Abstract
The Fas/Fas ligand (FasL) system participates in regulation of the immune system through the apoptotic process. However, the extent to which abnormalities in this system are involved in the loss of self-tolerance and development of autoimmune disease not associated with Fas/FasL mutations remains unknown. The present study addresses this issue in Fas/FasL-intact, systemic lupus erythematosus (SLE)-prone (NZB × NZW) (NZB/W) F1 mice. While splenic B cells from 2-month-old mice before overt SLE expressed Fas poorly, in vitro stimulation with an agonistic anti-CD40 mAb up-regulated their Fas expression, thus revealing the existence of two populations: one was Fashigh and highly susceptible to anti-Fas mAb-induced apoptosis, and the other was Faslow and apoptosis-resistant. The Faslow cells were included in the CD5+ B cell subpopulation and contained most of the cells that produced IgM anti-DNA antibodies. The isotype of anti-DNA antibodies switches from IgM to IgG in NZB/W F1 mice at ages beginning at about 6 months. These IgG anti-DNA antibodies were produced almost exclusively by a subpopulation of splenic B cells that spontaneously expressed low levels of Fas in vivo and were apoptosis-resistant. The findings indicate that precursor B cells for autoantibody production and presumably autoantibody-secreting cells in these mice are relatively resistant to Fas-mediated apoptosis, a finding supporting the concept that abnormalities of Fas-mediated apoptotic process are involved in the development of autoreactive B cells in Fas/FasL-intact autoimmune disease.
Apoptosis mediated by interaction between Fas and Fas ligand (FasL) is an important mechanism in the establishment of tolerance-of-self and autoimmunity. It operates in (i) negative selection of autoreactive T and B cells in central and peripheral immune systems (1, 2), (ii) cytotoxic T cell-mediated target cell lysis in organ-specific autoimmune disease (3), and (iii) protection of immunologically privileged sites (4, 5).
Thus far the importance of the Fas/FasL system in establishing tolerance-of-self has been examined mainly in Fas-mutation (lpr) mice. The lpr mutation is an autosomal recessive Fas gene defect (6) that causes a lymphoproliferative disorder characterized by massive accumulation of unusual T cells in lymphoid organs and systemic lupus erythematosus (SLE)-like autoimmune disease in association with autoantibodies, including some to DNA (7, 8). Nevertheless, the effect of this mutation on the establishment of self-tolerance remains uncertain. Although subtle changes in thymocyte selection have been reported in lpr homozygous mice (9, 10), negative selection of autoreactive T cells occurs in them in an apparently normal manner (11, 12). As regards B cell abnormalities, transplantation of hemopoietic cells has revealed that only B cells carrying the lpr mutation produce autoantibodies, a finding indicative of an intrinsic B lineage defect (13, 14). However, when introduced into mouse strains other than MRL, the mutated Fas gene did not result in significant autoantibody production associated with florid autoimmune disease (15).
B cells responsible for autoantibody production also occur in autoimmune disease-prone strains not mutated at Fas/FasL; in fact, the majority of autoimmune diseases in which autoantibodies occur are not associated with mutation in this system. Autoreactive B cells are eliminated or rendered functionally inactive during their development in the bone marrow of Fas-intact mice transgenic for an anti-DNA antibody (16). Whether the Fas/FasL system is involved in this process is as yet unknown. In the present study, we address the question of whether abnormalities in the Fas-mediated apoptotic process can be detected in the development of autoreactive B cells in Fas-intact, SLE-prone (NZB × NZW) (NZB/W) F1 mice.
MATERIALS AND METHODS
Mice and Reagents.
NZB/W F1 and BALB/c mice were originally obtained from Shizuoka Laboratory Animal Center (Shizuoka, Japan) and maintained in our animal facilities. Only female mice were used in the present study. Rat mAbs to mouse CD4 (GK1.5), CD8 (53–6.7), Thy-1.2 (30-H12), CD5 (53–7.3), and 6B2(B220) (CD45R, RA3–6B2), and hamster mAb to mouse CD95 (Fas, Jo2) were purchased from PharMingen. Hamster mAb to mouse CD40 (HM40–3) was established as described (17).
Cell Preparation.
Single spleen cell suspensions were obtained by gently dispersing spleen tissue with a glass tissue grinder in RPMI 1640 medium with 3% FCS. Peritoneal lymphocytes were obtained by washing the peritoneal cavity with the above medium, followed by incubation of the cells in 24-well flat-bottom plates at 37°C for 60 min to remove adherent cells. After lysis of red blood cells by ammonium chloride treatment, the suspended cells were washed three times in RPMI 1640 with 3% FCS. To obtain a B cell-enriched population, the cells were treated with a mixture of the rat mAbs to CD4, CD8, and Thy-1.2 plus rabbit complement at 37°C for 45 min.
The 6B2(B220)+CD5+ B and 6B2(B220)+CD5− B cells from 2-month-old mice were sorted by FACStar (Becton Dickinson) after staining the B cell-enriched population with fluorescein isothiocyanate (FITC)-conjugated anti-6B2 and phycoerythrin (PE)-conjugated anti-CD5 mAbs. Faslow and Fas− splenic B cell subsets from 8-month-old NZB/W F1 mice were sorted after staining the B cell-enriched population with FITC-conjugated anti-6B2 and biotinylated anti-Fas mAbs followed by PE-labeled avidin. Purity of these FACS-sorted subpopulations exceeded 95%, as determined by flow cytometry analysis.
Cell Culture.
For in vitro activation of B cells, aliquots of 5 × 105 cells in 200 μl of medium consisting of RPMI 1640, 5 × 10−5 M 2-mercaptoethanol, penicillin (100 units/ml), streptomycin (100 μg/ml), and 10% FCS were cultured in 96-well flat-bottomed plates in the presence of agonistic anti-CD40 mAb (HM40–3, 10 μg/ml) at 37°C for 3 days. To examine antibody production, aliquots of 2–5 × 105 cells in 200 μl of culture medium were cultured in 96-well round-bottomed plates at 37°C for 6 days. Isotype-specific anti-DNA antibodies were measured by ELISA. The titer of anti-DNA antibodies is expressed in units, referring to a standard curve obtained by serial dilution of a standard serum pool from 4- to 7-month-old NZB mice for IgM class antibodies and from NZB/W F1 mice over 7 months of age for IgG class antibodies, both containing 1,000 units of activity per ml (17).
Flow Cytometry Analysis for Fas Expression.
For two-color flow cytometry analysis to examine Fas expression, aliquots of 5–10 × 105 cells in 20 μl of phosphate-buffered saline (pH 7.4) supplemented with 0.2% BSA and 0.05% NaN3 were incubated with FITC-conjugated anti-6B2 and biotinylated anti-Fas mAbs followed by PE-labeled avidin. All incubations were carried out for 30 min at 4°C. The stained cells were examined on a FACStar analyzer equipped with the FITC/PE filter system. Expression of Fas on FACS-sorted CD5+ B and CD5− B cells was examined by three-color flow cytometry analyses with a FACStarPLUS analyzer (Becton Dickinson), after staining with biotinylated anti-Fas mAb, followed by streptavidin-allophycocyanin (APC) (Becton Dickinson).
Induction of Apoptosis.
B cells were incubated with agonistic anti-Fas mAb (Jo2, 1 μg/ml) for 24 hr, and the percent dead cells was calculated by dye exclusion after staining with trypan blue solution. For flow cytometry analysis of Fas expression on living cells, B cells were incubated with biotinylated anti-Fas mAb for 24 hr and stained again with biotinylated anti-Fas mAb, followed by streptavidin-APC. The cells were then resuspended in medium containing 2 μg/ml propidium iodide (PI; Sigma) and 10% FCS to stain dead cells. PI-negative living cells were gated by forward vs. side scatter, and intensity of Fas expression on living cells was examined with a FACStarPLUS analyzer.
RESULTS
Fas-Mediated Apoptosis-Resistant B Cell Subpopulation.
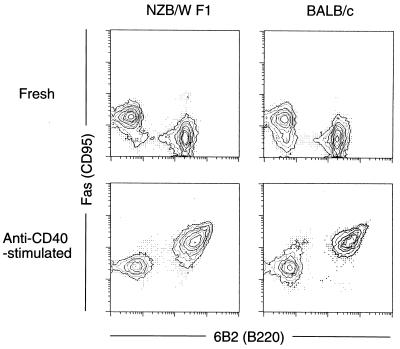
As shown in Fig. 1, 6B2(B220)+ splenic B cells from NZB/W F1 mice 2 months of age (i.e., before the onset of SLE) and age-matched BALB/c mice expressed Fas poorly, but in vitro stimulation with an agonistic anti-CD40 mAb markedly up-regulated their Fas expression, as observed also by others (18–20). To determine the sensitivity to Fas-mediated apoptosis of these B cells, the T cell-depleted B cell-enriched population in the spleen was first stimulated in the presence of anti-CD40 mAb for 3 days, followed by incubation with an agonistic anti-Fas mAb for 24 hr. The proportion of live cells remaining in the BALB/c splenic B cells was less than 2%, while the proportion in NZB/W F1 cells was 12%.
Figure 1.
Stimulation by anti-CD40 mAb markedly up-regulated Fas expression on B cells from both NZB/W F1 and BALB/c mice. Splenic lymphocytes from 2-month-old NZB/W F1 and BALB/c mice were cultured with anti-CD40 mAb for 3 days, and Fas expression was examined by two-color flow cytometry.
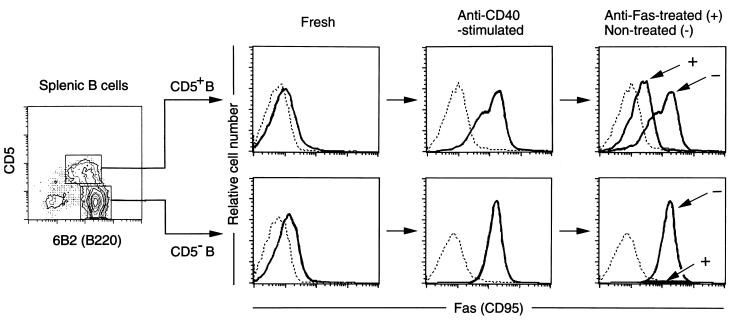
Apoptosis-Resistant B Cells Consist of a Subset of CD5+ B Cells.
Young NZB/W F1 mice have a larger proportion of CD5+ B cells in the spleen than BALB/c mice (21). We therefore compared the sensitivity to Fas-mediated apoptosis of FACS-sorted CD5+ and CD5− B cells from 2-month-old NZB/W F1 mice. Prior to culture, splenic B cells, irrespective of the expression of CD5, as expected expressed only low levels of Fas. On day 3 of culture in the presence of anti-CD40 mAb, the mode of Fas expression differed in the CD5+ and CD5− populations (Fig. 2). CD5− B cells showed a high level of Fas with a single peak in their FACS profile, while the profile of CD5+ B cells was biphasic, with low (Faslow) and high (Fashigh) level-expressing subpopulations. The differences were not due to differing levels of CD40 expression, since CD5+ and CD5− B cells show almost the same level of expression, with a single peak in the FACS profile in both cases (17). We then asked whether the difference affected Fas-mediated apoptosis. When the anti-CD40-stimulated B cell populations were cultured with anti-Fas mAb for 24 hr, the proportions of dead cells in CD5+ and CD5− populations were 66% and 99%, respectively, figures which correspond to the proportion of Fashigh cells in each population. All the remaining living cells, also shown in Fig. 2, belonged to the Faslow population, thus indicating that only the Fashigh population underwent Fas-mediated apoptosis. This was confirmed by experiments in which, when FACS-sorted Faslow and Fashigh CD5+ B cells were cultured in vitro in the presence of anti-Fas mAb, only Fashigh B cells underwent apoptosis (data not shown). Studies on peritoneal CD5+ B cells from 2-month-old NZB/W F1 and BALB/c mice showed that the profile of Fas expression was also biphasic, but most were composed of the Faslow population, which was resistant to Fas-mediated apoptosis (data not shown).
Figure 2.
Comparisons of Fas expression levels and sensitivity to Fas-mediated apoptosis between CD5+ and CD5− B cells. Splenic CD5+ and CD5− B cells from 2-month-old NZB/W F1 mice were FACS-sorted and stimulated with anti-CD40 mAb for 3 days, followed by incubation in the presence (Anti-Fas-treated) or absence (Non-treated) of anti-Fas mAb for 24 hr. Fas expression levels on PI-negative living cells were examined by three-color flow cytometry. Dotted line depicts autofluorescence.
As CD40 ligation up-regulated uniformly the expression of other cell surface activation molecules, such as major histocompatibility complex class II, CD23, CD54, CD80, and CD86, on both CD5+ and CD5− B cells (17), the low level of Fas expression was the only property that distinguished the apoptosis-resistant from the apoptosis-sensitive B cell subpopulation.
Anti-DNA Antibody-Producing B Cells Are Resistant to Fas-Mediated Apoptosis.
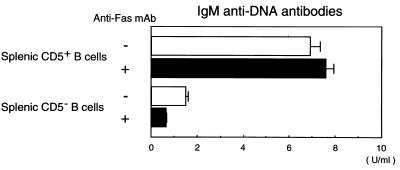
CD5+ B cells in the spleen from young NZB/W F1 mice without overt SLE are the major source of IgM anti-DNA low-affinity antibodies (22–24). To investigate the sensitivity of these cells to Fas-mediated apoptosis, FACS-sorted CD5+ and CD5− B cells from the spleen of 2-month-old NZB/W F1 mice were first stimulated in vitro with anti-CD40 mAb and were then further cultured for 6 days in the presence or absence of anti-Fas mAb. As shown in Fig. 3, IgM anti-DNA antibodies were mainly produced by CD5+ B cells. The finding that the amount of antibodies produced was almost equal in cultures with or without added anti-Fas mAb indicates that the apoptosis-resistant Faslow B cells were largely responsible for producing these antibodies. In the same experiments, FACS-sorted peritoneal CD5+ B cells from 2-month-old NZB/W F1 mice, irrespective of the presence or absence of anti-Fas mAb, also produced IgM anti-DNA antibodies, but to a lesser extent (approximately one-third of the titers produced by splenic CD5+ B cells). Peritoneal CD5+ B cells from 2-month-old BALB/c mice produced little IgM anti-DNA antibodies (data not shown).
Figure 3.
In vitro IgM anti-DNA antibody production by Faslow and apoptosis-resistant CD5+ B cell subpopulation from the spleen of NZB/W F1 mice. Splenic CD5+ and CD5− B cells from 2-month-old NZB/W F1 mice were FACS-sorted and stimulated with anti-CD40 mAb for 3 days, followed by incubation in the presence or absence of anti-Fas mAb for 6 days. Amounts of IgM anti-DNA antibodies in culture supernatants were measured by ELISA. Data represent the mean and SD of triplicate cultures. Similar results were obtained in three independent experiments.
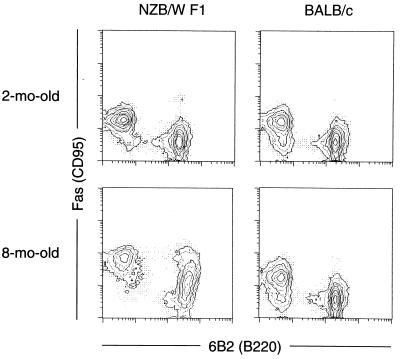
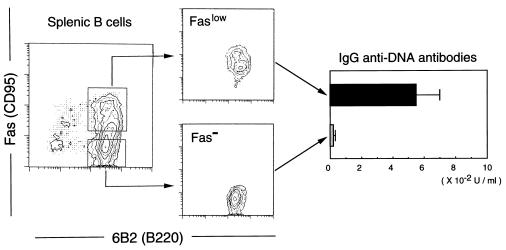
The serum anti-DNA antibodies switch from low-affinity IgM isotype to high-affinity IgG in NZB/W F1 mice at about 6 months of age, at a time when severe SLE begins to develop (22–24). As shown in Fig. 4, in contrast to the findings in 2-month-old NZB/W F1 mice, splenic B cells from 8-month-old NZB/W F1 mice, but not BALB/c mice at comparable ages, contained a considerable proportion of B cells spontaneously expressing the Faslow phenotype. In 6-day cultures, B cell-enriched spleen cells from 8-month-old NZB/W F1 mice produced IgG anti-DNA antibodies, irrespective of the presence or absence of anti-Fas mAb (data not shown). Fig. 5 shows that the FACS-sorted Faslow, but not Fas−, population of B cells from 8-month-old NZB/W F1 mice produced IgG anti-DNA antibodies in cultures in the presence of anti-Fas mAbs. These findings indicate that the subpopulation of splenic B cells in aged NZB/W F1 mice that spontaneously expresses the Faslow phenotype is resistant to Fas-mediated apoptosis and is almost entirely responsible for the observed IgG anti-DNA antibody synthesis. Peritoneal B cells from both NZB/W F1 and BALB/c mice, irrespective of the ages, expressed little Fas and did not produce IgG anti-DNA antibodies in cultures (data not shown).
Figure 4.
Splenic B cells from 8-month-old NZB/W F1, but not BALB/c, mice contained a considerable proportion of B cells spontaneously expressing the Faslow phenotype. Fas expression levels on splenic lymphocytes from 2- and 8-month-old NZB/W F1 and BALB/c mice were examined by two-color flow cytometry.
Figure 5.
In vitro IgG anti-DNA antibody production by the subpopulation of splenic B cells in aged NZB/W F1 mice that spontaneously expresses the Faslow phenotype. FACS-sorted splenic Faslow and Fas− B cells from 8-month-old NZB/W F1 mice were cultured for 6 days in the presence of anti-Fas mAb, and amounts of IgG anti-DNA antibodies in culture supernatants were measured using ELISA. Data represent the mean and SD of triplicate cultures. Similar results were obtained in three independent experiments.
DISCUSSION
The present study provides evidence that the development of autoreactive B cells in Fas/FasL-intact autoimmune disease is related to down-regulation of their Fas expression and an associated resistance to Fas-mediated apoptosis. At least two mechanisms operate in the establishment of self-tolerance among potentially autoreactive B cells. First, deletion of autoreactive B cell clones occurs in the bone marrow (25, 26), much as it does in the thymus for T cells. Although the role of the Fas system in this process remains unclear, studies on Fas-deficient mice suggest that an intact Fas system is not required (27). Second, autoreactive B cells that escape elimination in the bone marrow are rendered functionally inactive in the periphery (28). Using transgenic mice expressing hen egg lysozyme, and carrying both specific antibody and specific T cell receptor transgenes, Rathmell et al. (29) demonstrated that peripheral B cells chronically exposed to soluble antigen became functionally inactive (anergic B cells) in a Fas-independent manner, but could then be eliminated through cognate interaction with activated antigen-specific CD4+ T cells in a Fas-dependent manner. On the other hand, Fas-deficient anergic B cells were not eliminated by CD4+ T cells and were instead triggered to proliferate. The present study identified a mechanism that allows pathogenic autoantibodies to be made in Fas/FasL-intact NZB/W F1 mice, through autoreactive B cells down-regulating their Fas expression and thus escaping Fas-mediated elimination.
The Faslow, apoptosis-resistant B cell precursors of low-affinity IgM anti-DNA antibody producers in young NZB/W F1 mice were almost exclusively included in a subpopulation of CD5+ B cells. CD5+B cells produce low-affinity IgM antibodies that are polyreactive to a variety of antigens, including autoantigens (30, 31), and thus participate in providing natural immunity. Part at least of the CD5+ B cell repertoires appears to be selected and maintained by internal antigens (32, 33). CD5+ B cells selected is this way appear to protect themselves from elimination by bystander activated T cells through down-regulation of their Fas level. This form of protection appears to operate even in CD5+ B cells of normal individuals, because there exists a Fas-mediated apoptosis-resistant subpopulation of CD5+ B cells in normal healthy BALB/c mice.
At least three steps determine the fate of autoreactive B cells in NZB/W F1 mice. First, naive autoreactive B cells chronically exposed to self antigens switch their signal transduction pathways so as to down-regulate Fas expression, even if exposed to CD40-mediated signaling; consequently they escape Fas-mediated elimination. Second, these apoptosis-resistant B cells proceed to proliferate and mature so as to produce low-affinity IgM autoantibodies. Although the first step occurs even in normal healthy strains, the second step is unique in these mice and is genetically controlled (34, 35). Third, in response to signals from autoreactive T cells generated in these mice as they age (36), these autoreactive B cells are induced to express Fas, but to a lesser extent than do naive unstimulated B cells. Hence they are not eliminated, but instead proceed to clonal proliferation, affinity maturation, and class switching. Because splenic, but not peritoneal, B cells produce IgG anti-DNA antibodies in these mice, this process proceeds only in the microenvironment where effective T–B cell interactions can take place. Affinity-selected IgG autoreactive B cells were reported to localize predominantly in the splenic red pulp or outer periarteriolar lymphoid sheaths in SLE-prone (SWR × NZB)F1 mice (37).
There is a possibility that primary IgM anti-DNA antibody-producing cells and secondary IgG B cells are generated from separate precursor subpopulations (38). Studies of our own (23) and of others (39), however, have provided evidence that low-affinity IgM anti-DNA clones with little, if any, somatic mutations in Ig V region genes are indeed the precursors of affinity-selected IgG anti-DNA clones in aged NZB/W F1 mice with overt SLE.
The mechanisms responsible for down-regulation of the Fas expression and the associated resistance to Fas-mediated apoptosis among autoreactive B cells are unknown. It is pertinent that deficiency in the src-related protein tyrosine kinase Lyn, known to mediate antigen receptor-mediated signals, is associated with impaired CD40L-induced Fas expression and Fas-mediated apoptosis of B cells (40), as well as hypergammaglobulinemia and immune complex-type glomerulonephritis (41, 42). Rothstein et al. (43) and Foote et al. (44) reported that antigen receptor engagement blocks CD40L-induced susceptibility of B cells to Fas-dependent Th1-mediated cytotoxicity by means of protein kinase C and calcium signals. In this system, however, the signaling did not alter Fas expression. Thus, a further clarification of the mechanism responsible for down-regulation of the Fas expression and the associated resistance to Fas-mediated apoptosis in autoreactive B cells is likely to provide a better understanding of the mechanism of loss of B cell self-tolerance, and thus a new approach to therapy of autoimmune disease.
Acknowledgments
We thank M. Ohara for critical comments. This work was supported in part by Core Research for Evolutional Science and Technology (CREST) of Japan Science and Technology Corporation (JST) and a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture, Japan.
ABBREVIATIONS
- FasL
Fas ligand
- SLE
systemic lupus erythematosus
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
- PI
propidium iodide
References
- 1.Krammer P H, Dhein J, Walczak H, Behrmann I, Mariani S, Matiba B, Fath M, Daniel P T, Knipping E, Westendorp M O, Stricker K, Bäumler C, Hellbardt S, Germer M, Peter M E, Debatin K-M. Immunol Rev. 1994;142:175–191. doi: 10.1111/j.1600-065x.1994.tb00889.x. [DOI] [PubMed] [Google Scholar]
- 2.Lynch D H, Ramsdell F, Alderson M R. Immunol Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 3.Yagita H, Hanabuchi S, Asano Y, Tamura T, Nariuchi H, Okumura K. Immunol Rev. 1995;146:223–239. doi: 10.1111/j.1600-065x.1995.tb00691.x. [DOI] [PubMed] [Google Scholar]
- 4.Griffith T S, Brunner T, Fletcher S M, Green D R, Ferguson T A. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 5.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke R C. Nature (London) 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe-Fukunaga R, Brannan C I, Copeland N G, Jenkins N A, Nagata S. Nature (London) 1993;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 7.Murphy E D, Roths J B. In: Genetic Control of Autoimmune Disease. Rose N R, Bigazzi P E, Warner N L, editors. New York: Elsevier/North Holland; 1978. pp. 207–220. [Google Scholar]
- 8.Andrews B S, Eisenberg R A, Theofilopoulos A N, Izui S, Wilson C B, McConahey P J, Murphy E D, Roths J B, Dixon F J. J Exp Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto K, Yoshikai Y, Asano T, Himeno K, Iwasaki A, Nomoto K. J Exp Med. 1991;173:127–136. doi: 10.1084/jem.173.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou T, Bluethmann H, Eldridge J, Brockhaus M, Berry K, Mountz J D. J Immunol. 1991;147:466–474. [PubMed] [Google Scholar]
- 11.Kotzin B, Babcock S K, Herron L R. J Exp Med. 1988;168:2221–2229. doi: 10.1084/jem.168.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer P A, Balderas R S, McEvilly R J, Bobardt M, Theofilopoulos A N. J Exp Med. 1989;170:1869–1877. doi: 10.1084/jem.170.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perkins D L, Glaser R M, Mahon C A, Michaelson J, Marshak-Rothstein A. J Immunol. 1990;145:549–555. [PubMed] [Google Scholar]
- 14.Sobel E S, Katagiri T, Katagiri K, Morris S C, Cohen P I, Eisenberg R A. J Exp Med. 1991;173:1441–1449. doi: 10.1084/jem.173.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izui S, Kelley V E, Masuda K, Yoshida H, Roths J B, Murphy E D. J Immunol. 1984;133:227–233. [PubMed] [Google Scholar]
- 16.Erikson J, Radic M Z, Camper S A, Hardy R R, Carmack C, Weigert M. Nature (London) 1991;349:331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko Y, Hirose S, Abe M, Yagita H, Okumura K, Shirai T. Eur J Immunol. 1996;26:3061–3065. doi: 10.1002/eji.1830261236. [DOI] [PubMed] [Google Scholar]
- 18.Garrone P, Neidhardt E-M, Garcia E, Galibert L, van Kooten C, Banchereau J. J Exp Med. 1995;182:1265–1273. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schatter E J, Elkon K B, Yoo D-H, Tumang J, Krammer P H, Crow M K, Friedman S M. J Exp Med. 1995;182:1557–1565. doi: 10.1084/jem.182.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Taniuchi I, Maekawa Y, Howard M, Cooper M D, Watanabe T. Eur J Immunol. 1996;26:92–96. doi: 10.1002/eji.1830260114. [DOI] [PubMed] [Google Scholar]
- 21.Hayakawa K, Hardy R R, Parks D R, Herzenberg L A. J Exp Med. 1983;157:202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okada T, Abe M, Takiura F, Hirose S, Shirai T. Autoimmunity. 1990;7:109–120. doi: 10.3109/08916939008993383. [DOI] [PubMed] [Google Scholar]
- 23.Taki S, Hirose S, Kinoshita K, Nishimura H, Shimamura T, Hamuro J, Shirai T. Eur J Immunol. 1992;22:987–992. doi: 10.1002/eji.1830220417. [DOI] [PubMed] [Google Scholar]
- 24.Hirose S, Wakiya M, Kawano-Nishi Y, Jiang Y, Sanokawa R, Taki S, Shimamura T, Kishimoto T, Tsurui H, Nishimura H, Shirai T. Eur J Immunol. 1993;23:2913–2820. doi: 10.1002/eji.1830231114. [DOI] [PubMed] [Google Scholar]
- 25.Nemazee D A, Bürki K. Nature (London) 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 26.Hartley S B, Crosbie J, Brink R, Kantor A A, Basten A, Goodnow C C. Nature (London) 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 27.Rathmell J C, Goodnow C C. J Immunol. 1994;153:2831–2842. [PubMed] [Google Scholar]
- 28.Goodnow C C. Annu Rev Immunol. 1992;10:489–518. doi: 10.1146/annurev.iy.10.040192.002421. [DOI] [PubMed] [Google Scholar]
- 29.Rathmell J C, Cooke M P, Ho W Y, Grein J G, Townsend S E, Davis M M, Goodnow C C. Nature (London) 1995;376:181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- 30.Kocks C, Rajewsky K. Annu Rev Immunol. 1989;7:537–559. doi: 10.1146/annurev.iy.07.040189.002541. [DOI] [PubMed] [Google Scholar]
- 31.Casali P, Notkins A L. Immunol Today. 1989;10:364–368. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- 32.Hardy R R, Hayakawa K. Adv Immunol. 1994;55:297–339. doi: 10.1016/s0065-2776(08)60512-x. [DOI] [PubMed] [Google Scholar]
- 33.Rickert R C, Rajewsky K, Roes J. Nature (London) 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- 34.Hirose S, Tsurui H, Nishimura H, Jiang Y, Shirai T. Int Immunol. 1994;6:1857–1864. doi: 10.1093/intimm/6.12.1857. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Y, Hirose S, Hamano Y, Kodera S, Tsurui H, Abe M, Terashima K, Ishikawa S, Shirai T. J Immunol. 1997;158:992–997. [PubMed] [Google Scholar]
- 36.Sekigawa I, Okada T, Noguchi K, Ueda G, Hirose S, Sato H, Shirai T. J Immunol. 1987;138:2890–2895. [PubMed] [Google Scholar]
- 37.Jacobson B A, Panka D J, Nguyen K-A, Erikson J, Abbas A K, Marshak-Rothstein A. Immunity. 1995;3:509–519. doi: 10.1016/1074-7613(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 38.Linton P-J, Decker D J, Klinman N R. Cell. 1989;59:1049–1059. doi: 10.1016/0092-8674(89)90761-7. [DOI] [PubMed] [Google Scholar]
- 39.Tillman D M, Jou N-T, Hill R J, Marion T N. J Exp Med. 1992;176:761–779. doi: 10.1084/jem.176.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Koizumi T, Watanabe T. J Exp Med. 1996;184:831–838. doi: 10.1084/jem.184.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hibbs M L, Tarlinton D M, Armes J, Grail D, Hodgson G, Maglitto R, Stacker S A, Dunn A R. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 42.Nishizumi H, Tanuichi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 43.Rothstein T L, Wang J K M, Panka D J, Foote L C, Wang Z, Stanger B, Cull H, Ju S-T, Marshak-Rothstein A. Nature (London) 1995;374:163–165. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- 44.Foote L C, Schneider T J, Fischer G M, Wang J K M, Rasmussen B, Campbell K A, Lynch D H, Ju S-T, Marshak-Rothstein A, Rothstein T L. J Immunol. 1996;157:1878–1885. [PubMed] [Google Scholar]