark A, Sherwin R S, De Camilli P, Solimena M. J Biol Chem. 1995;270:2241–2246. doi: 10.1074/jbc.270.5.2241. [DOI] [PubMed] [Google Scholar]
- 4.Christgau S, Schierbeck H, Aanstoot H J, Aagaard L, Begley K, Kofod H, Hejnaes K, Baekkeskov S. J Biol Chem. 1991;266:21257–21264. [PubMed] [Google Scholar]
- 5.Kaufman D L, Houser C R, Tobin A J. J Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reetz A, Solimena M, Matteoli M, Folli F, Takei K, De Camilli P. EMBO J. 1991;10:1275–1284. doi: 10.1002/j.1460-2075.1991.tb08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Namchuk M, Lindsay L, Turck C W, Kanaani J, Baekkeskov S. J Biol Chem. 1997;272:1548–1557. doi: 10.1074/jbc.272.3.1548. [DOI] [PubMed] [Google Scholar]
- 8.Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji F Y, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Biochem Biophys Res Commun. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- 9.Kash S F, Johnson R S, Tecott L H, Noebels J L, Mayfield R D, Hanahan D, Baekkeskov S. Proc Natl Acad Sci USA. 1997;94:14060–14065. doi: 10.1073/pnas.94.25.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kash S F, Tecott L H, Hodge C, Baekkeskov S. Proc Natl Acad Sci USA. 1999;96:1698–1703. doi: 10.1073/pnas.96.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hensch T K, Fagiolini M, Mataga N, Stryker M P, Baekkeskov S, Kash S F. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian N, Hwang T N, Copenhagen D R. J Neurophysiol. 1998;80:1327–1340. doi: 10.1152/jn.1998.80.3.1327. [DOI] [PubMed] [Google Scholar]
- 13.Dacey D M, Brace S. Visual Neurosci. 1992;9:279–290. doi: 10.1017/s0952523800010695. [DOI] [PubMed] [Google Scholar]
- 14.Penn A A, Wong R O, Shatz C J. J Neurosci. 1994;14:3805–3815. doi: 10.1523/JNEUROSCI.14-06-03805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacoby R, Stafford D, Kouyama N, Marshak D. J Neurosci. 1996;16:8041–8056. doi: 10.1523/JNEUROSCI.16-24-08041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xin D, Bloomfield S A. J Comp Neurol. 1997;383:512–528. doi: 10.1002/(sici)1096-9861(19970714)383:4<512::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Van der Kloot W, Molgó J. Physiol Rev. 1994;74:899–991. doi: 10.1152/physrev.1994.74.4.899. [DOI] [PubMed] [Google Scholar]
- 18.Asada H, Kawamura Y, Maruyama K, Kume H, Ding R G, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Proc Natl Acad Sci USA. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Condie B G, Bain G, Gottlieb D I, Capecchi M R. Proc Natl Acad Sci USA. 1997;94:11451–11455. doi: 10.1073/pnas.94.21.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosahl T W, Spillane D, Missler M, Herz J, Selig D K, Wolff J R, Hammer R E, Malenka R C, Südhof T C. Nature (London) 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- 21.Pieribone V A, Shupliakov O, Brodin L, Hilfiker-Rothenfluh S, Czernik A J, Greengard P. Nature (London) 1995;375:493–497. doi: 10.1038/375493a0. [DOI] [PubMed] [Google Scholar]
- 22.Jin Y, Jorgensen E, Hartwieg E, Horvitz H R. J Neurosci. 1999;19:539–548. doi: 10.1523/JNEUROSCI.19-02-00539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song H, Ming G, Fon E, Bellocchio E, Edwards R H, Poo M. Neuron. 1997;18:815–826. doi: 10.1016/s0896-6273(00)80320-7. [DOI] [PubMed] [Google Scholar]
- 24.Pothos E N, Davila V, Sulzer D. J Neurosci. 1998;18:4106–4118. doi: 10.1523/JNEUROSCI.18-11-04106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poulain B, Baux G, Tauc L. Proc Natl Acad Sci USA. 1986;83:170–173. doi: 10.1073/pnas.83.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacchi O, Perri V. J Gen Physiol. 1973;61:342–360. doi: 10.1085/jgp.61.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLachlan E M. J Physiol (London) 1975;253:477–491. doi: 10.1113/jphysiol.1975.sp011201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golan H, Grossman Y. J Neurophysiol. 1996;75:2089–2098. doi: 10.1152/jn.1996.75.5.2089. [DOI] [PubMed] [Google Scholar]
- 29.Cousin M A, Nicholls D G. J Neurochem. 1997;69:1927–1935. doi: 10.1046/j.1471-4159.1997.69051927.x. [DOI] [PubMed] [Google Scholar]
- 30.Blanton M G, Lo Turco J J, Kriegstein A R. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 31.Cohen G A, Doze V A, Madison D V. Neuron. 1992;9:325–335. doi: 10.1016/0896-6273(92)90171-9. [DOI] [PubMed] [Google Scholar]
- 32.Manabe T, Renner P, Nicoll R A. Nature (London) 1992;355:50–55. doi: 10.1038/355050a0. [DOI] [PubMed] [Google Scholar]
- 33.Otis T S, Staley K J, Mody I. Brain Res. 1991;545:142–150. doi: 10.1016/0006-8993(91)91280-e. [DOI] [PubMed] [Google Scholar]
- 34.Press W H, Flannery B P, Teukolsky S A, Vetterling W T. Numerical Recipes: The Art of Scientific Computing. New York: Cambridge Univ. Press; 1986. [Google Scholar]
- 35.Van der Kloot W. Prog Neurobiol. 1991;36:93–130. doi: 10.1016/0301-0082(91)90019-w. [DOI] [PubMed] [Google Scholar]