Abstract
Angioplasty procedures are increasingly used to reestablish blood flow in blocked atherosclerotic coronary arteries. A serious complication of these procedures is reocclusion (restenosis), which occurs in 30–50% of patients. Migration of coronary artery smooth muscle cells (CASMCs) to the site of injury caused by angioplasty and subsequent proliferation are suggested mechanisms of reocclusion. Using both cultured human CASMCs and coronary atherectomy tissues, we studied the roles of osteopontin (OPN) and one of its receptors, αvβ3 integrin, in the pathogenesis of coronary restenosis. We also measured the plasma levels of OPN before and after angioplasty and determined the effect of exogenous OPN on CASMC migration, extracellular matrix invasion, and proliferation. We found that cultured CASMCs during log phase of growth and smooth muscle cell layer of the coronary atherosclerotic tissues of patients express both OPN mRNA and protein at a significantly elevated level compared with controls. Interestingly, whereas the baseline plasma OPN levels in control samples were virtually undetectable, those in patient plasma were remarkably high. We also found that interaction of OPN with αvβ3 integrin, expressed on CASMCs, causes migration, extracellular matrix invasion, and proliferation. These effects were abolished when OPN or αvβ3 integrin gene expression in CASMCs was inhibited by specific antisense S-oligonucleotide treatment or OPN-αvβ3 interaction was blocked by treatment of CASMCs with antibodies against OPN or αvβ3 integrin. Our results demonstrate that OPN and αvβ3 integrin play critical roles in regulating cellular functions deemed essential for restenosis. In addition, these results raise the possibility that transient inhibition of OPN gene expression or blocking of OPN-αvβ3 interaction may provide a therapeutic approach to preventing restenosis.
Atherosclerosis (for review see refs. 1 and 2) is the principal cause of heart attacks, stroke, gangrene, and loss of function of extremities. It accounts for approximately 50% of all mortalities in the United States, Europe, and Japan (1). The present therapeutic strategies for severe atherosclerosis in coronary arteries include percutaneous transluminal coronary angioplasty, directional coronary atherectomy (DCA) or related angioplasty procedures, and coronary artery bypass surgery. Reocclusion (or restenosis) of these arteries occurs in 30–50% of the patients undergoing various angioplasty procedures. This serious complication has been suggested to occur, at least in part, as a result of local inflammation, thrombosis, and smooth muscle cell (SMC) migration (3) and proliferation (4, 5) within the intima of coronary arteries.
In 1979, a transformation-related phosphoprotein was identified (6), which was later named osteopontin (OPN) (7). It is a secreted noncollagenous, glycosylated phosphoprotein (8–11) that binds to cell surface integrins (12), a family of heterodimeric glycoprotein subunits designated α and β. These integrins act as cell surface receptors for many ligands, including OPN (13). OPN gene expression has been reported to be a distinctive feature of rat aortic SMC (14). Moreover, rat and bovine SMC migration is promoted by OPN (15). It also has been demonstrated that high levels of OPN mRNA and protein are detectable in the rat and human aorta and carotid arteries during neointima formation (16–19). OPN overexpression has been shown to associate with rat arterial SMC proliferation (20). Most interestingly, it has been demonstrated that subjecting cultured cells to intermittent compressive force, similar to the ones that may be produced by some angioplasty procedures, causes OPN overexpression (21).
The present integrated study was undertaken to gain insight into the possible role of OPN and one of its cell-surface receptors (i.e., αvβ3 integrin) in the development of human coronary artery reocclusion.
MATERIALS AND METHODS
Study Subjects.
Informed consent was obtained from all patients in whom atherectomy/angioplasty was clinically indicated. A clinical research protocol was approved by the Institutional Review Board to study the possible cause(s) of restenosis after DCA/percutaneous transluminal coronary angioplasty. We studied atherectomy specimens from coronary arteries of 13 patients undergoing DCA and samples of normal coronary arteries, obtained at autopsy, from six patients (ages 18–68) who died of noncardiac causes and had no evidence of atherosclerosis. The patient profiles are presented in Table 1.
Table 1.
Profile of patients and controls
Informed consent was obtained after the nature and possible consequences of the atherectomy procedure were explained.
No evidence of coronary atherosclerosis at autopsy: death due to noncardiac causes.
The atherectomy tissue samples, immediately after removal, were divided asceptically into three parts. One was used for RNA extraction and the other two for Western blot analysis and in situ hybridization, respectively. RNase-free equipment and reagents were used for collection and storage of tissues used for in situ hybridization and RNA extraction. The control samples were obtained at autopsy and used under the same conditions as mentioned above.
Cell Culture.
Human coronary artery SMCs (CASMCs) were obtained from Clonetics (San Diego) and grown in SMC basal medium (Clonetics) supplemented with insulin, human fibroblast growth factor, human epidermal growth factor, and 5% fetal calf serum in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
Reverse Transcription–PCR (RT-PCR).
The RNAs from DCA tissues and cultured CASMCs were extracted as previously described (22). Reverse transcription of total RNAs from DCA patients and controls and cDNA amplifications were performed according to the method described previously (23). The sequence of the antisense primer, hOPN-R (nucleotides 928–909) was 5′-CTA CAA CCA GCA TAT CTT CA-3′ and the sense primer, hOPN-L (nucleotides 418–437) was 5′-CAC CAG TCT GAT GAG TCT CA-3′. The PCR products were detected by using digoxigenin-labeled hOPN probe; hOPN-P1 (nucleotides 647–628) was 5′-TCC ATG TGT GAG GTG ATG TC-3′. For the amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA the sense primer, GAPDH-L (nucleotides 388–405) was 5′-CCA TGG AGA AGG CTG GGG-3′, and the antisense primer, GAPDH-R (nucleotides 582–563) was 5′-CAA AGT TGT CAT GGA TGA CC-3′. The probe GAPDH-P (nucleotides 549–531) was 5′-CTA AGC AGT TGG TGG TGC A-3′.
Western Blot Analysis.
The level of OPN in CASMCs and in coronary atherectomy and normal coronary artery specimens was detected by Western blot analysis (24). Briefly, the specimens were homogenized in lysis buffer (50 mM Tris⋅HCl, pH 7.5, containing 150 mM NaCl, 1% Nonidet P-40, 15 μg/ml leupeptin, and 0.5 μM phenylmethylsulfonyl fluoride), and centrifuged at 12,000 × g for 10 min. The supernatants were electrophoresed on a 4–20% gradient SDS/polyacrylamide gel and electrotransferred to nitrocellulose membrane. The membranes were blocked, incubated with rabbit anti-rat OPN antibody (1:250 dilution), and detected with 125I-labeled protein A (ICN), followed by autoradiography.
Immunoprecipitation.
When CASMCs were treated with phorbol 12-myristate 13-acetate (PMA) (250 nM) they were incubated at 37°C for 24 hr. The cell lysates were immunoprecipitated using a kit from Boehringer Mannheim according to manufacturer’s instructions. Briefly, the cells were lysed with lysis buffer and centrifuged, and the supernatant was incubated with rabbit OPN-antibody for 1 hr then with protein A-agarose at 4°C overnight. Bound complexes were pelleted by centrifugation, washed, and electrophoresed. The Western blot analysis was done as previously described (24).
Binding Studies.
Purified human OPN was radioiodinated by the chloramine-T method (25). Subconfluent cultures of CASMCs were incubated with 125I-OPN (3.3 × 105 cpm/well) in the absence or presence of varying concentrations of unlabeled OPN in 0.5 ml of Hanks’ balanced salt solution, pH 7.6, containing 0.1% BSA. After incubation at 37°C for 3 hr, the reactions were stopped by rapid removal of medium containing unbound radiolabeled OPN, and the cells were washed and solubilized with 2 M NaOH. The radioactivity was measured by gamma counter, and the specific binding was calculated by subtracting the nonspecific binding from the total binding. The Kd value was determined by Scatchard analysis by using the Ligand computer program (26).
Affinity Crosslinking Experiments.
Subconfluent CASMC was incubated with 125I-OPN (6.6 × 105 cpm/well) in 1 ml of Hanks’ balanced salt solution, pH 7.6 containing 0.1% BSA in the absence or presence of unlabeled OPN or Gly-Arg-Gly-Asp-Ser (GRGDS) peptide (1 μM) at 37°C for 3 hr. After washing, the cells were incubated with 0.20 mM disuccinimidyl suberate (DSS) in 1 ml of Hanks’ balanced salt solution, pH 7.6 at 37°C for 30 min. The cells were scraped, collected by centrifugation, and lysed in 40 μl of 1% Triton X-100 solution containing 1 mM phenylmethylsulfonyl fluoride, 20 μg/ml leupeptin, and 2 mM EDTA. The supernatants (30 μl) obtained by centrifugation were electrophoresed as described previously (27) and autoradiographed.
CASMC Migration Assay.
Migration of CASMC was performed using Transwell cell culture chambers with an 8-μm pore size polycarbonate membrane (Costar) as described previously (28). Briefly, subconfluent human CASMC were trypsinized, centrifuged, and resuspended in basal medium (SmBM) supplemented with 0.2% BSA. Then, 0.25 ml of cell suspension (5 × 104 cells) was added to the upper compartment of the chamber. The lower compartment contained 0.5 ml of basal medium supplemented with 0.2% BSA and either two different concentrations of OPN (0.68 or 1.36 μg/ml) or buffer. After incubation at 37°C for 24 hr, the nonmigrated cells on the upper surface of the filters were scraped and washed. The migrated cells were fixed in methanol, stained with Giemsa, counted under an inverted microscope and photomicrographed (120×) using a Zeiss photomicroscope (Axiovert 405 M). In separate experiments, cells in the upper compartment also were treated with mouse anti-human αvβ3-antibody (10 μg/ml) before being assayed for migration to ascertain whether this OPN-stimulated migration is mediated via αvβ3. Preimmune IgG treatment served as a nonspecific control.
Extracellular Matrix (ECM) Invasion Assay.
The ECM invasion assay was performed using a commercially available 24-well Matrigel-coated invasion chamber (Collaborative Biomedical Products, Bedford, MA) as described previously (29). Briefly, the confluent CASMC were trypsinized, centrifuged, and resuspended in basal medium supplemented with 0.1% BSA. The lower compartment of the invasion chamber was filled with fibroblast-conditioned medium (FCM), which served as a chemoattractant. The invasion assays were initiated by inoculating the upper chamber with cells (1 × 105/well), which were either untreated or treated with varying concentrations of OPN (0.5–2.0 μg/ml). After incubating at 37°C for 24 hr, the cells in the upper chamber were discarded, the matrigel was scraped clear, and the cells that had invaded the matrigel and migrated to the lower surface of the filter, were fixed, stained, counted, and photomicrographed (120×) as described above. The cells also were pretreated with mouse anti-human αvβ3 antibody (10 μg/ml) as described above to determine if the OPN-induced invasion is mediated via αvβ3. Preimmune IgG was used as a nonspecific control.
CASMC Proliferation Assay.
CASMCs were cultured as described above, and the cells were starved in serum-free media for 48 hr. The proliferation assays were performed as described previously (30). Briefly, the cells were incubated in the absence or presence of platelet-derived growth factor AB (PDGF-AB; 100 ng/ml) and increasing concentrations of OPN (1–6 μg/ml) at 37°C for 24 hr. In separate experiments, cells were pretreated with either mouse anti-human αvβ3-antibody (5 μg/ml), preimmune IgG, or GRGDS peptide (10 nM) followed by OPN (3.0 μg/ml). After 4 hr, [3H]thymidine (1 μCi/ml) was added, and the cells were maintained in culture for an additional 24 hr under the same culture conditions as described previously. After removing the supernatants, the cells were washed with basal medium and lysed in 50% trichloroacetic acid. The acid-precipitable cell-bound radioactivity was measured using a scintillation counter (Beckman).
In Situ Hybridization.
Sections of paraformaldehyde-fixed tissues were placed on ribonuclease-free polylysine-treated glass slides (American Histolabs, Rockville, MD), and in situ hybridization was carried out as previously described (31). The hOPN probe, hOPN-P2 (nucleotide 647–608) 5′-TCC ATG TGT GAG GTG ATG TCC TCG TCT GTA GCA TCA GGG T-3′) was 3′ end-labeled with digoxigenin-11-ddUTP (Boehringer Mannheim) as described previously (23). The slides were photomicrographed with a Zeiss Axiomat photomicroscope (magnification 400×).
Immunofluorescence.
The methodology for immunofluorescence has been described previously (32). Briefly, CASMCs during log phase of growth on microscopic slides were fixed in 4% buffered paraformaldehyde. Similarly, atherectomy and control coronary tissues also were fixed in buffered 4% paraformaldehyde and embedded in paraffin, and histological sections were prepared (American Histolabs). These cell and tissue samples were used for immunofluorescent detection of OPN, SMC-specific α-actin, and αvβ3 integrin. The rabbit OPN antiserum has been previously characterized (24). mAb (clone 1A4) to human SMC-specific α-actin was purchased from Sigma, and mouse mAb to human αvβ3 was obtained from Chemicon.
Determination of Plasma OPN Levels.
Plasma sample preparation for OPN detection by Western blotting has been described previously (33). The method for Western blotting is described above (24). Relative density of the Western-positive OPN protein bands were determined using an LKB Ultrascan LX-800 densitometer.
RESULTS
Expression of OPN mRNA and Protein in Cultured Human CASMCs.
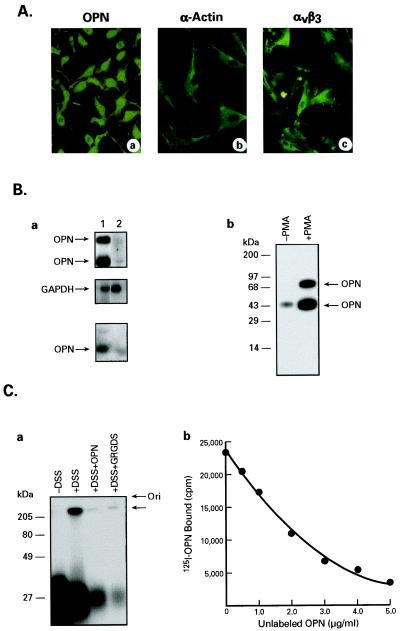
We first sought to determine the pattern of OPN mRNA and protein expression in cultured human CASMCs and to observe the effects of OPN treatment on the migration, ECM invasion, and proliferation of these cells. Accordingly, we obtained commercially available human CASMCs (Clonetics) and ascertained that these cells are 100% SMCs by immunofluorescence, which detected SMC-specific α-actin, OPN, and αvβ3 integrin. As shown in Fig. 1A, these cells express OPN (a), SMC-specific α-actin (b), and αvβ3 integrin (c). By using RT-PCR we also found that during log phase of growth these cells express elevated levels of OPN mRNA (Fig. 1Ba, top lane 1) compared with the confluent cultures (Fig. 1Ba, top lane 2). The GAPDH-mRNA signals were identical (Fig. 1Ba, middle lanes 1 and 2) in both nonconfluent and confluent cultures, demonstrating that these differences were not due to variability in gel loading or degradation of RNA during extraction. Western blot analysis of cell extracts also indicated that high levels of OPN are expressed during the log phase of growth (Fig. 1Ba, lower lane 1), compared with confluent cultures (Fig. 1Ba, bottom lane 2).
Figure 1.
Characterization of cultured CASMCs. (A) Detection of OPN (a), smooth muscle-specific α-actin (b), and integrin αvβ3 (c) in cultured CASMCs by indirect immunofluorescence. Note the cells express all three antigens. (B) (a) Expression of OPN mRNA and protein in CASMC by RT-PCR, and immunoprecipitation followed by Western blotting, respectively. Lane 1, CASMCs at log phase of growth. Lane 2, CASMCs from confluent cultures. The top, middle, and bottom panels are OPN mRNA, GAPDH mRNA, and OPN protein, respectively. Note that OPN mRNA and protein are easily detectable when the cells are in log phase of growth whereas the level is significantly lower when the cells reach confluence. (b) Immunoprecipitation and Western blot analysis of OPN production by semiconfluent cultures of CASMCs in response to PMA treatment. Lane 1, − PMA. Lane 2, + PMA (250 nM). (C) Affinity-crosslinking and competition binding of OPN to its receptor on cultured CASMCs. (a) 125I-OPN was incubated with CASMC in the absence or presence of unlabeled OPN or GRGDS peptide and then crosslinked with DSS as described below. Left to right: −DSS; +DSS; +DSS + unlabeled OPN; and +DSS + unlabeled GRGDS peptide. (b) 125I-OPN was incubated with CASMC using increasing concentrations of unlabeled OPN at 37°C for 3 hr. The data are an average of duplicate experiments.
Because PMA induces OPN gene expression, we stimulated the CASMCs with PMA and detected OPN production by immunoprecipitation of cell lysates followed by Western blotting. We found that the level of OPN protein in PMA-stimulated cells was markedly higher (Fig. 1Bb, right lane) than that of unstimulated cells (Fig. 1Bb, left lane). The two OPN bands (Fig. 1Bb, right lane), detected upon PMA stimulation, represent two isoforms of this protein. Because OPN may exert its effect on CASMCs by interacting with its cell-surface receptor, we also carried out 125I-OPN binding and affinity-crosslinking experiments. The 300-kDa protein band disappeared when the cells were pretreated with either OPN or GRGDS peptide and in the absence of DSS no such 300-kDa band was detected (Fig. 1Ca). The results indicate that OPN binds to a ≈300-kDa cell surface protein on CASMCs (Fig. 1Ca) with high specificity and affinity (Kd = 1 nM) (Fig. 1Cb). The results of immunoprecipitation with αvβ3 integrin antibody after binding and affinity-crosslinking of 125I-OPN with CASMCs established that the ≈300-kDa protein band is indeed αvβ3 integrin (data not shown).
Influence of OPN on CASMC Migration, ECM Invasion, and Proliferation.
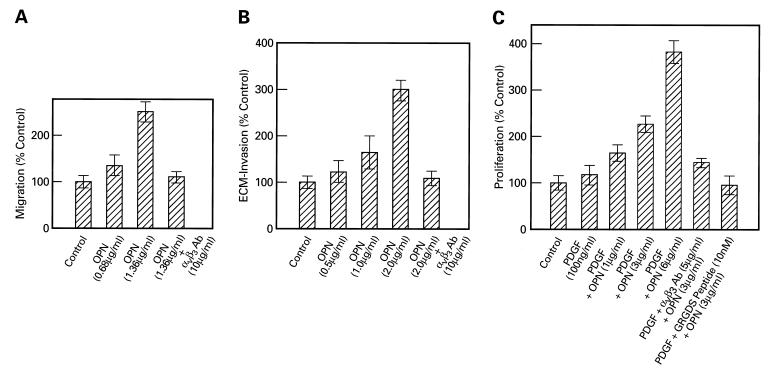
To determine the effects of OPN on CASMCs, we used established in vitro assay systems to evaluate the effects of this protein on cellular migration, ECM invasion, and proliferation. As shown in Fig. 2A, the rate of migration of CASMCs is enhanced with increasing concentrations of OPN used as chemoattractant. Similarly, OPN treatment of the cells also enhanced their invasiveness (Fig. 2B) when tested on Matrigel, an artificial ECM (Collaborative Biomedical Products), in a dose-dependent manner. The OPN-induced migration and ECM invasion were blocked when the cells were pretreated with αvβ3 integrin-antibody before performing each of these assays. A preimmune IgG, used as a control, failed to exert any inhibitory effect on OPN-induced migration and invasion (data not shown).
Figure 2.
(A) Effect of OPN on CASMC migration. These assays were performed by using either basal medium (Clonetics) containing 0.2% BSA or two different concentrations of OPN (0.68 and 1.36 μg/ml) in the lower chamber. In a separate experiment, the cells in the upper chamber were pretreated with either αvβ3 antibody or preimmune IgG (10 μg/ml), and OPN was added to the lower chamber at 1.36 μg/ml. The results are expressed as the mean of two determinations ± SEM. (B) OPN-induced ECM invasion by CASMCs. These assays were performed using untreated cells (controls) or cells treated with 0.5, 1.0, and 2.0 μg/ml of OPN, or cells pretreated either with αvβ3-antibody or preimmune IgG (10 μg/ml) and then treated with OPN (2.0 μg/ml). The results are expressed as the mean of two determinations ± SEM. (C) OPN-stimulated CASMC proliferation assay. The cells were untreated (control), or treated with PDGF-AB (100 ng/ml) alone or PDGF-AB and increasing concentrations of OPN (1.0, 3.0, and 6.0 μg/ml respectively). The cells also were pretreated with PDGF-AB followed by αvβ3-antibody (5 μg/ml) or PDGF-AB and preimmune IgG, or with PDGF-AB and GRGDS peptide (10 nM). They then were stimulated with OPN (3.0 μg/ml). The results are expressed as the mean of two determinations ± SEM.
OPN treatment of CASMCs also stimulated their proliferation in a dose-dependent manner (Fig. 2C). We carried out proliferation studies in the presence of PDGF-AB as it has been suggested that in vivo platelet activation may contribute to the pathogenesis of restenosis. While we observed that PDGF-AB alone has virtually no effect on CASMC proliferation, treatment of these cells with OPN had a dramatic dose-dependent effect when used in conjunction with 100 ng/ml of PDGF-AB (Fig. 2C). Treatment of the cells with OPN alone yielded a modest proliferative response (data not shown). Interestingly, treatment of CASMCs with GRGDS oligopeptide that corresponds to the cell adhesion sequence of OPN, or with αvβ3 antibody drastically inhibited OPN-induced proliferation (Fig. 2C). Moreover, transfection of CASMCs with lipofectin containing OPN-antisense S-oligonucleotide also showed the same effect (data not shown). Taken together, these results indicate that OPN gene expression is enhanced in proliferating, compared with contact-inhibited CASMCs, and that treatment of these cells with purified OPN stimulated their motility, ECM invasion, and proliferation. Moreover, these effects of OPN are transduced via αvβ3 integrin.
OPN mRNA and Protein Expression in Human Coronary Atherectomy Tissues.
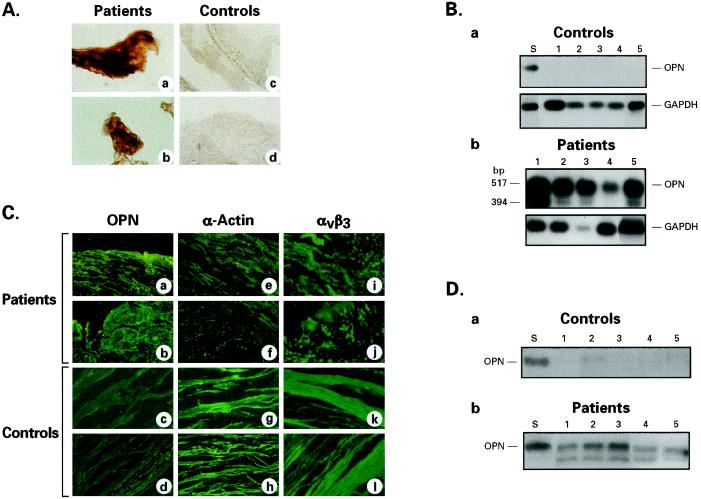
At this point, we were interested in learning whether a correlate of our in vitro results could be found in vivo. Accordingly, we sought to determine: (i) whether a clear distinction could be made between atherosclerotic and nonatherosclerotic coronary arterial tissues solely on the basis of OPN mRNA and protein expression patterns and if so, (ii) whether the arterial tissues that produce OPN also express one of its receptors, the αvβ3 integrin. Therefore, we studied coronary atherectomy tissues from 13 patients who participated in an approved clinical research protocol and in whom DCA was clinically indicated. Autopsy specimens of coronary arteries from six subjects, who had no evidence of atherosclerosis and had died of noncardiac causes, served as controls. A brief summary of profiles of patient and control subjects are presented in Table 1. The DCA and control tissues were used to detect OPN mRNA by in situ hybridization using a digoxigenin-labeled oligonucleotide probe derived from the sequence of human OPN cDNA. The results are shown in Fig. 3A. It is clear that atherosclerotic tissues obtained from DCA patients express very high levels of OPN mRNA (Fig. 3A a and b) while it is virtually undetectable in control samples (Fig. 3A, c and d). The results of RT-PCR using total RNA from control (Fig. 3Ba, lanes 1–5) and patient samples (Fig. 3Bb, lanes 1–5) corroborated the in situ hybridization results (Fig. 3A a–d). Because kidney is known to synthesize high levels of OPN constitutively, we standardized our assays on an OPN cDNA derived from human kidney RNA (CLONTECH) by RT-PCR (Fig. 3Ba, lane S). The apparent lack of OPN mRNA in control (autopsy) coronary arteries was not due to degradation of nucleic acids because the strong RT-PCR amplification of mRNA of a housekeeping gene, GAPDH, is virtually identical in each of these samples (Fig. 3B a and b, bottom). To determine whether the outer (adventitia), middle (media), or the inner (intima) tissue layers of the coronary arteries expressed OPN and αvβ3 integrin, we performed immunofluorescence of both DCA and control tissues using antibodies against OPN, SMC-specific α-actin, and αvβ3 integrin. In Fig. 3C (upper two rows) the immunofluorescence for OPN, SMC-specific α-actin, and αvβ3 integrin of coronary atherectomy tissues from two representative patients are shown. The lower two rows show the results from two representative control samples. It is clear that the patient tissues produced a high level of OPN-specific immunofluorescence (Fig. 3C a and b, OPN), while in the control tissues OPN immunofluorescence was virtually undetectable (Fig. 3C c and d). In both patient and control samples SMC-specific α-actin (Fig. 3C e–h) and the αvβ3 integrin (Fig. 3C i–l) were readily detectable. The Western blot analysis (Fig. 3D) of the control tissues showed a virtual lack of OPN (Fig. 3Da, lanes 1–5) compared with atherectomy samples in which appreciably higher levels of OPN were detected (Fig. 3Db, lanes 1–5). Purified OPN was used as a standard (Fig. 3D a and b, lane S). The SDS/PAGE and Western blotting of patient tissue extracts revealed two distinct OPN bands as noted previously.
Figure 3.
(A) Detection of OPN mRNA in coronary artery tissues of DCA patients and nonatherosclerotic subjects by in situ hybridization. a and b and c and d are bright-field photomicrographs of coronary atherectomy and normal coronary artery tissues, respectively (×200). Note the virtual absence of OPN mRNA signal in control tissues as compared with a very high level of the signal in atherectomy samples. (B) Detection of OPN mRNA by RT-PCR in normal coronary arteries (controls) and patient samples. (a) Lane S: RNA from human kidney (CLONTECH) used as positive control, and lanes 1–5, RNA from autopsy samples of five representative control subjects without evidence of coronary artery disease. Note the virtual absence of OPN mRNA signal in these samples. (b) Lanes 1–5, OPN expression in coronary atherectomy samples of five representative patients. GAPDH-mRNA expression in control and patient samples also are shown in a and b. (C) Detection of OPN, SMC-specific α-actin, and αvβ3 integrin by indirect immunofluorescence in coronary atherectomy tissues from two representative patients and normal coronary arteries. OPN: (a and b) patients; (c and d) controls. α-actin: (e and f) patients; (g and h) controls. αvβ3 integrin: (i and j) patients; (k and l) controls. Note high-intensity OPN-specific fluorescence on patient samples (a and b) compared with controls (c and d). The intensity of smooth muscle-specific α-actin and αvβ3 integrin fluorescence are very similar in both controls and patients. (D) Detection of OPN in control and coronary atherectomy tissues by Western blot analysis. (a) Autopsy samples from five apparently normal coronary arteries (lanes 1–5). (b) Samples from five atherectomy patients (lanes 1–5) and lane S represent purified OPN used as a standard. Note that while OPN is readily detectable in atherectomy samples, it is virtually absent from normal coronary arteries.
Plasma OPN Levels in Coronary Atherosclerotic Patients, Before and After Angioplasty.
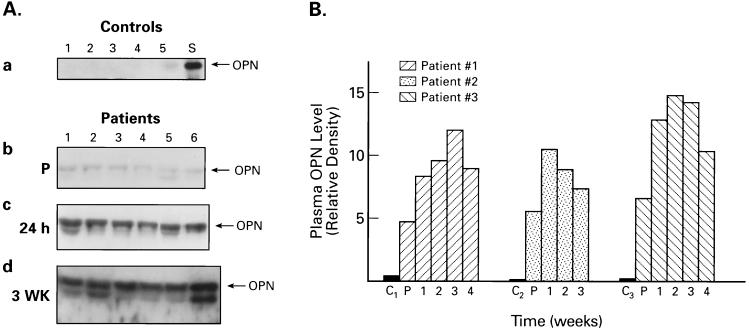
Because OPN is a secreted protein, we were interested in learning if its level in blood plasma changed after atherectomy. Accordingly, we obtained blood samples from DCA patients, the day before the procedure, 24 hr after, and on weekly intervals for 4 weeks after the DCA and subjected the plasma samples to analysis by SDS/PAGE and Western blotting. Equal amounts of total plasma proteins were loaded in each lane for electrophoresis. Semiquantitative, densitometric analysis of the OPN bands in Western blots also were performed. Plasma samples from healthy individuals, who had no clinical evidence of coronary artery disease, served as controls. Western blot analysis identified a clear difference in OPN levels between normal (Fig. 4Aa) and DCA patients (Fig. 4A b–d), respectively. As shown in Fig. 4Aa, the control plasma samples had virtually undetectable level of OPN (lanes 1–5), whereas those from DCA patients, collected 24 hr before the procedure (P in b), had readily visible OPN bands (lanes 1–6). OPN standard is designated S in a. What is more significant is the observation that plasma OPN levels dramatically increased 24 hr after DCA (c) and remained elevated even 3 weeks after the procedure (d). A follow-up of relative densities of OPN bands, resolved by SDS/PAGE and Western blotting of plasma samples of three representative DCA patients, collected over a 4-week period, are shown in Fig. 4B. In each case a separate control (lanes C1, C2, and C3) was used for comparison. It is clear that the baseline plasma OPN levels of the patients, even before the procedure, are appreciably higher than those of the healthy controls. Moreover, this elevated plasma OPN level was sustained for 4 weeks after DCA. Taken together, these results unambiguously demonstrate that OPN mRNA and protein expression in CASMCs of DCA tissues, as well as the baseline plasma OPN levels of these patients, are remarkably higher than those of the control subjects. Most interestingly, plasma levels of OPN were further elevated after DCA and remained high for the duration of this study.
Figure 4.
Western blot and densitometric analyses of OPN in plasma samples of patients and normal controls. (A) (a) Lanes 1–5, plasma OPN from five different controls; lane S: purified OPN standard. (b) Lanes 1–6, plasma samples of DCA patients obtained 24 hr before the procedure. (c) Lanes 1–6, DCA patients 24 hr after the procedure. (d) DCA patients, 3 weeks after DCA. Note the virtual absence of OPN in control samples (a) and highly conspicuous OPN bands in c and d. (B) Densitometric analysis of plasma OPN bands from three representative DCA patients. C1–3: controls; P: patients 24 hr before DCA. The numbers indicate time after the procedure in weeks. Note the sustained high levels of plasma OPN in patients.
DISCUSSION
The results of our in vitro experiments with cultured CASMCs and those derived from atherectomy tissues from patients suggest that there may be a cascade of events that lead to the development of restenosis after angioplasty and that OPN plays both autocrine and paracrine receptor-mediated roles, which critically affect the biology of CASMCs. OPN has been reported to have chemotactic properties (15) and has been demonstrated to induce proliferation in rat aortic SMCs (20). Thus, a likely scenario is that the inflammatory stimulus generated by the trauma of angioplasty is the triggering event that causes infiltration of monocytes and macrophages into the vascular smooth muscle layer. Because activated monocytes and macrophages are known to secrete OPN it could bind to αvβ3 integrin on CASMCs, which in turn respond by expressing more OPN. Secreted OPN then interacts with CASMCs in an autocrine or paracrine fashion and promotes their migration toward the intima where the angioplasty-induced injury has occurred. These cells then invade the ECM to arrive at the intima, where they proliferate to cause reocclusion.
It has been reported that vascular SMCs, when stimulated with vitronectin, undergo haptotaxis (34), a process in which the cells migrate toward an increasing gradient of a chemoattractant. More recently, Senger et al. (35, 36), have demonstrated that OPN and its GRGDS-containing thrombin cleavage fragment promote haptotaxis of tumor and vascular endothelial cells respectively, via the αvβ3 integrin. Our results in DCA patients indicate that plasma OPN levels dramatically increase after the procedure, which may create an increasing gradient of this protein from the media of the arterial wall (where the CASMCs are normally located) to the lumen of the artery, where the highest concentration of OPN may be found. This may explain why the CASMCs migrate from their original location in the arterial media toward the intima, which is in close proximity to the circulating high levels of OPN in the blood. CASMCs arriving at their destination in the intima proliferate due to OPN stimulation and, as a result, cause reocclusion. While there may be other factors involved in the pathogenesis of this complex disease process, our results raise a strong possibility that OPN and its αvβ3 integrin receptor play an essential role not only in stimulating the migration and ECM invasion but also proliferation of CASMCs. Our observation that transfection of CASMCs with lipofectin containing OPN-antisense S-oligonucleotides causes a drastic inhibition of proliferation of these cells may have potential clinical applications. The possibility of a short course of systemic OPN-antisense S-oligonucleotide treatment of patients after angioplasty or local administration of lipofectin containing S-oligonucleotides at the completion of the procedure may be worth studying in animal models as a means of preventing reocclusion.
Acknowledgments
We thank D. R. Senger and C. A. Perruzzi for generously providing pure human OPN used in this study. We also thank B. Mansfield, J. Chou, D. R. Senger, J. DeB. Butler, M. Nemir, and J. Sidbury, Jr. for critical review of the manuscript and helpful suggestions. We are grateful to Ms. Deborah Celantano for collecting and processing the patient samples. The expert photomicrographic assistance of Mr. Rick Dreyfuss and Ms. Shauna Everett of Medical Arts and Photography Branch, National Institutes of Health, is gratefully acknowledged. This project was supported in part by grants from Mallinckrodt Medical, and Guident Corporation, Washington, D.C.
ABBREVIATIONS
- CASMC
coronary artery smooth muscle cell
- SMC
smooth muscle cell
- OPN
osteopontin
- DCA
directional coronary atherectomy
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- DSS
disuccinimidyl suberate
- PMA
phorbol 12-myristate 13-acetate
- RT-PCR
reverse transcriptase–PCR
- ECM
extracellular matrix
- FCM
fibroblast conditioned medium
- GRGDS
glycine-arginine-aspartic acid- glycine-serine
- PDGF-AB
platelet-derived growth factor AB
References
- 1.Ross R. Nature (London) 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Hajjar D P, Nicholson A C. Amer Sci. 1995;83:460–467. [Google Scholar]
- 3.Ferrell M, Fuster V, Gold H K, Chesebro J H. Circulation. 1992;85:1630–1631. doi: 10.1161/01.cir.85.4.1630. [DOI] [PubMed] [Google Scholar]
- 4.Austin G E, Ratliff N B, Hollman J, Tabei S, Phillips D F. J Am Coll Cardiol. 1985;6:369–375. doi: 10.1016/s0735-1097(85)80174-1. [DOI] [PubMed] [Google Scholar]
- 5.Giraldo A A, Esposo O M, Meis J M. Arch Pathol Lab Med. 1985;109:173–175. [PubMed] [Google Scholar]
- 6.Senger D R, Wirth D F, Hynes R O. Cell. 1979;16:885–893. doi: 10.1016/0092-8674(79)90103-x. [DOI] [PubMed] [Google Scholar]
- 7.Franzen A, Heinegard D. Biochem J. 1985;232:715–724. doi: 10.1042/bj2320715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oldberg A, Franzen A, Heinegard D, Pierschbacher M, Ruoslahti E. J Biol Chem. 1988;263:19433–19436. [PubMed] [Google Scholar]
- 9.Nemir M, DeVouge M W, Mukherjee B B. J Biol Chem. 1989;264:18202–18208. [PubMed] [Google Scholar]
- 10.Craig A M, Bowden G T, Chambers A F, Spearman M A, Greenberg A H, Wright J A, Denhardt D T. Int J Cancer. 1989;46:133–137. doi: 10.1002/ijc.2910460124. [DOI] [PubMed] [Google Scholar]
- 11.Denhardt D T, Guo X. FASEB J. 1993;7:1475–1482. [PubMed] [Google Scholar]
- 12.Hynes R O. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 13.Oldberg A, Franzen A, Heinegard D. Proc Natl Acad Sci USA. 1986;83:8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giachelli C, Bae N, Lombardi D, Majesky M, Schwartz S. Biochem Biophys Res Commun. 1991;177:867–873. doi: 10.1016/0006-291x(91)91870-i. [DOI] [PubMed] [Google Scholar]
- 15.Liaw L, Almeida M, Hart C E, Schwartz S M, Giachelli C M. Circ Res. 1994;74:214–224. doi: 10.1161/01.res.74.2.214. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda T, Shirasawa T, Esaki Y, Yoshiki S, Hirokawa K. J Clin Invest. 1993;92:2814–2820. doi: 10.1172/JCI116901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giachelli G M, Bae N, Almeida M, Denhardt D T, Alpers C E, Schwartz S M. J Clin Invest. 1993;92:1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shanahan C M, Cary N R B, Metcalf J C, Weissberg P L. J Clin Invest. 1994;93:2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liaw L, Skinner M P, Raines E W, Ross R, Cheresh D A, Schwartz S M, Giachelli C M. J Clin Invest. 1995;95:713–724. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gadeau A-P, Campan M, Millet D, Candresse T, Desgranges C. Arterioscl Thromb. 1993;13:120–125. doi: 10.1161/01.atv.13.1.120. [DOI] [PubMed] [Google Scholar]
- 21.Kubota T, Yamauchi M, Onozaki J, Sato S, Suzuki Y, Sodek J. Arch Oral Biol. 1993;38:23–30. doi: 10.1016/0003-9969(93)90150-k. [DOI] [PubMed] [Google Scholar]
- 22.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 23.Peri A, Cordella-Miele E, Miele L, Mukherjee A B. J Clin Invest. 1993;92:2099–2109. doi: 10.1172/JCI116810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chackalaparampil I, Peri A, Nemir M, McKee M, Lin P H, Mukherjee B B, Mukherjee A B. Oncogene. 1996;12:1457–1467. [PubMed] [Google Scholar]
- 25.Hunter W M, Greenwood F C. Nature (London) 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- 26.Munson P J, Rodbard D. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Yue T-L, McKenna P J, Ohlstein E H, Farach-Carson M C, Butler W T, Johanson K, McDevitt P, Feuerstein G Z, Stadel J M. Exp Cell Res. 1994;214:459–464. doi: 10.1006/excr.1994.1282. [DOI] [PubMed] [Google Scholar]
- 29.Kundu G C, Mantile G, Miele L, Cordella-Miele E, Mukherjee A B. Proc Natl Acad Sci USA. 1996;93:2915–2919. doi: 10.1073/pnas.93.7.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monfardini C, Kieber-Emmons T, VonFeldt J M, O’Malley B, Rosenbaum H, Godillot A P, Kaushansky K, Brown C B, Voet D, McCallns D E, Weiner D B, Williams V W. J Biol Chem. 1995;270:6628–6638. doi: 10.1074/jbc.270.12.6628. [DOI] [PubMed] [Google Scholar]
- 31.Peri A, Dubin N, Dhanireddy R, Mukherjee A B. J Clin Invest. 1995;96:343–353. doi: 10.1172/JCI118040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peri A, Cowan B D, Bhartiya D, Miele L, Nieman L K, Nwaeze I O, Mukherjee A B. DNA Cell Biol. 1994;13:495–503. doi: 10.1089/dna.1994.13.495. [DOI] [PubMed] [Google Scholar]
- 33.Senger D R, Perruzzi C A, Gracey C F, Papadopoulos A, Tenen D G. Cancer Res. 1988;48:5770–5774. [PubMed] [Google Scholar]
- 34.Naito M, Hayashi T, Funaki C, Kuzuya M, Asai K, Yamada K, Kuzuya F. Exp Cell Res. 1991;194:154–156. doi: 10.1016/0014-4827(91)90145-k. [DOI] [PubMed] [Google Scholar]
- 35.Senger D R, Ledbetter S R, Claffey K P, Papadopoulos-Sergiou A, Perruzzi C A, Detmar M. Am J Pathol. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- 36.Senger D R, Perruzzi C A. Biochim Biophys Acta. 1996;1314:13–24. doi: 10.1016/s0167-4889(96)00067-5. [DOI] [PubMed] [Google Scholar]