Abstract
Increased cardiovascular mortality occurs in diabetic patients with or without coronary artery disease and is attributed to the presence of diabetic cardiomyopathy. One potential mechanism is hyperglycemia that has been reported to activate protein kinase C (PKC), preferentially the β isoform, which has been associated with the development of micro- and macrovascular pathologies in diabetes mellitus. To establish that the activation of the PKCβ isoform can cause cardiac dysfunctions, we have established lines of transgenic mice with the specific overexpression of PKCβ2 isoform in the myocardium. These mice overexpressed the PKCβ2 isoform transgene by 2- to 10-fold as measured by mRNA, and proteins exhibited left ventricular hypertrophy, cardiac myocyte necrosis, multifocal fibrosis, and decreased left ventricular performance without vascular lesions. The severity of the phenotypes exhibited gene dose-dependence. Up-regulation of mRNAs for fetal type myosin heavy chain, atrial natriuretic factor, c-fos, transforming growth factor, and collagens was also observed. Moreover, treatment with a PKCβ-specific inhibitor resulted in functional and histological improvement. These findings have firmly established that the activation of the PKCβ2 isoform can cause specific cardiac cellular and functional changes leading to cardiomyopathy of diabetic or nondiabetic etiology.
Activation of protein kinase C (PKC) has been postulated to be an important intracellular signaling pathway for modulating cardiac myocyte development, inotropic functions and cellular growth (1). PKC is composed of a family of serine-threonine kinases (2) whose isoform-specific functional role have not been clearly identified in vivo. In pathological states PKC activation, in particular the PKCβ isoforms, has been reported in diseased hearts removed from cardiac transplant recipients (3). Preferential activations of the PKCβ2 isoform in the heart, aorta, and retina and of PKCβ isoform in the renal glomeruli have also been observed in diabetic animals (4, 5). We have reported recently that the oral administration of a PKCβ isoform selective inhibitor (LY333531) ameliorated some of the early retinal and renal dysfunctions in diabetic rats (5). To test directly the hypothesis that sustained activation of PKCβ isoform can cause cardiac disease that resemble the cardiomyopathy observed in diabetes or in nondiabetic states, we generated transgenic mice with cardiac specific overexpression of PKCβ2.
MATERIALS AND METHODS
Construction of PKC Transgene and the Generation of Transgenic Mice.
A 2.1-kb BamHI fragment of mouse PKCβ2 cDNA (pPKCβ2-UC18-Bam; provided by C. L. Ashendel) (6) was inserted into BamHI site of PBK–CMV vector (Stratagene). The vector was then linearized with SalI (blunted) and ligated with 3.3-kb EagI–SalI fragment (blunted) of the plasmid containing rat myosin heavy chain (αMHC) promotor (p-2936++; provided by G. I. Fishman) (7). The resultant recombinant plasmid pαMHC–PKCβ–PBK was digested with SacI and MluI to generate 6.1-kb linear fragment and micro-injected into pronuclei of fertilized FVB mouse eggs (8). The genomic DNA (15 μg) extracted from the tail was digested with EcoRI and resolved by 1.0% agarose gel and transferred onto nylon membrane (ICN). The membrane was hybridized with 3.0-kb EcoRI fragment of pαMHC–PKCβ–PBK plasmid labeled with [α-32P]dATP (NEN) to ascertain gene transfer.
PKC Activity Assay.
The hearts from wild-type and transgenic mice were frozen in liquid N2, crushed into frozen powder, and homogenized in ice-cold buffer A (20 mM Tris⋅HCl, pH 7.5/2 mM EDTA/0.5 mM EGTA/1 mM phenylmethlysulfonyl fluoride/1 mM DTT/0.3 M sucrose/25 μg/ml leupeptin) with Polytron for 20 sec and then homogenized with a Dounce homogenizer (60 strokes). The homogenates were centrifuged at 1000 × g for 10 min, and the supernatant was ultracentrifuged at 100,000 × g for 30 min at 4°C. The resulting supernatant was retained as the cytosolic fraction and the pellets were resuspended with buffer B (buffer A without sucrose) and solubilized with 1% Triton X-100. After rotating for 45 min at 4°C, soluble membrane fractions were obtained by ultracentrifugation at 100,000 × g for 30 min. Both cytosolic and particulate membrane fractions were passed through 0.3 ml DEAE Sephacel (Pharmacia) column equilibrated with buffer B, washed twice in 2 ml of buffer B, and partially purified PKC fractions were eluted with 0.2 ml of buffer B containing 200 mM NaCl. PKC activities were measured as reported (9), and defined as the Ca2+, phosphatidylserine and diacylglycerol (Avanti Polar Lipids) stimulated transfer of 32P from [γ-32P]ATP (NEN) into the PKC-specific substrate (Arg-Lys-Arg-Thr-Leu-Arg-Arg-Leu).
Immunoblot Analysis.
Proteins from each fraction (75 μg for cytosol, 15 μg for membrane) were resolved by 0.8% SDS/PAGE and transferred electrically to polyvinylidene fluoride membrane (Schleicher & Schuell). After blocking with PBS containing 0.1% Tween-20 (PBS-T) and 3% BSA at 4°C overnight, the filter was incubated with isoform (α, β2, δ, ɛ) specific anti-PKC antibodies (GIBCO/BRL) at 1.0 μg/ml in PBS-T containing 1% BSA for 1 h at room temperature. After washing with PBS-T, the filter was incubated with peroxidase labeled anti-rabbit Ig antibody (1:4,000; Amersham) for 1 h, and the signals were detected by enhanced chemiluminescent system (Amersham). Partially purified PKC fraction from rat brain (20 μg) was used as an endogenous positive control. Protein concentration was determined by the method of Bradford.
Tissue Sections.
Cardiac histology was examined in 3- and 11-week-old wild-type and PKCβ2 transgenic mice with or without PKCβ inhibitor treatment. Fixed hearts were bisected transversely at the midventricular level. The apical halves were embedded conventionally in paraffin, sectioned from the cut surface at ≈5 μm, and stained with hematoxylin and eosin.
Echocardiographic Measurements.
Mice were anesthetized with Avertin (250 mg/g, i.p.), the chest was shaved, and electrocardiographic leads were attached to each limb with needle electrodes. M-mode studies were performed within Interspec–ATL (Ambler, PA) apogee X200 ultrasonograph using a dynamically focused 9-MHz annular array transducer applied to an offset coupled gel on the left hemithorax. M-mode and Doppler studies were analyzed as described (10). The study was performed blinded to the identity of the experimental groups.
Treatment of Control and Transgenic Mice with PKCβ Inhibitor.
Three-week-old mice (control and transgenic mice Tg4) were fed with mouse chow containing placebo or the PKCβ isoform-specific inhibitor LY333531 (5) at 200 mg/kg chow for additional 8 weeks. Echocardiographic measurements were performed in all mice at 11 weeks of age. Each group contained six mice (male/female ratio = 1).
Northern Blot Analysis and Probes.
Total RNA was isolated from both ventricles by the guanidinium isothiocyanate/phenol/chloroform method (11). Northern blot analysis was performed on 15 μg total RNA after 1% agarose-formaldehyde gel electrophoresis and subsequent capillary transfer to nylon membrane (ICN) and ultraviolet cross-linking using UV Stratalinker (Stratagene). cDNA probes (20 ng) were labeled with [α-32P]dATP (NEN) using the multiprime labeling system (Amersham), and oligonucleotide probes (2.5 pmol) were labeled with [γ-32P]ATP by T4 polynucleotide kinase (New England Biolabs). Blots were prehybridized, hybridized using Rapid-hyb buffer (Amersham) containing 100 μg/ml of salmon testes DNA (Sigma) and 106 cpm/ml of radiolabeled probe, and washed in 0.5× (2.0× for oligonucleotide probe) sodium chloride-sodium citrate (SSC), 5% SDS at 65°C with six changes. A 2.1-kb BamHI fragment of PKCβ cDNA was used for detecting PKCβ2 mRNA. Oligonucleotide probes for mouse αMHC and βMHC corresponding to specific 3′ untranslated region were synthesized (αMHC, 5′-CGA ACG TTT ATG TTT ATT GTG GAT TGG CCA CAG CGA GGG TCT GCT GGA GAG G-3′; βMHC, 5′-GCT TTA TTC TGC TTC CAC CTA AAG GGC TGT TGC AAA GGC TCC AGG TCT GAG GGC TTC-3′). Oligonucleotide probe for c-fos was purchased from Calbiochem. EcoRI and HindIII fragment (0.8 kb) of rat atrial natriuretic factor (ANF) cDNA (provided by D. G. Gardner, University of California, San Francisco), EcoRI fragment (0.3 kb) of mouse transforming growth factor β (TGFβ1) cDNA (provided by N. H. Ziyadeh) (12) and EcoRI, SalI fragment (1.8 kb) of mouse α1 (IV) collagen cDNA (provided by M. Lorenzi) (13) and EcoRI fragment of mouse α1 (VI) collagen cDNA (provided by M. L. Chu, Jefferson Medical College, Philadelphia) were prepared for probes, respectively. RNA loading difference was normalized using PstI fragment of human acidic ribosomal phosphoprotein P0 cDNA (36B4) as a control probe (14). Analysis employed PhosphorImager and computing densitometer (Molecular Dynamics).
Statistical Analysis.
Results are presented as the mean ± SD. Data were analyzed with Student t test or Student–Newman–Kuels test.
RESULTS
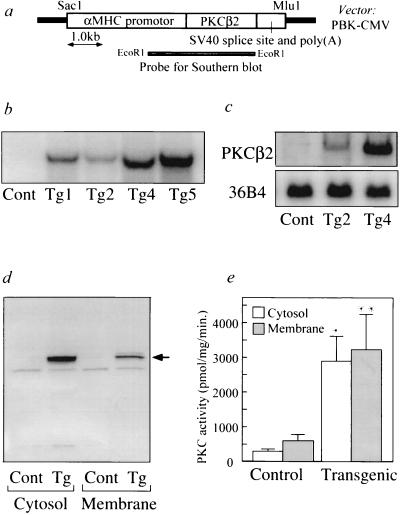
The transgene consisted of the adult cardiac myocyte-specific αMHC promotor (15) ligated to the entire coding sequence for the mouse PKCβ2 isoform (Fig. 1a). A SacI and MluI fragment of the recombinant plasmid was microinjected into pronuclei of fertilized FVB mice eggs and implanted into pseudopregnant FVB foster mothers (8). Success of gene transfer was identified by Southern blot analysis of genomic DNA extracted from mouse tail with αMHC–PKCβ2 transgene-specific probe (Fig. 1b). Five transgenic founder mice were obtained and four successfully transmitted the αMHC–PKCβ2 transgene to their progeny. The copy number of the αMHC–PKCβ2 transgene in each transgenic line were 3 copies in Tg1, 1 copy in Tg2, 8 copies in Tg4, and 12 copies in Tg5 (Fig. 1b). Heterozygous mice bearing the αMHC–PKCβ2 transgene were viable and reproduced normally; however mortality within 20 weeks of age was higher in transgenic mice [transgenic = 5.6% (4/70), control = 0% (0/87)]. The second generation of heterozygous transgenic mice and nontransgenic littermate control mice, 3–12 weeks of age, were used for all studies. Northern blot analysis using total RNA from the heart of control and transgenic mice revealed a single 3.6-kb transcript for all transgenic lines. Transgenic mice with high expression mouse line (Tg4) have 5 times more of the transcript than the low expression line (Tg2) (Fig. 1c). Endogenous mRNA of PKCβ2 isoform was detected in brain, spleen, lung, and kidney but not detected in the liver, muscle, and heart by Northern blot analysis using total RNA (15 μg) (data not shown). Immunoblot analysis performed with specific antibodies showed that the protein levels of PKCβ2 isoform in the subfractionated heart lysate was increased by 20- and 10-fold in the cytosol and membrane fractions, respectively, in the Tg4 transgenic mice compared with the wild type (Fig. 1d). There was more PKCβ2 isoform protein in the cytosolic fraction than in the membrane. Expression of the other isoforms of PKC as detected by immunoblot analysis showed PKCα isoform to be elevated by 2-fold only in cytosolic but not in the membrane fraction from heart of transgenic mice line (Tg4), where no changes were observed in δ and ɛ isoforms (data not shown). Total PKC activity was found to be increased in transgenic mouse (Tg4) by 9-fold in cytosolic fractions and 5-fold in membrane fractions of cardiac lysates (Fig. 1e).
Figure 1.
(a) Schematic representation of the PKCβ2 isoform transgene. The αMHC promotor includes 5′ regulatory element from rat αMHC gene and heterologous splice cassette. Simian virus 40 (SV40) sequence contains 3′ splicing signal and polyadenylation signal. Restriction enzymes used for generating fragments were as indicated. (b) Southern blot analysis of DNA from heterozygous transgenic mice (Tg1, Tg2, Tg4, and Tg5) and wild-type control (Cont). EcoRI digested mouse tail genomic DNA (15 μg) was hybridized with αMHC–PKCβ2 transgene-specific probe. Size of the positive band is 3.1kb. (c) Northern blot analysis of total RNA (15 μg) isolated from the heart of control (Cont), and transgenic mice (Tg2 and Tg4) hybridized with BamHI fragment of PKCβ2 cDNA. RNA loading differences were normalized using a control cDNA probe (36B4). (d) Immunoblot analysis of cytosolic and membrane fractions isolated from heart of control (Cont) and transgenic mice Tg4 (Tg) using antibodies to PKCβ2 isoform. Arrow indicates the position of endogenous PKCβ2 isoform corresponding to 80 kDa as estimated by prestained protein molecular weight marker (Bio-Rad). (e) PKC activity measurements in cytosolic and membrane fractions from hearts of control (Cont) and transgenic mice Tg4 (Transgenic). Results are the average of four separate experiments. ∗, P < 0.001; ∗∗, P < 0.005 versus nontransgenic control (Student t test).
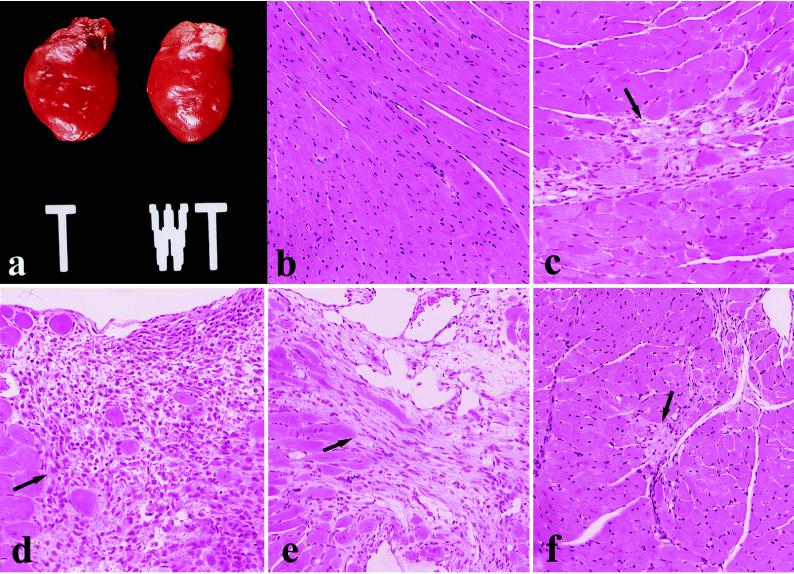
Hearts from 3-week-old transgenic mice line Tg4 had significantly increased heart weight (mg)/body weight (gm) ratios (Tg4 = 5.5 ± 0.6, control = 4.2 ± 0.2). At 11 weeks of age, hearts from transgenic mice Tg4 and Tg5 continued to exhibit increased heart/body weight ratios by 20% and 34%, respectively, as compared with age matched littermates (control = 4.4 ± 0.3, Tg4 = 5.3 ± 1.6 Tg5 = 5.9 ± 0.4, mean ± SD). Histology of the hearts of wild-type and transgenic mice (Tg4) at 3 and 11 weeks of age were examined. Wild-type mice all had normal cardiac configuration and myocardial histology, without hypertrophy, inflammation, necrosis, fibrosis, or calcification (Fig. 2 a and b). In contrast, transgenic mice overexpressing PKCβ2 (Tg4) at 11 weeks of age had cardiomegaly with global chamber dilatation (Fig. 2a), normal microscopic well-organized myocardial architecture without myofiber disarray, and diffuse myocyte hypertrophy with multifocal myocardial necrosis in various stages of healing with a mononuclear inflammatory infiltrate (Fig. 2 c–e). Lesions involved both right and left ventricular myocardium in subendocardial, mid-wall, and epicardial locations and were occasionally transmural, resulting in the thinning of the myocardial wall. These changes varied in location and severity among animals. Dystrophic calcifications were often noted in epipericardial lesions. Ongoing damage with inflammatory myocyte destruction was absent (i.e., there was no myocarditis) and areas of early necrosis without healing were not noted. Focal lesions were noted as early as 3 weeks of age. Vascular lesions were not identified in any cardiac sections of transgenic mice.
Figure 2.
Cardiac pathology in transgenic mice overexpressing PKCβ2 isoform at 11 weeks of age. (a) Gross photograph demonstrating global enlargement of typical transgenic mouse heart (T) compared with non transgenic littermate (WT). (b) Normal cardiac histology from nontransgenic control mouse. (c–e) Photomicrographs of myocardium in PKCβ2 transgenic mouse. (arrows). Microfocal evolving replacement fibrosis, indicative of healing myocyte necrosis (c). Larger epicardial focus of healing with mixed mononuclear inflammatory infiltrate and sharp borders (d). Transmural healing with wall thinning (e). (f) Representative histology of the heart in transgenic mouse treated with PKCβ inhibitor showing marked reduction in size and activity of myocardial lesions (arrow). All panels were stained with hematoxylin and eosin. (b, e, and f, ×120; c and d, ×150.)
To assess the functional consequences of PKCβ2 overexpression, M-mode echocardiography was performed (10) in a blinded fashion on 11-week-old transgenic (Tg4) and control mice. The result demonstrated that transgenic mice had thickening of interventricular septal wall, left ventricular posterior wall, and an increase of left ventricular mass (control = 30.4 ± 4.3 mg, Tg4 = 40.5 ± 9.9 mg, mean ± SD) indicating left ventricular hypertrophy (Table 1). Fractional shortening was decreased from 48% in control to 34% in transgenic mice due to impaired ventricular systolic performance. In these transgenic mice (Tg4) left ventricular end diastolic dimension was decreased by 15%, which coupled with increased wall thickness suggested the presence of reduced left ventricular chamber compliance. There was no significant difference in the systemic arterial blood pressure by tail cuff method between PKC transgenic mice (Tg4) and non transgenic control mice (systolic blood pressure; control = 100 ± 21 mmHg, transgenic = 89 ± 17 mmHg, mean ± SD).
Table 1.
Echocardiographic measurements in transgenic mice and non transgenic littermates with or without treatment of PKCβ inhibitor (LY333531)
| Nontreated
|
Treated with PKCβ inhibitor
|
|||
|---|---|---|---|---|
| Control (n = 6) | Transgenic (n = 6) | Control (n = 6) | Transgenic (n = 6) | |
| LVEDD, mm | 3.41 ± 0.29 | 2.91 ± 0.36* | 3.30 ± 0.26 | 3.36 ± 0.26 |
| LVESD, mm | 1.76 ± 0.24 | 1.93 ± 0.34 | 1.77 ± 0.16 | 2.01 ± 0.17 |
| FS, % | 48.4 ± 2.6 | 33.8 ± 7.5* | 46.3 ± 4.3 | 40.0 ± 2.3** |
| IVS, mm | 0.42 ± 0.03 | 0.61 ± 0.03* | 0.42 ± 0.03 | 0.39 ± 0.03 |
| PW, mm | 0.39 ± 0.02 | 0.64 ± 0.05* | 0.38 ± 0.05 | 0.38 ± 0.04 |
| LV mass, mg | 30.4 ± 4.3 | 40.5 ± 9.9* | 28.8 ± 6.3 | 27.8 ± 5.3 |
| Body weight, g | 26.2 ± 3.3 | 25.4 ± 3.1 | 23.0 ± 2.2 | 23.7 ± 2.5 |
| LV mass/body weight | 1.17 ± 0.15 | 1.61 ± 0.38* | 1.25 ± 0.26 | 1.18 ± 0.23 |
| Heart rate, b.p.m. | 405 ± 54 | 363 ± 34 | 434 ± 44 | 372 ± 49 |
LVEDD, left ventricular end diastolic dimension; LVESD, left ventricular end systolic dimension; IVS, interventricular septal wall thickness; PW left ventricular posterior wall thickness; FS, left ventricular fractional shortening = (LVEDD-LVESD)/LVEDD × 100.
*P < 0.05 vs. nontreated control.
**P < 0.05 vs. treated control (ANOVA with Student–Newman–Keuls test).
To determine whether and to what extent the abnormalities observed in cardiac function and histology of the transgenic mice were due to the activation of PKCβ isoform, the effect of a PKCβ isoform selective inhibitor LY333531 (5) was studied. Transgenic mice (Tg4) and nontransgenic littermates (control) at age of 3 weeks were randomly assigned to standard mouse chow or chow mixed with LY333531 at 200 mg/kg of chow for 8 weeks. Echocardiography and histological studies were performed on the hearts of control mice and transgenic mice with or without LY333531 treatment. Cardiac hypertrophy measured by left ventricular mass was normalized from 40.5 ± 9.9 mg to 27.8 ± 5.3 mg and fractional shortening substantially improved from 34% to 40% with PKCβ isoform inhibitor treatment (Table 1). Histological examination of transgenic mice treated by LY333531 showed markedly fewer and less active lesions, with reduction in the degree of left ventricular hypertrophy compared with nontreated transgenic mice (Fig. 2f). These results suggested that the morphological abnormalities in PKCβ isoform transgenic mice when inhibited soon after birth, can be largely prevented or reversed by inhibition of PKCβ2 isoform activity.
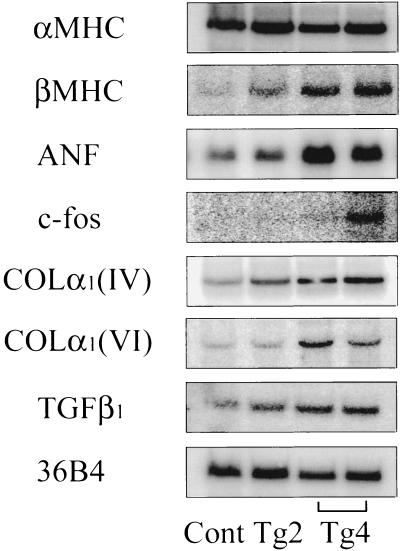
One of the early cardiac findings in transgenic mice overexpressing PKCβ2 isoform was cardiac myocyte hypertrophy, which has reported to express increased level of fetal pattern of genes coding for cardiac contractile proteins and a program of immediate-early genes (c-fos, Egr-1) (16, 17). Thus, the expression of α- and βMHC, ANF, and c-fos was quantitated by Northern blot analysis (Fig. 3). The expression of βMHC was increased 2-fold in Tg2 mice and 10-fold in Tg4 mice, whereas expression of αMHC did not alter between transgenic and control animals. The mRNA expressions of ANF was increased by 1.5-fold in Tg2 mice and 3-fold in Tg4 mice, and c-fos mRNA expression was increased 3- to 6-fold in Tg4 mice. Interestingly, c-fos protein expression detected in the hearts of nonobese diabetic (NOD) mice versus nondiabetic littermates also exhibited a 2- to 3-fold increase (data not shown).
Figure 3.
Expression of mRNAs for MHC isoforms (αMHC and βMHC), ANF, c-fos, collagen types IV and VI [COLα1 (IV, VI)], and TGFβ1 in PKCβ2 transgenic mice. Northern blot analysis of total heart RNA (15 μg) from control (Cont), transgenic mice (Tg2 and Tg4) at 8–12 weeks of age was performed. RNA loading differences were normalized using a control cDNA probe (36B4) (14).
The hypertrophied myocardium of diabetic and nondiabetic animals contains an excessive amount of extracellular matrix, including collagens as well as TGFβ1, which has been shown to stimulate extracellular matrix production in the vascular bed (18–21). The hearts of transgenic mice Tg2 and Tg4 showed increased mRNA expression of collagen α1(IV), collagen α1(VI), and TGFβ1 by 2- to 4-fold compared with control in accordance with the amount of PKC transgene incorporated and expressed (Fig. 3).
DISCUSSION
The functional role of PKC isoform activation and their specific actions have been postulated from cellular transfection studies and the discovery of an isoform-specific inhibitor to the PKCβ isoform (5, 22, 23). The present study on the overexpression of PKCβ isoform in the myocardium represents a successful in vivo overexpression of a PKC isoform in the cardiovascular system. The rationale for selecting PKCβ isoform was due to data suggesting that many of the microvascular and cardiovascular abnormalities observed in diabetic patients could be the result of the activation of PKCβ isoforms in the vasculature (4, 5, 24). In addition, a recent report has suggested that PKCβ isoform activation may be associated with end stage heart failure in general (3). The results of this study clearly demonstrated that excessive postnatal expression and activity of the PKCβ2 isoform in myocardium will induce severe structural and functional abnormalities of myocardium without changes in other organs or micro- and macrovasculatures.
Histological findings demonstrated that myocardial necrosis, healing, and dystrophic calcification were present as early as 3 weeks after birth, suggesting that the activation of PKCβ2 isoform has important regulatory effects on the growth, death, and calcium deposition in cardiac myocytes. The later findings of vastly thickened left and right ventricular walls were due to increases in the size and number of cardiomyocytes and interstitial extracellular matrix, which could be a direct effect of PKCβ isoform activation in the myocardium because hypertrophy was observed without evidence of hemodynamic overload. Echocardiographic functional studies supported the gross and histological morphology by showing massive thickened left and right ventricular walls as well as decreases in fractional shortening of the ventricles, suggesting load-independent depression of ventricular function. The findings of gross chamber dilatation, multifocal fibrosis, and areas of myocyte hypertrophy could be partly due to a compensatory response to the injured myocardium. The pathogenic role of PKCβ2 isoform was clearly demonstrated by the results of oral administration of a PKCβ isoform selective inhibitor (LY333531) because most of the histological and functional abnormalities in the PKCβ2 isoform transgenic mice were prevented or greatly ameliorated at a dose that we had previously reported to inhibit PKCβ isoform activity selectively (5). The data from the use of LY333531 are important since PKCα isoform protein was also increased in the heart of transgenic mice, probably secondary to PKCβ isoform activation or cardiac hypertrophy.
The analysis of the expression of various genes in the myocardium of the transgenic mouse provided evidence that the activation of PKCβ2 isoform has fundamentally changed the expression of various genes in the cardiac myocyte with increased expression of c-fos and βMHC and cytokines such as ANF and TGFβ1. This spectrum of changes in gene expression is similar to that found in fetal development and in heart failure induced by various causes such as infarction (16, 17, 25), again suggesting that activation of PKCβ2 isoform have fundamental effects on the growth and functions of the myocardium. Increases in the expression of extracellular matrix proteins such as types IV and VI collagen were most likely related to the increases in TGFβ1 level, which has been reported to be up regulated at transcription step by PKC activation (21).
PKCβ isoform activation appears to be associated with the cardiovascular complications of diabetes (4, 5, 24, 26). The present transgenic mouse model of targeted overexpression of PKCβ2 in the myocardium exhibited both similarity and dissimilarity to the pathology of diabetic cardiomyopathy. Similarities at the molecular and biochemical level included increased expression of c-fos, TGFβ1, and types IV and VI collagens as reported in various diabetic models (13, 14, 18, 19, 27). Findings from echocardiographic and pathological studies demonstrated decreased systolic and diastolic functions. Myocardial hypertrophy and fibrosis are also common to diabetic cardiomyopathy (28–31). There are some differences, however, such as myocardial necrosis and dystrophic calcification, which are generally not observed in diabetic cardiomyopathy. It is possible that these differences could be due to the fact that PKCβ2 isoform activation in the transgenic mouse was increased by 5- to 10-fold, whereas in diabetes PKCβ activities were only increased by 2- to 3-fold (4, 5, 24).
In summary, the successful overexpression of the PKCβ2 isoform in the myocardium of the transgenic mouse has clearly established that isoform-specific activation of PKC can cause alterations in the gene expression of the myocardium which will lead to severe abnormalities in cardiac myocyte growth, myocyte death, dystrophic calcification, hypertrophy, fibrosis, and hemodynamic dysfunction then resulted in increased mortality. The prevention of myocardial lesions and reversibility of dysfunction induced by specific PKCβ isoform inhibitor firmly established that these molecular, functional, and pathological changes were due to PKCβ isoform activation. These results provided the first direct evidence that excessive activation of PKC is involved in some of the structural and functional changes observed in diabetic cardiomyopathy and in other forms of heart failure of diverse etiology.
Acknowledgments
We thank M. Lipes and E. V. Boschetti at Transgenic Core, Joslin Diabetes Center, for generating transgenic mice. We also thank Joan C. Taylor for secretarial contribution. This study was supported by grants provided by the National Eye Institute, Specialized Center of Research in Heart Failure (HL52318), and the Diabetes Endocrinology Research Center (National Institute of Diabetes, Digestive, and Kidney Diseases) of National Institutes of Health.
ABBREVIATIONS
- PKC
protein kinase C
- MHC
myosin heavy chain
- ANF
atrial natriuretic factor
- TGF
transforming growth factor
- Tg
transgenic
References
- 1.Pucéat M, Brown J H. In: Protein Kinase C. Kuo J F, editor. Oxford: Oxford Univ. Press; 1994. pp. 249–268. [Google Scholar]
- 2.Nishizuka Y. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 3.Strasser R H, Briem S K, Vahl C F, Lang R, Hagl S, Kübler W. Circulation. 1996;94:I551. (abstr.). [Google Scholar]
- 4.Inoguchi T, Battan R, Handler E, Sportsman J R, Heath W, King G L. Proc Natl Acad Sci USA. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishii H, Jirousek M R, Koya D, Takagi T, Xia P, Clermont A, Bursell S E, Kern T S, Ballas L M, Heath W F, Stramm L E, Feener E P, King G L. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 6.Tang Y M, Ashendel C L. Nucleic Acids Res. 1990;18:5310. doi: 10.1093/nar/18.17.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fishman G I, Kaplan M L, Buttrick P M. J Clin Invest. 1994;93:1864–1868. doi: 10.1172/JCI117174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipes M A, Rosenzweig A, Tan K N, Tanigawa G, Ladd D, Seidman J G, Eisenbarth G S. Science. 1993;259:1165–1169. doi: 10.1126/science.8267690. [DOI] [PubMed] [Google Scholar]
- 9.Oliver F J, de la Rubia G, Feener E P, Lee M E, Loeken M R, Shiba T, Quertermous T, King G L. J Biol Chem. 1991;266:23251–23256. [PubMed] [Google Scholar]
- 10.Hoit B D, Khoury S F, Kranias E G, Ball N, Walsh R A. Circ Res. 1995;77:632–637. doi: 10.1161/01.res.77.3.632. [DOI] [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Sharma K, Ziyadeh F N. Am J Physiol. 1994;267:F1094–F1101. doi: 10.1152/ajprenal.1994.267.6.F1094. [DOI] [PubMed] [Google Scholar]
- 13.Cagliero E, Roth T, Roy S, Maiello M, Lorenzi M. J Biol Chem. 1991;266:14244–14250. [PubMed] [Google Scholar]
- 14.Liang P, Pardee A B. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 15.Subramaniam A, Jones W K, Gulick J, Wert S, Neumann J, Robbins J. J Biol Chem. 1991;266:24613–24620. [PubMed] [Google Scholar]
- 16.Chien K, Knowlton K U, Zhu H, Chien S. FASEB J. 1991;5:3037–3046. doi: 10.1096/fasebj.5.15.1835945. [DOI] [PubMed] [Google Scholar]
- 17.Izumo S, Nadal-Ginard B, Mahdavi V. Proc Natl Acad Sci USA. 1988;85:339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katayama S, Abe M, Negishi K, Takahashi K, Ishii J, Komeda K. Diabetes Res Clin Prac. 1994;26:163–169. doi: 10.1016/0168-8227(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 19.Spiro M J, He Q, D’Autilia M L. Diabetologia. 1995;38:430–436. doi: 10.1007/BF00410280. [DOI] [PubMed] [Google Scholar]
- 20.Villarreal F J, Dillmann W H. Am J Physiol. 1992;262:H1861–H1866. doi: 10.1152/ajpheart.1992.262.6.H1861. [DOI] [PubMed] [Google Scholar]
- 21.Massagué J. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 22.Clerk A, Bogoyevitch M A, Andersson M B, Sugden P H. J Biol Chem. 1994;269:32848–32857. [PubMed] [Google Scholar]
- 23.Watanabe T, Ono Y, Taniyama Y, Hazama K, Igarashi K, Ogita K, Kikkawa U, Nishizuka Y. Proc Natl Acad Sci USA. 1992;89:10159–10163. doi: 10.1073/pnas.89.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiba T, Inoguchi T, Sportsman R, Heath W F, Bursell S, King G L. Am J Physiol. 1993;265:E783–E793. doi: 10.1152/ajpendo.1993.265.5.E783. [DOI] [PubMed] [Google Scholar]
- 25.Pfeffer J M, Fischer T A, Pfeffer M A. Annu Rev Physiol. 1995;57:805–826. doi: 10.1146/annurev.ph.57.030195.004105. [DOI] [PubMed] [Google Scholar]
- 26.Craven P A, DeRubertis F R. J Clin Invest. 1989;83:1667–1675. doi: 10.1172/JCI114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreisberg J I, Radnik R A, Ayo S H, Garoni J, Saikumar P. Kidney Int. 1994;46:105–112. doi: 10.1038/ki.1994.249. [DOI] [PubMed] [Google Scholar]
- 28.Friedman N E, Levitsky L L, Edidin D V, Vitullo D A, Lacina S J, Chiemmongkoltip P. Am J Med. 1982;46:846–850. doi: 10.1016/0002-9343(82)90775-6. [DOI] [PubMed] [Google Scholar]
- 29.van Hoeven K H, Factor S M. Circulation. 1990;82:848–855. doi: 10.1161/01.cir.82.3.848. [DOI] [PubMed] [Google Scholar]
- 30.Regan T J, Lyons M M, Ahmed S S, Levinson G E, Oldewurtel H A, Ahmad M R, Haider B. J Clin Invest. 1977;60:885–899. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genda A, Mizuno S, Nunoda S, Nakayama A, Igarashi Y, Sugihara N, Namura M, Takeda R, Bunko H, Hisada K. Clin Cardiol. 1986;9:375–382. doi: 10.1002/clc.4960090804. [DOI] [PubMed] [Google Scholar]