Abstract
Recent data have identified leptin as an afferent signal in a negative-feedback loop regulating the mass of the adipose tissue. High leptin levels are observed in obese humans and rodents, suggesting that, in some cases, obesity is the result of leptin insensitivity. This hypothesis was tested by comparing the response to peripherally and centrally administered leptin among lean and three obese strains of mice: diet-induced obese AKR/J, New Zealand Obese (NZO), and Ay. Subcutaneous leptin infusion to lean mice resulted in a dose-dependent loss of body weight at physiologic plasma levels. Chronic infusions of leptin intracerebroventricularly (i.c.v.) at doses of 3 ng/hr or greater resulted in complete depletion of visible adipose tissue, which was maintained throughout 30 days of continuous i.c.v. infusion. Direct measurement of energy balance indicated that leptin treatment did not increase total energy expenditure but prevented the decrease that follows reduced food intake. Diet-induced obese mice lost weight in response to peripheral leptin but were less sensitive than lean mice. NZO mice were unresponsive to peripheral leptin but were responsive to i.c.v. leptin. Ay mice did not respond to subcutaneous leptin and were 1/100 as sensitive to i.c.v. leptin. The decreased response to leptin in diet-induced obese, NZO, and Ay mice suggests that obesity in these strains is the result of leptin resistance. In NZO mice, leptin resistance may be the result of decreased transport of leptin into the cerebrospinal fluid, whereas in Ay mice, leptin resistance probably results from defects downstream of the leptin receptor in the hypothalamus.
In mammals, the maintenance of energy stores in the form of triglycerides is of adaptive value, particularly in times of nutritional scarcity. Numerous studies of humans and other mammals have indicated that the size of the adipose tissue mass is tightly regulated (1–3). The identification of leptin and its receptors supports this finding and indicates that leptin is an afferent signal in a negative-feedback loop regulating body weight (4–6). The level of plasma leptin is positively correlated with adipose tissue mass, and injections of recombinant leptin reduce food intake and body fat content in normal animals (7–10). Leptin injections also attenuate some of the hormonal changes observed during starvation (11). While leptin treatment decreases weight in normal mice, some investigators have suggested that this is a pharmacological effect and that leptin’s principle function is to signal starvation (12, 13). Recent data have also suggested that the hypothalamus is an important target of leptin action and that leptin’s metabolic effects are qualitatively different from those of food restriction (6, 14–18).
Plasma leptin levels are significantly higher in obese animals and humans (7, 19, 20). These findings have led to the conclusion that obese individuals are relatively insensitive to endogenous leptin, and as a consequence become obese (7, 19). To directly test this hypothesis, the potency of subcutaneous and intracerebroventricular (i.c.v.) infusion of leptin was compared among lean and diet-induced obese AKR/J (DIO), New Zealand Obese (NZO), and Ay mice. The i.c.v. infusion was performed using a microsurgical technique for long-term infusion into the third ventricle of mice (21). AKR/J mice are lean on a chow diet but have a heritable predisposition toward developing obesity when fed a high-fat diet (22). NZO mice inherit a polygenic obesity, and Ay mice carry a single dominant mutation that results in obesity (23–25). These strains all develop an obese syndrome associated with high blood levels of leptin. The experiments reported here indicate that DIO, NZO, and Ay mice are relatively insensitive to leptin. However, in each case, the sensitivity differed, providing possible insights into the mechanisms of leptin resistance and obesity in these obese strains.
METHODS
Animal Maintenance.
Animals were purchased from The Jackson Laboratory (C57BL/6J, C57BL/6J Ay, AKR/J) or Charles River Breeding Laboratories (NZO) and maintained in individual cages or housed in groups of four (AKR/J). Mice were maintained on a 12-hr light:dark schedule, fed, weighed and, if necessary, injected between 1000 and 1200. All animals were cared for according to approved institutional guidelines.
Subcutaneous Pumps.
Pumps were filled aseptically with 0.2-μm-filtered PBS (pH 7.4), artificial cerebrospinal fluid (aCSF) (1 mM MgCl2/1 mM CaCl2/145 mM NaCl/3 mM KCl), or leptin in PBS (pH 7.4) and soaked in 0.9% NaCl at 37°C for 4 hr. Animals were anesthetized with methoxyflurane and the pump was implanted subcutaneously dorsally.
Intracerebroventricular (i.c.v.) Cannula.
Animals were anesthetized with ketamine and xylazine and placed on a stereotaxic apparatus (Kopf Instruments model 900 small animal stereotaxic). The calvaria was exposed, and two 0.5-mm-diameter holes were drilled posteriorly bilaterally. Self-threading pins (TMS, Whaledent) were screwed into each hole and a 1-mm-diameter hole was drilled over bregma. The cannula, a 30-gauge needle angled at 9°, was implanted into the third ventricle with the following coordinates: midline, −0.3 mm AP, 3 mm ventral (0 point bregma). The cannula was secured to the skull and the pins with acrylic and attached to 6 cm of Tygon tubing (0.01-inch diameter), which itself was attached to 1 cm of tubing (0.03-inch diameter). An osmotic pump was then attached to the end of this tubing, placed in the dorsal subcutaneous space.
Indirect Calorimetry.
Twenty-four-hour energy expenditure was measured by indirect calorimetry. The calorimeter system consists of a Magnos IV oxygen analyzer and a Uras 3G carbon dioxide analyzer (Harmann Braun; Frankfurt, Germany) and six Plexiglas metabolic chambers maintained in an environmental room separate from the analyzers. First-pass fresh air maintained at constant temperature and humidity is pulled through the chambers at a fixed rate by individual vacuum pumps located outside the environmental room. The sampled air is pulled through a condenser (Hartmann Braun) to remove humidity before being analyzed. Percentage of O2 consumed and CO2 produced is converted to ml/min by multiplying by the flow rate corrected to standard temperature and pressure. Energy expenditure was calculated from oxygen consumption using standard formulae (26). The analyzers are calibrated using a Westoff gas mixing pump (Digamix type m/300c; Bochum, Germany) and the entire system was tested by burning propane. Estimation error for the respiratory quotient is less than 1%.
Leptin Measurements.
Blood was collected from the retro-orbital venous plexus, using heparinized capillary tubes. Plasma was collected and 50 μl was used with a leptin RIA kit (Linco, St. Charles, MO) to determine serum leptin concentrations.
Body Composition Analysis.
Animals were dehydrated in an oven at 90°C for 4–7 days until constant mass was achieved. The dried carcass was homogenized, and lipid was extracted from 0.5-g aliquots for 3.5 hr in a Soxhlet apparatus with a 3:1 chloroform/methanol mixture. The extracted aliquot was dried overnight and weighed. The difference in weight following extraction represents lipid, and the remaining mass represents lean body mass.
Statistics.
All statistics were calculated using the paired Student’s t test. Values are expressed ±SD.
RESULTS
Peripheral Leptin Infusion.
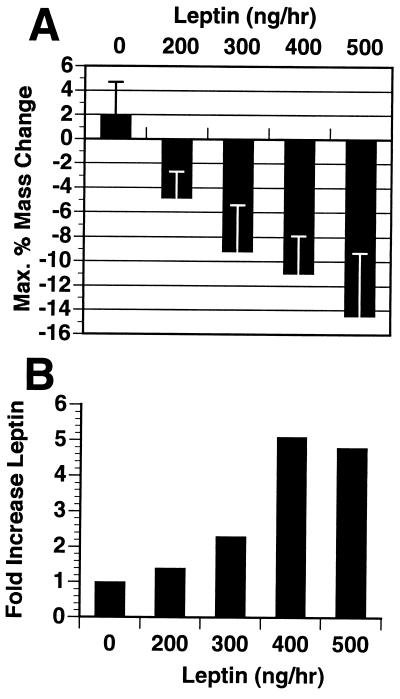
While it has been shown that intraperitoneal (i.p.) injections of leptin (12.5 mg/kg twice daily) into wild-type mice results in a significant reduction in food intake and body weight, supraphysiological doses were required (8). To assess whether leptin has weight reducing effects at physiological levels, leptin was infused into the subcutaneous tissue of wild-type mice by using osmotic infusion pumps (Alzet; Palo Alto, CA). The potency of the weight-reducing effects of leptin was directly related to the relative rise in plasma leptin concentration during the infusion period (Fig. 1). Increasing plasma leptin concentration above the endogenous level (5 ng/ml) resulted in a dose-dependent loss of body weight and body fat content. Subcutaneous infusion of leptin at a rate of 200 ng/hr led to a 1.4-fold increase in plasma leptin from 5 to 7 ng/ml and resulted in a 5% reduction in body weight, as compared to a 2% weight gain over the same period of time in animals receiving PBS. A 2.1-fold increase in leptin level led to a 9% reduction in weight. A loss of 15% of total weight and a complete loss of body fat was observed with a 5-fold increase in plasma leptin level (infusion rate of 500 ng/hr).
Figure 1.
Dose–response of wild-type C57BL/6J mice (n = 8 per group) to recombinant murine leptin delivered by 14-day subcutaneous infusion pumps (Alzet). (A) Maximum body mass change in response to leptin as a percentage of initial mass. (B) Fold increase in serum leptin levels achieved by each dose. Blood was collected at time of pump implantation by retro-orbital bleeding and again 10 days later at the time of maximum body mass change. Leptin levels were measured by RIA (Linco).
i.c.v. Leptin Infusion.
Previous data have suggested that the central nervous system, in particular the hypothalamus, is an important target of leptin action (6, 14–16, 18). If true, administration of leptin at low doses i.c.v. should replicate the effects of higher doses administered peripherally. To test this, the effect of chronic i.c.v. leptin infusion was compared with continuous subcutaneous infusion. Thirty-gauge cannula were surgically implanted into the third ventricle of mice and connected to an osmotic pump placed in the dorsal subcutaneous space. This procedure allows the infusion of leptin into the cerebrospinal fluid (CSF) at a constant rate for 4 weeks. i.c.v. infusion of leptin at 8 ng/hr resulted in a significant decrease in food intake and a 15% decrease in body weight as compared with controls (Fig. 2). This dose had no effect when infused peripherally (data not shown). Furthermore, i.c.v. infusion of 8 ng/hr of recombinant human leptin did not lead to the presence of human leptin in plasma as measured using a human-specific leptin RIA (data not shown).
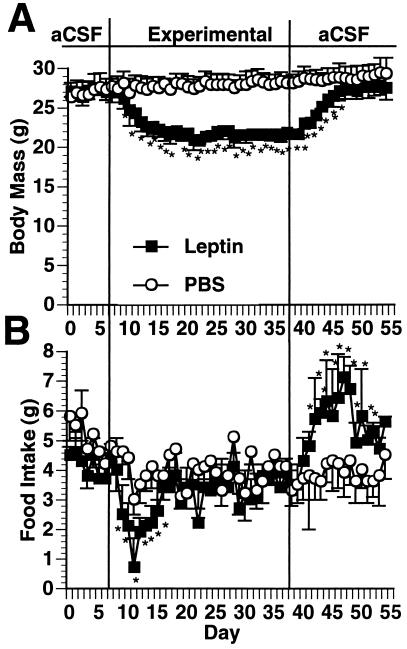
Figure 2.
Response of wild-type C57BL/6J mice to i.c.v. leptin infusion. The third ventricle was stereotaxially cannulated with a 30-guage needle connected to an osmotic pump. The animals were infused with aCSF for 1 week, after which they were infused with either recombinant murine leptin at a dose of 8 ng/hr (n = 6) or PBS vehicle control (n = 6). The animals were then followed for 30 days and body mass (A) and food intake (B) were measured. At the end of the 30-day experimental infusion the pumps were changed back to aCSF and the animals were followed for 14 more days. ∗, Significantly (P ≤ 0.05 different from PBS control.
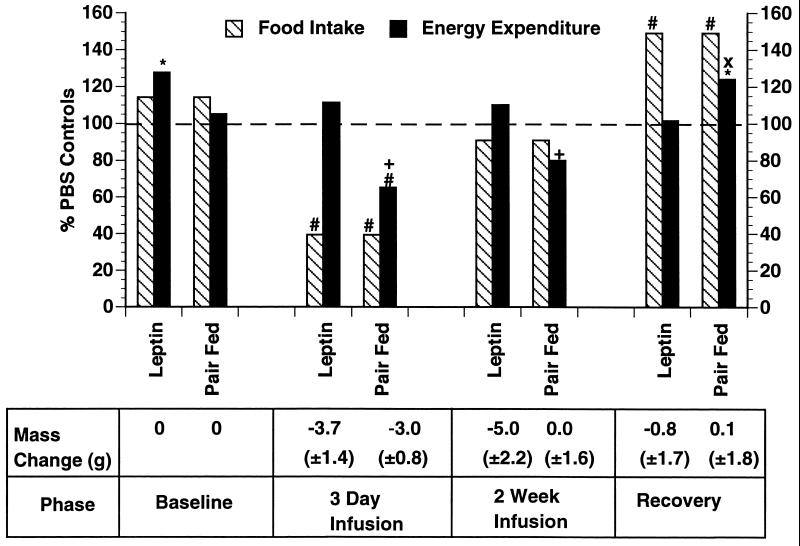
i.c.v. infusion of leptin caused a rapid reduction in body weight, with a maximal weight loss at 8 days of treatment which was stably maintained for the entire 30-day infusion period (Fig. 2). Food intake reached a nadir after 3 days of infusion but then returned to the level of PBS controls by the eighth day of infusion. Despite the return of food intake to normal, the reduced weight was maintained for the duration of the leptin infusion. After replacement of the leptin infusion with aCSF, the animals exhibited a marked hyperphagia and quickly recovered the lost weight. To further characterize the effect of i.c.v. leptin on energy balance, food intake was measured in treated mice and 24-hr energy expenditure was assessed by indirect calorimetry (Fig. 3). Measures were made at several time points in groups receiving i.c.v. leptin or i.c.v. PBS and in a third group of mice receiving i.c.v. PBS but pair-fed to the leptin-treated group. The data indicated that after 3 days of i.c.v. leptin infusion, the treated animals had entered a state of negative energy balance in which caloric intake was reduced by more than 50%, while energy expenditure remained unchanged. These findings at day 3 of the leptin infusion are different from those seen in the pair-fed group, in which the decreased food intake was associated with a 35% decrease in energy expenditure (P < 0.02). At 14 days, after maximal weight loss had been achieved, energy expenditure and food intake in the leptin-treated animals were equivalent to those of the PBS-infused and the pair-fed groups. Replacement of the leptin infusion by aCSF resulted in a state of positive energy balance in which caloric intake was increased while energy expenditure was unchanged. Positive energy balance was maintained until the weight of the leptin-treated group returned to that of the control group. In aggregate, these data suggest that addition of exogenous leptin adjusts the set point for body weight to a lower but stable level by reducing food intake without the compensatory reduction in energy expenditure observed in equivalently food-restricted animals.
Figure 3.
Effect of i.c.v. leptin infusion vs. pair-feeding on 24-hr energy expenditure. Three groups of mice were cannulated as before and placed in metabolic chambers (n = 6 per group). One group was infused with leptin at 8 ng/hr, a control group was infused with PBS, and a final control group was infused with PBS and pair-fed to the leptin-infused group. Food intake was measured daily and energy expenditure was measured at four different times: baseline, 3 days following leptin infusion or PBS control, 2 weeks after beginning leptin infusion or PBS control, and 10 days following infusion with aCSF during the recovery phase. Energy expenditure and food intake are graphed as a percentage of the PBS control group. Mass change refers to the mass change relative to the baseline mass at day 0. ∗, P < 0.05 vs. PBS; #, P < 0.005 vs. PBS; +, P < 0.005 vs. leptin; ×, P < 0.05 vs. leptin.
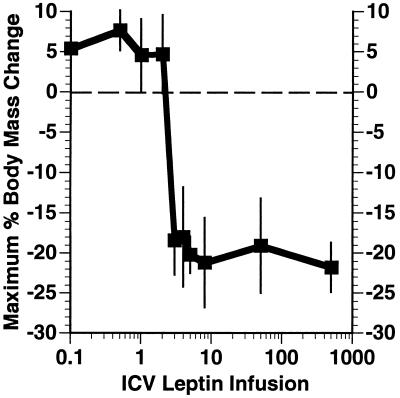
The dose–response relationship of i.c.v. leptin was evaluated (Fig. 4). In these studies, infusion of leptin at several doses between 3 ng/hour and 500 ng/hour led to an equivalent degree of weight loss and a complete loss of body fat. There was a tendency (statistically nonsignificant) for the groups receiving the higher infusion rate to lose weight more quickly. However, all groups eventually lost equivalent amounts of weight. Leptin infusion at rates of 2 ng/hr or lower were without effect. Thus, the dose–response curve for i.c.v. leptin is very steep, a finding also observed after administration of a single dose of i.c.v. leptin (27).
Figure 4.
Dose–response of wild-type C57BL/6J to i.c.v. leptin. The animals’ maximal body mass change in response to i.c.v. infused leptin at doses ranging from 0 to 500 ng/hr was measured and is shown as a percentage of the initial body mass at the beginning of leptin infusion (n = 6 for all doses but 500 ng/hr, where n = 4).
Leptin Treatment of Obese Mice.
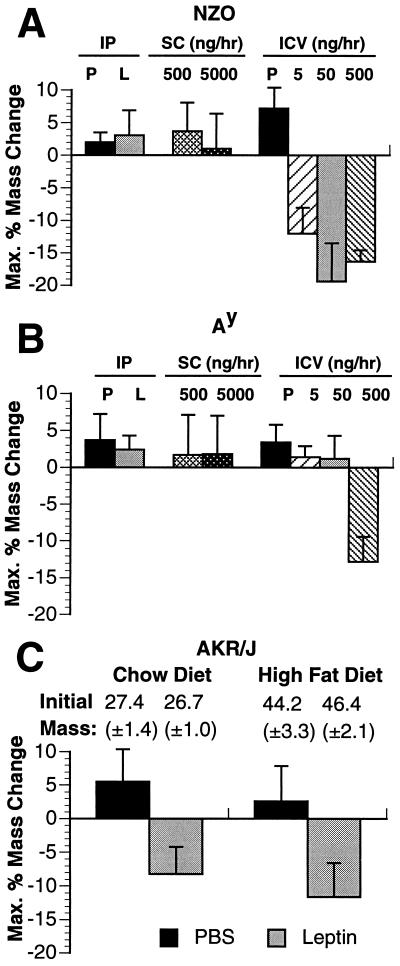
All murine models of obesity tested, with the exception of ob/ob, have high circulating leptin levels, suggesting that they may be resistant to its weight-reducing effects (unpublished data) (7, 20). To test this, peripheral or i.c.v. leptin was administered to three strains of obese animals: DIO, NZO, and Ay. AKR/J mice are lean on a standard chow diet but become obese when fed a high-fat diet (45% calories of fat) (22). NZO mice exhibit a polygenic form of obesity, whereas Ay mice carry a single dominant mutation in the promoter of the agouti gene (23, 24, 28). Both Ay and NZO failed to respond to high dose administration of subcutaneous leptin at doses as high as 5 μg/hr, which is 10-fold higher than that required for a maximal response in lean mice (Fig. 5A and B). In addition, these animals did not respond to high doses of intraperitoneal leptin (12.5 mg/kg, twice daily. In contrast, the DIO mice did respond to high-dose intraperitoneal leptin but to a lesser extent than AKR/J mice fed a chow diet; the DIO mice lost 30.5% (±14.1%) of their body fat vs. 82.7% (±19.7%) of body fat in the lean group on a chow diet (Fig. 5C). The effects of i.c.v. infusion of leptin were tested in both Ay and NZO mice which did not respond to high-dose peripheral leptin. i.c.v. leptin infusion of the NZO mice led to weight loss at a low dose of 5 ng/hr (Fig. 5A). In Ay mice, infusion of leptin i.c.v. did not result in demonstrable weight loss at doses as high as 50 ng/hr (Fig. 5B). Infusion of leptin in Ay mice at a rate of 500 ng/hr did lead to a modest but significant weight loss. Thus, while i.c.v. leptin in NZO mice resulted in significant depletion of adipose tissue at a dose of 5 ng/hr, only a partial response was seen in Ay mice even at doses 100-fold higher. The correct placement and functional status of the cannulas in the Ay mice receiving low doses of i.c.v. leptin were tested by measuring water intake after infusion of angiotensin II at 3 ng/hr (21). The animals that did not respond to i.c.v. leptin but that subsequently received i.c.v. angiotensin II showed an increased water intake from 10.4 (±1.4) ml to 31.8 (±1.7) ml (>300% increase), confirming that the cannulas were properly positioned.
Figure 5.
Effect of peripheral and central leptin in different murine models of obesity. (A and B) NZO mice (A) and Ay (B) mice were administered recombinant leptin or PBS control (P) by three different methods: (i) high-dose intraperitoneal (IP) injection (12.5 mg/kg twice daily) (n = 8; female, 20 weeks); (ii) subcutaneous (SC) infusion pump (n = 5 for each dose; female, 12 weeks); and (iii) i.c.v. (ICV) infusion (n = 5 at each dose; female, 12 weeks) at the doses indicated. (C) AKR/J mice were tested for their sensitivity to peripheral recombinant leptin at a high dose of 12.5 mg/kg twice daily. One group of mice (n = 16; female, 14 weeks) was lean on a standard chow diet and half this group received leptin intraperitoneally while the other half received vehicle PBS control intraperitoneally. Another group (n = 16; female, 14 weeks) was obese after being on a high-fat diet (45% calories from fat; Teklad, Madison, WI) for 10 weeks. Half of this group received leptin intraperitoneally at a dose of 12.5 mg/kg twice daily, while the other group received vehicle PBS control intraperitoneally.
DISCUSSION
Obesity is associated with important psychological and medical morbidities, the latter including hypertension, hyperlipidemia, and non-insulin-dependent diabetes mellitus (NIDDM) (29). Several lines of evidence have suggested that obese individuals have a higher “set point” for body weight relative to lean subjects (1–3). The identification of leptin has suggested a possible mechanism for the maintenance of different weights in different individuals. Thus, a higher set point present in obese individuals may be the result of a relative or absolute insensitivity to leptin. Indeed, both obese rodents and obese humans generally have high plasma levels of leptin, suggesting that they are relatively insensitive to its effects (unpublished data) (7, 20). Resistance to leptin’s effects on adipose tissue homeostasis would be predicted to lead to a temporary state of positive energy balance and an increase in adipose mass with stable maintenance of a new higher body weight. Although the high leptin levels seen in obese animals suggested they may be relatively resistant to its weight-reducing effects, this possibility had not been formally tested. The data presented here demonstrate that, among three strains of obese mice, there are variable degrees of leptin resistance. It is anticipated that similar variation in leptin sensitivity will be observed among obese humans.
Studies of the leptin sensitivity of obese rodents required that the dose–response relationship be established in wild-type animals. Previous data have indicated that supraphysiologic doses of intraperitoneal leptin are required for weight loss in wild-type animals (8–10, 27). The data presented here suggest that this high dosage requirement was the result of the suboptimal pharmacokinetics of the intraperitoneal mode of administration. In the current studies, a clear weight-reducing effect of leptin at physiologic levels was observed after subcutaneous infusion. It has recently been suggested that leptin’s principle role is to suppress the physiologic response to starvation (11–13). The data presented here confirm that leptin also plays an important role in the response to weight gain and suggests that a physiologic increase in endogenous leptin levels in lean animals acts to return an animal’s weight to its “set point.”
The measurement of energy balance in animals receiving i.c.v. leptin demonstrates that leptin acts to blunt the reduced energy expenditure that typically follows reduced caloric intake. This effect is different from that observed in ob/ob mice, which show an increase in energy expenditure after leptin treatment (8, 9). These and other data indicate that leptin’s effects are qualitatively different from those that accompany food restriction. For example, leptin-induced weight loss is specific for the adipose tissue mass, whereas equivalent food restriction alone reduces both adipose and lean body mass (8). The physiological basis for the observed differences in energy expenditure in leptin-treated and pair-fed mice is unknown but could include differential effects on brown fat and/or locomotor activity (9, 30). Finally, it should be noted that animals receiving leptin either i.c.v. or subcutaneously initially enter a state of negative energy balance which returns to normal once adipose stores have been depleted. This is in contrast to wild-type or ob/ob mice parabiosed to db/db animals, which enter a persistent state of negative energy balance and eventually die, presumably due to starvation. It is possible the death of the parabiotic animal is partly a result of stress from the surgery and subsequent parabiotic union coupled to a state of persistent hyperleptinemia. Alternatively, the death of animals parabiosed to db/db mice may reflect the presence of factors in addition to leptin in db/db mice.
The increased potency of leptin in lean mice when administered i.c.v. is consistent with other data indicating that the central nervous system, in particular the hypothalamus, is the principle site of leptin action (6, 10, 14, 18, 27). Indeed, chronic infusions of very low doses of leptin into the third ventricle replicate the weight-reducing effects of leptin administered peripherally at much higher doses. The effects of i.c.v. leptin are specific, as higher doses of the same preparation do not have any observable effect on Ay mice. Several other reports have suggested that leptin also has effects on peripheral tissues such as ovary and pancreatic β cells (31, 32). The potency of i.c.v. leptin suggests that these peripheral effects are not necessarily required for leptin’s weight-reducing effects. Curiously, the dose–response relationship of leptin is different between the central and peripheral modes of administration, a finding also seen after single doses of i.c.v. leptin (27). The basis for this apparent difference in the dose–response relationship between central and peripheral leptin is not clear. The data may suggest that the transport across the blood–brain barrier can be a rate-limiting step in the response to leptin. A saturable transport system for leptin has been identified in mice and in brain capillaries from humans (33, 34). This finding could also reflect cooperativity of leptin binding or reflect coordinate effects on the action potential of many neurons. The cellular basis for the response to i.c.v. leptin is unknown and requires further investigation.
While the sensitivity to both peripheral and centrally administered leptin is highly reproducible in wild-type mice, each of the obese strains tested shows a decreased sensitivity to leptin and implicates leptin resistance in the pathogenesis of these forms of obesity. The extent of this resistance is variable among the DIO, NZO, and Ay mice. Whereas DIO mice respond to peripheral leptin, NZO and Ay mice do not respond at the high doses tested. A similar study also concluded that DIO obesity is the result of leptin resistance, although in that report the animals did not respond at all to peripheral leptin (35). A third study has shown a reduction in food intake in DIO mice treated with peripheral leptin (10). The basis for these differences is unclear.
Leptin-induced weight loss in Ay mice requires a substantially higher i.c.v. dose of leptin relative to that required in lean and NZO mice. These data suggest that the leptin resistance in Ay mice is the result of a defect in the neural pathway activated by leptin. Recent studies suggest that obesity in these mice is the result of competitive inhibition of binding of α melanocyte-stimulating hormone (αMSH) to the MC-4 receptor by the agouti protein (36–39). The insensitivity of Ay mice to leptin strongly suggests that normal function of the MC-4 receptor is required for the response to exogenous leptin. The elucidation of the anatomic relationship between neurons expressing the signaling form of the leptin receptor, Ob-Rb, and those expressing proopiomelanocortin (the αMSH precursor) or the MC-4 receptor will be of great importance in further studies to establish leptin’s mechanism of action (6).
NZO mice respond to i.c.v. leptin with sensitivity similar to wild-type mice. This may suggest that transport of leptin into the CSF is diminished in NZO mice, a possibility that can now be directly tested. Normal responsiveness to i.c.v. leptin has also been reported in DIO mice, and decreased transport of leptin into the CSF has also been suggested in the pathogenesis of this form of obesity (35). A saturable transport system across the blood–brain barrier for leptin has been identified, and decreased levels of CSF leptin are seen in some obese patients (34, 40). The basis for the resistance of NZO and DIO mice to peripheral leptin could have important implications for understanding the pathogenesis of human obesity.
Acknowledgments
We thank Susan Korres for preparing the manuscript and Amgen for kindly providing recombinant mouse and human leptin. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK49853 (C.B.), the Robert J. Jr. and Helen C. Kieberg Foundation (J.B.W.), and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK41096 (J.M.F.).
ABBREVIATIONS
- i.c.v.
intracerebroventricular(ly)
- CSF
cerebrospinal fluid
- aCSF
artificial CSF
- DIO
diet-induced obese AKR/J
- NZO
New Zealand Obese
References
- 1.Adolph E F. Am J Physiol. 1947;151:110–125. doi: 10.1152/ajplegacy.1947.151.1.110. [DOI] [PubMed] [Google Scholar]
- 2.Hervey G R. Nature (London) 1969;222:629–631. doi: 10.1038/222629a0. [DOI] [PubMed] [Google Scholar]
- 3.Leibel R L, Rosenbaum M, Hirsch J. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 5.Tartaglia L A, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards G J, Campfield L A, Clark F T, Deeds J, Muir C, Sanker S, Moriarty A, Moore K, Smutko J S, Mays G G, Woolf E A, Monroe C A, Tepper R I. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 6.Lee G, Proenca R, Montez J, Carroll K, Darvishzadeh J, Lee J, Friedman J. Nature (London) 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 7.Maffei M, Halaas J, Ravussin E, Pratley R E, Lee G H, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern P A, Friedman J M. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 8.Halaas J L, Gajiwala K, Maffei M, Cohen S, Chait B T, Rabinowitz D, Lallone R, Burley S, Friedman J M. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 9.Pelleymounter M A, Cullen M J, Baker M B, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 10.Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 11.Ahima R S, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier J S. Nature (London) 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 12.Flier J S, Elmquist J K. Nat Biotechnol. 1997;15:20–21. doi: 10.1038/nbt0197-20. [DOI] [PubMed] [Google Scholar]
- 13.Spiegelman B, Flier J. Cell. 1996;87:377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 14.Maffei M, Fei H, Lee G H, Dani C, Leroy P, Zhang Y, Proenca R, Negrel R, Ailhaud G, Friedman J M. Proc Natl Acad Sci USA. 1995;92:6957–6960. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods A, Stock M. Nature (London) 1996;381:745. doi: 10.1038/381745a0. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz M, Baskin D, Bukowski T, Kuijper J, Foster D, Lasser G, Prunkard D, Porte D, Woods S, Seeley R, Weigle D. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 17.Levin N, Nelson C, Gurney A, Vandlen R, Sauvage F D. Proc Natl Acad Sci USA. 1996;93:1726–1730. doi: 10.1073/pnas.93.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaisse C, Halaas J L, Horvath C, Darnell J E, Stoffel M, Friedman J M. Nat Genet. 1996;13:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 19.Considine R V, Sinha M K, Heiman M L, Kriauciunas A, Stephens T W, Nyce M R, Ohannesian J P, Marco C C, McKee L J, Bauer T L, Caro J F. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 20.Frederich R C, Hamann A, Anderson S, Lollmann B, Lowell B B, Flier J S. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 21.Denton D A, Blair-West J R, McBurnie M, Osborne P G, Tarjan E, Williams R M, Weisinger R S. Am J Physiol. 1990;259:R729–R735. doi: 10.1152/ajpregu.1990.259.4.R729. [DOI] [PubMed] [Google Scholar]
- 22.West D B, Waguespack J, York B, Goudey-Lefevre J, Price R A. Mamm Genome. 1994;5:546–552. doi: 10.1007/BF00354928. [DOI] [PubMed] [Google Scholar]
- 23.Bielschowsky M, Bielschowsky F. Proc Univ Otago Med Sch. 1953;31:29–31. [Google Scholar]
- 24.Bateson W. Proc Zool Soc London. 1903;2:71–99. [Google Scholar]
- 25.Danforth C. Proc Soc Exp Biol Med. 1926;24:69–71. [Google Scholar]
- 26.Brouwer E. In: Animal and Human Calorimetry. McLean J, Tobin G, editors. Cambridge, U.K.: Cambridge Univ. Press; 1987. pp. 302–305. [Google Scholar]
- 27.Stephens T W, Basinski M, Bristow P K, Bue-Valleskey J M, Burgett S G, Craft L, Hale J, Hoffmann J, Hsiung H M, Kriauciunas A, MacKellar W, Rosteck P R, Jr, Schoner B, Smith D, Tinsley F C, Zhang X-Y, Heiman M. Nature (London) 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 28.Miller M W, Duhl D M J, Brieling H, Cordes S P, Ollmann M M, Winkes B M, Barsh G S. Genes Dev. 1993;7:454–467. doi: 10.1101/gad.7.3.454. [DOI] [PubMed] [Google Scholar]
- 29.Grundy S M, Barnett J P. DM, Dis-Mon. 1990;36:645–696. [PubMed] [Google Scholar]
- 30.Collins S, Kuhn C M, Petro A E, Swick A G, Chrunyk B A, Surwit R S. Nature (London) 1996;380:677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 31.Zachow R, Magoffin D. Endocrinology. 1997;138:847–850. doi: 10.1210/endo.138.2.5035. [DOI] [PubMed] [Google Scholar]
- 32.Emilsson V, Liu Y-L, Cawthorne M A, Morton N M, Davenport M. Diabetes. 1997;46:313–316. doi: 10.2337/diab.46.2.313. [DOI] [PubMed] [Google Scholar]
- 33.Golden P, Maccagnan T, Pardridge W. J Clin Invest. 1997;99:14–18. doi: 10.1172/JCI119125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banks W A, Kastin A J, Huang W, Jaspan J B, Maness L M. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 35.Heek M V, Compton D S, France C F, Tedesco R P, Fawzi A B, Graziano M P, Sybertz E J, Strader C D, Davis H R. J Clin Invest. 1997;3:385–390. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huszar D, Lynch C A, Fairchild-Huntress V, Dunmore J H, Fang Q, Berkemeier L R, Gu W, Kesterson R, Boston B A, Cone R D, Smith F J, Campfield L A, Burn P, Lee F. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 37.Friedman J M. Nature (London) 1997;385:119–120. doi: 10.1038/385119a0. [DOI] [PubMed] [Google Scholar]
- 38.Fan W, Boston B A, Kesterson R A, Hruby V J, Cone R D. Nature (London) 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 39.Mountjoy K, Mortrud M, Low M, Simerly R, Cone R. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz M W, Peskind E, Raskind M, Boyko E J, Daniel Porte J. Nat Med. 1996;2:589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]