Abstract
Recent studies indicate that CTLA-4 interaction with B7 ligands transduces an inhibitory signal to T lymphocytes. Mice homozygous for a null mutation in CTLA-4 have provided the most dramatic example of the functional importance of CTLA-4 in vivo. These animals develop a fatal lymphoproliferative disorder and were reported to have an increase in CD4+ and CD8+ thymocytes and CD4−CD8− thymocytes, and a decrease in CD4+CD8+ thymocytes. Based on these observations, it was proposed that CTLA-4 is necessary for normal thymocyte development. In this study, CTLA-4-deficient mice carrying an insertional mutation into exon 3 of the ctla-4 gene were generated. Although these mice display a lymphoproliferative disorder similar to previous reports, there was no alteration in the thymocyte profiles when the parathymic lymph nodes were excluded from the thymi. Further, thymocyte development was normal throughout ontogeny and in neonates, and there was no increase in thymocyte production. Finally, T cell antigen receptor signaling, as assessed by proximal and distal events, was not altered in thymocytes from CTLA-4−/− animals. Collectively, these results clearly demonstrate that the abnormal T cell expansion in the CTLA-4-deficient mice is not due to altered thymocyte development and suggest that the apparent altered thymic phenotype previously described was due to the inclusion of parathymic lymph nodes and, in visibly ill animals, to the infiltration of the thymus by activated peripheral T cells. Thus it appears that CTLA-4 is primarily involved in the regulation of peripheral T cell activation.
Optimal T cell stimulation requires, in addition to the T cell antigen receptor (TCR) signal, a second costimulatory signal. CD28 is the primary costimulatory molecule on T cells (1). Conversely, the CD28-homologue CTLA-4 recently has been shown to have a negative regulatory role in T cell activation (2). CD28 and CTLA-4 recognize common ligands, namely B7.1 and B7.2. Blocking CTLA-4/B7 interactions with anti-CTLA-4 antibody or anti-B7 antibodies in the presence of TCR and CD28 signaling was found to augment T cell proliferation (3, 4). Further, crosslinking TCR, CD28, and CTLA-4 resulted in a dramatic inhibition of T cell proliferation and cytokine secretion, due to the prevention of progression from G1 to S/G2 stages of the cell cycle (5, 6). These results suggested that CTLA-4 regulated immune responses by inhibiting proliferation of activated T cells and/or by attenuating the TCR and CD28/B7 signals during the initiation of T cell activation. The critical role for CTLA-4 in down-regulating T cell responses is demonstrated in the CTLA-4−/− mice, which develop a rapid and fatal T cell lymphoproliferative disorder (2, 7, 8). A large proportion of the splenic and lymph node T cells in these mice have properties of activated cells: They are CD69+, CD25+, CD44hi, CD62Llo, and CD45RBlo, and proliferate and secrete cytokines spontaneously ex vivo (2, 7, 8).
Dramatic alterations in the thymocyte subpopulations, including an increase in the CD4+ and CD8+ single positive (SP) thymocytes and CD4−CD8− double negative (DN) thymocytes, and a decrease in the percentage of CD4+CD8+ double positive (DP) thymocytes also were reported to occur in CTLA-4−/− mice (7, 8). It was proposed that alterations in thymocyte development, possibly affecting the TCR repertoire and/or the TCR signaling, result in altered production of mature thymocytes, and ultimately lead to the phenotype in the periphery of the CTLA-4−/− mice. Alternatively, these abnormalities may be secondary to the peripheral T cell expansion, resulting in infiltration of the activated peripheral T cells into the thymus and/or stress-induced death of immature thymocytes. It is important to distinguish between these possibilities to determine the etiology of the lymphoproliferative disorder in the CTLA-4−/− mice and to fully understand the function of CTLA-4.
Immature thymocytes undergo positive and negative selection upon TCR ligation, which results in clonal survival or apoptosis, respectively, to generate the peripheral TCR repertoire. Maturation of the TCR/CD3 signal to generate a proliferative rather than an apoptotic response also occurs during thymic development (9). Because costimulatory molecules have a critical influence on the outcome of TCR engagement on mature T cells, their possible role in thymic development is an intriguing question. CD28 is expressed on the surface of murine thymocytes (10), suggesting that it may play a role in thymic selection and/or maturation. CD28/CTLA-4 ligands, B7–1 and B7–2, also are expressed in the thymus, particularly on the medullary epithelial cells and thymic dendritic cells throughout development (11–14). A role for CD28/B7 costimulatory interactions in thymocyte differentiation has been shown by some (13, 15–18), but not others (19–22). Also, no overt defects in thymocyte development were detected in CD28−/− (23) and B7–1−/− (24) animals. On balance, it appears that CD28/B7 interactions are not essential for thymocyte development.
Because CTLA-4 transduces an inhibitory signal on peripheral T cells, it could play a novel role in thymocyte development, possibly by dampening the TCR signal transduced by TCR’s with high affinity for self-major histocompatibility complex antigens. Alternatively, CTLA-4 engagement during thymocyte development may ensure that TCR signaling provides a maturation versus an activation signal. There has been some uncertainty concerning CTLA-4 expression in the thymus. CTLA-4 mRNA transcripts have been detected by Northern blot analysis in thymocytes ex vivo (ref. 25; M. Krummel and J.P.A., unpublished data) and in thymocytes activated in vitro (25). Although cell surface protein expression has been difficult to detect, CTLA-4 recently has been reported to be expressed at low levels on thymocytes ex vivo (26). In any event, CTLA-4 is largely restricted to intracellular sites (27, 28), and cell surface expression is not an indicator of the presence of functionally relevant levels (2, 5).
In this study we have investigated the etiology of the apparent phenotypic alterations in the thymocytes of CTLA-4−/− animals. We first assessed the stage of T cell development at which abnormal T cell activation occurs in the CTLA-4−/− mice. Analysis of fetal and newborn thymi demonstrated that there was no alteration of the thymocyte subsets, and that the onset of the lymphoproliferative disorder in the periphery occurred before any phenotypic changes in the thymus. Further, if the grossly enlarged parathymic lymph nodes were separated from the thymic tissue, no phenotypic alterations in the thymocytes were seen until the animals were extremely ill. Second, we tested to see if an increased production of SP thymocytes contributed to the expansion of the peripheral T cell compartment. Incorporation of thymidine analog bromodeoxyuridine (BrdU) in vivo demonstrated that there was no alteration in the production of thymocytes in the CTLA-4−/− animals. Third, we tested whether the TCR-mediated signals were normal in the immature and mature thymocytes. The ability of the immature DP and mature CD4+ SP thymocytes to respond to TCR signaling, as determined by anti-CD3-induced apoptosis and the modulation intracellular calcium, was not modified in CTLA-4−/− thymocytes. These results indicate that thymocyte differentiation is normal in CTLA-4-deficient mice and suggest that CTLA-4 does not play a critical role in thymocyte development. Rather, its primary function appears to be to modulate T cell activation in mature T cells and maintain T cell homeostasis in the periphery.
MATERIALS AND METHODS
Targeting Construct and Generation of CTLA-4−/− Animals.
The CTLA-4 gene was cloned from isogenic 129 genomic DNA library, using the 1.4-kb BamHI fragment of the cDNA as the probe. The 5′ 4.3-kb BamHI and 3′ 2.2-kb fragments were subcloned into pKSNT. Neomycin phosphotransferase cDNA driven by the pgk promoter (29) was cloned into the BamHI site of exon 3 in the opposite transcriptional orientation. Pgk-thymidine kinase cDNA (30) was inserted 5′ of the region of homology. The vector was introduced into embryonic stem cell R1, as described (31), and cells containing the homologous recombinant event were identified by Southern blot analysis (data not shown). Animals homozygous for the mutation were generated and maintained in microisolators in accordance with the Animal Care Regulations of the University of California at Berkeley. The day of plug was designated day 0 of gestation (E0) for timed pregnancies.
Reagents.
BrdU was purchased from Sigma, and Indo-1acetoxy-methyl ester and 4′-6-diamidino-2-phenylindole were obtained from Molecular Probes. Anti-CD4-PE, anti-CD44-PE/FITC, H57-TC, anti-CD32-PE, anti-CD69-FITC/biotin, anti-CD25-FITC, anti-CD40-PE, anti-CD28-biotin, anti-CD5-biotin, anti-HSA-biotin, anti-thy1.2-PE/FITC, anti-CD117-biotin, anti-CD62L-PE, anti-CD45RB-FITC (all from PharMingen), anti-B220-FITC/biotin, anti-CD8-TC, Streptavidin (SA)-TC, SA-PE (Caltag, South San Francisco, CA), anti-CD4–613 (GIBCO), anti-BrdU-FITC (Becton Dickinson), H57-FITC (32), and anti-γδ TCR (GL-3) were used for FACS analysis. Antibodies anti-CD3 (500A2; ref. 33), nonspecific hamster control (560–31; ref. 4) and rabbit anti-hamster antibodies (Pierce) were used for functional assays.
FACS Analysis.
Single cell suspensions and FACS samples were prepared as described (5). The samples were washed and run on an XL (Coulter), and 40,000 events/sample gated on live cells were collected.
In Vivo BrdU Labeling.
Newborn mice were injected with 0.1 ml of 10 mg/ml BrdU i.p. twice (9 hr apart) and killed 12 hr later. BrdU incorporation was detected as described (34).
Calcium Flux.
Thymocytes (8 × 106 cells/ml) were incubated with Indo-1 (3 μM) for 30 min at 37°C. The cells were labeled with anti-CD4-PE, CD8-TC, and anti-CD3 or control antibody 560. Once the baseline fluorescence ratio was established, the calcium flux in response to anti-CD3 or 560 crosslinked by anti-hamster antibody (55 μg/ml) was determined. Indo-1 was excited by UV light on an ELITE IV flow cytometer, with ratio of FUV405 (Ca2+-chelated)/FUV525 (free) vs. time was recorded and data (≥ 80,000 events/sample) was collected for 5 min.
DNA Analysis.
Thymocytes (4 × 106 cells/well) were incubated in 6-well plates coated with anti-CD3 (10 μg/ml) or control antibody 560 for 16–18 hr at 37°C. The cells were labeled with anti-CD4 and anti-CD8 antibodies, fixed with 70% ethanol and stained with 4′-6-diamidino-2-phenylindole (10 μg/ml in PBS, 0.6% Nonidet P-40), which binds to A and T nucleotides. The samples were analyzed using UV excitation, and measurements were made at 460 nm. Fifty thousand events/sample were collected and analyzed using the multicycle program.
RESULTS
Generation of CTLA-4-Deficient Mice.
Mice homozygous for the mutation in the third exon of CTLA-4 developed a lymphoproliferative disorder (2), similar to previous reports (7, 8). The majority of the T cells are activated in vivo, and the mice die at 3 to 4 weeks of age, presumably due to the infiltration of lymphocytes into the vital organs.
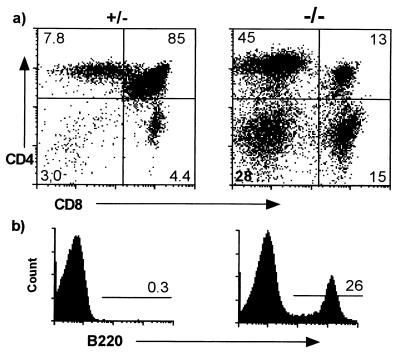
One of the striking phenotypic changes originally described in these animals was the apparent increase in the SP and DN thymocytes and decrease in the immature DP thymocytes (7, 8), as shown in Fig. 1a. Interestingly, a large percentage of the DN cells are B220+ (Fig. 1b), CD5−, CD40+, Thy-1− (data not shown), indicating the presence of B cells in these cell preparations. Because normal thymic B220+ B cells express CD5 (35), these are probably conventional B cells.
Figure 1.
Apparent thymocyte phenotype in CTLA-4−/− animals. FACS analysis of all tissue initially believed to be thymus from 18-day-old CTLA-4−/− and littermate control mice. (a) Total thymocytes. (b) DN thymocytes.
Apparent Alteration in Thymocytes Is Due to Enlarged Parathymic Lymph Nodes.
Upon visual inspection the thymi of CTLA-4−/− animals older than 12 days of age appeared to have an altered morphology. Also, the presence of conventional B cells in the cell suspensions (Fig. 1b) is reminiscent of lymph nodes. Because the peripheral LNs are enlarged by this age, it is possible that the parathymic LNs also increase in size. To determine whether this occurred, intraperitoneal injection of India ink 20 min before euthanasia was used to distinguish the thymus from the parathymic LNs (36). There is a dramatic increase in the size of the parathymic LNs in the CTLA-4−/− mice (Fig. 2A). In fact, the thymic subsets in the CTLA-4−/− mice are completely normal if the parathymic LNs are dissected away (Fig. 2B). Further, thymi with only a 2- to 3-fold increase in SP populations were observed in animals >3 weeks of age. These results indicate that the earlier reports of phenotypic alterations in the thymi (7, 8) were probably due to the inclusion of parathymic LN. In extremely sick mice, there was an increase in the percentage of SP T cells even in the thymus proper (data not shown), probably due to infiltration of peripheral lymphocytes. Decreased thymic cellularity also was observed in the visibly ill animals, although it was not possible to accurately determine the cell numbers due to the difficulties in completely dissecting away the parathymic LN from the thymic lobes at this stage.
Figure 2.
Enlarged parathymic lymph nodes skews the analysis of thymocytes. (A) Parathymic LN uptake of India ink in wild-type (Upper) and CTLA-4−/− (Lower) mice. (B) FACS analysis of the thymus proper that excludes versus the parathymic LNs that take up the ink was performed. Representative data is shown for 16-day-old CTLA-4−/− and littermate control mice. The DP population in the parathymic LN is due to thymocytes.
Normal Thymocyte Development During Fetal Ontogeny and in Neonates and Young CTLA-4−/− Mice.
These results suggested that there was no primary defect in thymocyte development. To investigate this further, thymic development was examined from day 14.5 of gestation (E14.5) until 3–4 weeks of age. Fetal thymi obtained from timed pregnancies were analyzed individually for the expression of diagnostic surface antigens and genotyped by Southern blot analysis. Fetal liver progenitor cells seed the fetal thymus at E12, and differentiate into progenitor T cells (DN, CD3−, CD44+, c-kit+, CD25+; ref. 37). These cells become CD44−, CD25 −, commence TCR gene rearrangement, and undergo lineage commitment (38). γδTCR+ thymocytes are the first detectable TCR/CD3+ thymocytes (39). The majority of the thymocytes become committed to the αβTCR lineage and up-regulate CD4 and CD8. By E16, DP thymocytes are actively rearranging the αβTCR receptors and become TCRlo. The cells undergo selection, mature to CD4SP or CD8SP αβTCRhi and begin to emigrate from the thymus at rates similar to adults by approximately day 3–5 after birth (40).
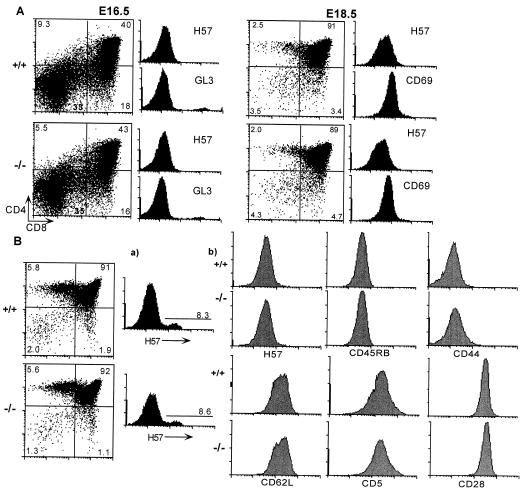
Fetal thymi from litters at E14.5, E16.5, and E18.5 were analyzed. There were no alterations during early thymic differentiation at any of the major developmental check points, as defined by antibodies to cell surface markers including CD44, CD25, and CD117 (data not shown), CD4, CD8, CD69, and γδ TCR, αβ TCR expression in the CTLA-4−/− and CTLA-4+/− embryos compared with the wild-type controls (representative data shown in Fig. 3A). Thymocytes from newborn (4–10 days of age) and young (11–28 days of age) mice also were examined. The distribution of the cells in the thymic subsets remained normal until approximately 11–13 days of age (as assessed by the expression of Sca-1, CD4, CD8, CD69, CD25, CD5, αβTCR, CD44, CD62L, CD28, and CD45RB; representative data shown for 11-day-old animals in Fig. 3B) by which time the peripheral T cells already were undergoing dramatic activation and expansion. There was no alteration in the αβTCR repertoire of the thymocytes or peripheral T cells between the littermates and the CTLA-4−/− animals (assessed by anti-TCR Vβ2, 3-, 5-, 6-, 7-, 8-, 9-, 11-, 13-, and 14-specific antibodies; data not shown).
Figure 3.
Thymic development in CTLA-4−/− mice is normal during ontogeny and in newborns. Single cell suspensions were prepared from the thymi and examined by FACS analysis. (A) Fetal thymocytes at E16.5 and E18.5. The expression of αβTCR and γδTCR on the total thymocytes is shown. (B) Thymocytes from 11-day-old animals were stained with antibodies to CD4, CD8, and the indicated cell surface markers. The expression on (a) total thymocytes and (b) DP thymocytes is shown. The number of embryos/animals analyzed at each time point were: E14.5 +/+ n = 2, +/− n = 3, −/− n = 4 (data not shown); E16.5 +/+ n = 1, +/− n = 5, −/− n = 4; E18.5 +/+ n = 2, +/− n = 3, −/− n = 4; D11 +/+ and +/− n = 9, −/− n = 8.
Cell Division of Thymocyte Subsets Is Unchanged in the Absence of CTLA-4.
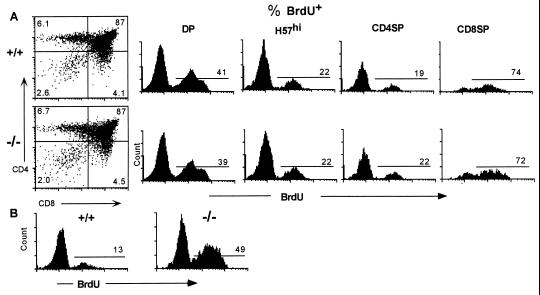
Although the thymic subsets appeared normal phenotypically in the CTLA-4−/− mice, an increased rate of production of thymocytes could contribute to the expansion of the peripheral T cell compartment. To investigate this possibility, in vivo cell division analysis was performed by measuring BrdU incorporation. There was a high incorporation of BrdU in the DP thymocytes of 11-day-old wild-type and CTLA-4−/− mice (Fig. 4A), consistent with previous reports that DP cells have a high rate of division (34, 41, 42). Also, there was an extremely high rate of cell division in the CD8 SP thymocytes, due to the high proliferative rate of the CD3−CD4−CD8+ progenitor T cells present in this population (42, 43). Interestingly, there was no difference in the percentage of BrdU+ cells in any of the thymocyte populations in the CTLA-4−/− versus littermate control animals, including the mature TCRhi SP population (Fig. 4A). Also, thymocyte turnover was unaltered in the CTLA-4−/− animals, as determined by BrdU pulse–chase experiments (unpublished data). Conversely, there was a dramatic 3- to 4-fold increase in the BrdU+ splenic T cells in these CTLA-4−/− mice compared with the littermate controls (Fig. 4B).
Figure 4.
The absence of CTLA-4 does not alter thymocyte cell division. (A) Percentage of BrdU+ cells in the DP, total H57hi thymocytes, and SP (CD4SP >96% αβTCRhi; CD8SP 45% αβTCRhi) thymocytes in 11-day-old CTLA-4−/− mice and littermate controls. (B) Percentage of BrdU+ splenic αβTCR+ T cells in these animals.
TCR-Elicited Calcium Flux Is Normal in CTLA-4−/− Thymocytes.
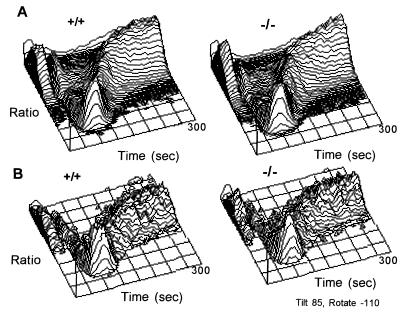
TCR crosslinking initiates a number of biochemical events, including a transient increase in intracellular calcium concentrations (44). Further, the ability to modulate intracellular calcium changes during thymocyte maturation (45). To determine if there was an alteration in the TCR signaling in these thymocytes and to assess if the maturation of the TCR signal was altered in thymocytes from CTLA-4−/− animals, the TCR-signaling capacity of the thymocytes was assessed by proximal and distal responses, namely anti-CD3-induced calcium flux and apoptosis, respectively. CD3-crosslinking resulted in a similar intracellular calcium flux in the CTLA-4−/− thymocytes as compared with the littermate control animals (representative data from five independent experiments are shown in Fig. 5). This was true for both the DP thymocytes (Fig. 5A), which include cells undergoing selection and mature CD4SP T cell lineage (αβTCRhi) (Fig. 5B), which have undergone thymic selection and will emigrate to the periphery. The calcium responses were comparable in latency, magnitude, and duration.
Figure 5.
Calcium flux. Thymocytes were loaded with Indo-1 and incubated with anti-CD4-PE and anti-CD8-TC and anti-CD3 (500A2) or 560 antibodies. The ratio of Indo-1-chelated intracellular calcium versus free calcium was measured over time in CTLA-4−/− and littermate control (A) DP thymocytes and (B) CD4 SP thymocytes from 11-day-old animals.
Anti-CD3-Induced Apoptosis of Thymocytes.
Anti-CD3-induced apoptosis was examined to further determine if TCR signaling was altered in the immature and mature thymocytes from the CTLA-4−/− animals. Representative results of four independent experiments are shown in Table 1. DNA analysis by 4′-6-diamidino-2-phenylindole labeling demonstrated that there was no difference in the percentage of the thymocytes in cell cycle ex vivo, supporting the results with BrdU incorporation in vivo. Further, there was no difference in the percentage of DP or CD4SP thymocytes that underwent apoptosis upon CD3 crosslinking between the CTLA-4−/− and littermate control mice (Table 1). Overnight incubation with immobilized anti-CD3 antibody also induced CD5 and CD69 expression and down-regulated CD4 and CD8 expression (15, 46–48) to equivalent levels on CTLA-4−/− and control thymocytes (data not shown), further demonstrating that the TCR-induced responses are not altered in the CTLA-4-deficient thymocytes.
Table 1.
Anti-CD3-Induced apoptosis of thymocytes from CTLA-4−/− and control mice
| Mice | % of total thymocytes
|
|||
|---|---|---|---|---|
| Apoptotic | G1 | G2 | S | |
| DP | ||||
| Ex vivo control | — | 83 | 0.2 | 17 |
| −/− | — | 87 | 0.2 | 13 |
| Anti-CD3 control | 45 | 45 | 0.3 | 9.4 |
| −/− | 46 | 44 | 0.2 | 9.7 |
| CD4SP | ||||
| Ex vivo control | — | 92 | 0.2 | 7.6 |
| −/− | — | 94 | 0.2 | 6.0 |
| Anti-CD3 control | 59 | 39 | 0.2 | 2.2 |
| −/− | 54 | 43 | 1.1 | 2.3 |
Thymocytes from 11-day-old littermate mice were incubated on anti-CD3 antibody or control antibody 560-coated plates for 16–18 hr at 37°C, as described above. Representative data from four independent experiments. Less than 8% of the cells were apoptotic when incubated with the control antibody.
DISCUSSION
Previous reports have suggested an important role for CTLA-4 in thymocyte differentiation, based on the phenotypic changes in the thymocytes in CTLA-4−/− mice. There are at least two explanations for these observations: CTLA-4 is critical for normal thymocyte differentiation; or, alternatively, the apparent abnormalities in the thymic subset distribution are secondary events resulting from the thymus being infiltrated by activated peripheral T cells or impairment of thymocyte development by stress-induced alterations in the thymic milieu. Our results clearly show that there is no primary defect in thymic development in the absence of CTLA-4, either at the phenotypic or functional levels. Further, we show that the reported early changes of the thymocyte subsets may have been due to inclusion of parathymic LNs. The results also suggest that infiltration of peripheral T cell blasts into the thymi is principally responsible for the phenotypic changes observed in visibly ill CTLA-4−/− mice. This infiltration may be nonspecific, because activated T cells also are found in a number of nonlymphoid tissues, including the lung, heart, liver, and skin. Alternatively, activated T cells may preferentially home to the thymus as has been shown to occur in the thymi of normal animals (49). It has been proposed that blast cell thymic homing may function to introduce novel self-antigens into the thymus (50) or provide a feedback mechanism to decrease the production of SP thymocytes (51).
Approximately 1 × 106 SP thymocytes per day normally emigrate from the thymus and contribute to the pool of mature resting T cells (52). These recent thymic emigrants undergo further maturation in the periphery before becoming mature naive peripheral T cells (34, 53). The requirements for this maturation and specifically, the role of costimulatory molecules in this process has not been established. Our results suggest that there is no difference between the CD4 SP thymocytes in the CTLA-4−/− and the control animal, yet activated T cells can be detected as early as 5 days of age (unpublished result), suggesting that the T cells become activated almost immediately once in the periphery. It will be important to determine if there is an inherent difference in the mature naive T cells from CTLA-4−/− compared with littermate control animals in their ability to respond to TCR/CD3 signals. To date, attempts to analyze resting naive mature peripheral T cells from the CTLA-4-deficient mice have been difficult because the majority of the T cells are activated in vivo.
It is becoming increasingly evident that TCR-mediated signals in peripheral T cells are modified by positive and negative signals, and that the resultant response is a culmination of these signals. Much less is known concerning the possible accessory signals influencing TCR signaling, particularly inhibitory signals, during thymocyte development. Recently, it was demonstrated that CD5-deficient thymocytes are hyperresponsive to TCR-mediated responses. Peripheral T cells, on the other hand, appeared normal in these animals (54). Results presented here demonstrate that CTLA-4 may influence the TCR signals in an opposing manner to that of CD5. Thus, molecules modifying TCR signals during T cell development may be distinct from those regulating peripheral T cell activation.
Collectively, these results show that thymic development proceeds normally in the CTLA-4-deficient mice and strongly suggest that the lymphoproliferative disorder evident in these mice primarily initiates in the peripheral T cell compartment. It remains to be determined if the abnormal T cell activation is due to the inability to regulate ongoing antigen-specific T cell responses, or if the absence of CTLA-4 alters the TCR threshold necessary to initiate activation of mature T cells. It will be important to identify the stage in T cell development/maturation at which TCR signals and T cell activation can be influenced by the CD28- and CTLA-4-mediated signals and how these signals are integrated with the TCR signaling pathway.
Acknowledgments
We wish to thank P. Schow for expert assistance with flow cytometry, S. Grell for antibody purification, and Dr. J. Kang for helpful discussions and critical review of the manuscript. C.A.C. is a recipient of a Human Frontiers Science Program Organization Fellowship. This work was supported by National Institutes of Health Grant CA40041.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: BrdU, 5-bromo-2-deoxyuridine; DP, double positive; DN, double negative; E, gestational day; LN, lymph node; SP, single positive; TCR, T cell antigen receptor.
References
- 1.Lenschow D J, Walunas T L, Bluestone J A. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Chambers C A, Krummel M F, Boitel B, Hurwitz A A, Sullivan T J, Fournier S, Cassell D, Brunner M, Allison J P. Immunol Rev. 1996;153:27–46. doi: 10.1111/j.1600-065x.1996.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 3.Walunas T L, Lenschow D J, Bakker C Y, Linsley P S, Freeman G J, Green J M, Thompson C B, Bluestone J A. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 4.Krummel M F, Allison J P. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krummel M F, Allison J P. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walunas T L, Bakker C Y, Bluestone J A. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waterhouse P, Penninger J M, Timms E, Wakeham A, Shahinian A, Lee K P, Thompson C B, Griesser H, Mak T W. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 8.Tivol E A, Borriello F, Schweitzer A N, Lynch W P, Bluestone J A, Sharpe A H. Immunity. 1995;3:541–546. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 9.Smith C A, Williams G T, Kingston R, Jenkinson E J, Owens J J T. Nature (London) 1989;337:181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- 10.Gross J A, Callas E, Allison J P. J Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
- 11.Reiser H, Freeman G J, Razi-Wolf Z, Gimmi C D, Benacerraf B, Nadler L M. Proc Natl Acad Sci USA. 1992;89:271–275. doi: 10.1073/pnas.89.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson A J, Hosier S, Brady W, Linsley P S, Farr A G. J Immunol. 1993;151:2453–2461. [PubMed] [Google Scholar]
- 13.Degermann S, Surh C D, Glimcher L H, Sprent J, Lo D. J Immunol. 1994;152:3254–3263. [PubMed] [Google Scholar]
- 14.Inaba K, Witmer-Pack M, Inaba M, Hathcock K S, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley P S, Ikehara S, Muramatsu S, Hodes R J, Steinman R M. J Exp Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Punt J A, Osbourne B A, Takahama Y, Sharrow S O, Singer A. J Exp Med. 1994;179:709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Radvanyi L G, Sharma A, Shaw P, Green D R, Miller R G, Mills G B. J Immunol. 1995;155:1829–1837. [PubMed] [Google Scholar]
- 17.Kishimoto H, Cai Z, Brunmark A, Jackson M R, Peterson P A, Sprent J. J Exp Med. 1996;184:531–537. doi: 10.1084/jem.184.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walunas T L, Sperling A I, Khattri R, Thompson C B, Bluestone J A. J Immunol. 1996;156:1006–1013. [PubMed] [Google Scholar]
- 19.Jones L A, Izon D J, Nieland J D, Linsley P S, Kruisbeek A M. Int Immunol. 1993;5:503–512. doi: 10.1093/intimm/5.5.503. [DOI] [PubMed] [Google Scholar]
- 20.Page D M, Kane L P, Allison J P, Hedrick S M. J Immunol. 1993;151:1868–1880. [PubMed] [Google Scholar]
- 21.Vukmanovic S, Stella G, King P D, Dyall R, Hoquist K A, Harty J T, Nikolic-Zugic J, Bevan M J. J Immunol. 1994;152:3814–3823. [PubMed] [Google Scholar]
- 22.Jenkinson E J, Anderson G, Moore N C, Smith C A, Owen J J. Dev Immunol. 1994;3:265–271. doi: 10.1155/1994/75434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahinian A, Pfeffer K, Lee K P, Kundig T M, Kishihara K, Wakeham A, Kawai K, Ohashi P S, Thompson C B, Mak T W. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 24.Sharpe A H. Curr Opin Immunol. 1995;7:389–395. doi: 10.1016/0952-7915(95)80115-4. [DOI] [PubMed] [Google Scholar]
- 25.Brunet J-F, Denizot F, Luciani M-F, Roux-Dosseto M, Suzan M, Mattei M-G, Golstein P. Nature (London) 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 26.Wagner D H, Hagmna J, Linsley P S, Hodsdon W, Freed J H, Newell M K. J Exp Med. 1996;184:1631–1638. doi: 10.1084/jem.184.5.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung H T, Bradshaw J, Cleaveland J S, Linsley P S. J Biol Chem. 1995;270:1–8. doi: 10.1074/jbc.270.42.25107. [DOI] [PubMed] [Google Scholar]
- 28.Linsley P S, Bradshaw J, Greene J, Peach R, Bennett K L, Mittler R S. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 29.Adra C N, Boer P H, McBurney M W. Gene. 1987;60:65–74. doi: 10.1016/0378-1119(87)90214-9. [DOI] [PubMed] [Google Scholar]
- 30.Tybulewicz V L, Crawford C E, Jackson P K, Bronson R T, Mulligan R C. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 31.Chambers C A, Kang J, Pawling J, Huber B, Hozumi H, Nagy A. Proc Natl Acad Sci USA. 1994;91:1138–1142. doi: 10.1073/pnas.91.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubo R T, Born W, Kappler J W, Marrack P, Pigeon M. J Immunol. 1989;142:2736–2742. [PubMed] [Google Scholar]
- 33.Allison J P, Havran W L, Poenie M, Kimura J, Degraffenreid L, Ajami S, Duwe G, Weiss A, Tsien R. In: The T Cell Receptor, UCLA Symposia on Molecular and Cellular Biology, New Series. Kappler J, Davis M, editors. New York: Liss; 1987. pp. 33–45. [Google Scholar]
- 34.Tough D F, Sprent J. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyama-Inaba M, Kuma S, Inaba K, Ogata H, Iwai H, Yasumizu R, Muramatsu S, Steinman R, Ikehara S. J Exp Med. 1988;168:811–816. doi: 10.1084/jem.168.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishell B B, Shiigii S M. Selected Methods in Cellular Immunology. San Francisco: Freeman; 1980. pp. 8–9. [Google Scholar]
- 37.Ikuta K, Uchida N, Friedman J, Weissman I L. Annu Rev Immunol. 1992;10:759–783. doi: 10.1146/annurev.iy.10.040192.003551. [DOI] [PubMed] [Google Scholar]
- 38.Kang J, Raulet D H. Semin Immunol. 1997;9:171–179. doi: 10.1006/smim.1997.0069. [DOI] [PubMed] [Google Scholar]
- 39.Havran W L, Allison J P. Nature (London) 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 40.Kelly K A, Scollay R. Eur J Immunol. 1992;22:329–334. doi: 10.1002/eji.1830220207. [DOI] [PubMed] [Google Scholar]
- 41.Egerton M R, Scollay R, Shortman K. Proc Natl Acad Sci USA. 1990;87:2579–2582. doi: 10.1073/pnas.87.7.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huesmann M, Scott B, Kisielow P, von Boehmer H. Cell. 1991;66:533–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- 43.Nikolic-Zugic J, Bevan M. Proc Natl Acad Sci USA. 1988;85:8663–8666. doi: 10.1073/pnas.85.22.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finkel T H, Marrack P, Kappler J, Kubo R T, Cambier J C. Nature (London) 1987;330:179–181. doi: 10.1038/330179a0. [DOI] [PubMed] [Google Scholar]
- 45.Hedin K E, Appleby M W, Clapham D E. Immunology. 1995;84:183–192. [PMC free article] [PubMed] [Google Scholar]
- 46.Swat W, Dessing M, von Boehmer H, Kisielow P. Eur J Immunol. 1993;23:739–746. doi: 10.1002/eji.1830230326. [DOI] [PubMed] [Google Scholar]
- 47.Page D M, Kane L P, Allison J P, Hedrick S M. J Immunol. 1993;151:1868–1880. [PubMed] [Google Scholar]
- 48.Curnow S J, Schmidt-Verhulst A-M. Eur J Immunol. 1994;24:2401–2409. doi: 10.1002/eji.1830241021. [DOI] [PubMed] [Google Scholar]
- 49.Agus D B, Surh C D, Sprent J. J Exp Med. 1991;173:1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webb S R, Sprent J. Science. 1990;248:1357–1363. doi: 10.1126/science.1694041. [DOI] [PubMed] [Google Scholar]
- 51.Kelly K A, Pircher H, von Boehmer H, Davis M M, Scollay R. Eur J Immunol. 1993;23:1922–1928. doi: 10.1002/eji.1830230829. [DOI] [PubMed] [Google Scholar]
- 52.Sprent J, Tough D F. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 53.Kelly D A, Scollay R. Int Immunol. 1990;2:419–425. doi: 10.1093/intimm/2.5.419. [DOI] [PubMed] [Google Scholar]
- 54.Tarakhovsky A, Kanner S B, Hombach J, Ledbetter J A, Muller W, Killeen N, Rajewsky K. Science. 1995;269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]