Abstract
Proteinase-activated receptor 2 (PAR-2) is a recently characterized G-protein coupled receptor that is cleaved and activated by pancreatic trypsin. Trypsin is usually considered a digestive enzyme in the intestinal lumen. We examined the hypothesis that trypsin, at concentrations normally present in the lumen of the small intestine, is also a signaling molecule that specifically regulates enterocytes by activating PAR-2. PAR-2 mRNA was highly expressed in the mucosa of the small intestine and in an enterocyte cell line. Immunoreactive PAR-2 was detected at the apical membrane of enterocytes, where it could be cleaved by luminal trypsin. Physiological concentrations of pancreatic trypsin and a peptide corresponding to the tethered ligand of PAR-2, which is exposed by trypsin cleavage, stimulated generation of inositol 1,4,5-trisphosphate, arachidonic acid release, and secretion of prostaglandin E2 and F1α from enterocytes and a transfected cell line. Application of trypsin to the apical membrane of enterocytes and to the mucosal surface of everted sacs of jejunum also stimulated prostaglandin E2 secretion. Thus, luminal trypsin activates PAR-2 at the apical membrane of enterocytes to stimulate secretion of eicosanoids, which regulate multiple cell types in a paracrine and autocrine manner. We conclude that trypsin is a signaling molecule that specifically regulates enterocytes by triggering PAR-2.
Proteases in the intestinal lumen are traditionally considered digestive enzymes. Trypsin is one of the most important digestive proteases. Trypsinogen is a major component of pancreatic juice and, once activated in the intestinal lumen by enterokinase, trypsin digests dietary proteins and activates other zymogens. However, recent observations suggest that trypsin is a also a signaling molecule that specifically regulates cells by cleaving and triggering proteinase-activated receptor 2 (PAR-2) (1, 2).
The PARs are a new family of G-protein-coupled receptors for serine proteases. Thrombin activates PAR-1 and PAR-3 (3, 4), and trypsin and mast cell tryptase activate PAR-2 (1, 5). Proteases cleave within the extracellular N terminus of PARs, exposing tethered ligand domains that bind and activate the cleaved receptors. Synthetic peptides corresponding to the tethered ligands of PAR-1 and PAR-2 directly bind and activate the receptors and are useful reagents for investigating receptor functions without the use of proteases, which may have nonspecific effects (1, 3).
PAR-1 and PAR-3 are expressed by platelets, fibroblasts, and endothelial cells, and mediate many of the effects of thrombin (3, 4, 6). PAR-2 is expressed in the gastrointestinal tract, pancreas, kidney, liver, airway, ovary, and eye (1, 2) and is found in epithelial and endothelial cell lines, smooth muscle, T cell lines, and tumor cell lines (2, 7–10). The proteases that activate PAR-2 in these locations and the physiological function of PAR-2 are unknown. Trypsin in the intestinal lumen could activate PAR-2 if the receptor is expressed at the apical membrane of enterocytes. This may stimulate the secretion of eicosanoids, since trypsin induces prostaglandin (PG) secretion from hepatocytes (11). Furthermore, PAR-1 activates cytoplasmic phospholipase A2 (cPLA2) and stimulates arachidonic acid release and PG secretion (12–15). Eicosanoids act locally in the intestinal wall to regulate epithelial transport, growth, and blood flow, and to mediate inflammation and cytoprotection (16). Thus, luminal trypsin may influence multiple processes in the intestine through activation of PAR-2 and secretion of eicosanoids.
Trypsin at concentrations of <10 nM activates PAR-2 in transfected cells (2), yet the concentration of trypsin in pancreatic juice and in the intestinal lumen of the rat is 1–40 μM (17, 18). We hypothesized that trypsin, at concentrations normally present in the intestinal lumen, regulates enterocytes by activating PAR-2. The aims were to (i) localize PAR-2 in intestinal epithelium, (ii) determine if physiological concentrations of trypsin activate PAR-2 on enterocytes, and (iii) examine if this activation stimulates arachidonic acid release and PG secretion.
METHODS
Cell Lines.
Generation of a transfected sarcoma virus transformed rat kidney epithelial (KNRK)–PAR-2 cell line has been described (2, 19). hBRIE 380 cells, a polarized, differentiated enterocyte cell line from rat small intestine, were from G. Aponte (University of California, Berkeley) (20). Cells were passed with No-Zyme (GIBCO) to avoid trypsin and plated 24–48 h before use. KNRK cells were plated on poly-d-lysine coated glass coverslips for microscopy or plastic plates for assays of inositol 1,4,5-trisphosphate (InsP3) generation, arachidonic acid release, and PG secretion. hBRIE cells were plated on plastic plates for these assays or on 3-μm pore Transwell filters (PTFE membrane; Corning Costar) for microscopy and to permit selective application of agonists to the apical or basolateral membranes. Plates and filters were coated with 1.5–2% Matrigel or collagen for polarized growth of hBRIE cells (20). Cells grown on filters formed a confluent layer of one and occasionally two to three cells thick. hBRIE cells on filters formed tight functions, as determined by immunofluorescence using an antibody to the tight junction protein ZO1. Cells were assayed in serum-free medium containing 0.1% BSA at 37°C.
Northern Blot Analysis.
Total RNA was extracted from rat intestinal mucosa (scraped from submucosa) and cell lines, separated on a 1.0% formaldehyde-agarose gel, transferred to nylon filters, and crosslinked. A 320-bp probe to transmembrane domain II and extracellular loop II of human PAR-2, and a 230-bp probe to the N terminus of rat PAR-2 were generated by PCR (2, 9). Probes were labeled with [α-32P]dCTP and hybridized with filters (2). Filters were also hybridized with a cDNA probe to β-actin. The intensity of hybridization was measured using a PhosphorImager (Molecular Dynamics).
Immunofluorescence.
A polyclonal antiserum (B5) was raised in rabbits to a peptide corresponding to rat PAR-2 (30GPNSKGR↓SLIGRLDT46P-YGGC) coupled to keyhole limpet hemocyanin (↓ trypsin cleavage site, YGGC for conjugation). An affinity-purified rabbit polyclonal antiserum (PAR-2C) to human PAR-2 (↓37SLIGKVDGTSHVTGKG53V) was from C. Derian (R. W. Johnson Pharmaceutical Research Institute, Spring House, PA). The molecular weight of PAR-2 is unknown, but human PAR-2 has a calculated mass of ≈44 kDa and one potential glycosylation site. Antisera recognized major proteins of ≈44 and ≈73 kDa on Western blots of KNRK–PAR-2 cells, and signals were suppressed by preabsorption with PAR-2 fragments (data not shown). Cells were fixed in 4% paraformaldehyde for 20 min at 4°C (21). Rats fasted for 16 h were fixed by transcardial perfusion with paraformaldehyde (21). PAR-2 was localized in 10-μm frozen sections of small intestine, 5-μm paraffin sections of hBRIE cells on filters, and KNRK–PAR-2 cells on coverslips by indirect immunofluorescence and confocal microscopy (21).
InsP3 Generation.
InsP3 was measured using a competitive binding assay (22). hBRIE cells were preincubated with 10 mM LiCl for 10 min to prevent metabolism of InsP3. Results were calculated as pmols InsP3/106 cells.
Arachidonic Acid Release.
Cells were incubated in medium containing 10% serum and 0.5 μCi/ml [3H]arachidonic acid (180 Ci/mmol, DuPont/New England Nuclear; 1 Ci = 37 GBq) for 6 h for KNRK–PAR-2 cells (57% incorporation of label) and 12 h for hBRIE cells (55% incorporation). Cells were incubated with agonists, and the medium was aspirated, centrifuged, and counted.
PG Secretion.
PGE2 and PGF1α secretion from cells was measured by RIA (DuPont/New England Nuclear). Results were calculated as pg of PG secreted per 106 cells. PG secretion was measured from everted sacs of jejunum. Rats were fasted for 16 h and anesthetized with ketamine and xylazine (87 and 13 mg/kg, i.m., respectively). To remove endogenous trypsin from the intestine, a catheter was placed into the bile-pancreatic duct to divert secretion, and the duodenum and jejunum were perfused with NaCl containing 2 mg/ml soybean trypsin inhibitor (SBTI) at 6 ml/min for 2 h. Everted sacs (2 cm) of jejunum were prepared, ligated, washed, and incubated in 2 ml oxygenated Krebs buffer at 37°C. Tissue was incubated in buffer for 10 min, and agonists, or carrier (control) were added to the mucosal solution for 30 min. Basal PGE2 secretion after 10 min and stimulated secretion after 30 min were measured in the mucosal solution. Results were calculated as PGE2 secreted per gram of tissue.
Statistical Analyses.
Observations on cell lines were made in duplicate from n > 3 experiments. Observations on rats were made on n > 3 animals. Results are expressed as mean ± SE. Differences were examined by ANOVA and Student–Newman–Keuls test.
RESULTS
Expression of PAR-2 by Epithelial Cells.
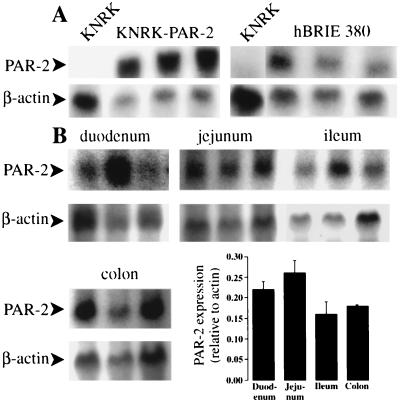
PAR-2 was expressed at very low levels by untransfected KNRK cells but was highly expressed by KNRK–PAR-2 and hBRIE cells (Fig. 1A). PAR-2 was highly expressed at similar levels in duodenal, jejunal, ileal, and colonic mucosa (Fig. 1B). A transcript of ≈3 kb, of similar size to that found in mouse and human (1, 2), was detected in cells and tissues.
Figure 1.
Detection of PAR-2 and β-actin mRNA by Northern blot analysis. Blots (30 μg RNA/lane) were hybridized with probes to human PAR-2 (KNRK–PAR-2 cells) or rat PAR-2 (untransfected KNRK cells, hBRIE cells, rat tissues). (A) Cell lines. (B) Intestinal mucosa; each lane from a different rat. The bar graph shows the ratio of the intensity of hybridization of PAR-2 and β-actin probes, determined using a PhosphorImager (mean ± SE, n = 3 rats).
Localization of PAR-2 in Epithelial Cells.
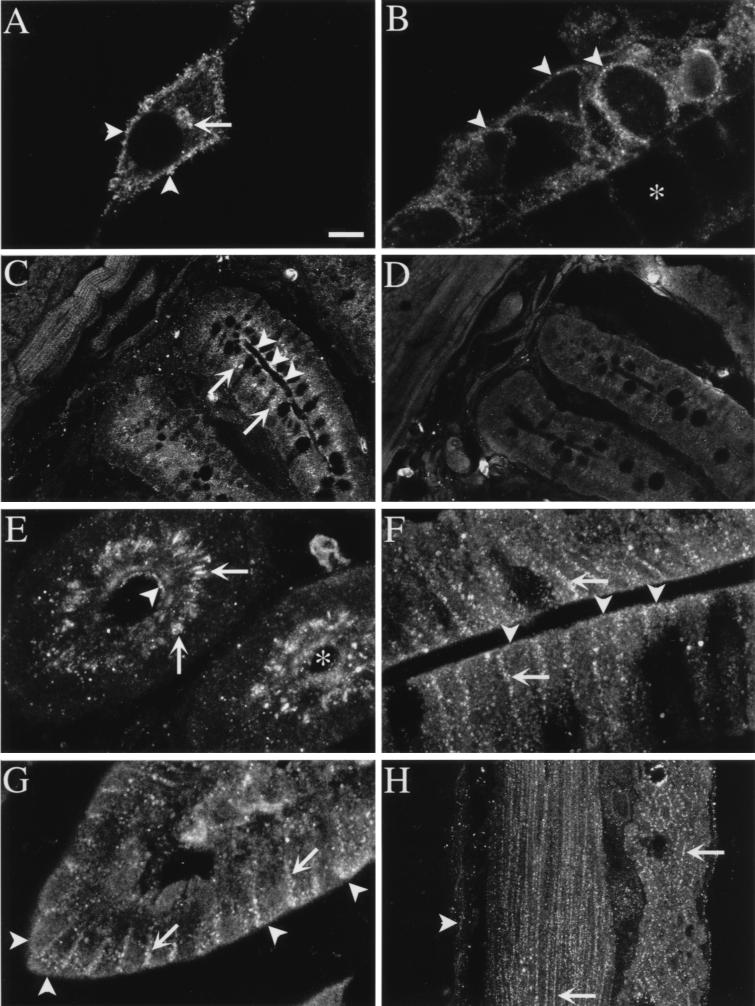
Antisera to two different epitopes gave similar results. In KNRK–PAR-2 cells, PAR-2 was detected at the plasma membrane, in the Golgi apparatus, and in vesicles (Fig. 2A, refs. 2 and 19). In hBRIE cells grown to confluence on filters, PAR-2 was detected at the apical and basolateral membrane, in the Golgi apparatus, and in vesicles (Fig. 2B). PAR-2 was prominently localized to enterocytes of the villi and crypts of Lieberkühn of the small intestine, where it was present at the plasma membrane and in an intracellular compartment (Fig. 2 C and E–G). This compartment was stained with an antibody to mannosidase II, and is thus the Golgi apparatus (19). PAR-2 was detected at the apical and basolateral membranes of enterocytes in the crypts (Fig. 2 C and E), and villi (Fig. 2 F and G). PAR-2 was found at the plasma membrane of myocytes in muscularis externa and muscularis mucosa (Fig. 2H). Staining was abolished by preabsorption of antisera with 1–10 μM of the receptor fragments (Fig. 2D).
Figure 2.
Localization of PAR-2 by immunofluorescence and confocal microscopy. (A) KNRK–PAR-2 cells (arrowheads, plasma membrane; arrow, Golgi apparatus). (B) Section of hBRIE cells on filter (arrowheads, apical membrane; asterisk, filter). (C and D) Jejunum muscle and mucosa (arrowheads, apical membrane of enterocytes; arrows, Golgi apparatus). (E) Transverse section through jejunal crypt (arrowhead, apical membrane of enterocytes; arrows, Golgi apparatus; asterisk, lumen). (F) Duodenum villus. (G) Jejunum, villus tip (for F and G, arrowheads, apical membrane of enterocytes; arrows, basolateral membrane of enterocytes). (H) Jejunum muscle (arrowhead, muscularis mucosa; arrows, muscularis externa). PAR-2 was localized using antiserum B5 (1:1,000, 24 h, 4°C; A and C–F) or PAR-2 C (2–4 μg/ml, 24 h, 4°C; B, F, and G). D is a control with B5 preabsorbed with 10 μM receptor fragment. Images are composites of two to three optical sections at 0.5–1.0 μm. [Bar = 7 μm (A), 5 μm (B), 25 μm (C and D), 8 μm (E–G), 10 μm (H).]
InsP3 Generation.
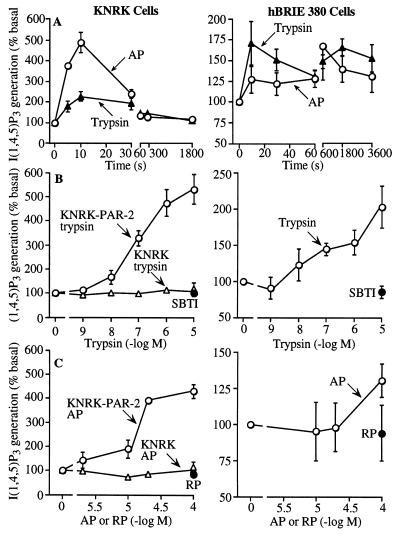
Trypsin (Worthington) and activating peptide corresponding to the tethered ligand of rat PAR-2 (AP, SLIGRL-NH2) stimulated InsP3 generation in KNRK–PAR-2 and hBRIE cells, which peaked within 10 s (Fig. 3A). Trypsin was considerably more potent and slightly more efficacious than AP, and trypsin that was inactivated by preincubation with 10 mg/ml SBTI for 60 min and reverse peptide (RP, LRGILS-NH2) had no effect (Fig. 3 B and C). Trypsin and AP had no effect in untransfected KNRK cells (Fig. 3 B and C). The potency of trypsin was similar in KNRK–PAR-2 and hBRIE cells.
Figure 3.
InsP3 generation by KNRK–PAR-2, untransfected KNRK, and hBRIE cells. (A) Time course to 100 nM trypsin and 100 μM AP. (B and C) Effects of graded concentrations of trypsin and AP. SBTI-inhibited trypsin and RP were assayed in KNRK–PAR-2 and hBRIE cells. Concentration response curves were generated at 10 s for KNRK–PAR-2 and KNRK cells, and at 30 min in hBRIE cells in the presence of LiCl. Results are expressed as % basal generation (n = 3–4 experiments).
Arachidonic Acid Release.
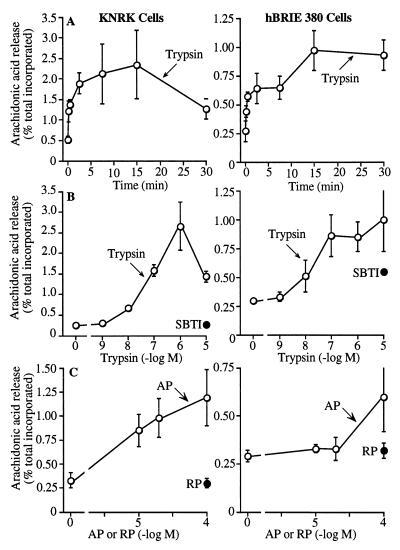
Trypsin and AP stimulated maximal release of radioactivity from KNRK–PAR-2 and hBRIE cells loaded with [3H]arachidonic acid after 15 min (Fig. 4A). Trypsin was more potent and efficacious than AP (Fig. 4 B and C). SBTI-treated trypsin and RP had no effect. The potency of trypsin was similar in KNRK–PAR-2 and hBRIE cells, and was comparable to the potency for stimulation of InsP3 generation.
Figure 4.
Radioactivity release from KNRK–PAR-2 and hBRIE cells loaded with [3H]arachidonic acid. (A) Time course to 100 nM trypsin. (B and C) Effects of graded concentrations of trypsin and AP. SBTI-inhibited trypsin and RP were assayed in KNRK–PAR-2 and hBRIE cells. Concentration response curves were generated at 30 min. Results are expressed as % total incorporation of [3H]arachidonic acid (n = 3–4 experiments).
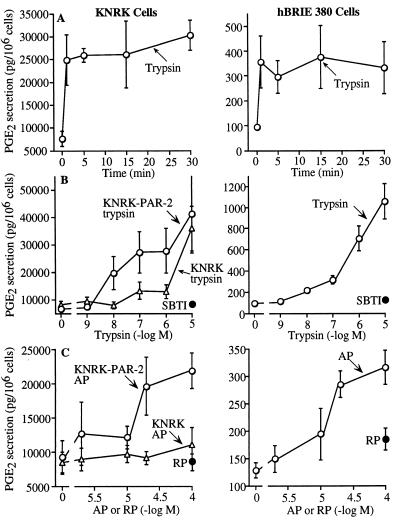
PG Secretion.
PGE2 and PGF1α are important paracrine agents in the intestine (16). Basal PGE2 secretion was far higher from KNRK–PAR-2 than hBRIE cells, but trypsin and AP stimulated a greater fold increase in secretion from hBRIE cells. PGE2 secretion was maximal at 5–15 min (Fig. 5A), and trypsin was more potent and efficacious than AP (Fig. 5 B and C). SBTI-treated trypsin and RP had no effect. In untransfected KNRK cells, trypsin did not stimulate PGE2 release except at high concentrations (10 μM) and AP had no effect. The potency of trypsin was similar in KNRK–PAR-2 and hBRIE cells and was comparable to the potency for stimulation of InsP3 generation and arachidonic acid release. In KNRK–PAR-2 cells, basal PGF1α secretion (pg PGF1α/106 cells) was 716 ± 133, and 1 μM trypsin and 100 μM AP stimulated secretion to 1,757 ± 83 and 1,193 ± 51 at 15 min, respectively. In hBRIE cells, basal PGF1α secretion was 777 ± 85, and 1 μM trypsin and 100 μM AP stimulated secretion to 1,998 ± 324 and 1,677 ± 371 at 15 min, respectively.
Figure 5.
PGE2 secretion by KNRK–PAR-2, untransfected KNRK, and hBRIE cells. (A) Time course to 100 nM trypsin. (B and C) Effects of graded concentrations of trypsin and AP. SBTI-inhibited trypsin and RP were assayed in KNRK–PAR-2 and hBRIE cells. Concentration response curves were generated at 15 or 30 min. Results are expressed as pg of PGE2 secreted per 106 cells (n = 3–4 experiments).
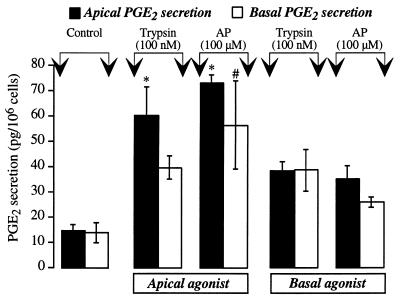
Trypsin and AP were applied to the apical or basal surface of hBRIE cells on filters, and PGE2 secretion was measured to provide functional evidence for localization of PAR-2 at the apical or basolateral membrane. Application of 100 nM trypsin or 100 μM AP to the apical membrane for 30 min stimulated a >4-fold secretion of PGE2 into the apical solution and a >2-fold secretion into the basal solution (Fig. 6). Basal application of trypsin and AP stimulated a >2-fold increase in PGE2 secretion into apical and basal solutions.
Figure 6.
PGE2 secretion by hBRIE cells grown on filters. Trypsin, AP, or carrier (control) were applied to the apical or basal membranes. After 30 min, the apical and basal solutions were removed and PGE2 was measured. Results are expressed as pg PGE2 secreted per 106 cells (n = 3 experiments). ∗ and #, P < 0.05 compared with apical and basal controls.
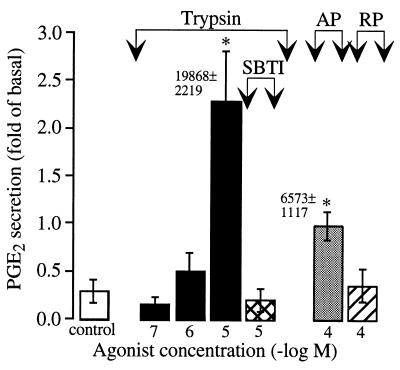
To determine if luminal trypsin stimulated PGE2 secretion from the intact intestine, trypsin and AP were applied to the mucosal surface of everted sacs of jejunum and PGE2 secretion into the mucosal solution was measured. Application of 10 μM trypsin or 100 μM AP to the mucosa for 30 min stimulated PGE2 secretion by >7-fold and >3-fold over basal levels, respectively (Fig. 7). SBTI-treated trypsin and RP had no effect.
Figure 7.
PGE2 secretion by everted sacs of rat jejunum. Everted sacs of jejunum were incubated in buffer for 10 min (basal), and trypsin, AP, RP, or carrier (control) were added for 30 min. Results are expressed as the fold increase in secretion above basal (n = 4–13 rats). ∗, P < 0.05 compared with controls. Numbers indicate increase over basal in PGE2 secretion per gram of tissue to 10 μM trypsin and 100 μM AP.
DISCUSSION
Trypsin, at concentrations normally present in the intestinal lumen, activates PAR-2 at the apical membrane of enterocytes and stimulates the generation of eicosanoids, which may influence multiple functions of the intestine. Our results show that PAR-2 is highly expressed by enterocytes, where it is found at the apical and basolateral membranes. Trypsin activates PAR-2 in enterocytes to stimulate InsP3 generation, arachidonic acid release, and PG secretion. PGs act in a paracrine and autocrine manner in the intestine to regulate multiple processes (16). Thus, luminal trypsin is a signaling molecule that specifically regulates enterocytes by activating PAR-2.
Several observations indicate that PAR-2 is expressed by enterocytes. First, we detected abundant PAR-2 mRNA in the intestinal mucosa by Northern blot analysis. In support of this observation, we previously localized PAR-2 mRNA to enterocytes by in situ hybridization (2). Second, we localized immunoreactive PAR-2 to the apical and basolateral membranes and the Golgi apparatus of enterocytes in the villi and crypts of small intestine. PAR-2 was also detected at the apical and basolateral membranes of hBRIE cells grown on filters under conditions that permit polarized growth and formation of tight junctions (20), which we verified by microscopy. We have previously detected epitope-tagged PAR-2 at the plasma membrane and Golgi apparatus of KNRK–PAR-2 cells (2, 19), which supports our current results. Staining of cells and tissues was abolished by preincubation of antisera with fragments used for immunization, confirming specificity. Luminal trypsin may activate PAR-2 at the apical membrane of enterocytes, or at the basolateral membrane if the epithelium is damaged. Trypsin induces internalization and degradation of PAR-2 in lysosomes, and the Golgi pool is required to replenish PAR-2 at the plasma membrane and permit cells to maintain responsiveness to repeated stimulation by trypsin (19). The prominent Golgi store of PAR-2 may permit enterocytes to recover their capacity to respond to trypsin between meals. Finally, trypsin and AP stimulated InsP3 generation, arachidonic acid release, and PG secretion from enterocytes, confirming expression of functional PAR-2. The findings that application of trypsin and AP to the apical membrane of hBRIE cells on filters and to mucosal surface of everted sacs of intestine stimulated PGE2 secretion provides functional evidence for the presence of PAR-2 at the apical membrane of enterocytes.
Trypsin and AP stimulated InsP3 generation, arachidonic acid release and PG secretion in KNRK–PAR-2 and hBRIE cells. Trypsin and AP mobilize Ca2+ in many cell lines (2, 7, 8, 10). In support of our results is the report that trypsin stimulates PG secretion from hepatocytes by a G-protein-coupled receptor (11) that is probably PAR-2. Activation of PAR-1 also stimulates arachidonic acid release and eicosanoid secretion (12–14). The release of arachidonic acid is important because its availability is the rate-limiting step in eicosanoid generation (16). PAR-2 may activate cPLA2, which releases arachidonic acid from membrane phospholipids. A Ca2+-dependent membrane-binding domain targets cPLA2 to its substrates in the plasma membrane (14, 23), and protein kinase C and mitogen-activated protein kinase phosphorylate and activate cPLA2 (14, 24, 25). PAR-2 increases intracellular Ca2+ concentration and stimulates protein kinase C and mitogen-activated protein kinase in enterocytes (ref. 19, N.W.B., unpublished data), suggesting a mechanism for the activation of cPLA2. The rapidity with which PG secretion follows activation of PAR-2 suggests that PG generation depends on cyclooxygenase 1, rather than inducible cyclooxygenase 2 (16).
Several observations indicate that the responses to trypsin are mediated by PAR-2. First, trypsin did not stimulate InsP3 generation or arachidonic acid release in untransfected KNRK cells. Only very high concentrations of trypsin stimulated PG secretion from these cells, probably due to the low levels of endogenous PAR-2 expression. Second, all of the effects of trypsin on cell lines and the everted sacs were mimicked by PAR-2 AP. We cannot be sure that AP only activates PAR-2, but it does not activate the PAR-1 (26). Trypsin was usually more potent and efficacious than AP, as it is in other systems (1, 2). Thrombin is also more potent and sometimes more efficacious than peptides corresponding to the PAR-1-tethered ligand (3, 27). The reason for these differences is not known, but it could be explained by a more favorable presentation of a tethered ligand than the free peptide to the receptor binding site. AP may also be degraded by enterocyte proteases. Aminopeptidases rapidly inactivate PAR-1 AP (28), and the N-terminal Ser is critical for activity of PAR-2 AP (26). Trypsin may also activate a receptor that does not interact with AP.
The concentration of trypsin in pancreatic juice (≈40 μM) and intestinal fluid (≈1–10 μM) exceeds that required to activate PAR-2 in cell lines (≈10 nM) (17, 18). Higher trypsin concentrations (10 μM) were required to stimulate PG secretion from everted sacs, although this is still in the physiological range. This may be due to the presence of mucus, which coats the epithelium and protects it from proteases. Thus, activation by trypsin may be more effective if the mucus layer is disrupted. The production of trypsin inhibitors, and the fact that PGs measured in the organ bath probably underestimate concentrations in the extracellular fluid, may also explain the lack of effects of lower trypsin concentrations.
PAR-2 was also detected at the basolateral membranes of enterocytes and in myocytes. PAR-2 is also expressed in gastric muscle (9). Enzymes other than trypsin probably activate PAR-2 in these locations. One possibility is mast cell tryptase, which activates PAR-2 in several cell types, including enterocytes and myocytes (ref. 5, N.W.B., unpublished data). Mast cells containing tryptase are normally found in the intestine, and their number increases during inflammation (29), suggesting that PAR-2 mediates some effects of mast cell proteases. PAR-2 is up-regulated by interleukin 1α and tumor necrosis factor, supporting its role in inflammation (30).
We conclude that pancreatic trypsin activates PAR-2 at the apical membrane of enterocytes and regulates the intestinal epithelium. Thus, in addition to its digestive function, trypsin should also be considered an intestinal signaling molecule that specifically regulates enterocytes through PAR-2.
Acknowledgments
We thank S. Mokashi for the PAR-2 B5 antibody and Dr. B. Al-Ani for rat PAR-2 cDNA. This work was supported by the National Institutes of Health and the Medical Research Council of Canada.
ABBREVIATIONS
- PAR
proteinase-activated receptor
- AP
rat activating peptide (SLIGRL-NH2)
- RP
reverse peptide (LRGILS-NH2)
- InsP3
inositol 1,4,5-trisphosphate
- cPLA2
cytoplasmic phospholipase A2
- PG
prostaglandin
- KNRK
sarcoma virus transformed rat kidney epithelial cells
- SBTI
soybean trypsin inhibitor
References
- 1.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Proc Natl Acad Sci USA. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Böhm S K, Kong W, Brömme D, Smeekens S P, Anderson D C, Connolly A, Kahn M, Nelken N A, Coughlin S R, Payan D G, Bunnett N W. Biochem J. 1996;314:1009–1016. doi: 10.1042/bj3141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vu T K, Hung D T, Wheaton V I, Coughlin S R. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 4.Ishihara H, Connolly A, Zeng D, Kahn M, Zheng Y, Timmons C, Tram T, Coughlin S. Nature (London) 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- 5.Molino M, Barnathan E S, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie J, Schechter N, Woolkalis M, Brass L F. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 6.Grand R J, Turnell A S, Grabham P W. Biochem J. 1996;313:353–368. doi: 10.1042/bj3130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santulli R J, Derian C K, Darrow A L, Tomko K A, Eckardt A J, Seiberg M, Scarborough R M, Andrade-Gordon P. Proc Natl Acad Sci USA. 1995;92:9151–9155. doi: 10.1073/pnas.92.20.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwa J J, Ghibaudi L, Williams P, Chintala M, Zhang R, Chatterjee M, Sybertz E. Circ Res. 1996;78:581–588. doi: 10.1161/01.res.78.4.581. [DOI] [PubMed] [Google Scholar]
- 9.Saifeddine M, Al-Ani B, Cheng C, Wang L, Hollenberg M. Br J Pharmacol. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mari B, Guerin S, Far D F, Breitmayer J P, Belhacene N, Peyron J F, Rossi B, Auberger P. FASEB J. 1996;10:309–316. doi: 10.1096/fasebj.10.2.8641564. [DOI] [PubMed] [Google Scholar]
- 11.Levine L. Prostaglandins. 1994;47:437–449. doi: 10.1016/0090-6980(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 12.Bills T K, Smith J B, Silver M J. J Clin Invest. 1977;60:1–6. doi: 10.1172/JCI108745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weksler B B, Ley C W, Jaffe E A. J Clin Invest. 1978;63:923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer R, Roberts E, Manetta J, Hyslop P, Jakubowski J. J Biol Chem. 1993;268:26796–26804. [PubMed] [Google Scholar]
- 15.Bartoli F, Lin H, Ghomashchi F, Gelb M, Jain M, Apitz-Castro R. J Biol Chem. 1994;269:15625–15630. [PubMed] [Google Scholar]
- 16.Eberhart C, Dubois R. Gastroenterology. 1995;109:285–301. doi: 10.1016/0016-5085(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 17.Green G, Nasset E S. Gastroenterology. 1980;79:695–702. [PubMed] [Google Scholar]
- 18.Miyasaka K, Green G M. Gastroenterology. 1984;86:114–119. [PubMed] [Google Scholar]
- 19.Böhm S K, Khitin L M, Grady E F, Aponte G, Payan D G, Bunnett N W. J Biol Chem. 1996;271:22003–22016. doi: 10.1074/jbc.271.36.22003. [DOI] [PubMed] [Google Scholar]
- 20.Aponte G W, Keddie A, Hallden G, Hess R, Link P. Proc Natl Acad Sci USA. 1991;88:5282–5286. doi: 10.1073/pnas.88.12.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grady E F, Gamp P D, Baluk P, McDonald D M, Payan D G, Bunnett N W. Neuroscience. 1996;16:1239–1254. doi: 10.1016/0306-4522(96)00357-0. [DOI] [PubMed] [Google Scholar]
- 22.Garland A M, Grady E F, Lovett M, Vigna S R, Frucht M M, Krause J E, Bunnett N W. Mol Pharmacol. 1996;49:438–446. [PubMed] [Google Scholar]
- 23.Clark J, Lin L, Kriz R, Ramesha C, Sultzman L, Lin A, Milona N, Knopf J. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 24.Parker J, Daniel L, Waite M. J Biol Chem. 1987;262:5385–5393. [PubMed] [Google Scholar]
- 25.Lin L, Wartmann M, Lin A, Knopf J, Seth A, Davis R. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 26.Blackhart B D, Emilsson K, Nguyen D, Teng W, Martelli A J, Nystedt S, Sundelin J, Scarborough R M. J Biol Chem. 1996;271:16466–16471. doi: 10.1074/jbc.271.28.16466. [DOI] [PubMed] [Google Scholar]
- 27.Kramer R M, Roberts E F, Hyslop P A, Utterback B G, Hui K Y, Jakubowski J A. J Biol Chem. 1995;270:14816–14823. doi: 10.1074/jbc.270.24.14816. [DOI] [PubMed] [Google Scholar]
- 28.Coller B S, Ward P, Ceruso M, Scudder L E, Springer K, Kutok J, Prestwich G D. Biochemistry. 1992;31:11713–11720. doi: 10.1021/bi00162a007. [DOI] [PubMed] [Google Scholar]
- 29.Castro G, Powell D. In: Physiology of the Gastrointestinal Tract. Johnson L R, editor. New York: Raven; 1994. pp. 709–750. [Google Scholar]
- 30.Nystedt S, Ramakrishnan V, Sundelin J. J Biol Chem. 1996;271:14910–14915. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]